Abstract
Background
Primary prevention implantable cardioverter defibrillator (ICD) reduce all-cause mortality by reducing sudden cardiac death. There are conflicting data regarding whether patients with more advanced heart failure derive ICD benefit owing to the competing risk of nonsudden death.
Methods
We performed a patient-level meta-analysis of New York Heart Association (NYHA) class II/III heart failure patients (left ventricular ejection fraction ≤35%) from 4 primary prevention ICD trials (MADIT-I, MADIT-II, DEFINITE, SCD-HeFT). Bayesian-Weibull survival regression models were used to assess the impact of NYHA class on the relationship between ICD use and mortality.
Results
Of the 2,763 patients who met study criteria, 68% (n = 1,867) were NYHA II and 52% (n = 1,435) were randomized to an ICD. In a multivariable model including all study patients, the ICD reduced mortality (hazard ratio [HR] 0.65, 95% posterior credibility interval [PCI]) 0.40–0.99). The interaction between NYHA class and the ICD on mortality was significant (posterior probability of no interaction = .036). In models including an interaction term for the NYHA class and ICD, the ICD reduced mortality among NYHA class II patients (HR 0.55, PCI 0.35–0.85), and the point estimate suggested reduced mortality in NYHA class III patients (HR 0.76, PCI 0.48–1.24), although this was not statistically significant.
Conclusions
Primary prevention ICDs reduce mortality in NYHA class II patients and trend toward reducing mortality in the heterogeneous group of NYHA class III patients. Improved risk stratification tools are required to guide patient selection and shared decision making among NYHA class III primary prevention ICD candidates. (Am Heart J 2017;191:21–29.)
Sudden cardiac death (SCD) is the third leading cause of death in the United States, claiming >325,000 lives annually.1,2 The risk of SCD is increased in the presence of certain types of structural heart disease including a reduced left ventricular ejection fraction (LVEF).2 This observation led to multiple randomized controlled trials of the primary prevention implantable cardioverter defibrillator (ICD) in different populations of patients with a severely reduced LVEF. Importantly, most of these trials demonstrated that the primary prevention ICD reduced mortality among patients with a severe ischemic3–6 or nonischemic cardiomyopathy.3,7 Whereas the primary results have led to widespread use of the ICD for primary prevention, subgroup analyses have led to questions regarding ICD efficacy in patients with more advanced heart failure (HF).3
Primary prevention ICDs reduce all-cause mortality by reducing SCD due to arrhythmic causes. However, increasing HF severity is associated with an increased risk of death due to pump failure.8 Although data from the Metoprolol CR/XL Randomized Intervention Trial in-Congestive Heart Failure (MERIT-HF) study show that >50% of patients with New York Heart Association (NYHA) class III HF symptoms die suddenly,8 these patients did not appear to derive survival benefit from the ICD in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).3 Subgroup analyses from other randomized controlled trials (RCTs) of primary prevention ICDs however did not yield consistent results. Notably, a meta-analysis of published data from the RCTs of primary prevention ICDs showed a trend toward survival benefit from the ICD in patients with NYHA class III symptoms; however, this meta-analysis included a cardiac resynchronization therapy trial and a trial of ICD use immediately after a myocardial infarction, limiting applicability to contemporary patients.9 Given these conflicting findings, we sought to examine the efficacy of the ICD by NYHA class using patient-level data from pivotal primary prevention ICD trials.
Methods
Study population
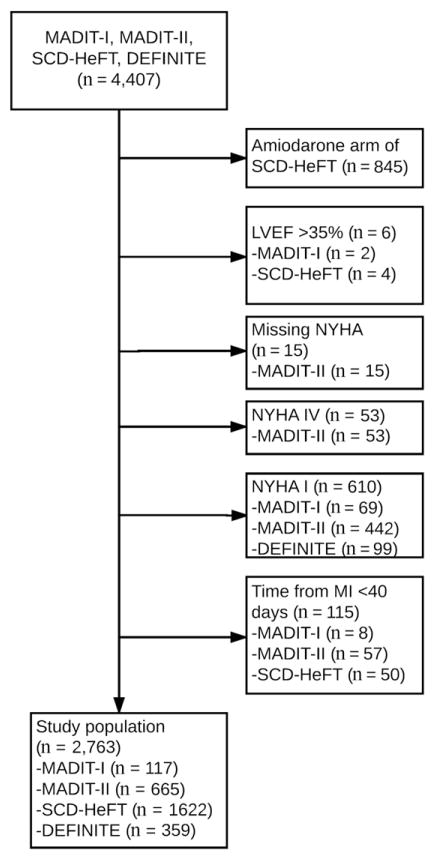
Our study population consisted of patients included in primary prevention ICD trials. Patients from Multicenter Automatic Defibrillator Implantation Trial I (MADIT-I),5 MADIT-II,6 SCD-HeFT,3 and Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE)7 were considered for the analysis. Multicenter Unsustained Tachycardia Trial (MUSTT)4 patients were not included because of the lack of data on key comorbidities. Patient inclusion criteria included LVEF ≤35%, NYHA II or III symptom class, and either (1) no prior myocardial infarction or (2) a time from myocardial infarction to randomization of at least 40 days. We excluded individuals randomized to the amiodarone arm of SCD-HeFT. Individuals with NYHA I symptoms were not studied because they were not included in SCD-HeFT and such patients were a distinct minority in MADIT-II.
