Abstract
Lynch Syndrome is a hereditary cancer syndrome caused by a germline mutation in a DNA mismatch repair gene, usually MLH1, MSH2, MSH6, or PMS2. The most common cancers associated with Lynch Syndrome are colorectal adenocarcinoma and endometrial carcinoma. Identification of women with Lynch Syndrome-associated endometrial cancer is important, as these women and their affected siblings and children are at-risk of developing these same cancers. Germline testing of all endometrial cancer patients is not cost effective, and screening using young age of cancer diagnosis and/or presence of family history of syndrome-associated is underutilized and ineffective. Therefore, most groups now advocate for tumor tissue testing to screen for Lynch Syndrome, with germline testing targeted to women with abnormal tissue testing results. Immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 is used in many clinical laboratories for this tumor screening step, as immunohistochemistry is relatively inexpensive and is technically more accessible for smaller clinical labs. PCR-based tissue testing, while technically more challenging, does play an important role in the identification of these patients. MLH1 methylation analysis identifies women with tumor MLH1 loss who likely have sporadic endometrial cancer and do not need heightened cancer prevention surveillance. High levels of microsatellite instability have been identified in tumors with retained positive expression of mismatch repair proteins. Somatic sequencing of mismatch repair genes from tumor DNA, while not currently available in most clinical laboratories, is helpful in resolution of cases in which germline sequencing fails to identify a mutation in a mismatch repair gene. The tumor tissue testing approach can help to identify most women at-risk for germline mutations in a Lynch Syndrome gene, but not all patients will be captured using this approach. Clinical suspicion can still play a pivotal role in accurately identifying a subset of these patients.
Keywords: Endometrial cancer, Lynch Syndrome, microsatellite instability, MLH1 methylation, mismatch repair gene sequencing
Introduction
Lynch Syndrome (LS), also known as hereditary non-polyposis colorectal cancer (HNPCC), is an autosomal dominant, hereditary cancer syndrome typified by increased prevalence of colorectal and endometrial adenocarcinomas among affected probands. In addition to these two cancers, other Lynch Syndrome associated tumors (LATs) include small intestine, hepatobiliary tract, ovarian, urinary tract, brain and skin.1 For women with germline mutations, the lifetime risk of developing either colorectal cancer or endometrial cancer is 73.4%.2 Additionally, women with germline mutations are at least equally as likely to present with a gynecologic cancer as their sentinel cancer diagnosis as they are colorectal cancer.3 Individuals with Lynch Syndrome harbor a mutation in DNA mismatch repair (MMR) genes, MLH1, MSH2, MSH6, PMS2 and EPCAM. In an unaffected individual, the DNA MMR system identifies and repairs DNA mismatches post-replication.4 An EPCAM mutation causes secondary methylation of the MSH2 gene, causing loss of MSH2 protein. Identifying an individual with Lynch Syndrome at the time of his/her sentinel cancer diagnosis allows for enhanced screening for other Lynch Syndrome associated tumors and risk-reducing surgeries, such as hysterectomy for women.5 In addition, once a Lynch Syndrome mutation is identified, family members can be tested and undergo enhanced screening to prevent or detect cancers early.
Screening Criteria
Prior to the availability of tumor-based screening tests, clinically based screening criteria were developed to help identify individuals at increased risk for having Lynch Syndrome. These criteria have largely emerged from information collected via familial colorectal cancer registries and rely heavily on the presence of colon cancer among probands and their families. Young age at cancer diagnosis, significant family history of colorectal or endometrial cancer, and right-sided colon cancer tumors are key clinical traits incorporated into many of the existing criteria.6 The two most commonly used clinical screening criteria are Amsterdam II and the Revised Bethesda Criteria (Table 1). The Amsterdam II criteria were intended to identify individuals with Lynch Syndrome based on clinical history alone, do not fully recognize extra-colonic tumors, and maximize specificity (78–91%) at the cost of sensitivity (39–72%).7–10 The Revised Bethesda Criteria were developed to identify which patients would benefit from proceeding with tumor-based screening and have a higher sensitivity (82–94%) and lower specificity (25–41%) for detecting germline mutations.7,8,11–13 In 2007, the Society of Gynecologic Oncology (SGO) published a statement with clinical criteria for which gynecologic cancer patients would benefit from further evaluation for Lynch Syndrome, which was similar to Amsterdam II and Revised Bethesda criteria (Table 2).14 A study performed by our group evaluated 412 sequential, unselected endometrial cancer patients, comparing SGO clinical screening criteria to universal tumor testing, and found the sensitivity and specificity for detecting tumors at elevated risk for Lynch Syndrome were 32.6% and 77%, respectively.15 The clinically based screening criteria performed better at identifying women with increased risk for MLH1 and MSH2 mutations and performed poorly at identifying tumors with increased risk for MSH6 and PMS2 mutations. Other studies have also shown that individuals with germline mutations in MSH6 and PMS2 are more likely to have an older age at diagnosis and family histories that are dominated by gynecologic cancer or have less prevalent colon cancers.15–18 In addition to the limitations of these available screening criteria, patients with concerning family histories for Lynch Syndrome are not being universally screened by providers.19
Table 1.
Summary of Amsterdam II and Revised Bethesda Criteria for the Evaluation of Lynch Syndrome.13
| Amsterdam II (All criteria must be met) |
Revised Bethesda |
|---|---|
| At least 3 relatives with a Lynch Syndrome associated cancer; one should be a first-degree relative of the other 2 | Colorectal cancer diagnosed at age < 50 |
| Familial adenomatous polyposis has been ruled out | Synchronous, metachronous or other Lynch Syndrome related tumors |
| Two affected generations | MSI-H colorectal cancer in a pt <60 |
| At least one Lynch Syndrome associated cancer should be diagnosed < age 50 | Colorectal cancer in ≥ 1 first-degree relatives with a Lynch Syndrome related tumor, one occurring at age <50 |
| Colorectal cancer in ≥ 2 first- or second-degree relatives with Lynch Syndrome related tumors |
Table 2.
Society of Gynecologic Oncology (SGO) Criteria for those at 5–10% and 20–25% risk of having a germline mutation in MLH1, MSH2, MSH6, or PMS2.14
| SGO 5–10% Criteria | SGO 20–25% Criteria |
|---|---|
| Endometrial or colorectal cancer diagnosed before age 50 | Probands meeting Amsterdam II Criteria |
| Endometrial or ovarian cancer with a synchronous or metachronous colorectal cancer or other Lynch Syndrome associated tumor at any age | Synchronous or metachronous endometrial or colorectal cancer first cancer occurring before age 50 |
| Endometrial or colorectal cancer and a first degree relative with Lynch Syndrome associated tumor diagnosed before age 50 | Synchronous or metachronous ovarian cancer or colorectal cancer, first cancer occurring before age 50 |
| Endometrial or colorectal cancer and ≥ 2 first or second degree relatives with Lynch Syndrome associated tumors | Endometrial or colorectal cancer with tumor testing suggestive of Lynch Syndrome (IHC or MSI-H) |
| Proband with first- or second- degree relatives who meet these criteria | First or second degree relative with a known germline mutation |
Immunohistochemistry
Immunohistochemistry (IHC) is an efficient and relatively inexpensive technique to screen carcinomas for the presence or absence of MLH1, MSH2, MSH6, and PMS2 protein expression.20 Light microscopy is performed to evaluate for nuclear protein expression, with stromal cells serving as an internal positive control. Absence of mismatch repair protein expression in tumor cells with retained expression in adjacent stromal cells indicates a defect in DNA mismatch repair (Figure 1A, 1B; Figure 2A, 2B). Individuals with mutations in MLH1 will exhibit loss of MLH1 and PMS2 protein expression, while patients with MSH2 and EPCAM mutations will have IHC loss of MSH2 and MSH6, due to the dominant role of MLH1 and MSH2 in heterodimer formation of these proteins during mismatch repair. Individuals with germline mutations in MSH6 or PMS2 typically show only IHC loss of the corresponding MMR protein.21,22 The greatest strengths of the IHC approach are that it is easily accessible, cost effective, and informative results point to the gene of interest (Table 3).
Figure 1.
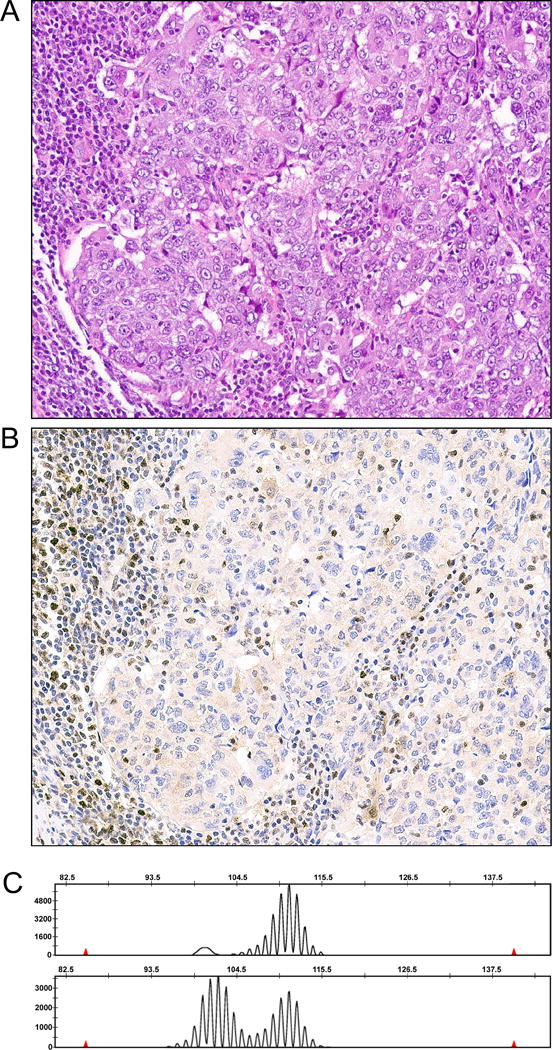
MSI-high endometrial carcinoma with PMS2 loss by immunohistochemistry. (A) H&E of tumor, demonstrating a poorly differentiated endometrial carcinoma with adjacent tumor infiltrating lymphocytes. (B) PMS2 immunohistochemistry, showing that tumor infiltrating lymphocytes and stromal cells adjacent to the tumor have retained nuclear expression for PMS2. Tumor cells have faint cytoplasmic expression of PMS2, but no nuclear expression for this mismatch repair protein. (C) A representative microsatellite tracing for BAT40 from this MSI analysis. Note that tumor tracing has more peaks than the tracing for normal. In this case, normal ovary was used as a source of normal. This tumor had allelic shift in 4 other microsatellites as well (not shown).
Figure 2.
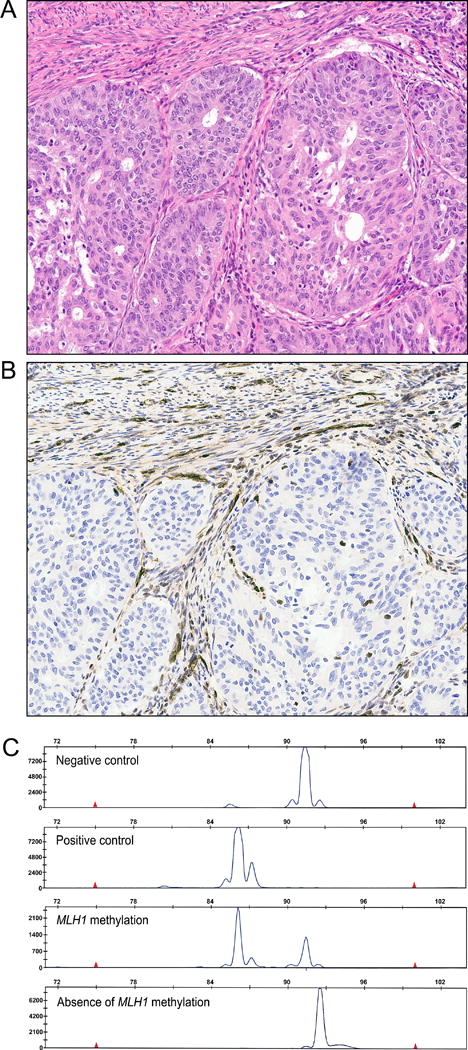
MLH1 methylation analysis. (A) H&E for an endometrial carcinoma subsequently shown to have MLH1 protein loss due to methylation of the MLH1 gene. This tumor is typical in that routine examination of H&E stained slides would not have identified that this tumor would have MLH1 protein loss. (B) MLH1 immunohistochemistry, demonstrating that this endometrial carcinoma has IHC loss of MLH1. Adjacent myometrial cells and stromal cells have intact positive expression for MLH1. (C). This tumor had presence of MLH1 gene methylation (third panel) and was therefore considered sporadic. For each analysis, negative control and positive control cell lines are used as controls. Most tumors with MLH1 methylation will have two peaks detected, one corresponding to a methylated peak and one corresponding to an unmethylated peak. The presence of the unmethylated peak is due to contamination by stromal cells and inflammatory cells that lack MLH1 methylation.
Table 3.
Probability of identifying a germline Lynch Syndrome mutation based on microsatellite instability testing or immunohistochemistry result.14,17,23,32
| Mircosatellite Instability/Immunohistochemistry Result | Probability of identifying a germline mutation | Advantages | Disadvantages |
|---|---|---|---|
| MSI-High | 21% |
|
|
| Any type of IHC loss | 21% |
|
|
| IHC loss of MLH1/PMS2 (and absence of MLH1 promoter methylation) | 33% | ||
| IHC loss of MSH2/MSH6 | 67% | ||
| IHC loss of MSH6 | 24% | ||
| IHC loss of PMS2 | 62% | ||
The sensitivity and specificity of IHC alone for the detection of germline Lynch Syndrome mutations overall is 21% but varies based on mutation (Table 3). For tumors with IHC loss of MLH1/PMS2 and the absence of MLH1 promoter methylation, the probability of identifying a germline mutation is 33%. For tumors exhibiting IHC loss of MSH2 with or without loss of MSH6, the probability is 67%. For tumors with IHC loss of MSH6 or PMS2, the probability of identifying a germline mutation is 24% and 62%, respectively.17,23
Addition of PCR-based MLH1 Promoter Methylation to Immunohistochemistry
An estimated 15–20% of all endometrial and colorectal adenocarcinomas will exhibit epigenetic silencing of the MLH1 gene promoter secondary to methylation, a sporadic event in carcinogenesis.24–27 When using solely IHC to screen tumors for Lynch Syndrome, all patients that exhibit IHC loss of MLH1/PMS2 would be recommended referral to a genetic counselor for consideration of germline testing. The addition of the PCR-based MLH1 promoter methylation assay to the assessment of these tumors has the potential to identify sporadic carcinomas and limit the number of patients referred for germline testing.27 To identify the presence of MLH1 promoter methylation, DNA is isolated from formalin-fixed, paraffin-embedded tissue sections, microdissected with a scalpel blade, and treated with bisulfite to convert methylated cytosine to uracil. DNA is then amplified using fluorescently labeled PCR primers specific for methylated (M) or the unmethylated (U) versions of MLH1 and amplified PCR products are then detected using capillary electrophoresis and GeneScan software. Chromatograms for the tumor are then compared to known positive and negative control cell lines (Figure 2C).27
65–96.9% of endometrial tumors exhibiting IHC loss of MLH1 protein expression will have methylation at the MLH1 promoter region.27–31 Prior studies have shown that IHC loss of MLH1 is a poor predictor for a germline mutation, and clinically based risk factors such as young age at diagnosis and family history of Lynch Syndrome associated tumors cannot reliably predict which tumors with IHC loss of MLH1 will have MLH1 promoter methylation.27,32 In a retrospective study of 337 endometrial cancer patients, 54 (16%) were found to have IHC loss of protein expression for MLH1, and 45/54 (74%) of these had methylation of the MLH1 promoter.27 Comparing the individuals with and without MLH1 methylation did not reveal statistically significant differences amongst histology, FIGO stage, endometrioid grade, lymphatic/vascular space invasion, tumor location, tumor size, or those meeting SGO clinical criteria. Specifically, when using SGO clinical criteria alone for triaging tumors with IHC loss of MLH1, 22/54 (41%) would require genetic counseling referral, subjecting 12 women to unnecessary referrals and germline testing; an additional 4 women with tumors lacking MLH1 methylation would not be referred to genetic counseling. Costly genetic testing can be avoided by incorporating this PCR-based assay as part of a tumor-based Lynch Syndrome screening program, but it is not yet a universally available technique.33–36 The reasons for this are not entirely clear, but one reason may be is that many community hospitals do not have clinical laboratories with access to PCR-based tumor testing.
Microsatellite Instability Analysis
DNA microsatellites consist of multiple, tandem repeats of mono-, di-, and tri- nucleotides that are susceptible to errors during DNA replication resulting in a change in this number of tandem repeats. A change in the number of tandem repeats is termed microsatellite instability (MSI).37 The Bethesda Panel is a set of microsatellite sites recommended for PCR-based MSI analysis which includes BAT25, BAT26, D5S346, D2S123 and D17S250, BAT40 and TGF-βR2.13 Similar to MLH1 methylation analysis, formalin-fixed, paraffin-embedded tumor sections are microdissected, and extracted DNA is amplified and analyzed with an ABI genetic analyzer. DNA from normal tissue is also needed for this analysis. Any tissue source can be used for this normal, including the buffy coat from a peripheral blood draw. A tumor exhibiting allelic shift in 3 or more markers is defined as MSI-high (MSI-H), 1–2 markers as MSI-low (MSI-L), and no allelic shift as microsatellite stable (MSS) (Figure 1C). Lynch Syndrome associated cancers are typically MSI-H, while sporadic tumors with no defects in DNA MMR are typically MSS. MSI-L represents somewhat of a clinical conundrum.38 Nearly all MSI-L colorectal carcinomas are sporadic; however, women with endometrial carcinomas and known Lynch Syndrome, often with germline MSH6 mutations, have been found to have MSI-L or MSS tumors.38 The optimal management strategy for tumors with MSI-L is not clear in the endometrial cancer population.
PCR-based Screening for BRAF mutations
In colorectal cancer, up to 10% of MSI-H tumors will be associated with a somatic mutation in the BRAF gene, a gene involved in the MAPK pathway.39 For tumors with IHC loss of MLH1 and a BRAF mutation, no further Lynch Syndrome screening needs to be performed as BRAF mutation is associated with sporadic MSI-H. The underlying reason for this interesting association is unknown. BRAF mutations are exceedingly rare in endometrial cancer, so this test is of minimal utility in the pathologic algorithms for LS evaluation in this population.29,34,40 BRAF mutation analysis may therefore be useful in patients with colorectal cancers with IHC loss of MLH1, when an MLH1 methylation assay is not available.
Immunohistochemistry vs. Microsatellite Instability Analysis
Because IHC is relatively inexpensive and less technically challenging to perform, IHC assessment of the mismatch repair proteins MLH1, MSH2, MSH6, and PMS2 has become the major tissue testing strategy to screen patients for Lynch Syndrome. Furthermore, many interpret this IHC assessment as interchangeable with PCR-based MSI testing, rationalizing that both tests are unnecessary because they are presumed to be equivalent. Several large studies, however, have effectively demonstrated that there are clinically significant discordances between IHC and MSI testing. In a retrospective analysis of 591 colorectal and endometrial cancer patients who had undergone tissue testing, it was noted that amongst 102 MSI-high cancers, 12 (12%) had intact positive expression of the four mismatch repair proteins.20 Eight of these patients subsequently had germline testing performed, with germline mutations detected in Lynch Syndrome genes in four patients. These four patients would not have been captured if the tissue testing approach only employed IHC for mismatch repair proteins.20 In the Gynecologic Oncology Group’s large retrospective study of 938 women with endometrioid-type endometrial cancer, 33 non-MSS tumors (14 MSI-high, 19 MSI-Low) had intact positive IHC expression of the four mismatch repair proteins. Tumors with intact positive expression of mismatch repair proteins by IHC represented 6% of the women with MSI-high endometrial cancers.16 From their data, they estimated that 1/150 women with endometrial cancer-associated Lynch Syndrome have tumors with intact positive IHC expression of mismatch repair proteins.16 A small subset of patients with MSI-high tumors with retained positive expression of mismatch repair proteins may have germline mutations in MLH1 that result in expression of a defective protein, but explanations for most of these have remained elusive. The advantages and disadvantages of both IHC and MSI testing are summarized in Table 3.
Microsatellite instability panels in use in most clinical laboratories are derived from years of experience and validation of Lynch Syndrome testing in colorectal cancer patients. It has been long assumed that these same panels would be useful in the Lynch Syndrome tissue testing evaluation of women with endometrial cancer and other cancer types. There is some evidence, however, that the incidence and pattern of MSI-high in cancer types outside of the colon may be very different. Using an extended panel of 12 microsatellites, including 4 in our current panel (BAT25, BAT26, BAT40, and TGF-βRII), MSI was analyzed in 44 colorectal cancers and 57 endometrial cancers from 8 families with known germline mutations in MLH1 or MSH2.38 The incidence of MSS was higher in the endometrial cancers (23%) than in the colorectal cancers (11%). The colorectal cancers frequently displayed instability at the TGF-βRII locus, but this was infrequent in the endometrial cancers. This is also observed in our clinical practice (Broaddus, R.R. unpublished data). Alternatively, the endometrial cancers frequently displayed instability at the PTEN locus, but this microsatellite locus is not present in the panels employed by most clinical labs. Amongst the MSI-high tumors, the endometrial cancers had a significantly lower proportion of unstable loci.38 We have seen a MSS adrenocortical carcinoma with MSH2 IHC loss from a patient who also had a MSI-high colorectal cancer with MSH2 IHC loss; the family had a known germline MSH2 mutation.41 Also, we have seen members of a family with germline mutation in MSH2 and endometrial cancer, colon cancer, and anaplastic carcinoma of the thyroid. All three cancers displayed IHC loss of MSH2. The colon and endometrial cancers were MSI-high, while the thyroid anaplastic carcinoma was MSS.41
Detection of Somatic Mutations in Lynch Syndrome-Associated Cancers
Immunohistochemistry for DNA mismatch repair proteins and PCR-based MSI analysis and MLH1 methylation analysis have proven to be excellent techniques to identify endometrial and colorectal cancers with defects in DNA mismatch repair. There are numerous publications in the peer-reviewed literature that attest to the sensitivity and specificity of this tissue testing approach.16,38,42–44 It is well-known, however, that not all MSI-high tumors with loss of immunohistochemical expression of a mismatch repair protein and free of MLH1 methylation will have germline mutations in a mismatch repair gene identified. In a large retrospective study, 365 endometrial cancer patients underwent tissue testing, with 51/365 (14%) of patients having tissue testing results suggestive of Lynch Syndrome.45 A germline mutation in a mismatch repair gene was only identified in 6%, however, indicating that less than 50% of tissue testing positive cases have an associated germline mutation.45 In a larger GOG cooperative group retrospective study examining tissue testing in 938 women with endometrioid-type endometrial carcinomas, tissue testing results suggestive of Lynch Syndrome were identified in 11% of patients, but germline testing identified a deleterious mutation in approximately 43% of these, again indicating that less than half of endometrial cancer patients with tissue testing results suggestive of Lynch Syndrome will have a germline mutation identified.16
Patients with positive tissue testing results, yet no mismatch repair gene mutation identified in the germline, represent a clinical conundrum and genetic counseling challenge.46 Some authorities believe that these patients do have Lynch Syndrome, but they have complex mismatch repair gene alterations that cannot be detected by the usual methods. For example, a novel inversion of exons 1–7 of the MSH2 gene has been reported in patients with tumors with MSH2 immunohistochemical loss but no germline MSH2 mutation identified by conventional sequencing.47 Screening recommendations for tissue testing positive/germline testing negative patients and their family members is complex. Should all siblings and children receive heightened cancer prevention screening? Some advocate that at least the index patient should receive heightened colon cancer screening, if they have not yet developed this malignancy.
Recent data suggest another explanation for at least a subset of these patients. Different groups have identified the presence of somatic mutations in mismatch repair genes for endometrial and colorectal carcinomas that had tissue testing results (IHC loss of a DNA mismatch repair protein, no MLH1 methylation detected, and MSI-high) suggestive of Lynch Syndrome, but no germline mutation was identified.48–51 Some of these cases are associated with an ultra-mutated genotype and germline or somatic mutations in the genes POLE or POLD1. The identification of such somatic mutations is important, as it eliminates the need for cancer screening surveillance in these patients and helps to decrease patient anxiety about the possibility of having a hereditary cancer syndrome. Unfortunately, these sequencing assays are not yet available in most clinical laboratories. Many clinical laboratories, especially those supporting large cancer patient populations, do have next-generation sequencing (NGS) capabilities, but the mismatch repair genes, POLE, and POLD1, are not present on most of these panels, which are typically designed with cancer therapeutics in mind. Currently, these genes are not considered therapeutic targets for the cancer clinical trials employing targeted therapy approaches.
The Clinical Lab Does not Have all the Answers
IHC, MSI analysis, and MLH1 methylation analysis have proven to be highly specific and sensitive in their abilities to detect endometrial cancers with defective DNA mismatch repair. Adding somatic testing for MLH1, MSH2, MSH6, PMS2, POLE, and POLD would help to close the loop on at least a subset of cases in which a germline mutation is not identified despite tissue testing results suggestive of Lynch Syndrome.
Despite the implementation of these excellent clinical tests, there still remain a few patients who defy the best efforts of the clinical laboratory. In our previous study of 365 endometrial cancer patients with both tissue testing and germline analyses performed, 2/20 (10%) women with germline mutations in a Lynch Syndrome gene had intact positive expression of the four DNA mismatch repair proteins. Each of these women had tumors that were too small to support MSI analyses, so it is uncertain if the detection of MSI-high would have captured these patients. These patients had germline mutations in PMS2 and MSH6, each was older than 50 years of age, and each had no family history of a Lynch-associated cancer.45 Outside of a study, we have recently identified an endometrial cancer patient older than age 50 with no family history of a Lynch-associated cancer whose tumor had intact positive expression of mismatch repair proteins and was MSI-low. The patient’s gynecologic oncologist was suspicious of the possibility of Lynch Syndrome due to her tumor arising from the lower uterine segment. Tumors with this anatomic location represent 3% of all endometrial cancers but nearly 30% are associated with Lynch Syndrome.52 Subsequent germline testing indeed identified a deleterious MSH6 mutation. Therefore, while laboratory testing can help to identify most women at-risk for germline mutations in a Lynch gene, it is important to realize that not all patients will be captured using this approach, and clinical suspicion stills plays a pivotal role in identifying these cases.
Acknowledgments
NIH 2P50 CA098258-06 SPORE in Uterine Cancer (RRB)
Footnotes
Conflict of Interest Disclosures: None for all authors.
References
- 1.Watson P, Lynch HT. The tumor spectrum in HNPCC. Anticancer Res. 1994;14:1635–9. [PubMed] [Google Scholar]
- 2.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–7. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105:569–74. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 4.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–87 e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. The New England journal of medicine. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 6.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. The New England journal of medicine. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 7.Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000;37:641–5. doi: 10.1136/jmg.37.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsoekh D, Wagner A, van Leerdam ME, et al. A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting. Gut. 2008;57:1539–44. doi: 10.1136/gut.2008.156695. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 10.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 11.Resnick KE, Hampel H, Fishel R, