Introduction
Patients with familial hypercholesterolemia (FH, OMIM # 143890) have lifelong elevations in LDL-cholesterol (LDL-C) that result in deposition of cholesterol in tendons – referred to as tendon xanthomas and occurring in roughly 19% of heterozygous FH patients1 – and an increased risk of premature cardiovascular disease. FH is an autosomal dominant disorder resulting from mutations in the low-density lipoprotein receptor (LDLR), apolipoprotein B (APOB), or proprotein convertase subtilisin-like kexin type 9 (PCSK9) genes.2
Heterozygous mutations in LDLR are the most common cause of FH. In addition to elevated LDL-C, patients harboring LDLR mutations may have lower levels of high-density lipoprotein-cholesterol (HDL-C) and only mild, if any, elevations in triglycerides. 3,4, 5 Severe hypertriglyceridemia is rarely seen in FH patients.5 We report an alcoholic patient who presented with severely elevated serum triglycerides and both tuberous and tendon xanthomas in whom the diagnosis of FH was confirmed by genetic testing.
Clinical Case
A 75-year-old Hispanic man was referred to us for genetic testing as part of a larger research protocol.6 His past medical history included 3-vessel coronary artery bypass graft at age 47 and a myocardial infarction (MI) at age 60, hypothyroidism, osteoporosis, gastro-esophageal reflux, hypertension, recurrent major depressive disorder with a history of a suicide attempt, heavy alcohol and tobacco abuse, and Alzheimer’s dementia (initially diagnosed at age 72 with superimposed frontal lobe damage from prior alcohol use).
Hyperlipidemia was first diagnosed at age 34, although his wife noted that he had Achilles tendon xanthomas and arcus senilis since age 22 (when they first met). He had been tried on multiple medications (he could not recall exact names), and ultimately underwent ileal bypass surgery at age 41 for treatment. The ileal bypass was reversed at age 60 due to multiple episodes of intestinal obstruction. At age 66, he was hospitalized for rhabdomyolysis. His serum creatine kinase level was found to be >40,000 Units per Liter (U/L) (reference range: 52–336 U/L) while taking simvastatin 40 milligrams (mg) twice daily, gemfibrozil 600 mg twice daily and niacin 500 mg three times daily. He had been on simvastatin for 15 years, gemfibrozil for 4 years and niacin for 18 months prior to this hospitalization. After this hospitalization he was referred to a specialty lipid clinic for further evaluation and treatment.
His mother suffered a lethal MI at age 34 and several maternal uncles had hypercholesterolemia. His sister had a history of a coronary artery bypass graft and also had a lethal MI at age 55, and his brother committed suicide at age 19. He had 4 daughters, one of which was known to have hypercholesterolemia.
The patient smoked 1.5 packs of cigarettes per day for more than 30 years and consumed 4–8 glasses of wine per day. His wife reported a memory disturbance after the reversal of the ileal bypass, which was progressive and worse when he drank alcohol.
After hospitalization for rhabdomyolysis, his lipid medications had been adjusted to atorvastatin 20 mg daily, fish oil 5 capsules three times daily, colesevelam 1875 mg twice daily, and sitostanol margarine 2 tablespoons daily. He had extreme elevations in both serum total cholesterol (TC) of 611 milligrams per deciliter (mg/dL) (reference range: <200 mg/dL) and triglycerides 725 mg/dL (reference range: 50–150 mg/dL). HDL-C was 42 mg/dL (reference range: 40–60 mg/dL). A few available lipid panels prior to admission showed serum total cholesterol ranging from 239–461 mg/dL, triglycerides ranging from 192–499 mg/dL, and HDL-C 43–62 mg/dL, and LDL-C 157–240 mg/dL. His serum TSH level was normal (3.77 micro-international units per milliliter (μIU/mL), reference range: 0.35–5.5 μIU/mL).
Other medications included aspirin 325 mg daily, folic acid 1 mg daily, isosorbide mononitrate 20 mg twice daily, calcium carbonate 500 mg daily, donepezil 10 mg daily, duloxetine 60 mg daily, ergocalciferol 50,000 International Units (IU) monthly, esomeprazole 40 mg daily, levothyroxine 25 micrograms daily, ramipril 5 mg daily, and risedronate 10 mg weekly.
