Abstract
Objectives
To test whether napping was associated with two inflammatory markers with known relationships to cardiovascular disease: high sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6). Because IL-6 is known to impact central inflammatory processes that relate to sleep regulation, including subjective fatigue, we tested whether this relationship was moderated by sleep duration, sleep efficiency, and self-reported sleep quality.
Design
Cross-sectional.
Participants
A community sample of black and white men (N=253) completed a week of actigraphy and diary measures of sleep and napping and provided a fasting blood sample.
Measurements/Analysis
Napping was measured as the proportion of days with at least 30 minutes napped and the average minutes napped per day. Linear regressions adjusted for race, socioeconomic status, employment, BMI, smoking, medications that affect sleep or inflammation, working the nightshift, and day-sleeping status, followed by interaction terms between napping and sleep duration, efficiency, and quality, respectively.
Results
There were no significant main effects of actigraphy- or diary-measured napping on IL-6 or hsCRP. Moderation analyses indicated elevated IL-6 values among men who napped more days (by actigraphy) and demonstrated short sleep duration (p=.03). Moderation analyses also indicated elevated IL-6 among men who demonstrated greater average minutes napped (by actigraphy) and short sleep duration (p<.001), low efficiency (p=.03), and poor quality (p=.03). Moderation analyses involving diary napping or hsCRP were not significant.
Conclusions
Actigraphy-assessed daytime napping is related to higher IL-6 in men who demonstrate worse sleep characteristics. Daytime napping may pose additional risk for inflammation, beyond the known risk conferred by short sleep.
Keywords: napping, fatigue, sleep duration, inflammation, men
Introduction
Sleep characteristics are associated with cardiovascular morbidity and mortality in epidemiological studies of adults. Meta-analyses support associations between self-reported short sleep and increased risk for morbidity and mortality from coronary heart disease and stroke (1), as well as all-cause mortality (2,3). Additionally, meta-analytic evidence demonstrates positive associations between self-reported daily daytime napping and increased risk of all-cause mortality (4–6) and risk of cardiovascular disease (4).
Inflammation is an important process in the etiology and pathogenesis of CVD (7,8). Common markers of inflammation include C-reactive protein (CRP), an acute phase reactant marker of inflammation synthesized in hepatocytes (9), and interleukin (IL)-6, a proinflammatory cytokine that plays a role in coordinating local and systemic inflammatory responses (10). Circulating concentrations of CRP (11) and IL-6 (12) are associated with atherosclerosis, a disease process involving the accumulation of plaque within artery walls and resulting obstruction of blood flow, which may result in clinical CVD events (e.g., heart attack, stroke) (13). High-sensitivity CRP (hsCRP) prospectively predicts cardiovascular events in both healthy subjects and those with coronary disease (14), while IL-6 is associated with risk of coronary heart disease (CHD), particularly nonfatal myocardial infarction and fatal CHD (15).
Moreover, inflammation is increasingly studied in relation to nocturnal sleep in community samples. A recent meta-analysis found associations between continuous measures of actigraphy- or polysomography-assessed short sleep with elevated IL-6, but not with CRP (16). However, comparisons of short sleep duration (< 7 hours) versus a “normal” (as defined by the study authors) sleep reference (7 to 8 hours) showed no association with IL-6 or CRP (16). In contrast, long sleep duration (> 8 hours) compared to normal sleep was associated with higher CRP and IL-6 (16). Overall, the extant literature suggests associations between both short and long sleep duration with inflammatory markers, although results depend on how sleep duration is measured. Taken together, these associations mirror the literature on sleep and other indicators of poor health, such as diabetes (17) and depressive symptoms (18).
One behavioral response to short sleep is daytime napping. However, there is limited evidence concerning associations between napping and inflammation. In older adults, one study reported a positive association between self-reported daily napping and CRP, particularly among those who reported spending either the fewest or the most hours in bed at night (19); however, a second study reported no association between self-reported napping and circulating levels of IL-6 (20). In young adults (mean age = 29 years), evidence suggested a positive association between self-reported nap frequency across the past week and CRP, particularly among those who napped every day and slept short (< 5 hours) at night (21). Finally, in a sample of healthy high school students, more actigraphy-assessed proportion of days napped across a weeklong study period was associated with elevated serum IL-6 but not CRP; though, notably, almost all students in this study had short nocturnal sleep (22). In summary, the available evidence largely suggests a positive relationship between napping and inflammation, and the possibility that short sleep may potentiate the effects of napping on inflammatory outcomes. However, there are few studies in this area and current evidence is clearly limited by a lack of objective measurement of napping.
The current study investigated the association between napping and inflammation in a sample of Black and White men in their early 30’s. There were two main objectives. First, we assessed associations between napping frequency and duration with CRP and IL-6, two widely-examined markers of inflammation that are associated with the development of CVD. Second, because IL-6 is known to impact central inflammatory processes that relate to sleep regulation, including subjective fatigue (10), we tested whether the relationship between napping and inflammation was stronger among men who reported poorer subjective sleep quality and experienced shorter or less efficient nocturnal sleep. We hypothesized that (1) greater napping would be associated with elevated inflammatory markers, and (2) associations between napping and inflammatory markers, particularly IL-6, would be stronger among those with shorter sleep duration, lower sleep efficiency, and poorer subjective sleep quality. Finally, given meta-analytic evidence that blacks demonstrate shorter and more fragmented sleep using both polysomography and self-report measures (23), we conducted exploratory analyses to examine whether relationships between napping and inflammation varied by race.
Methods
Participants
Data for the current study came from the population-based Pittsburgh Youth Study (PYS). Initiated in 1987, PYS is an ongoing study of Black and White men originally recruited from Pittsburgh Public schools when they were in either first or seventh grade. Approximately half the sample was recruited based on a screen for risk for anti-social behavior. Further information about the PYS is available elsewhere (24).
A subsample from the original PYS study was recruited to participate in the Pathways to Healthy Hearts Study. Men were ineligible if they were: incarcerated, lived more than 75 miles away from Pittsburgh and were not planning to return, were severely mentally disabled, had withdrawn from the original PYS study, or were deceased. Recruitment began with the younger cohort. Of the 322 eligible men in the younger cohort, 272 participated in all or some portion of the study. Because the target sample size was 300 men for the measurement of cardiovascular risk, we recruited 40 additional participants from potentially eligible men in the older cohort. Overall, 307 men provided a fasting blood draw and completed a health behavior interview, and of those, 285 participated in the sleep protocol (n=37 were from the older cohort). This study was approved by the Institutional Review Board at the University of Pittsburgh and all participants provided informed consent. The analytic sample was similar to both the younger cohort and the potentially eligible men from the older cohort in proportion of Blacks and high risk screening group (ps>.17).
Overview of Procedure
Participants were scheduled to complete a laboratory visit and a seven-day sleep and daily diary protocol. Participants had a venous blood draw in a recumbent position. The blood draw was performed in the morning by trained personnel after verifying that participants had been fasting for at least eight hours, had not used tobacco products or engaged in strenuous physical activity for at least three hours, and had not taken medications for infectious disease or used any illicit drugs (i.e., marijuana) for at least 24 hours. Participants who reported having a cold, flu, or allergies within three days of the blood draw were rescheduled. For the sleep assessment, participants wore an actigraph continuously for seven days and nights. They completed handwritten diaries each morning and evening to report sleep duration and napping, respectively. Days on which the participants reported feeling ill during the sleep assessment were removed from analyses (< 1% of total study days across all participants). The blood draw occurred for 93.2% of the sample on the first day of the sleep protocol, and for 3.6% during the week of the sleep protocol. The remaining 3.2% (n=8) of the sample completed the blood draw more than one week before the sleep protocol (range = 9 to 46 days prior) due to actigraph malfunction or participant non-compliance, and subsequent participant agreement to re-do the sleep protocol.
Measures
Actigraphy
Actigraph devices are worn on the non-dominant wrist and record movements/accelerations; using activity counts and diary records of bedtime and wake time, periods of sleep and wake can be estimated. The Mini-mitter actiwatch models AW-16, AW-64 and Actiwatch 2 (Phillips Respironics, Bend, OR) were used to collect actigraphy data continuously over seven days and nights. Stored data were downloaded into the Actiware software program (version 5.61) for processing and analysis. The watches were configured to collect data over a 1-minute epoch. The medium threshold (default) was selected to detect one major sleep period of at least 3 hours in duration, and as many minor sleep periods as occurred, based upon sleep onset and offset using the 10-minute criterion. Sleep periods occurring within 30 minutes of the major (generally nocturnal) sleep interval (either 30 minutes prior to sleeping or after waking) were combined with the major sleep interval. All subsequent sleep variables were then calculated from data within these set rest periods. Sleep duration was calculated as the time (in hours) spent asleep between sleep onset and sleep offset, excluding periods of wakefulness throughout the night. A measure of duration was calculated for the one major sleep period, as well as for the sum of all minor sleep periods (naps) across each twenty-four hour day. The procedure for calculating duration was identical for participants whose major sleep period regularly occurred during the day (i.e., after 5 a.m.). Sleep efficiency was calculated as the percentage of time attempting to sleep that was actually spent asleep, such that higher values reflect greater efficiency. The actiwatch has been used extensively in research studies, and has been validated against polysomnography measures for nocturnal sleep episodes (25,26).
Given that there is no accepted criterion for scoring actigraphy-assessed naps, we designated periods of napping (in minutes) by the same criterion used to determine sleep periods, i.e., ten minutes of quiescence. Actigraphy-assessed daytime naps were detected using a software algorithm that ‘automatically’ creates minor rest intervals based on when the participant appears to be napping (i.e., participants’ reports were not used to set rest interval parameters). The program searched for minor rest intervals that were 10 minutes or longer in duration. In a comparison of naps of at least 15 minutes in duration measured concurrently by actigraphy (using the medium threshold setting) and by polysomnography (PSG) in healthy adults aged 18 to 35 years, results suggested good accuracy, sensitivity, and specificity for the detection of nap and non-nap (resting wake) periods (27); the accuracy for nap and non-nap periods, respectively, was 85% and 77%. Two napping variables were used in primary analyses: 1) proportion of days napped across the study period (i.e., total number of days when they napped at least 30 minutes or more, divided by number of days with actigraphy data) and 2) average minutes napped per day across the study period (i.e., total number of minutes napped across the study period divided by the number of days with actigraphy data). The average minutes napped per day variable was positively skewed and underwent square root transformation prior to analysis.
Daily diary
Participants completed sleep diaries each morning after awakening and each night before going to bed. In the morning diary, participants were asked to report the time they got into bed the previous night, the time they actually tried to go to sleep, perceived number of minutes it took them to fall asleep, wake time, time they got out of bed to start their day, number and total duration of awakenings after sleep, experience of pain/discomfort during sleep, and sleep quality. The times reflecting when the participant went to sleep and awoke were used to compute a diary-reported sleep duration variable, excluding minutes of wake after sleep onset. They reported sleep quality by responding to the question: “How would you rate the quality of your sleep last night?” using a five-point scale (0=very poor to 4=very good). In the evening diary, participants were asked to respond to the prompt: “Today, I napped for a total of (blank) minutes.” Diary-reported naps were not confirmed by actigraphy, which is consistent with the extant literature on napping, which relies solely on self-report of napping and sleep. Diary-reported variables were calculated consistent with actigraphy measures reported above: proportion of days with at least 30 minutes of napping and average minutes napped per day. Both variables were positively skewed and underwent square root transformation prior to analysis.