Statistical analysis
We combined patient-level data from 4 primary prevention ICD trials. Missing baseline data were imputed using empirical frequencies stratified by trial and cardiomyopathy etiology. For example, if smoking status was missing from a SCD-HeFT patient with ischemic heart disease, the empirical frequency of smoking among SCD-HeFT patients with ischemic heart disease would be used to guide imputation. Creatinine clearance was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.10
We described the overall baseline characteristics of the entire study population and then compared these characteristics among patient subgroups when categorized by NYHA symptom class (II or III) using proportions for categorical variables and means with SDs for continuous variables. Baseline characteristics were compared among NYHA III patients across trials because of notable trial-specific differences in outcomes among NYHA III. Differences between groups were tested using the χ2 test for categorical variables and t tests for continuous variables.
The primary end point for this study was all-cause mortality. Event rates were compared among those randomized to ICD versus no ICD after stratification by NYHA symptom class by constructing Kaplan-Meier curves. Cox proportional hazards models were constructed for univariate analyses, and Bayesian-Weibull survival regression models11 were constructed for multivariable analyses. Multivariable models were constructed to ascertain whether any observed relationships between NYHA class and ICD efficacy were related to HF severity (as assessed by symptom class) versus other comorbidities that may be more common among those with more advanced HF. Unless otherwise specified, all multivariable models adjusted for age, sex, race, LVEF, QRS duration >120 milliseconds, ischemic heart disease, antiarrhythmic drug use, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, smoking, and diabetes. We assessed the interaction term between NYHA symptom classes and receipt versus nonreceipt of ICD on all-cause mortality, and included this term in the overall final model. Notably, Bayesian statistical methods have been used successfully in analyses of the pooled database of patient-level data from primary prevention ICD trials.12–15 Bayesian methods have the advantage of being able to borrow data from across trials. For example, when assessing ICD efficacy among SCD-HeFT criteria patients, the Bayesian approach draws from SCD-HeFT type patients from across all ICD trials and not just patients who were specifically enrolled in SCD-HeFT.
Rates of sudden and nonsudden death were calculated using the cumulative incidence function to account for competing causes of death. Comparisons of the cumulative incidence functions were performed using the Gray’s test16.
Exploratory frequentist analyses were performed to understand the relationship between cardiomyopathy etiology, NYHA symptom class, all-cause mortality, and cause-specific mortality. Cox proportional hazard models were used for analyses where the end points were all-cause mortality. To account for competing risks, Fine-Gray models were used to assess cause-specific mortality.
Statistical analyses were performed on deidentified data using R version 3.3.1.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents. Primary funding was provided by the Agency for Healthcare Research and Quality (5 R01 HS018505–03).
Results
Patient characteristics
A total of 2,763 patients were included after applying inclusion and exclusion criteria (Figure 1), and baseline characteristics are described in Table I. Patients were predominantly older (60 ± 12 years), male (79%), smokers (79%), with ischemic cardiomyopathy (58%), and with moderate to severely reduced LVEF (23 ± 7%) and were frequently treated with β-blockers (67%) and ACE inhibitors (89%). Of these patients, 68% (n = 1,867) had NYHA II symptoms and 52% (n = 1,435) were randomized to an ICD. All trials contributed more NYHA II patients compared with NYHA III patients: DEFINITE, 263 versus 96; MADIT-I, 84 versus 33; MADIT-II, 394 versus 271; and SCD-HeFT, 1,126 versus 496. Compared with NYHA class II patients, NYHA class III patients were older, were more often female, and demonstrated higher comorbidity burden, including higher rates of ischemic cardiomyopathy, hypertension, diabetes, chronic kidney disease, atrial fibrillation (AF), and pulmonary disease. NYHA class III patients had a lower LVEF and were less often treated with β-blockers, but the rates of ACE inhibitor use were comparable (Table I). A comparison of baseline characteristics of patients when stratified by NYHA class and treatment (ICD vs no ICD) is included in Supplemental Table 1.
Figure 1.
Consort diagram depicting derivation of the study population.
Table I.