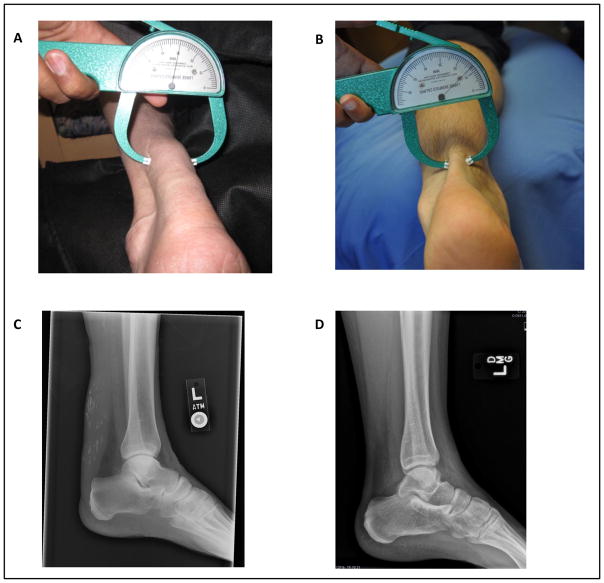
On physical exam, he had normal body mass index (24 kg/m2). He had bilateral arcus senilis, calcified appearing tuberous xanthomas over his knees, and large xanthomas over his Achilles tendons (Figure 1A).
Figure 1.
Achilles tendon xanthomas. A, Image of patient’s Achilles tendon with caliper measuring thickness of 30 mm. B, Image of a normal Achilles tendon with width of 13 mm. C, Lateral plain radiograph demonstrating thickness of the patient’s Achilles tendon with scattered calcifications. D, Lateral plain radiograph with normal Achilles tendon.
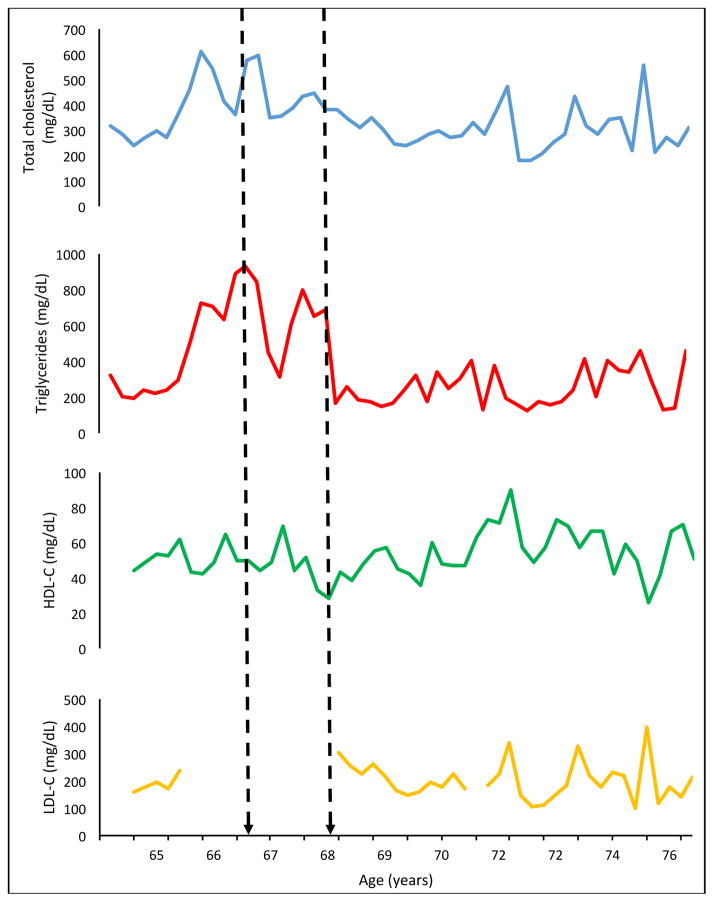
His initial and repeated lipid panels demonstrated high triglycerides and total cholesterol. He was tried on multiple lipid-lowering regimens and was counseled on the need to abstain from alcohol. Of note, when he abstained from alcohol on two occasions (Figure 2), his serum triglycerides improved to 164 mg/dL. However, he resumed alcohol, often drinking 6–8 cups of wine per day and was “intoxicated” every day per his wife.
Figure 2.
Graphs depicting lipid levels over 10 years of follow-up. Dashed arrows indicate time points where the patient had stopped drinking alcohol, but with resumption during time points in-between.
His best lipid panel over follow-up has been TC of 181 mg/dL, TG 125 mg/dL, HDL-C 49 mg/dL, and LDL-C 107 mg/dL while adherent to ezetimibe 10 mg daily, fish oil 5 capsules twice daily, colesevelam 1875 mg twice daily, and rosuvastatin 20 mg daily (Figure 2).