High sensitivity C-reactive protein (hsCRP)
CRP was measured turbidimetrically by measuring increased absorbance when the CRP in the sample reacts with anti-CRP antibodies. The intra- and inter-assay coefficients of variation were 5.5% and 3.0%, respectively. Due to skewness, hs-CRP was log transformed after adding 1.
Interleukin-6 (IL-6)
IL-6 was determined using a high-sensitivity enzyme-linked immunosorbent assay (ELISA). The intra- and inter-assay coefficients of variation were 6.6% and 4.9%, respectively. Due to skewness, IL-6 was log transformed after adding 1.
Covariates
Participants reported race (black = −0.5, white = 0.5), employment status (0=not currently employed, 1=working part-time and/or full-time), and nightshift worker status, defined as working between 11 p.m. and 6 a.m. (0=no, 1=yes). Socioeconomic status (SES) was measured via the two-factor Hollingshead index (1975), which incorporates self-reported educational attainment and occupational status. Participants reported their current medications, which were coded for whether they affected sleep or inflammation (for each type of medication: 0=no medication, 1=currently taking medication), and reported whether they smoked cigarettes in the past year (0=no, 1=yes). Participants were also coded as day-sleepers if their major sleep period regularly occurred after 5 a.m. (0=no, 1=yes). Staff obtained measures of height and weight, which were used to calculate body mass index (BMI); due to skewness, BMI underwent log transformation prior to analysis.
Analytic Plan
Of the 307 men who participated in the laboratory protocol, 44 men were excluded for one or a combination of the following reasons: participant was blind (n=1), reported extremely late wake times (i.e., woke up after 3 p.m.; n=2), treated apnea (n=6), demonstrated hsCRP values > 10 mg/dl prior to log transformation (n=17); reported being sick within three days of the blood draw (n=17) or missing blood draw data (n=1). An additional 10 men were removed from diary analyses for having fewer than four days of diary data, and 18 men were removed from actigraphy analyses due to missing actigraphy data or equipment malfunction (n=10) or having fewer than four days of actigraphy data (n=8). Thus, the final analytic sample included 253 and 245 men in analyses involving diary or actigraphy, respectively; see Table 1 for characteristics of the full analytic sample.
Table 1.
Sample Characteristics by Race
| N | Full Sample | Black (n = 138) | White (n = 115) | |
|---|---|---|---|---|
| Age, years; M (SD) | 253 | 33.0 (2.6) | 33.2 (2.6) | 32.9 (2.5) |
| Socioeconomic status, M (SD)a | 251 | 31.3 (14.3) | 28.5 (13.8) | 34.7 (14.1) |
| Employed (at least part time), N (%) | 253 | 199 (78.7%) | 105 (76.1%) | 94 (81.7%) |
| Smokers in past year, N (%) | 253 | 143 (56.5%) | 84 (60.9%) | 59 (51.3%) |
| Taking sleep medications, N (%)a | 253 | 35 (13.8%) | 12 (8.7%) | 23 (20.0%) |
| Taking anti-inflammatory medications, N (%) | 253 | 9 (3.6%) | 4 (2.9%) | 5 (4.3%) |
| Nightshift worker, N (%) | 253 | 18 (7.1%) | 8 (5.8%) | 10 (8.7%) |
| Daysleeper, N (%) | 253 | 18 (7.1%) | 10 (7.2%) | 8 (7.0%) |
| Actigraphy Measures | ||||
| Sleep duration, hours; M (SD)a | 243 | 5.9 (1.2) | 5.5 (1.1) | 6.3 (1.2) |
| Sleep efficiency %; M (SD)a | 243 | 79.2 (6.9) | 78.0 (7.5) | 80.6 (5.9) |
| Proportion of days napped; M (SD) | 243 | .32 (.24) | .32 (.24) | .31 (.24) |
| Average minutes napped per day; Mdn (IQR)b | 243 | 25.0 (10.0, 45.4) | 27.5 (11.3, 48.4) | 19.3 (8.4, 43.7) |
| Diary Measures | ||||
| Sleep duration, hours; M (SD)a | 253 | 7.2 (1.2) | 6.9 (1.2) | 7.4 (1.2) |
| Sleep quality; M (SD) | 252 | 2.3 (0.6) | 2.4 (.67) | 2.2 (.57) |
| Proportion of days napped; Mdn (IQR)b | 253 | .14 (0.0, .29) | .14 (0.0, .29) | .14 (0.0, 0.29) |
| Average minutes napped per day; Mdn (IQR)b | 253 | 8.6, (0.0, 23.0) | 8.6 (0.59, 26.38) | 7.1 (0.0, 20.0) |
| Cardiovascular Risk Factors | ||||
| BMI; Mdn (IQR)b | 253 | 28.3 (25.2, 32.6) | 28.4 (24.9, 33.0) | 27.9, (25.9, 31.7) |
| hsCRP, mg/L; Mdn (IQR)b | 253 | 1.42 (.61, 2.80) | 1.4 (.55, 2.60) | 1.43 (.70, 3.19) |
| IL-6, pg/mL; Mdn (IQR) b | 253 | 1.49 (1.06, 2.36) | 1.60 (1.10, 2.27) | 1.41 (1.04, 2.55) |
Note. BMI = body mass index; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin 6; IQR = Interquartile Range (25th, 75th percentiles); Mdn = Median. Socioeconomic status is rated on the two-factor Hollingshead scale (38).