Baseline characteristics among the entire study population and among subgroups defined by NYHA symptom class
| All (N = 2763) | NYHA class II (n = 1867) | NYHA class III (n = 896) | P value* | |
|---|---|---|---|---|
| ICD treatment | 1435 (51.9) | 951 (50.9) | 484 (54.0) | .14 |
| Age (y), mean (SD) | 60.19 (11.8) | 59.74 (11.7) | 61.15 (11.9) | .004 |
| Male sex | 2169 (78.5) | 1492 (79.9) | 677 (75.6) | .01 |
| Race | .622 | |||
| Black | 462 (16.7) | 310 (16.6) | 152 (17.0) | |
| White | 2162 (78.3) | 1468 (78.6) | 694 (77.5) | |
| Other | 139 (5.0) | 89 (4.8) | 50 (5.6) | |
| Ischemic cardiomyopathy | 1613 (58.4) | 1032 (55.3) | 581 (64.8) | <.001 |
| Hypertension | 1314 (54.7) | 847 (52.8) | 467 (58.5) | .01 |
| Diabetes | 848 (30.7) | 531 (28.5) | 317 (35.4) | <.001 |
| Hyperlipidemia | 38 (40.0) | 29 (40.9) | 9 (37.5) | .962 |
| LVEF, mean (SD) | 23.21 (6.6) | 23.66 (6.4) | 22.26 (6.7) | <.001 |
| Prior CABG | 889 (37.0) | 568 (35.4) | 321 (40.1) | .027 |
| Prior MI | 1491 (54.0) | 950 (50.9) | 541 (60.4) | <.001 |
| Prior PCI | 662 (27.6) | 420 (26.3) | 242 (30.3) | .041 |
| LBBB | 504 (21.4) | 336 (21.3) | 168 (21.7) | .885 |
| QRS duration (ms), mean (SD) | 120.10 (31.3) | 118.86 (31.1) | 122.66 (31.6) | .003 |
| Antiarrhythmic drug | 80 (2.9) | 52 (2.8) | 28 (3.1) | .706 |
| β-Blocker | 1854 (67.1) | 1311 (70.2) | 543 (60.6) | <.001 |
| ACE inhibitor | 2450 (88.7) | 1664 (89.1) | 786 (87.7) | .305 |
| GFR* (mL/min/1.73 m2) | <.001 | |||
| ≥60 | 1508 (63.2) | 1082 (68.0) | 426 (53.7) | |
| <60 to ≥30 | 793 (33.2) | 472 (29.7) | 321 (40.4) | |
| <30 | 85 (3.6) | 38 (2.4) | 47 (5.9) | |
| AF | 195 (9.8) | 118 (8.5) | 77 (13.0) | .003 |
| Peripheral vascular disease | 373 (18.8) | 23 (6.6) | 7 (5.4) | .789 |
| Pulmonary disease | 377 (18.1) | 225 (16.2) | 148 (25.0) | <.001 |
| Smoking | 2177 (79.2) | 1478 (79.7) | 699 (78.3) | .425 |
CABG, Coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention; LBBB, left bundle-branch block; GFR, glomerular filtration rate (measured by Chronic Kidney Disease Epidemiology Collaboration formula).
Comparison limited to trials with available data.
ICD implantation and sudden versus nonsudden death
Because of previously reported differences in ICD efficacy among NYHA class III patients by trial (ie, no ICD efficacy among NYHA III patients in SCD-HeFT), we compared baseline characteristics among NYHA class III patients by trial (Table II). As expected, SCD-HeFT patients were less likely to have ischemic cardiomyopa-thy and a history of revascularization, consistent with inclusion of nonischemic patients. In accordance with enrollment criteria, there were no DEFINITE patients with a history of ischemic cardiomyopathy or revascularization. SCD-HeFT and DEFINITE were more contemporary trials, and patients in these studies were more likely to be on contemporary HF medications (β-blocker, ACE inhibitor) compared with the older MADIT trials.
Table II.
A comparison of baseline characteristics among NYHA class III patients in 4 primary prevention ICD randomized controlled trials
| DEFINITE (N = 96) | MADIT I (N = 33) | MADIT II (N = 271) | SCD-HeFT (N = 496) | P value* | |
|---|---|---|---|---|---|
| ICD treatment | 47 (49.0) | 15 (45.5) | 171 (63.1) | 251 (50.6) | .004 |
| Age (y), mean (SD) | 57.83 (14.1) | 63.58 (8.6) | 64.42 (10.7) | 59.84 (11.9) | .787 |
| Male sex | 65 (67.7) | 31 (93.9) | 225 (83.0) | 356 (71.8) | <.001 |
| Race | <.001 | ||||
| Black | 25 (26.0) | 3 (9.1) | 24 (8.9) | 100 (20.2) | |
| White | 66 (68.9) | 29 (87.9) | 237 (87.5) | 362 (73.0) | |
| Other | 5 (5.2) | 1 (3.0) | 10 (3.70) | 34 (6.9) | |
| Ischemic cardiomyopathy | 0 (0) | 33 (100) | 271 (100) | 277 (55.9) | <.001 |
| Hypertension | 0 (NA) | 16 (48.5) | 151 (55.9) | 300 (60.5) | .234 |
| Diabetes | 28 (29.2) | 3 (9.1) | 100 (36.9) | 186 (37.5) | .005 |
| Hyperlipidemia | 0 (NA) | 9 (37.5) | 0 (NA) | 0 (NA) | NA |
| LVEF, mean (SD) | 20.02 (6.4) | 22.45 (6.7) | 21.40 (5.8) | 23.15 (7.1) | <.001 |
| Prior CABG | 0 (NA) | 19 (57.6) | 162 (59.8) | 140 (28.2) | <.001 |
| Prior MI | 0 (0) | 33 (100) | 271 (100) | 237 (47.8) | <.001 |
| Prior PCI | 0 (NA) | 7 (21.2) | 113 (42.0) | 122 (24.6) | <.001 |
| LBBB | 0 (NA) | 4 (12.9) | 54 (21.7) | 110 (22.2) | .477 |
| QRS duration (ms), mean (SD) | 116.7 (30.5) | 125.9 (31.7) | 126.6 (33.8) | 121.5 (30.4) | .612 |
| Antiarrhythmic drug | 8 (8.3) | 9 (27.3) | 11 (4.1) | 0 (0) | <.001 |
| β-Blocker | 73 (76.0) | 3 (9.1) | 166 (61.3) | 301 (60.7) | <.001 |
| ACE inhibitor | 82 (85.4) | 20 (60.6) | 214 (79.0) | 470 (94.8) | <.001 |
| GFR* (mL/min/1.73 m2) | .013 | ||||
| ≥60 | 0 (NA) | 11 (35.5) | 137 (50.7) | 278 (56.4) | |
| <60 to ≥30 | 0 (NA) | 19 (61.3) | 109 (40.4) | 193 (39.2) | |
| <30 | 0 (NA) | 1 (3.2) | 24 (8.9) | 22 (4.5) | |
| AF | 31 (32.3) | 0 (NA) | 0 (NA) | 46 (9.3) | <.001 |
| Peripheral vascular disease | 1 (1.04) | 6 (18.2) | 0 (NA) | 0 (NA) | .900 |
| Pulmonary disease | 16 (16.7) | 0 (NA) | 0 (NA) | 132 (26.6) | .053 |
| Smoking | 98 (96.9) | 22 (73.3) | 214 (79.0) | 370 (74.6) | <.001 |
NA, not available.
Comparison limited to trials with available data. NA denotes that the indicated trial was not included in the comparison because the relevant data were not collected at baseline.
When comparing the baseline characteristics of NYHA III SCD-HeFT patients by treatment group, patients who were randomized to ICD implantation compared with those randomized to placebo only were found to be significantly older (61.4 ± 11.9 vs 58.2 ± 11.7 years, P = .004), were more likely to have a history of AF (12% vs 6%, P = .03), and trended toward a lower likelihood of being on ACE inhibitors (93% vs 97%, P = .08) (Supplemental Table 2).”
ICD implantation and all-cause mortality
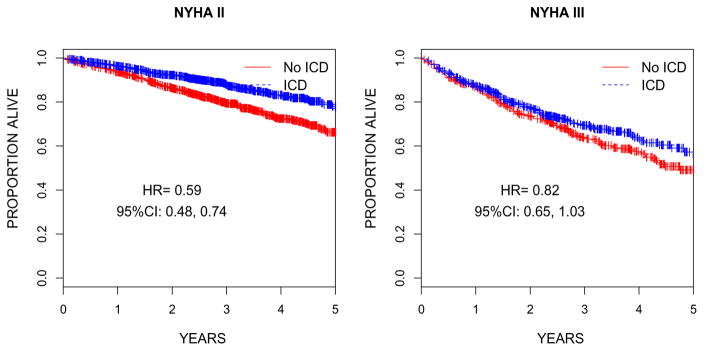
ICD efficacy by NYHA class was subsequently assessed using unadjusted Cox proportional hazards models and graphically depicted with Kaplan-Meier curves (Figure 2). The ICD led to a significant reduction in mortality among NYHA class II patients (hazard ratio [HR] 0.59, CI 0.48–0.74) and a borderline significant reduction in mortality among NYHA class III patients (HR 0.82, CI 0.65–1.03).
Figure 2.
Kaplan-Meier curves depicting differences in survival among patients randomized to ICD versus no ICD among (A) NYHA class II and (B) NYHA class III patients. The differences in the risk of death by treatment group were assessed using unadjusted Cox proportional hazards models.
We subsequently completed a series of multivariable adjusted Bayesian-Weibull survival regression models to account for heterogeneity between trials. In a pooled analysis of NYHA II and III patients from all trials, the ICD led to a reduction in mortality (HR 0.65, 95% posterior credibility interval 0.40–0.99), a finding that was consistent across trials (Table III). In multivariable adjusted Bayesian-Weibull survival regression models including an interaction term for the NYHA class and the ICD, we observed that the ICD demonstrated a reduction in mortality among NYHA class II patients (HR 0.55, 95% posterior credibility interval 0.35–0.85) but not NYHA class III patients (HR 0.76, 95% posterior credibility interval 0.48–1.24) (Table IV). We found a significant interaction between NYHA class and ICD use on mortality (posterior probability of no interaction = .036).
Table III.
Bayesian analysis* of ICD efficacy among NYHA II and III patients
| Group | HR | Lower PCI | Upper PCI |
|---|---|---|---|
| DEFINITE | 0.65 | 0.42 | 0.93 |
| MADIT I | 0.59 | 0.31 | 0.81 |
| MADIT II | 0.61 | 0.44 | 0.80 |
| SCD-HeFT | 0.72 | 0.61 | 0.87 |
| Overall | 0.65 | 0.40 | 0.99 |
PCI, 95% posterior credible interval.
Adjusted for age, sex, race, ejection fraction, QRS duration >120 milliseconds, ischemic heart disease, antiarrhythmic drug use, β-blockers, ACE inhibitors, smoking, and diabetes.
Table IV.
Bayesian analysis* of ICD efficacy by NYHA class
| NYHA class | Group | HR | Lower PCI | Upper PCI |
|---|---|---|---|---|
| II | DEFINITE | 0.55 | 0.36 | 0.81 |
| MADIT I | 0.50 | 0.28 | 0.71 | |
| MADIT II | 0.52 | 0.36 | 0.69 | |
| SCD-HeFT | 0.61 | 0.49 | 0.79 | |
| Overall | 0.55 | 0.35 | 0.85 | |
| III | DEFINITE | 0.77 | 0.50 | 1.20 |
| MADIT I | 0.68 | 0.37 | 1.00 | |
| MADIT II | 0.71 | 0.50 | 0.99 | |
| SCD-HeFT | 0.84 | 0.66 | 1.08 | |
| Overall | 0.76 | 0.48 | 1.24 |
Adjustment for age, sex, race, ejection fraction, QRS duration >120 milliseconds, ischemic heart disease, antiarrhythmic drug use, β-blockers, ACE inhibitors, smoking, and diabetes and an interaction term for NYHA class and ICD use. Posterior probability of no interaction = .036.
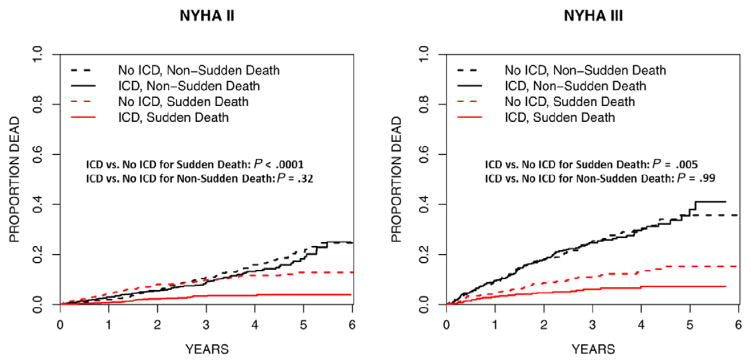
To understand whether the competing risks of nonsudden death may impact ICD efficacy among NYHA III patients, we performed a series of analyses comparing sudden versus nonsudden death by NYHA class and treatment. Figure 3 depicts the stratified rates of sudden versus nonsudden death for patients with NYHA class II or class III symptoms by treatment (ICD vs no ICD). Among NYHA class II patients, the ICD led to a lower rate of SCD (10% vs 3%, P < .001) but not non-SCD (11% vs 9%, P = .32). Similarly, among NYHA III patients, the ICD led to a lower rate of SCD (11% vs 6%, P = .005) but not non-SCD (25% vs 25%, P = .99).
Figure 3.
Cumulative incidence functions depicting sudden versus nonsudden death among patients randomized to ICD versus no ICD when stratified by NYHA class. NYHA II patients are compared in the left pane; NYHA III patients are compared in the right pane.
Given the observed differences in ICD efficacy by NYHA class and trial, we assessed for differences in the risk of sudden versus nonsudden death across these strata in the control arms. We observed significant differences in rates of sudden (P = .01) and nonsudden (P < .001) death across trials among NYHA III patients; differences were also found among NYHA II patients (P = .01 for both comparisons). NYHA class III patients (vs NYHA class II patients) demonstrated substantially more across--trial variability in the relative frequency of sudden and nonsudden deaths at 3 years (Table V). When considering all trials, the proportion of nonsudden deaths was substantially higher in NYHA class III patients compared with NYHA class II patients.
Table V.
Three-year rates of sudden and nonsudden death among control patients stratified by trial
| DEFINITE | MADIT I | MADIT II | SCD-HeFT | P value* | Overall | |
|---|---|---|---|---|---|---|
| NYHA II | ||||||
|
|
||||||
| Sudden death, % | 5.2 (5/139) | 20.6 (8/46) | 12.6 (14/151) | 9.5 (54/580) | .01 | 10.0 (81/916) |
| Nonsudden death, % | 7.7 (9/139) | 21.9 (9/46) | 16.0 (13/151) | 9.4 (52/580) | .01 | 11.0 (83/916) |
| NYHA III | ||||||
| Sudden death, % | 6.8 (3/49) | 6.0 (1/18) | 24.8 (15/100) | 9.3 (22/245) | .01 | 11.0 (41/412) |
| Nonsudden death, % | 33.2 (12/49) | 67.2 (11/18) | 30.1 (18/100) | 20.7 (49/245) | <.001 | 25.0 (90/412) |
Reflects Gray test statistic which was used to assess differences between cumulative incidence functions. Three-year event rates and counts of death (in parentheses) were reported for illustrative purposes.
We hypothesized that cardiomyopathy etiology might partially explain differences in ICD efficacy by NYHA class and subsequently performed a series of exploratory frequentist analyses of ICD efficacy (Supplemental Table 3). In adjusted analyses including NYHA class II/III patients with stratification by cardiomyopathy etiology, the ICD reduced mortality among both ischemics (HR 0.66, CI 0.54–0.89) and nonischemics (HR 0.71, CI 0.53–0.95). Notably, the interaction between NYHA class, ICD use, and mortality was significant among ischemic (P = .0046) but not nonischemic (P = .62) patients. In adjusted analyses of the ischemic subgroup including an interaction term for ICD efficacy by NYHA class, the ICD reduced mortality among NYHA class II patients (HR 0.50, CI 0.38–0.67) and only trended toward reducing mortality among NYHA class III patients (HR 0.85, CI 0.65–1.11). Among ischemic NYHA class III patients, point estimates suggested ICD efficacy among patients in MADIT I (HR 0.44, CI 0.16–1.17) and MADIT II (HR 0.52, CI 0.33–0.84) but not among SCD-HeFT (HR 1.30, CI 0.92–1.84) patients. In adjusted analyses of the relatively smaller nonischemic subgroup including an interaction term for ICD and NYHA class, point estimates are consistent with ICD benefit for NYHA II (HR 0.75, CI 0.52–1.09) and NYHA III (HR 0.65, CI 0.41–1.03) patients (Pinteraction = .64).
When considering cause-specific mortality, the ICD led to a reduction in sudden death among both ischemic (HR 0.37, CI 0.26–0.53) and nonischemic subgroups (HR 0.32, CI 0.17–0.61); nonsudden death rates were not modified in either group (Supplemental Table 3). There was no significant interaction between treatment (ICD vs no ICD) and NYHA class within the ischemic or nonischemic subgroups for either end point (sudden vs nonsudden death).
Discussion
This study demonstrates a number of key findings regarding the relationship between primary prevention ICD efficacy and HF severity as assessed by NYHA functional class. Although the ICD reduced mortality among the pooled population of NYHA class II/III patients, efficacy was only firmly demonstrated among the NYHA class II subgroup. Although the point estimate suggested ICD efficacy (HR 0.76) among NYHA class III patients, the posterior credible intervals were wide and not statistically significant, in part because of a heterogeneous patient population. The ICD significantly reduced sudden death among all patients regardless of NYHA class and cardiomyopathy etiology. We identified variability in competing causes of nonsudden death and cardiomyopathy etiology as potential explanations for the observed differences in ICD efficacy among patients with NYHA class III symptoms versus those with NYHA class II symptoms. Additionally, the relatively fewer NYHA III patients (compared with NYHA II patients) may have resulted in reduced power to detect a true difference in all-cause mortality among those with and without an ICD. Through the merger of patient-level data from 4 pivotal ICD trials, this study represents the largest study of the relationship between HF severity and ICD efficacy, providing key insights into this relationship, as well as important implications for guidelines, patient care, and future research.
Current primary prevention ICD guidelines rely on NYHA class assessment for appropriate patient selection in efforts to reflect the inclusion and exclusion criteria of the pivotal ICD trials and to help identify patients who are most likely to benefit from a primary prevention ICD17. Primary prevention ICD implantation is considered a Class I indication among NYHA class II and III HF patients with LVEF ≤35% due to an ischemic or nonischemic cause.17 The results of the current study strongly support the current guidelines regarding NYHA class II patients and suggest that a Class I recommendation for NYHA class III patients may be appropriate. Although the width of the posterior credible intervals may partially reflect a relatively smaller number of NYHA III patients compared with NYHA II patients in our study (896 vs 1,867), we believe that the width of the posterior credible intervals may be due to substantial patient heterogeneity. This underscores the need for improved risk stratification in patients with NYHA class III symptoms to better identify patients who may not benefit from the ICD because of to an increased risk of nonsudden death.
ICD benefit will be more likely among patients with a relatively high risk for SCD and a relatively low risk of dying from nonsudden death (ie, the fatal events that the ICD cannot prevent). A crude comparison of the relative rates of 3-year sudden versus nonsudden deaths (Table V) in the control arms demonstrates that, with the exception of MADIT II, the proportion of nonsudden deaths increased substantially with increasing HF severity. Notably, the rates of SCD among NYHA class III patients remained high, and the ICD significantly reduced the risk of sudden death in this subset, suggesting that there is indeed an important role for the ICD in a key subset of NYHA class III patients. Although improvements in medical therapy for HF since publication of these pivotal ICD trials may reduce pump failure–related deaths and increase the number of HF patients who are poised to benefit from ICDs, HF exacerbations are tightly linked to ventricular arrhythmias,18 making it difficult to make conclusive statements on the basis of this complex relationship.