Materials and Methods
The protocol was approved by the Institutional Review Board of UT Southwestern, and the patient provided written informed consent. Genomic DNA was isolated from whole blood. All 18 exons and the flanking intronic regions of LDLR were amplified and sequenced using Applied Biosystems 3730xl DNA Analyzer (Applied Biosystems, Carlsbad, California, USA) as previously described.7 Apolipoprotein E genotyping was done as previously described.7
Results
LDLR sequencing revealed the patient harbored a heterozygous p.(Q448*);c.1342C>T mutation on exon 9. The patient also was heterozygous for a single nucleotide polymorphism in LDLR intron 16 (rs2738460; c.2389+46C>T with a minor allele frequency of 0.23 in subjects of European origin; from dbSNP – accessed May 2017). Apolipoprotein E genotyping revealed he was wild-type (E3/E3).
Discussion
Our case demonstrates that patients with FH can develop a unique lipid phenotype – due to chronic alcoholism – causing a diagnostic conundrum and highlighting the utility of a detailed social history, physical examination, and confirmatory genetic testing. While the presence of tendon xanthomas, arcus senilis, and a strong family history suggested FH, severe hypertriglyceridemia and tuberous xanthomas on the knees suggested an alternative diagnosis of dysbetalipoproteinemia (type 3 hyperlipoproteinemia, OMIM # 107741). In light of genetic testing confirming the diagnosis of FH, heavy alcohol intake likely changed his phenotype from a classical FH phenotype to one consistent with dysbetalipoproteinemia (increased cholesterol and triglycerides).8 Alcohol ingestion is known to increase plasma triglyceride concentrations by increasing triglyceride synthesis and production of very low density lipoprotein (VLDL) particles in the liver.9
Typically, plasma triglyceride levels in heterozygous FH patients are not significantly elevated. In a US registry of FH patients (mostly diagnosed clinically without genetic confirmation), the median (IQR) plasma triglyceride level was 116 mg/dL (82–170, n = 1867).10 Furthermore, patients with mutation-confirmed FH – like our patient – have slightly lower triglycerides than mutation-negative FH patients: in a Spanish population of FH patients, the median (IQR) triglyceride level was 97 (68–139, n = 459) in those with LDLR or APOB mutations while the median (IQR) triglyceride level was 122 mg/dL (n = 366, range of 86–189 mg/dL) in those without an identifiable mutation.11
Influence from incidental genes may lead to severe hypertriglyceridemia in FH patients. Recently, a mutation-confirmed FH patient – similar to our case – was reported with triglycerides of 951 mg/dL and tendon xanthomas on physical examination.12 Targeted next-generation DNA sequencing was done and although it failed to identify any disease-causing mutations in hypertriglyceridemia-associated genes (e.g. lipoprotein lipase, apolipoprotein c2), the patient did harbor a high polygenic burden of single nucleotide polymorphisms that raise serum triglyceride levels.
Based on our patient’s lipid profile, FH may not be on the differential diagnosis unless a thorough physical examination was done and a detailed medical history was obtained. Once FH was suspected, diagnostic criteria for FH (Simon-Broome, MedPed, and the Dutch Lipid Clinic Network) could have been used to give a diagnosis. However, these criteria have been called into question in recent manuscripts as having poor sensitivity and specificity to predict pathogenic LDLR mutations.13
For FH, genetic testing has an unclear role in clinical care. In this case, the patient participated in a larger research study that involved genetic testing. Although the results may not benefit him directly (other than to provide a definitive diagnosis), they may be useful for his daughters and grandchildren. Recent data indicates that a positive genetic test has prognostic implications for cardiovascular risk beyond just LDL-C levels 6, 14, 15, for which his relatives should be counseled.