Race main effect from ANOVAS or logistic regression, p<.05
Raw values are shown in table: actigraphy-assessed average minutes napped and diary-assessed proportion of days napped and average minutes napped were square root transformed prior to analysis; BMI, hsCRP, and IL-6 values were natural log transformed prior to analyses.
Preliminary analyses examined race differences and unadjusted correlations among sleep, napping, and inflammation variables. Actigraphy-assessed napping and diary-reported napping were treated as independent constructs. Risk score at screening was unrelated to actigraphy- or diary-measured napping and inflammatory outcomes (ps>.12), thus, it was not included as a covariate in primary analyses. For primary analyses, hierarchical linear regressions were conducted to measure associations between inflammatory markers and two separate nap variables: proportion of days with at least 30 minutes napped and average minutes napped; nap variables were measured by actigraphy and daily diary. All primary analyses were analyzed using separate hierarchical regression models adjusting for race, BMI, smoking, SES, employment status, use of medications known to affect sleep or inflammation, night shift workers, and day-sleepers (Step 1), followed by the nap variable of interest (Step 2). Additional analyses explored moderation of napping and inflammation relationships by sleep duration, sleep efficiency, subjective sleep quality, and BMI, each entered separately into Step 3 of the regression models. In the case of significant moderation, variables were analyzed via the method of Aiken and West (28) for continuous variables. Simple slopes (i.e., associations between napping and inflammation) were examined at values representing top tertile, median, and bottom tertile of the moderating variable. Exploratory analyses examined race differences with interaction terms between race and the nap variable of interest predicting IL-6 and CRP, respectively. P-values were considered statistically significant at p <.05.
Results
Sample Characteristics
The analytic sample was composed of 138 black males and 115 white males, with a mean age of 33 years (Table 1). Approximately 79% of the sample was employed at least part time. As shown in Table 1, participants were overweight (median BMI = 28.3). At the time of the sleep study, 57% of the sample reported smoking cigarettes in the past year, 4% reported current use of anti-inflammatory medications, 14% reported current use of medications that affect sleep, and 7.1% reported being nightshift workers or daysleepers.
Significant race differences emerged for current use of medications that affect sleep, such that more whites reported using these medications, relative to blacks (Table 1). Blacks had lower Hollingshead Index scores than whites. There were no race differences regarding other characteristics.
Sleep and Napping Characteristics
As demonstrated by objective actigraphy measures, men slept on average 5.9 hours (range: 2.2–10.4) across the weeklong study period and demonstrated average sleep efficiency of 79.2% (range: 55.0–92.7). As demonstrated by self-report diary measures, men slept on average 7.2 hours (range: 3.4–11.6) across the weeklong study period, and average sleep quality was between “average” and “good” (M = 2.3; range: 0.9–4.0). There were significant race differences in actigraphy-assessed and self-reported sleep duration across the week, with blacks reporting shorter duration relative to whites. Blacks also demonstrated poorer actigraphy-assessed sleep efficiency, relative to whites. There were no race differences regarding subjective sleep quality.
Napping was a common behavior (Table 1). As demonstrated by actigraphy measures, 95% of men demonstrated at least one nap across the week-long study period (range: 0–29 naps). Men napped approximately 32% of the 7 days sampled (range: 0–100%) and 25.0 minutes per day (range: 0–216.0 minutes). With regard to diary-reported napping, 70% percent of men reported at least one nap in their diary during the weeklong study period (range: 0–8 naps). Men napped approximately 14% of days (range: 0–86%) and an average of 8.6 minutes per day (range: 0–120.0 minutes) by diary self-report. Unlike sleep, no race differences emerged in actigraphy-assessed or diary-reported napping.
Bivariate Correlations
Correlations among sleep, napping, and moderator variables are presented in Table 2. Longer actigraphy-assessed sleep duration was associated with higher sleep efficiency, longer diary-reported sleep duration, and fewer diary-reported days napped and average minutes napped across the week. Longer diary-reported sleep duration and better sleep quality were associated with fewer diary-reported days napped and fewer minutes napped. Diary-reported napping variables were highly correlated. There were moderate correlations among actigraphy- and diary-assessed nap variables (rs = .40-.48, ps <.01).
Table 2.
Bivariate Correlations Among Primary Study Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Actigraphy sleep duration | -- | ||||||||||
| 2. Actigraphy sleep efficiency | .52* | -- | |||||||||
| 3. Actigraphy prop. days napped | −.12 | −.15* | -- | ||||||||
| 4. Actigraphy average minutes napped | −.09 | −.13* | .86* | -- | |||||||
| 5. Diary sleep duration | .54* | −.03 | −.01 | −.04 | -- | ||||||
| 6. Diary good sleep quality | .08 | .09 | −.01 | .02 | .09 | -- | |||||
| 7. Diary prop. days napped | −.23* | −.13* | .40* | .43* | −.21* | −.24* | -- | ||||
| 8. Diary average minutes napped | −.24* | −.15* | .43* | .48* | −.21* | −.17* | .91* | -- | |||
| 9. Race | .30* | .19* | −.03 | −.06 | .20* | −.11 | −.11 | −.09 | -- | ||
| 10. BMI | −.05 | −.02 | −.01 | −.08 | .01 | .06 | −.14* | −.11 | −.02 | -- | |
| 11. IL-6 | −.06 | −.05 | .13* | .14* | −.003 | .09 | −.05 | .01 | −.02 | .26* | -- |
| 12. hsCRP | −.02 | −.01 | −.03 | −.03 | .01 | .06 | −.04 | −.03 | .09 | .45* | .46* |
Note. BMI = body mass index; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin 6; Prop. days napped = proportion of days with at least one nap of 30 minutes or more.
