Abstract
Objectives
To identify variables associated with successful elective extubation, and to determine neonatal morbidities associated with extubation failure in extremely preterm neonates.
Study design
This study was a secondary analysis of the National Institute of Child Health and Human Development Neonatal Research Network’s Surfactant, Positive Pressure, and Oxygenation Randomized Trial that included extremely preterm infants born at 240/7 to 276/7 weeks’ gestation. Patients were randomized either to a permissive ventilatory strategy (continuous positive airway pressure group) or intubation followed by early surfactant (surfactant group). There were prespecified intubation and extubation criteria. Extubation failure was defined as reintubation within 5 days of extubation.
Results
Of 1316 infants in the trial, 1071 were eligible; 926 infants had data available on extubation status; 538 were successful and 388 failed extubation. The rate of successful extubation was 50% (188/374) in the continuous positive airway pressure group and 63% (350/552) in the surfactant group. Successful extubation was associated with higher 5-minute Apgar score, and pH prior to extubation, lower peak fraction of inspired oxygen within the first 24 hours of age and prior to extubation, lower partial pressure of carbon dioxide prior to extubation, and non-small for gestational age status after adjustment for the randomization group assignment. Infants who failed extubation had higher adjusted rates of mortality (OR 2.89), bronchopulmonary dysplasia (OR 3.06), and death/bronchopulmonary dysplasia (OR 3.27).
Conclusions
Higher 5-minute Apgar score, and pH prior to extubation, lower peak fraction of inspired oxygen within first 24 hours of age, lower partial pressure of carbon dioxide and fraction of inspired oxygen prior to extubation, and nonsmall for gestational age status were associated with successful extubation. Failed extubation was associated with significantly higher likelihood of mortality and morbidities.
Trial registration
ClinicalTrials.gov: NCT00233324.
Mechanical ventilation support is needed for most extremely preterm (EPT) infants (gestational age [GA] <28 weeks] to maintain adequate oxygenation and ventilation.1 The coexistence of lung immaturity, weak respiratory drive, excessively compliant chest wall, and surfactant deficiency often contribute to dependency on mechanical ventilation during the first days or weeks after birth. Prolonged mechanical ventilation is associated with high mortality and morbidities including ventilator-associated pneumonia, pneumothorax, and bronchopulmonary dysplasia (BPD).1–3 Each additional week of mechanical ventilation is associated with an increase in the risk of neurodevelopmental impairment.1 Reduction in the need and duration of invasive mechanical ventilation may potentially improve outcome of preterm infants. This goal may be achieved by the use of noninvasive respiratory support and, among intubated infants, by reducing the duration of mechanical ventilation by successful extubation as early as possible. Large randomized controlled trials have demonstrated that the outcome of neonates supported initially with noninvasive support (such as continuous positive airway pressure [CPAP]) is comparable with those intubated and given early endotracheal surfactant.4,5 Weaning from the ventilator in preterm neonates is quite variable and inconsistent among centers and clinicians who have limited ability to predict extubation readiness.6 The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network’s (NRN) randomized controlled Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) provided specific extubation and reintubation criteria for the 2 randomization groups of permissive ventilation (CPAP group) or ventilation and early surfactant (surfactant group). The variables established for the SUPPORT trial were targeted to minimize ventilation time for the CPAP group, and allow for more time intubated for the surfactant group. At present, there is a paucity of data on the incidence of failed elective extubation and its association with the clinical outcome of preterm infants. Rates of extubation failure may be as high as 40%–50% among extremely preterm infants in some centers.7–9 The incidence of extubation failure varies significantly in studies because of lack of uniform definitions and criteria for extubation and reintubation.
Extubation failure has been independently associated with increased mortality, longer hospitalization, and more days on oxygen and ventilatory support.10–12 It is critical, therefore, to attempt extubation early and at a time when successful extubation is likely. Identifying factors associated with successful extubation may help reduce the duration of mechanical ventilation, improve outcomes, as well as help in designing future research studies to improve the outcomes of ventilated preterm infants.
We used a cohort of EPT infants enrolled in the SUPPORT randomized controlled trial of permissive ventilation strategy (CPAP) vs endotracheal intubation followed by surfactant (surfactant) to identify clinical variables that were associated with a successful first elective extubation and to evaluate mortality and short-term morbidities associated with extubation failure after elective extubation. The primary hypothesis was that perinatal and peri-extubation characteristics are associated with successful extubation among EPT infants. The secondary hypothesis was that failed extubation would be associated with higher mortality and neonatal morbidities among these infants.
Methods
This was a secondary analysis of pre-existing data from the SUPPORT trial conducted at the participating sites of NICHD NRN (ClinicalTrials.gov: NCT00233324). Institutional review board approval and parental consent was obtained for the main SUPPORT trial. Patients were eligible for the SUPPORT trial if they were (1) 240/7 to 276/7 weeks by best obstetric estimate; (2) born without known malformations; and (3) if a decision had been made to provide full resuscitation for them after written informed consent had been obtained from a parent. Enrolled subjects were randomized before delivery to either noninvasive respiratory support and a permissive ventilatory strategy (CPAP group) or intubation followed by early surfactant (surfactant group). Infants were also randomized to different oxygen saturation targets (85%–89% and 91%–95%). For the current study, all infants enrolled in the SUPPORT trial who were endotracheally intubated within the first 24 hours of postnatal age were included. Infants who died before an extubation attempt, had accidental extubation, or were transferred/discharged home prior to an elective extubation attempt were excluded.
Detailed data were collected for baseline characteristics (GA, birth weight, sex, race, hypertensive disorders of pregnancy, acute chorioamnionitis, prolonged rupture of membrane [>18 hours], mode of delivery, multiple births, antenatal steroids administration, resuscitation in the delivery room), and respiratory support on day 1. Successful extubation was defined as survival without the need for respiratory support with an endotracheal tube for more than 5 days. Peri-extubation characteristics were collected, including age at extubation, ventilator support (fraction of inspired oxygen [FiO2], pH, and partial pressure of carbon dioxide [PCO2] prior to extubation), and postextubation respiratory support. An electronically altered pulse oximeter (Masimo Radical Pulse Oximeter; Masimo, Radical, Yorba, California), which had a maximum variation of 3%, was used in SUPPORT trial until 36 weeks’ postmenstrual age (PMA) for both lower (85%–89%) and higher (91%–95%) target saturation groups.
Criteria for intubation, extubation, and reintubation were different in the 2 study groups during the first 2 weeks of age. Infants in the CPAP group could be intubated if they met any of the following criteria: FiO2 >0.50 required to maintain oxygen saturation at or above 88% using the study electronically altered pulse oximeter, PCO2 >65 mm Hg, or hemodynamic instability. Intubation could also be performed at any time for repetitive apnea requiring positive pressure ventilation, clinical shock, sepsis, and/or the need for surgery. Extubation of an infant in the CPAP group was to be attempted within 24 hours after the infant met all of the following criteria: a PCO2 below 65 mm Hg with a pH higher than 7.20; an oxygen saturation at or above 88% using the altered study pulse oximeter with an FiO2 less than or equal to 0.50; a mean airway pressure of less than 10 cm H2O; a ventilator rate of less than or equal to 20 breaths per minute; an amplitude of less than twice the mean airway pressure if high-frequency oscillatory ventilation was being used; hemodynamic stability; and the absence of clinically significant patent ductus arteriosus. Criteria for reintubation were the same as those for initial intubation.
All patients in the surfactant group were to be intubated in the delivery room and receive surfactant within 1 hour of birth. The infants were to be extubated within 24 hours after meeting the following criteria: a PCO2 of less than 50 mm Hg and a pH greater than 7.30; an FiO2 ≤0.35 with a blood oxygen saturation level of 88% or higher using the altered study pulse oximeter; a mean airway pressure less than 8 cm H2O; a ventilator rate of 20 breaths per minute or less; an amplitude of less than twice the mean airway pressure if high-frequency oscillatory ventilation was being used; and hemodynamic stability without evidence of clinically significant patent ductus arteriosus. Infants randomized to the surfactant group could be reintubated by standard of care guidelines of the participating institution.
The primary outcome was successful extubation for greater than 5 days. Secondary outcomes were BPD, late onset sepsis, death and/or BPD, length of hospital stay, days on mechanical ventilation and days on supplemental oxygen among survivors. BPD was defined as the use of supplemental oxygen at 36 weeks’ PMA or discharge.13 Sepsis was defined as a positive blood culture after 72 hours of age and antibiotic administration for minimum of 5 days, or less if the patient died. Severe intracranial hemorrhage (ICH) was defined as ventricular enlargement with concurrent or prior blood in the ventricles and/or a cerebral parenchymal bleed.
Statistical Analyses
To study the incidence and predictors of successful extubation, we included only the first extubation attempt. We compared the perinatal and peri-extubation characteristics of successfully extubated infants with those who failed extubation. Categorical characteristics were compared between the 2 groups using χ2 or Fisher exact tests, and continuous characteristics were analyzed using t tests or Wilcoxon rank sum tests. A generalized linear mixed model was created to determine factors associated with successful extubation. Independent variables in the model were selected based on clinical and statistical association with extubation failure (P values of <.10 in bivariate tests). Center was included as a random effect. Surfactant or CPAP group assignment was also included as an independent variable. We also performed a subgroup analysis and created a generalized linear mixed model to determine factors associated with successful extubation for the CPAP and surfactant groups separately.
Patients in the successful and failed extubation groups were compared for neonatal morbidities including death, BPD, death/BPD, severe ICH, sepsis, length of hospital stay, days on mechanical ventilation with endotracheal tube and days on oxygen support (among survivors). Among those neonates who failed extubation, we explored the timing of extubation failure using a Kaplan-Meier survival curve.
Results
Of 1316 infants in the SUPPORT trial, 1071 (81%) infants were intubated in the first 24 hours after birth, and 926 infants had data available for extubation success/failure outcome. Of the 926 infants, 538 (58%) of the extubations were successful and 388 (41.9%) failed. Fifty percent (188/374) of those who were in the CPAP group and were intubated, and 63% (350/552) of those in the surfactant group were successfully extubated.
Neonates in the failed extubation group had lower birth weight (mean ± SD 764 ± 177 vs 882 ± 180 g), GA (25.8 ± 1.0 vs 26.5 ± 1.0 week), rate of prolonged rupture of membranes (50% vs 61%), 5-minute Apgar score (median [IQR], 7 [5–8] vs 7 [6–8], higher FiO2 in first 24 hours of age [36% vs 29%], surfactant doses (2 ± 1.15 vs 1.5 ± 0.89), randomization to CPAP group (48% vs 35%), and were more likely to be small for gestational age (SGA) (Table I).
Table I.
Characteristics of infants in successful and failed extubation groups
| Characteristics Mean (SD), median (25th, 75th percentile), or n/N (%) |
Successful extubation n = 538 | Failed extubation n = 388 | P value |
|---|---|---|---|
| Birth weight (g) | 882 (180) | 764 (177) | <.01 |
| GA (wk) | 26.5 (1.04) | 25.8 (1.04) | <.01 |
| Male | 295/538 (55%) | 237/388 (61%) | .058 |
| SGA (<10th percentile on Alexander growth curve) | 20/538 (3.7%) | 39/388 (10.1%) | <.01 |
| Antenatal steroids | |||
| None | 25/538 (4.7%) | 13/388 (3.4%) | .042 |
| Partial | 155/538 (29%) | 87/388 (22%) | |
| Complete course | 358/538 (67%) | 288/388 (74%) | |
| Prolonged rupture of membranes | 194/317 (61%) | 105/210 (50%) | .011 |
| DR chest compression | 23/538 (4.3%) | 38/385 (9.9%) | <.01 |
| DR epinephrine | 11/538 (2.0%) | 17/385 (4.4%) | .038 |
| Surfactant doses | 1.50 (0.89) | 2.04 (1.15) | <.01 |
| Highest FiO2—first 24h | 0.29 (0.14) | 0.36 (0.18) | <.01 |
| Randomization group (Surfactant) | 350/538 (65%) | 202/388 (52%) | <.01 |
| Apgar score—5 min | 7 (6, 8) | 7 (5, 8) | <.01 |
DR, delivery room.
More neonates received delivery room chest compression and epinephrine in the failed extubation group.
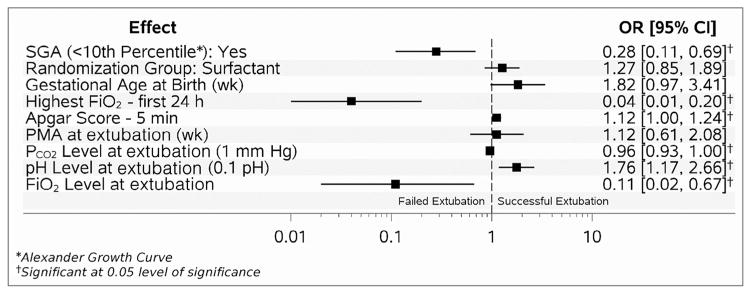
Table II presents the unadjusted comparison of peri-extubation clinical and respiratory variables in the failed and successful extubation groups. Neonates in the successful extubation group had higher PMA, pH, and lower PCO2 and FiO2 prior to extubation compared with neonates in the failed group. Multivariable analysis showed that higher 5-minute Apgar score (OR 1.12; 95% CI 1.00, 1.24), and higher pH prior to extubation (OR 1.76; 95% CI 1.17, 2.66) were associated with successful extubation after controlling for clinical center and study randomization group. SGA status (OR 0.28; 95% CI 0.11, 0.69), higher FiO2 at 24 hours after birth (OR 0.04; 95% 0.01, 0.20), higher FiO2 prior to extubation (OR 0.11; 95% CI 0.02, 0.67), and higher PCO2 prior to extubation (OR 0.96; 95% CI 0.93, 1.00) were associated with failed extubation (Figure 1). The logistic regression model had an area under the curve of 0.84.
Table II.
Peri-extubation characteristics of successful vs failed extubation groups
| Characteristics Mean (SD), median (25th, 75th percentile), or n/N (%) |
Successful extubation n = 538 | Failed extubation n = 388 | P value |
|---|---|---|---|
| PMA at extubation (wk) | 27.5 (1.79) | 27.0 (2.12) | <.01 |
| Age at extubation (d) | 2 (2, 6) | 3 (2, 9) | <.01 |
| Pre-extubation characteristics: | |||
| FiO2 | 0.26 (0.11) | 0.38 (0.22) | <.01 |
| pH | 7.37 (.07) | 7.29 (0.13) | <.01 |
| pCO2 (mm Hg) | 40.1 (9.19) | 50.1 (16.6) | <.01 |
| First postextubation level of support (nasal IMV) | 74/536 (13.8%) | 78/332 (23.5%) | <.01 |
IMV, intermittent mechanical ventilation.
Figure 1.
Adjusted markers for successful extubation (AUC 0.84). AUC, area under the curve.
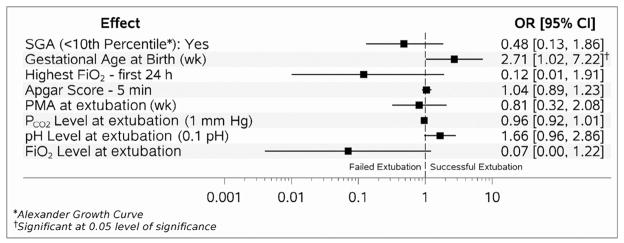
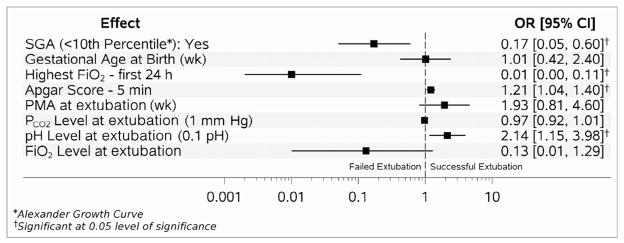
Factors associated with extubation success in the multivariable analysis performed for the surfactant and CPAP group separately are shown in Figures 2 and 3 (available at www.jpeds.com).
Figure 2.
Adjusted markers for successful extubation for surfactant group (AUC 0.85).
Figure 3.
Adjusted markers for successful extubation for CPAP group (AUC 0.84).
In the surfactant group, only GA was significantly associated with the likelihood of successful extubation (OR 2.71; 95% CI 1.02, 7.22) (Figure 2). In the CPAP group, higher 5-minute Apgar score (OR 1.21; 95% CI 1.04, 1.40) and pH prior to extubation (OR 2.14; 1.15, 3.98) were associated with higher likelihood of successful extubation, and higher peak FiO2 within first 24 hours of age (OR 0.01; 95% 0.00, 0.11) and SGA status (OR 0.17; 95% CI 0.05, 0.60) were associated with lower likelihood of successful extubation (Figure 3).
Among those who failed extubation within 5 days, most (75%) were reintubated within 2 days (Figure 4). Among the successful group (n = 538) that included infants who remained extubated for at least 5 days, 243 (45%) were ultimately reintubated. The median and IQR of the reintubation time (in days) were 11.5 (7.4, 18.6).
Figure 4.
Kaplan-Meier curve of time to failed extubation (days).
In bivariate analysis, extubation failure was associated with a 5-fold increase in mortality, higher rate of BPD, BPD or death, severe ICH, and sepsis (Table III). Among survivors, infants in the failed extubation group had longer hospital stays and more days on oxygen and mechanical ventilation compared with the successful extubation group. After adjustment for SGA status, randomization group (CPAP vs surfactant group), postextubation respiratory support, GA, FiO2 at 24 hours of age, 5-minute Apgar score, and PMA at extubation, failed extubation was associated with a higher rate of mortality (OR 2.89; 95% CI 1.73, 4.83), BPD (OR 3.06; 95% CI 2.11, 4.44), combined outcome of BPD or death (OR 3.27, 95% CI 2.31,4.64), and severe ICH (OR 2.28; 95% CI 1.42,3.66).
Table III.
Neonatal outcomes of infants in successful vs failed extubation groups
| Outcomes | Successful extubation n = 538 (n/N [%] or mean [SD]) | Failed extubation n = 388 (n/N [%] or mean [SD]) | Unadjusted OR or mean difference and 95% CI | aOR or mean difference and 95% CI |
|---|---|---|---|---|
| Death before discharge | 31/538 (5.7%) | 109/388 (28%) | 6.59 (4.28, 10.16) | 2.89 (1.73, 4.83) |
| BPD | 156/514 (30%) | 188/295 (64%) | 4.04 (2.92, 5.59) | 3.06 (2.11, 4.44) |
| Death or BPD | 180/538 (33%) | 281/388 (72%) | 5.34 (3.95, 7.22) | 3.27 (2.31, 4.64) |
| ICH grade III/IV | 40/536 (7.5%) | 86/375 (23%) | 3.68 (2.43, 5.57) | 2.28 (1.42, 3.66) |
| Length of time (d) on oxygen (among survivors) | 48.9 (34.6) | 79.5 (33.8) | −29.3 (−34.1, −24.4) | −17.9 (−22.3, −13.5) |
| Length of time (d) on mechanical ventilation (among survivors) | 14.5 (18.8) | 39.9 (27.7) | −23.3 (−26.5, −20.1) | −16.2 (−19.0, −13.5) |
| Length of hospital stay (among survivors) | 93.9 (35.8) | 117.4 (43.3) | −23.7 (−29.4, −18.0) | −11.8 (−17.3, −6.3) |
| Late onset sepsis | 172/538 (32%) | 157/367 (43%) | 1.62 (1.22, 2.16) | 1.14 (0.82, 1.59) |
Data on the occurrence and timing of severe ICH was collected for all infants till 14 days of age. Of those infants who were extubated within the first 14 days of age (n = 778), 36 had severe ICH diagnosed on or prior to the day of extubation: 7 (2%) in the successful extubation group, compared with 29 (9%) in the failed group. Forty-two infants had severe ICH diagnosed after the day of extubation: 13 (3%) in the successful group compared with 29 (9%) in the failed group.
Discussion
In a cohort of extremely preterm infants who participated in the NICHD NRN SUPPORT study, we noted a high rate of failed elective first extubation (41.9%). Higher 5-minute Apgar score, and pH prior to extubation, lower peak FiO2 within first 24 hours of age, lower PCO2 and FiO2 prior to extubation, and non-SGA status were associated with successful extubation. The area under the receiver operator characteristic curve for a model for extubation success was moderate (0.84). Because of lack of good evidence-based prediction tools for extubation success, weaning ventilatory variables and the decision to extubate are currently based on clinician preference and influenced by clinical evaluation, blood gas variables, oxygen needs, and level of ventilator support.14 Preterm neonates may fail extubation because of a combination of reasons including increased work of breathing, significant apnea and bradycardia, low oxygen saturation, respiratory acidosis, and upper airway narrowing.12
Our results are consistent with the recent study by Manley et al9 of extremely preterm infants (n = 174, <28 weeks GA), which showed that higher GA and lower pre-extubation PaCO2 predicted extubation success.9 Lower GA, prolonged ventilation (>2 weeks), low 5-minute Apgar score, low pre-extubation blood pH, and extubation from higher ventilatory settings and failed spontaneous breathing test have been noted to be associated with extubation failure.12,15,16
In comparison with published literature, our study found that the need for lower FiO2 at 24 hours of age and non-SGA-status were also associated with extubation success. We speculate that high FiO2 requirement on day 1 of life could be a marker of severity of respiratory distress syndrome and brain immaturity, and SGA-status could be associated with poor lung growth. We noted that blood gas pH was significantly predictive of extubation readiness in the CPAP group, but not in the surfactant group. For clinicians following an aggressive extubation strategy, such as in the CPAP group of the SUPPORT trial, blood gas pH prior to extubation is an important marker for extubation readiness in addition to intrinsic infant characteristics.
The definition of extubation failure in previous studies has been quite variable among investigators ranging from 2 to 7 days after extubation.6 Among neonates who experienced extubation failure within 5 days, most (75%) were reintubated less than 2 days after extubation (Figure 4). Restriction to 3–5 days to define the incidence of extubation failure may capture most failed extubations and avoid inclusion of new onset morbidities as a cause of extubation failure.
We also noted that infants who failed extubation had higher rates of neonatal morbidities, including BPD, death, and combined outcome of BPD/death. Among survivors, the failed extubation group had longer hospital stay and more days on oxygen and mechanical ventilation compared with the successful extubation group.
Our results are consistent with a study by Baisch et al11 on older children (n = 130) in pediatric intensive care unit settings that found failed extubation to be associated with longer hospital stay and ventilation courses. Manley et al9 also noted that among extremely preterm (GA <28 weeks, n = 174) infants, those who failed extubation were more likely to die and to need prolonged respiratory support and hospitalization.
In addition to higher respiratory morbidity among those who failed extubation, we noted a higher rate of severe ICH. The relationship between failed extubation and this morbidity is not known. We do not know whether severe ICH contributed to the extubation failure, occurred because of extubation failure, or was just an association.
Whether failed extubation produces a respiratory setback because of ineffective breathing that causes lung atelectasis or is just a marker of an inherent baseline difference in the groups of failed and successful infants cannot be determined from our data. Failed extubation has been associated with requirement of significantly higher respiratory support 24 hours after reintubation compared with pre-extubation support among EPT neonates.12 Endotracheal reintubation is not an easy or benign process. Recent studies have reported rates of successful intubation between 60% and 73% in preterm neonates, as well as long time (51 ± 28 seconds) needed for intubation.17,18 Endotracheal intubation is associated with discomfort to the patient and may result in malposition of the tube, trauma to the airway, and hemodynamic instability.17,19–22 Endotracheal intubations have also been associated with alterations in brain function as monitored by electroencephalography.23
As extubation failure is associated with significant respiratory setback, neonatal morbidities, including BPD and higher mortality, the decision to electively extubate an extremely preterm infant needs to be carefully considered. Clinical status, blood gas variables including pH, PCO2, and level of respiratory support needed may provide guidance on the probability of successful extubation. Elective extubation in a patient not ready for extubation may lead to reintubation and an increase in neonatal morbidities.
At present, there is paucity of data on predictors of successful extubation and its effect on the clinical outcome of preterm infants. This study provides data in a large cohort of patients to reduce the knowledge gap in this area. Understanding clinical factors associated with extubation failure (pH, FiO2, and PCO2 prior to extubation) may provide guidance to clinicians to decide extubation readiness of extreme preterm infants. Understanding the mortality and morbidities associated with extubation failure stresses the need to identify neonates ready for extubation, as well as the need for research to explore the interventions to improve the success of extubation among preterm infants needing mechanical ventilation.
There are limitations in the current study. Even though we adjusted for important and significant clinical variables, there may be unknown differences in the failed and successful extubation groups, which may be associated with both failed extubation as well as the neonatal morbidities. There were differences in the intubation, extubation, and reintubation criteria in the 2 randomized groups. We adjusted for the randomization group in our analysis. We are not able to provide a cutoff for pCO2, pH, or FiO2 prior to extubation to predict extubation success as these factors were part of the intubation and extubation criteria specified for the SUPPORT CPAP and surfactant groups. This study focuses only on the initial extubation failure; in clinical practice, infants may have repeated intubation and extubation episodes. We did not have information on some pre-extubation respiratory support characteristics or on the use of caffeine before and after extubation in the study cohort. A recent systematic review noted that prophylactic methylxanthines reduce the rate of extubation failure in premature infants.24 We noted higher use of noninvasive positive pressure ventilation (NIPPV) in the group that failed extubation (23.5%) compared with the successful extubation group (13.8%). Use of NIPPV has been associated with higher rates of successful extubation in randomized controlled trials.24 As the current study did not control the use of respiratory support postextubation, we think that the higher use of NIPPV among the failed extubation group was related to the level of immaturity and sickness of the patients. Whether the association between extubation failure and morbidities such as severe ICH were related to the extubation procedure itself could not be determined from these data.
The strengths of the study include the size of the study population and use of prospectively collected data with well-defined, a priori definitions of various morbidities in a multicenter randomized controlled trial. The guidelines provided for extubation and reintubation in the study protocol helped to minimize variation in the approach of clinicians and allowed for the identification of other factors associated with extubation failure. Data from the current study provide potentially useful information on the associations between early clinical factors and extubation failure, along with an indication of their relative significance.
In conclusion, extubation failure is common (42%) in extremely preterm infants. Successful extubation for a minimum of 5 days in extremely preterm infants who participated in SUPPORT trial was associated with non-SGA status, higher 5-minute Apgar score, lower peak FiO2 within the first 24 hours of age, lower FiO2 prior to extubation, and higher pH and lower PCO2 at the time of extubation. Infants who failed extubation had significantly higher rates of mortality and neonatal morbidities, including BPD, BPD/death, and severe ICH. The latter findings underscore the need for further research to define extubation practices that result in the earliest possible extubation while minimizing extubation failure; our data can guide design of such studies.
Acknowledgments
Supported by The National Institutes of Health (NIH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Heart, Lung, and Blood Institute (NHLBI), the National Center for Research Resources, and the National Center for Advancing Translational Sciences. Full financial support information available at www.jpeds.com (Appendix). Participating Neonatal Research Network (NRN) sites collected and transmitted data to RTI International, the data coordinating center (DCC) for the network, which stored and managed the data for this study.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. One behalf of the NRN, Drs Abhik Das (DCC Principal Investigator) and Marie Gantz (DCC Statistician) had full access to all of the data in the study, and with the NRN Center Principal Investigators, take responsibility for the integrity of the data and accuracy of the data analysis.
Glossary
- BPD
Bronchopulmonary dysplasia
- CPAP
Continuous positive airway pressure
- EPT
Extremely preterm
- FiO2
Fraction of inspired oxygen
- GA
Gestational age
- ICH
Intracranial hemorrhage
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- PCO2
Partial pressure of carbon dioxide
- PMA
Postmenstrual age
- SGA
Small for gestational age
- SUPPORT
Surfactant, Positive Pressure, and Oxygenation Randomized Trial
Appendix
The following investigators are additional members of the National Institute of Child Health and Human Development Neonatal Research Network, National Institutes of Health, Bethesda, MD:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011);
Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women and Infants Hospital of Rhode Island (U10 HD27904)—Abbot R. Laptook, MD; William Oh, MD; Angelita M. Hensman, RN BSN; Dan Gingras, RRT; Susan Barnett, RRT; Sarah Lillie, RRT; Kim Francis, RN; Dawn Andrews, RN; Kristen Angela, RN.
Case Western Reserve University Rainbow Babies and Children’s Hospital (U10 HD21364, M01 RR80)—Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center University of Cincinnati Hospital and Good Samaritan Hospital (U10 HD27853, M01 RR8084)—Kurt Schibler, MD; Edward F. Donovan, MD; Vivek Narendran, MD MRCP; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN.
Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30)—Ronald N. Goldberg, MD; Kathy J. Auten, MSHS; Kimberly A. Fisher, PhD, FNP-BC, IBCLC; Katherine A. Foy, RN; Gloria Siaw, BSN CRA.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, CTSA UL1 RR25008, M01 RR39)—Barbara J. Stoll, MD; Susie Buchter, MD; Anthony Piazza, MD; David P. Carlton, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development—Stephanie Wilson Archer, MA.
Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750)—Brenda B. Poindexter, MD MS; James A. Lemons, MD; Faithe Hamer, BS; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie D. Wilson, BSN CCRC.
National Heart, Lung, and Blood Institute—Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD.
RTI International (U10 HD36790)—W. Kenneth Poole, PhD (deceased); Margaret Cunningham, BS; Betty K. Hastings; Amanda R. Irene, BS; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W. Pickett II, BS; Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University Lucile Packard Children’s Hospital (U10 HD27880, CTSA UL1 RR25744, M01 RR70)—Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC, Melinda S. Proud, RCP.
Tufts Medical Center Floating Hospital for Children (U10 HD53119, M01 RR54)—Ivan D. Frantz III, MD; John M. Fiascone, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32)—Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. Vivien A. Phillips, RN BSN.
University of California—San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461)—Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Wade Rich, RRT; Kathy Arnell, RNC; Renee Bridge, RN; Clarence Demetrio, RN.
University of Iowa (U10 HD53109, CTSA UL1 RR24979, M01 RR59)—Edward F. Bell, MD; John A. Widness, MD; Jonathan M. Klein, MD; Karen J. Johnson, RN BSN.
University of Miami Holtz Children’s Hospital (U10 HD21397, M01 RR16587)—Shahnaz Duara, MD; Ruth Everett-Thomas, RN MSN.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997)—Kristi L. Watterberg, MD; Robin K. Ohls, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN.
University of Rochester Medical Center Golisano Children’s Hospital (U10 HD40521, M01 RR44)—Dale L. Phelps, MD; Nirupama Laroia, MD; Linda J. Reubens, RN CCRC; Erica Burnell, RN.
University of Texas Southwestern Medical Center at Dallas Parkland Health and Hospital System and Children’s Medical Center Dallas (U10 HD40689, M01 RR633)—Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; James Allen, RRT; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Lepps, RN; Melissa Martin, RN; Nancy A. Miller, RN; Araceli Solis, RRT; Diana M Vasil, RNC-NIC; Kerry Wilder, RN.
University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (U10 HD21373)–Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Brenda H. Morris, MD; Beverly Foley Harris, RN BSN; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Patti L. Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64)—Bradley A. Yoder, MD; Roger G. Faix, MD; Jill Burnett, RN; Jennifer J. Jensen, RN BSN; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN BSN.
Wake Forest University Baptist Medical Center Brenner Children’s Hospital and Forsyth Medical Center (U10 HD40498, M01 RR7122)—T. Michael O’Shea, MD MPH; Nancy J. Peters, RN CCRP.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385)—Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Mary Johnson, RN BSN.
Yale University Yale-New Haven Children’s Hospital and Bridgeport Hospital (U10 HD27871, CTSA UL1 RR24139, MO1 RR125, M01 RR6022)—Richard A. Ehrenkranz, MD; Harris C. Jacobs, MD; Vineet Bhandari, MD DM; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN.
Footnotes
The authors declare no conflicts of interest.
Portions of this study were presented during the Pediatric Academic Societies, Baltimore, MD, April 30–May 3, 2016.
References
- 1.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Bancalari E, Gerhardt T. Bronchopulmonary dysplasia. Pediatr Clin North Am. 1986;33:1–23. doi: 10.1016/s0031-3955(16)34967-7. [DOI] [PubMed] [Google Scholar]
- 3.Strong RM, Passy V. Endotracheal intubation. Complications in neonates. Arch Otolaryngol. 1977;103:329–35. doi: 10.1001/archotol.1977.00780230051006. [DOI] [PubMed] [Google Scholar]
- 4.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 5.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Mandari H, Shalish W, Dempsey E, Keszler M, Davis PG, Sant’Anna G. International survey on periextubation practices in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2015;100:F428–31. doi: 10.1136/archdischild-2015-308549. [DOI] [PubMed] [Google Scholar]
- 7.Stefanescu BM, Murphy WP, Hansell BJ, Fuloria M, Morgan TM, Aschner JL. A randomized, controlled trial comparing two different continuous positive airway pressure systems for the successful extubation of extremely low birth weight infants. Pediatrics. 2003;112:1031–8. doi: 10.1542/peds.112.5.1031. [DOI] [PubMed] [Google Scholar]
- 8.Hermeto F, Bottino MN, Vaillancourt K, Sant’Anna GM. Implementation of a respiratory therapist-driven protocol for neonatal ventilation: impact on the premature population. Pediatrics. 2009;123:e907–16. doi: 10.1542/peds.2008-1647. [DOI] [PubMed] [Google Scholar]
- 9.Manley BJ, Doyle LW, Owen LS, Davis PG. Extubating extremely preterm infants: predictors of success and outcomes following failure. J Pediatr. 2016;173:45–9. doi: 10.1016/j.jpeds.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–92. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 11.Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med. 2005;6:312–8. doi: 10.1097/01.PCC.0000161119.05076.91. [DOI] [PubMed] [Google Scholar]
- 12.Chawla S, Natarajan G, Gelmini M, Kazzi SN. Role of spontaneous breathing trial in predicting successful extubation in premature infants. Pediatr Pulmonol. 2013;48:443–8. doi: 10.1002/ppul.22623. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 14.Kamlin CO, Davis PG, Morley CJ. Predicting successful extubation of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F180–3. doi: 10.1136/adc.2005.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermeto F, Martins BM, Ramos JR, Bhering CA, Sant’Anna GM. Incidence and main risk factors associated with extubation failure in newborns with birth weight <1,250 grams. J Pediatr (Rio J) 2009;85:397–402. doi: 10.2223/JPED.1922. [DOI] [PubMed] [Google Scholar]
- 16.Costa AC, de Schettino RC, Ferreira SC. Predictors of extubation failure and reintubation in newborn infants subjected to mechanical ventilation. Rev Bras Ter Intensiva. 2014;26:51–6. doi: 10.5935/0103-507X.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bismilla Z, Finan E, McNamara PJ, LeBlanc V, Jefferies A, Whyte H. Failure of pediatric and neonatal trainees to meet Canadian Neonatal Resuscitation Program standards for neonatal intubation. J Perinatol. 2010;30:182–7. doi: 10.1038/jp.2009.152. [DOI] [PubMed] [Google Scholar]
- 18.Kamlin CO, O’Connell LA, Morley CJ, Dawson JA, Donath SM, O’Donnell CP, et al. A randomized trial of stylets for intubating newborn infants. Pediatrics. 2013;131:e198–205. doi: 10.1542/peds.2012-0802. [DOI] [PubMed] [Google Scholar]
- 19.Walner DL, Loewen MS, Kimura RE. Neonatal subglottic stenosis—incidence and trends. Laryngoscope. 2001;111:48–51. doi: 10.1097/00005537-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesh V, Ponnusamy V, Anandaraj J, Chaudhary R, Malviya M, Clarke P, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011;170:223–7. doi: 10.1007/s00431-010-1290-8. [DOI] [PubMed] [Google Scholar]
- 21.Schuman TA, Jacobs B, Walsh W, Goudy SL. Iatrogenic perinatal pharyngoesophageal injury: a disease of prematurity. Int J Pediatr Otorhinolaryngol. 2010;74:393–7. doi: 10.1016/j.ijporl.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Schedlbauer EM, Todt I, Ernst A, Seidl RO. Iatrogenic tracheal rupture in children: a retrospective study. Laryngoscope. 2009;119:571–5. doi: 10.1002/lary.20107. [DOI] [PubMed] [Google Scholar]
- 23.Shangle CE, Haas RH, Vaida F, Rich WD, Finer NN. Effects of endotracheal intubation and surfactant on a 3-channel neonatal electroencephalogram. J Pediatr. 2012;161:252–7. doi: 10.1016/j.jpeds.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson KN, Roberts CT, Manley BJ, Davis PG. Interventions to improve rates of successful extubation in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 2016;171:165–74. doi: 10.1001/jamapediatrics.2016.3015. [DOI] [PubMed] [Google Scholar]