Our patient’s mutation, the stop mutation p.(Q448*), has been previously reported in a homozygous FH patient from Spain as well as a heterozygous FH patient from Mexico.16, 17 However, phenotype data on lipid and lipoprotein values was not reported for these subjects. This mutation is not in the ExAC database18 or in ClinVar (accessed March 2017).19
Although, our patient’s baseline and post-surgical serum lipids and lipoprotein values were not available, he may have benefited from the intestinal bypass surgery with regards to not just LDL-C lowering, but improving his lifespan. Before FDA approval of statins in 198720, only bile acid sequestrants, niacin and fibrates were available for lipid lowering. For those FH patients who were unresponsive to these therapies, partial ileal bypass was recommended. Partial ileal bypass is effective at reducing LDL-C and death due to CHD: Buchwald et al showed that the procedure reduced LDL cholesterol by 38% at 5 years compared to controls, and death due to CHD and confirmed nonfatal myocardial infarction was 35% lower in those undergoing partial ileal bypass surgery (n=421) compared to controls (n=417) (125 vs. 82 events, respectively; p<0.001).21 Common side effects of partial ileal bypass include diarrhea, kidney stones, gallstones, and intestinal obstruction.21 Our patient had multiple episodes of intestinal obstruction and thus had the procedure reversed.
Our patient had remarkable Achilles tendon xanthomas. Tendinous xanthomas form movable hard nodules that can infiltrate tendons, tendon attachments, ligaments, fascia and the periosteum.22 These are suggestive of FH, but can also be seen in other rare genetic disorders such as cerebrotendinous xanthomatosis (CTX) and sitosterolemia. To distinguish between these other genetic disorders, the clinical history, genetic testing, and serum sterol profiles are important as the xanthomas may appear similar.22 In CTX, individuals may have neurological or cognitive symptoms, normal cholesterol levels, and high plasma levels of cholestenol.23 In sitosterolemia, individuals have premature cardiovascular disease associated with very high plasma cholesterol levels (up to 1,000 mg/dL) and increased plasma concentrations of plant sterols.24 Other lesions can appear similarly, but can be differentiated based on lipid levels. In FH, lesions are commonly found on the Achilles tendons, dorsum of the hands, and extensor surfaces of the knees and elbows. On lateral plain radiographs, xanthomas on the Achilles tendon appear as abnormal thickening of the tendon or as noncalcified soft-tissue masses.25, 26 Our patient had rare atypical xanthomata involving the bilateral Achilles tendons that appeared calcified on radiographs (Figure 1B). Our patient also had tuberous xanthomas over his knees. Tuberous xanthomas are flat or slightly elevated yellow appearing nodules in the dermis and subcutaneous tissue.22 These lesions are commonly found in the skin over the joints or on the buttocks. They are most often described in patients with dysbetalipoproteinemia but can also occur in patients with FH, CTX, or sitosterolemia.22
Our patient was on a very high dose of simvastatin in addition to gemfibrozil at the time of his episode of rhabdomyolysis. Since FH patients present with severe hypercholesterolemia, they usually do not require fibrates. However, due to this patient’s presentation with severe hypertriglyceridemia, he was initiated on a combination of simvastatin and gemfibrozil which resulted in rhabdomyolysis. Rhabdomyolysis is a known rare side-effect of statin use, exacerbated by interactions with concomitant medications such as gemfibrozil.27 The FDA has now classified the drug interaction between simvastatin and gemfibrozil as Risk Rating X with the recommendation to avoid the combination. The reported time course for the development of rhabdomyolysis with this drug combination varies widely, ranging from 5–900 days.27 Our patient was reportedly on this combination for approximately 4 years, however he was known to be poorly adherent to his medications so the exact number of days he was compliant with this therapy remains unknown.
Conclusions
The diagnosis of FH is made clinically, however laboratory findings are not always as expected and other medical conditions may cause secondary changes in the lipid profile. Genetic testing may play a role in cases such as ours, where the clinical course and physical examination findings suggest FH but lipid levels may not be consistent.
Acknowledgments
Sources of Funding: The work was supported by grants from the Southwest Medical Foundation, Center for Human Nutrition at UT Southwestern and from the National Institutes of Health (NIH) K23 HL114884 and CTSA Grant UL1TR001105 for REDCap.
Footnotes
Disclosures: Z.A. has received honorarium for speaker bureau for Amgen and advisory board meetings sponsored by Regeneron and Akcea. A.G. received research grants from Ionis and Quintiles, and is a consultant for Aegerion, Akcea, Ionis, Nordic Biotech and Venture Point.
References
- 1.deGoma EM, Ahmad ZS, O’Brien EC, Kindt I, Shrader P, Newman CB, et al. Treatment Gaps in Adults With Heterozygous Familial Hypercholesterolemia in the United States: Data From the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9:240–9. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973;70:2804–8. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. European Atherosclerosis Society Consensus Panel on Familial H. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–57. doi: 10.1093/eurheartj/ehu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–70. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JLHHH, Brown MS. Familial Hypercholesterolemia. The Online Metabolic and Molecular Bases of Inherited Disease. 1995 [Google Scholar]
- 6.Ahmad Z, Adams-Huet B, Chen C, Garg A. Low Prevalence of Mutations in Known Loci for Autosomal Dominant Hypercholesterolemia in a Multi-Ethnic Patient Cohort. Circ Cardiovasc Genet. 2012;5:666–75. doi: 10.1161/CIRCGENETICS.112.963587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad Z, Adams-Huet B, Chen C, Garg A. Low prevalence of mutations in known loci for autosomal dominant hypercholesterolemia in a multiethnic patient cohort. Circ Cardiovasc Genet. 2012;5:666–75. doi: 10.1161/CIRCGENETICS.112.963587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahm AH, RA . Primary Hypertriglyceridemia. In: Garg A, editor. Dyslipidemias. Humana Press; 2015. pp. 205–220. [Google Scholar]
- 9.Hannuksela ML, Ramet ME, Nissinen AE, Liisanantti MK, Savolainen MJ. Effects of ethanol on lipids and atherosclerosis. Pathophysiology. 2004;10:93–103. doi: 10.1016/j.pathophys.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad ZS, Andersen RL, Andersen LH, O’Brien EC, Kindt I, Shrader P, et al. US physician practices for diagnosing familial hypercholesterolemia: data from the CASCADE-FH registry. J Clin Lipidol. 2016;10:1223–9. doi: 10.1016/j.jacl.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Civeira F, Ros E, Jarauta E, Plana N, Zambon D, Puzo J, et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol. 2008;102:1187–93. 1193 e1. doi: 10.1016/j.amjcard.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Najera SD, Hegele RA. Heterozygous familial hypercholesterolemia presenting as chylomicronemia syndrome. J Clin Lipidol. 2017;11:294–296. doi: 10.1016/j.jacl.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016:354. doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad Z, Li X, Wosik J, Mani P, Petr J, McLeod G, et al. Premature coronary heart disease and autosomal dominant hypercholesterolemia: Increased risk in women with LDLR mutations. J Clin Lipidol. 2016;10:101–8. e1–3. doi: 10.1016/j.jacl.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–89. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozas P, Castillo S, Tejedor D, Reyes G, Alonso R, Franco M, et al. Molecular characterization of familial hypercholesterolemia in Spain: identification of 39 novel and 77 recurrent mutations in LDLR. Hum Mutat. 2004;24:187. doi: 10.1002/humu.9264. [DOI] [PubMed] [Google Scholar]
- 17.Vaca G, Vazquez A, Magana MT, Ramirez ML, Davalos IP, Martinez E, et al. Mutational analysis of the LDL receptor and APOB genes in Mexican individuals with autosomal dominant hypercholesterolemia. Atherosclerosis. 2011;218:391–6. doi: 10.1016/j.atherosclerosis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–5. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:484–93. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH) N Engl J Med. 1990;323:946–55. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 22.Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181–8. doi: 10.5507/bp.2014.016. [DOI] [PubMed] [Google Scholar]
- 23.Bjorkhem I. Cerebrotendinous xanthomatosis. Curr Opin Lipidol. 2013;24:283–7. doi: 10.1097/MOL.0b013e328362df13. [DOI] [PubMed] [Google Scholar]
- 24.Yoo EG. Sitosterolemia: a review and update of pathophysiology, clinical spectrum, diagnosis, and management. Ann Pediatr Endocrinol Metab. 2016;21:7–14. doi: 10.6065/apem.2016.21.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dussault RG, Kaplan PA, Roederer G. MR imaging of Achilles tendon in patients with familial hyperlipidemia: comparison with plain films, physical examination, and patients with traumatic tendon lesions. AJR Am J Roentgenol. 1995;164:403–7. doi: 10.2214/ajr.164.2.7839978. [DOI] [PubMed] [Google Scholar]
- 26.Narvaez JA, Narvaez J, Ortega R, Aguilera C, Sanchez A, Andia E. Painful heel: MR imaging findings. Radiographics. 2000;20:333–52. doi: 10.1148/radiographics.20.2.g00mc09333. [DOI] [PubMed] [Google Scholar]
- 27.Chang JT, Staffa JA, Parks M, Green L. Rhabdomyolysis with HMG-CoA reductase inhibitors and gemfibrozil combination therapy. Pharmacoepidemiol Drug Saf. 2004;13:417–26. doi: 10.1002/pds.977. [DOI] [PubMed] [Google Scholar]