Proportion of days napped and average minutes napped (by actigraphy and diary measures) were square root transformed prior to analysis; BMI, hsCRP, and IL-6 values were natural log transformed prior to analyses.
p ≤ .05.
Higher IL-6 was associated with more actigraphy-assessed days napped and average minutes napped across the week, but was not related to any other sleep variables. Higher BMI was associated with fewer diary-reported proportion of days napped, but was unrelated to all other sleep or napping variables. hsCRP was not related to any sleep duration or napping variables. IL-6 and hsCRP were moderately correlated with each other (r = .46, p < .001) and with BMI.
Association of Actigraphy-Assessed Napping and Inflammatory Outcomes
As shown in Table 3, there were no significant associations between actigraphy-assessed proportion of days with at least 30 minutes napped or average minutes napped across the seven-day study period and hsCRP or IL-6 (all ps > .56). However, several variables (i.e., sleep duration, efficiency, and quality) emerged as moderators of the association between actigraphy-assessed napping and IL-6, such that each moderator strengthened the positive effect of napping on IL-6 (described below). Additionally, as expected, both BMI (p<.001) and being a smoker (p<.01) were significantly associated with higher IL-6. Finally, the association between actigraphy-assessed napping and IL-6 did not vary by race, and the association between actigraphy-assessed nap variables and hsCRP did not vary by any of the moderators tested (all ps > .38).
Table 3.
Hierarchical Linear Regressions Showing the Contributions of Demographics, BMI, Medications, and Napping to the Prediction of Log Transformed Inflammatory Markers
| Outcome: | Actigraphy | Diary | ||
|---|---|---|---|---|
|
| ||||
| hsCRP | IL-6 | hsCRP | IL-6 | |
| Step 1 | ||||
| (Constant) | .06 (.12) | .23 (.07)* | .08 (.12) | .23 (.07)* |
| Race (−.5=white, .5=black) | .20 (.13) | .00 (.08) | .19 (.13) | .01 (.08) |
| Socioeconomic status | −.01 (.01) | −.01 (.00) | −.01 (.01) | −.01 (.003) |
| Employment (0=no, 1=part-time and/or full-time) | −.18 (.19) | .02 (.11) | −.19 (.19) | .02 (.11) |
| ln BMI (mean centered) | 2.56 (.32)* | .92 (.19)* | 2.40 (.31)* | .88 (.18)* |
| Smoker (0=no, 1=yes) | .25 (.15)† | .28 (.09)* | .22 (.15) | .28 (.09)* |
| Sleep medications (0=no, 1=yes) | .51 (.19)* | .13 (.11) | .49 (.19)* | .13 (.11) |
| Anti-inflammatory medications (0=no, 1=yes) | .44 (.38) | .37 (.23) | .27 (.37) | .35 (.21) |
| Daysleeper (0=no, 1=yes) | .12 (.29) | .20 (.17) | .10 (.30) | .15 (.17) |
| Nightshift worker (0=no, 1=yes) | −.08 (.28) | .11 (.17) | −.03 (.30) | .19 (.17) |
| Step 2 of separate models | ||||
| Proportion of days with at least 30 minutes napped | −.23 (.27) | .24 (.16) | .24 (.22) | .02 (.13) |
| Average minutes napped | −.01 (.02) | .03 (.01)† | .03 (.02) | .01 (.01) |
| Step 3 of separate models (interaction terms) | ||||
| Proportion of days with at least 30 minutes napped | ||||
| X average sleep duration | −.21 (.24) | −.29 (.14)* | −.12 (.17) | −.06 (.10) |
| X average sleep efficiency | −.01 (.04) | −.02 (.02) | −.05 (.03)† | −.03 (.02) |
| X sleep quality | .21 (.46) | −.40 (.27) | −.23 (.35) | −.02 (.20) |
| X BMI | 1.10 (1.31) | .12 (.77) | .72 (.98) | −.66 (.56) |
| X race | −.04 (.53) | .09 (.31) | −.33 (.42) | −.07 (.25) |
| Average minutes napped | ||||
| X average sleep duration | −.02 (.02) | −.04 (.01)* | −.02 (.02) | −.01 (.01) |
| X average sleep efficiency | −.003 (.003) | −.004 (.002)* | −.004 (.004) | −.003 (.002) |
| X sleep quality | .00 (.04) | −.05 (.02)* | −.02 (.03) | −.01 (.02) |
| X BMI | .09 (.12) | −.05 (.07) | .12 (.11) | −.03 (.07) |
| X race | .02 (.05) | .02 (.03) | −.01 (.05) | .001 (.03) |
Note. BMI = body mass index; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin 6.
Values reflect unstandardized coefficient (standard error); Proportion of days napped and average minutes napped (by actigraphy and diary measures) were square root transformed prior to analysis; BMI, hsCRP, and IL-6 values were natural log transformed prior to analyses.
p ≤ .10.
p ≤ .05.