Many studies have identified NYHA class III HF as a risk factor for mortality among ICD recipients.19,20 Although existing data support the notion that not all NYHA class III patients are poised to benefit from ICD implantation, relatively few studies suggest potential solutions for risk stratification in this population. Our study demonstrates the urgency with which additional research and guideline recommendations are needed on the topic of risk stratification in NYHA class III patients, particularly those with an ischemic cardiomyopathy. A compelling secondary analysis of SCD-HeFT demonstrated that longer 6-minute walk distance, a more objective measure than NYHA class, was a potent predictor of ICD benefit.21 Notably, 297 of the 692 NYHA class III patients in the SCD-HeFT analysis had baseline 6-minute walk distances predictive of probable ICD benefit, suggesting important prognostic value among NYHA III patients. The Seattle Heart Failure Model is based on readily available clinical variables and may represent an important tool for identifying NYHA class III patients with a high likelihood of ICD benefit; when applied to the SCD-HeFT population, it demonstrated excellent discriminative ability and showed that among patients with a predicted annual mortality of >20%, ICD benefit was highly unlikely.22 Notably, the Seattle Heart Failure Model identified 449 (of 751) NYHA class III patients in SCD-HeFT as being likely to derive benefit from an ICD. Recent studies have suggested that the Seattle Proportional Risk Model, which predicts risk of sudden cardiac death, can be used in combination with the Seattle Heart Failure Model to predict potential ICD benefit.23,24 In addition to these clinical characteristics, a recent analysis of primary prevention ICD recipients demonstrated that biomarkers of inflammation, myocardial fibrosis, and HF may have the potential to identify patients with a high risk of nonsudden death.25 Because the optimal method for risk stratification of NYHA class III patients is not clear, research in this area should be a high priority.
The use of Bayesian methodology for this study allowed for the opportunity to better understand the finding that, within SCD-HeFT, NYHA III patients did not appear to derive benefit from ICD implantation.3 The results from our study provide evidence suggesting that this finding may be related to some imbalances in the baseline characteristics of NYHA III patient in the ICD versus the medical therapy groups. When comparing the baseline characteristics of NYHA III patients by treatment group, we found that patients randomized to the ICD were older and more commonly had AF, 2 findings that would suggest a higher risk of nonsudden death (and lower likelihood of ICD benefit). In our unadjusted analyses of the SCD-HeFT NYHA III patients, we found a nonsignificant relationship between ICD implantation and all-cause mortality with a point estimate suggesting possible harm (HR 1.30, CI 0.92–1.84). However, when we tested ICD efficacy among SCD-HeFT criteria NYHA III patients in Bayesian models that borrowed patients from other trials, we identified an HR (0.84) and posterior credibility interval (0.66–1.08) suggesting benefit in an underpowered analysis. These conclusions support the use of ICDs among NYHA III patients who meet SCD-HeFT criteria and illustrate that the randomization of slightly sicker NYHA III patients to ICD implantation in SCD-HeFT may be an important part of the explanation for this frequent source of confusion and debate. Of note, it is plausible that the statistically significant difference among placebo versus ICD NYHA III SCD-HeFT patients could be related to chance in the setting of multiple testing (which was not corrected for given the exploratory nature of these analyses).
Another possibility regarding the lack of apparent ICD benefit among the NYHA III SCD-HeFT patients may be related to the comparator group. SCD-HeFT, in contrast to other landmark trials, compared the ICD to a placebo. Although the mechanisms linking placebo use to improved mortality remain controversial,26 it is possible that “placebo benefit” led to improved outcomes in the placebo arm, further limiting the ability to detect a signal for ICD benefit. Notably, among the NYHA III patients across the trials who were not randomized to an ICD, the SCD-HeFT patients had the lowest annual mortality rate (Table V).
Limitations
Although the patients included in this analysis were prospectively enrolled in 1 of 4 randomized trials, this analysis is a nonprespecified retrospective analysis and is subject to the inherent limitations of retrospective studies; however, the data were collected prospectively in the most robust RCTs of primary prevention ICDs. In addition, many patients in this study were implanted before the widespread use of certain commonly used HF therapies, including aldosterone antagonists and cardiac resynchronization therapy as well as contemporary ICD programming, potentially limiting the generalizability of the findings to contemporary HF patients. Additionally, use of β-blockers in these trials was relatively low compared with contemporary standards. Although our study provides valuable data regarding the complexities of ICD use in NYHA class III patients, our adjustment variables were limited to those captured across all trials, and as such, we were unable to include biomarkers, objective measures of exertional capacity (eg, 6-minute walk distance), and HF-related quality of life measures (eg, Kansas City Cardiomyopathy Questionnaire). Our study included fewer NYHA class III patients compared with NYHA class II patients, and as such, it is difficult to determine the extent to which reduced power versus subpopulation heterogeneity contributed to our study findings. Because of the noninclusion of NYHA I patients in SCD-HeFT, our analysis necessarily focused solely on NYHA class II and III patients. Finally, NYHA class assessment is an inherently subjective classification, and class assignment may be prone to several types of biases.
Clinical implications
Our study has a number of important clinical implications. Our study strongly supports the use of primary prevention ICDs in NYHA class II patients who meet guideline-based criteria. The heterogeneous outcomes after primary prevention ICD implants among NYHA class III patients underscore the diversity of patients with NYHA III HF who meet ICD criteria, and the need for improved risk stratification and patient-centered shared decision making for this challenging cohort. The results of this study should not change the current ICD implantation patterns in NYHA III patients, but they highlight the need for a more nuanced discussion about risk and benefit of ICD implantation with NYHA III patients and their caregivers. Finally, the results underscore the importance of careful patient selection and an emerging need to incorporate additional factors (eg, more objective measures of functional status, biomarkers, detailed risk calculators) into the decision-making process.
Conclusions
In a patient-level meta-analysis including patients from 4 pivotal primary prevention ICD trials, primary prevention ICDs reduced mortality in NYHA class II patients and trended toward reducing mortality in NYHA class III patients. The heterogeneity in outcomes among NYHA class III patients appears to be related to differential risk of sudden versus nonsudden death and cardiomyopathy etiology. Improved risk stratification tools are needed to enhance patient selection and shared decision-making strategies among NYHA class III primary prevention ICD candidates.
Supplementary Material
Acknowledgments
Primary funding was provided by the Agency for Healthcare Research and Quality (5 R01 HS018505-03). Drs Friedman and Zeitler were funded by NIH T-32 training grant HL069749. The funding sources had no role in the design, analysis, or interpretation of the data or in the decision to submit the article for publication.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ahj.2017.06.002.
Footnotes
Disclosures: Dr Friedman has received modest research grants from Boston Scientific, significant research grants from the National Cardiovascular Data Registry, modest educational grants from St. Jude Medical and Boston Scientific, and salary support from the NIH T-32 training grant HL069749. All other authors report no relevant disclosures.
References
- 1.Kong MH, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 7.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 8.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-H-F) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 9.Al-Khatib SM, Sanders GD, Mark DB, et al. Implantable cardioverter defibrillators and cardiac resynchronization therapy in patients with left ventricular dysfunction: randomized trial evidence through 2004. Am Heart J. 2005;149:1020–34. doi: 10.1016/j.ahj.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 12.Hess PL, Al-Khatib SM, Han JY, et al. Survival benefit of the primary prevention implantable cardioverter-defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–86. doi: 10.1161/CIRCOUTCOMES.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess PL, Laird A, Edwards R, et al. Survival benefit of primary prevention implantable cardioverter-defibrillator therapy after myocardial infarction: does time to implant matter? A meta-analysis using patient-level data from 4 clinical trials. Heart Rhythm. 2013;10:828–35. doi: 10.1016/j.hrthm.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pun PH, Al-Khatib SM, Han JY, et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. Am J Kidney Dis. 2014;64:32–9. doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail. 2014;2:623–9. doi: 10.1016/j.jchf.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 17.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Singh JP, Hall WJ, McNitt S, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) J Am Coll Cardiol. 2005;46:1712–20. doi: 10.1016/j.jacc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 19.Bilchick KC, Stukenborg GJ, Kamath S, et al. Prediction of mortality in clinical practice for Medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–55. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Guallar E, Blasco-Colmenares E, et al. Clinical and serum-based markers are associated with death within 1 year of de novo implant in primary prevention ICD recipients. Heart Rhythm. 2015;12:360–6. doi: 10.1016/j.hrthm.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbein DP, Hellkamp AS, Mark DB, et al. Use of the 6-min walk distance to identify variations in treatment benefits from implantable cardioverter-defibrillator and amiodarone: results from the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2014;63:2560–8. doi: 10.1016/j.jacc.2014.02.602. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–42. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy WC, Li Y, Reed SD, et al. Does the implantable cardioverter-defibrillator benefit vary with the estimated proportional risk of sudden death in heart failure patients? JACC Clin Electrophysiol. 2017;3:291–8. doi: 10.1016/j.jacep.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadman R, Poole JE, Dardas TF, et al. A novel method to predict the proportional risk of sudden cardiac death in heart failure: derivation of the Seattle Proportional Risk Model. Heart Rhythm. 2015;12:2069–77. doi: 10.1016/j.hrthm.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Zhang Y, Blasco-Colmenares E, et al. Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE-ICD) Circ Arrhythm Electrophysiol. 2014;7:1084–91. doi: 10.1161/CIRCEP.113.001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson IB. Adherence, placebo effects, and mortality. J Gen Intern Med. 2010;25:1270–2. doi: 10.1007/s11606-010-1530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.