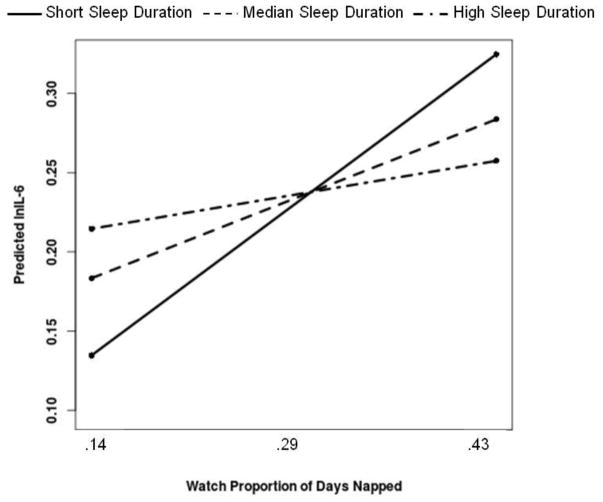
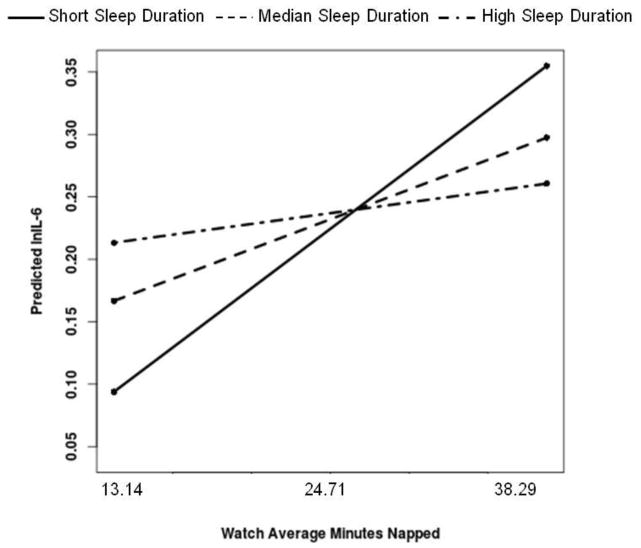
Regarding moderation analyses, actigraphy-assessed sleep duration moderated the association between actigraphy-assessed proportion of days napped and IL-6 [B(SE)=−.29(.14), p=.034]. Simple slope analyses revealed that more days napped was significantly associated with higher IL-6 among men who demonstrated the shortest sleep (bottom tertile=less than 5.30 hours) [ B(SE)=.40(.18), p=.026], but not for men at the median (duration=5.94 hours) [B(SE)=.21(.16), p=.19] or men who demonstrated the longest sleep (top tertile=greater than 6.35 hours) [ B(SE)=.09(.17), p=.61]; see Figure 1. Actigraphy-assessed sleep duration moderated the association between actigraphy-assessed average minutes napped and IL-6 [B(SE)= −.04(.01), p<.001]. Simple slope analyses revealed that more minutes napped was significantly associated with higher IL-6 among men who demonstrated the shortest sleep (bottom tertile) [B(SE)=.05(.02), p<.01], but not for men at the median [B(SE)=.02(.01), p=.08] or men who demonstrated the longest sleep (top tertile) [B(SE)=.01(.01), p=.53]; see Figure 2.
Figure 1.
More actigraphy-assessed days napped is associated with higher IL-6 among men who demonstrate short actigraphy-assessed sleep duration. Both proportion of days napped and sleep duration were treated as continuous variables in the regression equation, but were grouped for graphical purposes. Simple slopes showed that more days napped is significantly associated with higher IL-6 among men in the bottom tertile of sleep duration, but not for men at the median or in the top tertile of sleep duration.
Figure 2.
More actigraphy-assessed minutes napped is associated with higher IL-6 among men who demonstrate short actigraphy-assessed sleep duration. Both average minutes napped and sleep duration were treated as continuous variables in the regression equation, but were grouped for graphical purposes. Simple slopes showed that more minutes napped is significantly associated with higher IL-6 among men in the bottom tertile of sleep duration, but not for men at the median or in the top tertile of sleep duration.
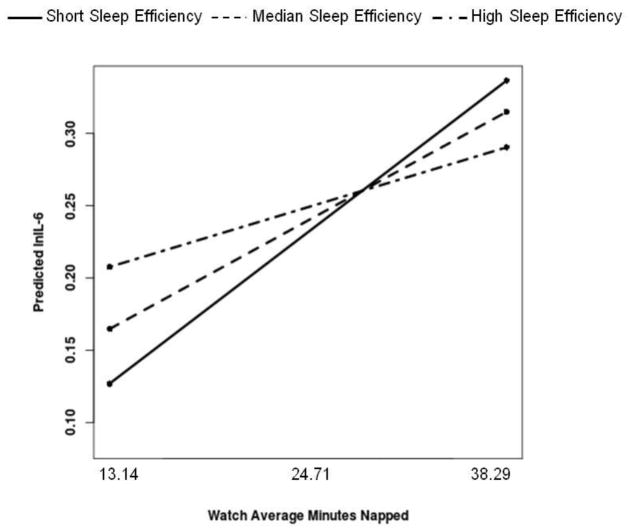
Actigraphy-assessed sleep efficiency moderated the association between actigraphy-assessed average minutes napped and IL-6 [B(SE)= −.004(.002), p=.029]. Simple slope analyses revealed that more minutes napped was significantly associated with higher IL-6 among men with the lowest efficiency (bottom tertile=77.3%) [B(SE)=.04(.02), p<.01] and men at the median of sleep efficiency [efficiency=80.0%) [B(SE)=.03(.01), p=.05], but not for men who demonstrated the highest sleep efficiency (top tertile=83.1%) [ B(SE)=.01(.02), p=.37]; see Figure 3.
Figure 3.
More actigraphy-assessed minutes napped is associated with higher IL-6 among men who demonstrate low actigraphy-assessed sleep efficiency. Both average minutes napped and sleep efficiency were treated as continuous variables in the regression equation, but were grouped for graphical purposes. Simple slopes showed that more minutes napped is associated with higher IL-6 among men in the bottom tertile and at the median of sleep efficiency, but not for men who demonstrated the highest sleep efficiency.
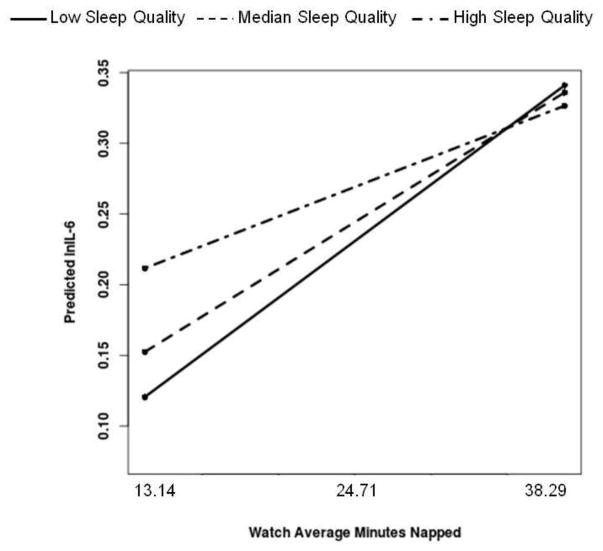
Finally, self-reported sleep quality moderated the association between actigraphy-assessed average minutes napped and IL-6 [B(SE)= −.05(.02), p=.03]. Simple slope analyses revealed that more minutes napped was significantly associated with higher IL-6 among men who demonstrated the poorest sleep quality (bottom tertile=2.00) [B(SE)=.04(.02), p=.009] as well as men at the median of sleep quality (quality=2.14) [B(SE)=.03(.01), p=.02], but not for men who reported the best sleep quality (top tertile = 2.43) [B(SE)=.02(.01), p=.184]; see Figure 4.
Figure 4.
More actigraphy-assessed minutes napped is associated with higher IL-6 among men who demonstrate low diary-reported perceived sleep quality. Both average minutes napped and sleep quality were treated as continuous variables in the regression equation, but were grouped for graphical purposes. Simple slopes showed that more minutes napped is associated with higher IL-6 among men in the bottom tertile and at the median of sleep quality, but not for men who demonstrated the highest sleep quality.
Association of Diary-Reported Napping and Inflammatory Outcomes
As shown in Table 3, there were no significant associations between diary-reported proportion of days with at least 30 minutes napped or the average minutes napped across the study period and hsCRP or IL-6 outcomes (all ps > .26).
The association between diary-reported napping and hsCRP or IL-6 also did not vary by diary-reported short sleep or sleep quality, sleep efficiency (by actigraphy) or race (all ps ≥ .10).
Discussion
This study used actigraphy-assessed and self-report daily diary measures of daytime napping and sleep over one week to evaluate whether napping was associated with elevated inflammatory markers in a community sample of black and white men. We did not find a main effect of napping with inflammation. However, consistent with previous work (21), results revealed that actigraphy-assessed aspects of sleep moderated the association between actigraphy-assessed napping and IL-6. More napping was associated with higher IL-6 among men who had short sleep duration, low sleep efficiency, or poor perceived sleep quality. Together, our results and those of Mantua and Spencer (21) raise the possibility that short sleep may not be “corrected” by napping and it may be better to lengthen sleep duration.
In light of evidence that napping causes disruptions in the sleep-wake cycle (29) and that more napping temporally predicts decreased same-day nocturnal sleep duration, efficiency, and quality (30,31), our data support the hypothesis that the combination of more napping and poor sleep may have multiplicative associations with IL-6 levels. Another way to interpret the data is that men with the shortest or least efficient sleep demonstrated the lowest levels of inflammation, but only if they did not nap throughout the weeklong study period, an unexpected finding that deserves future study. However, this group (short/less efficient sleep + no naps) represents a small portion of the sample and it is unclear how these results would generalize to larger samples, given that the majority (95%) of our sample did demonstrate at least one nap during the study period. Importantly, the pattern of results regarding actigraphy-assessed napping and IL-6 was consistent across race. Finally, we did not find evidence of associations between daytime napping and hsCRP.
There are a number of reasons why napping may be associated with IL-6, but not with hsCRP. As previously described, research suggests that IL-6 is a “sleep factor” and its concentration in peripheral circulation shows circadian variation (32–34). Further, IL-6 also appears to play a role in communicating with the central nervous system (CNS) to result in “sickness behaviors” that include sleep disruption and fatigue (10). In contrast, CRP levels do not show circadian variation (35) and CRP does not cross the blood brain barrier to influence CNS processes, except following brain injury. Thus, although they are both peripheral markers of inflammation, IL-6 in particular is indicated in sleep behaviors and disruption. Given its association with sleep loss and sleepiness, Vgontzas and colleagues (33) have suggested that IL-6 is a marker of sleep need. It is possible that individuals nap when IL-6 levels are highest, particularly during the afternoon, which may explain why IL-6 was associated with actigraphy-assessed napping but not with sleep duration in the current sample.
The present study found that actigraphy, but not diary, measures of napping were associated with inflammatory markers. The present findings are similar to results in older adults, which also did not find an association between self-reported napping and IL-6 (20). Our results are also consistent with data in both adolescents (22) and adults (36) that demonstrate more napping by actigraphy, relative to diary measures. However, our results are not consistent with evidence in younger (21) and older adults (19) that suggest an association between self-reported napping and elevated CRP, perhaps due to differences in self-report measures of napping. Our study averaged daily self-report of napping frequency and duration across one week of sleep diaries, while the aforementioned studies measured napping using single item questions; for example, “How often have you napped in the previous week” (21) and “Do you normally take a nap during the day?” (19). Thus it is possible that these single-item questions reflect habitual napping behavior and are more prone to retrospective bias, while our daily diary measures more accurately reflect the actual behavior of napping.
The present study has several limitations. First, inflammatory markers were measured at one time point; thus, we cannot link within-individual variation in napping to within-individual variation in IL-6 for example. Second, the cross-sectional nature of the study precludes causal inference, and it is certainly possible that the association between napping and IL-6 is bidirectional (10). Third, results may not be generalizable to women. Finally, since actigraphy involves measurement of accelerations (37), we cannot be certain that some periods recorded as “sleep” were not actually very still moments in wake (e.g., watching TV). Thus, it is possible that the aforementioned relationship between actigraphy-assessed napping and IL-6 reflects periods of low activity and IL-6, and consequently our results could reflect broader associations between sedentary behaviors and inflammatory markers. Future studies could use additional measures, such as metabolic equivalents, that may more accurately differentiate periods of low activity from periods of napping. Fourth, although long sleep has also been associated with inflammation (defined as > 8 hours in a review by (16)), we were unable to test curvilinear associations in our sample, given that only nine men slept longer than 8 hours by actigraphy. Fifth, because our goal was to examine relationships between napping and inflammation in the context of normative sleep, we excluded men with treated sleep apnea from analyses (n=6). However, since both sleep apnea and hsCRP are known risk markers for CVD, it is possible that napping may be associated with hsCRP in a sample that did not exclude individuals with sleep disorders.
To our knowledge, the present study is the first to use both objective and subjective measures of daytime napping to investigate associations between napping and inflammatory markers in black and white men. Actigraphy-assessed napping was associated with elevated IL-6 in men, but only if they had shorter, less efficient, or poorer-quality nocturnal sleep. Additionally, the association was independent of known correlates of inflammation, particularly race, medications for sleep and inflammation, BMI, and smoking. These findings provide initial evidence that actigraphy-assessed napping is associated with increased levels of a marker of inflammation that is known to predict cardiovascular risk, and provide the important caveat that the association between napping and IL-6 is significantly potentiated under conditions of poor sleep.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01HL111802 and T32HL07560). Data collection for the Pittsburgh Youth Study from which the present participants were drawn wasfunded by the National Institute on Drug Abuse (DA411018), National Institute on Mental Health (MH48890, MH50778), Pew Charitable Trusts, and the Office of Juvenile Justice and Delinquency Prevention (96-MU-FX-0012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. SLEEP. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime Napping and the Risk of Cardiovascular Disease and All-Cause Mortality: A Prospective Study and Dose-Response Meta-Analysis. Sleep. 2015;38(12):1945–1953. doi: 10.5665/sleep.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong G, Wang Y, Tao T, Ying J, Zhao Y. Daytime napping and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Sleep Med. 2015;16(7):811–819. doi: 10.1016/j.sleep.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Zhang Q, Shang X. Meta-analysis of self-reported daytime napping and risk of cardiovascular or all-cause mortality. Med Sci Monit. 2015;21:1269–1275. doi: 10.12659/MSM.893186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis — an inflammatory disease. New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.de Ferranti S, Rifai N. C-reactive protein, inflammation, and coronary risk. Cardiology Clinics. 2002;21:315–325. doi: 10.1016/s0733-8651(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 10.Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2012;26(6):859–865. doi: 10.1016/j.bbi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. Journal of Internal Medicine. 2002;2002(252):283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 12.Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22(3):147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Medicine. 2008;5 doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneita Y, Ohida T, Uchiyama M, et al. The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. Journal of Clinical Psychiatry. 2006;67:196–203. doi: 10.4088/jcp.v67n0204. [DOI] [PubMed] [Google Scholar]
- 19.Leng Y, Ahmadi-Abhari S, Wainwright NW, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open. 2014;4(11):e006071. doi: 10.1136/bmjopen-2014-006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okun ML, Reynolds CF, 3rd, Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantua J, Spencer RM. The interactive effects of nocturnal sleep and daytime naps in relation to serum C-reactive protein. Sleep Med. 2015;16(10):1213–1216. doi: 10.1016/j.sleep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowski KP, Hall MH, Marsland AL, Matthews KA. Is daytime napping associated with inflammation in adolescents? Health Psychology. 2016;35:1298–1306. doi: 10.1037/hea0000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Loeber R, Farrington DP, Stouthamer-Loeber M, White HR. Violence and serious theft: development and prediction from childhood to adulthood. New York, NY: Routledge; 2008. [Google Scholar]
- 25.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 26.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnagraphic, and subjective assessment of sleep parameters in sleep-disorders patients. Sleep Medicine. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 27.Kanady JC, Drummond SP, Mednick SC. Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res. 2011;20(1 Pt 2):214–222. doi: 10.1111/j.1365-2869.2010.00858.x. [DOI] [PubMed] [Google Scholar]
- 28.Aiken SG, West LS. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 29.Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 30.Owens JF, Buysse DJ, Hall M, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. Journal of Clinical Sleep Medicine. 2010;6:330–335. [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubowski KP, Hall MH, Lee L, Matthews KA. Temporal relationships between napping and nocturnal sleep in healthy adolescents. Behavioral Sleep Medicine. 2016;14:1–13. doi: 10.1080/15402002.2015.1126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav Immun. 2013;27(1):38–41. doi: 10.1016/j.bbi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12(3):131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 34.Vgontzas AN, Dimitris AP, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. Journal of Clinical Endocrinology and Metabolism. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 35.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clinical Chemistry. 2001;47:426–430. [PubMed] [Google Scholar]
- 36.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. Journal of Sleep Research. 1999;8:175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 37.van Wouwe NC, Valk PJL, Veenstra BJ. Sleep monitoring: a comparison between three wearable instruments. Military Medicine. 2011;176:811–816. doi: 10.7205/milmed-d-10-00389. [DOI] [PubMed] [Google Scholar]
- 38.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]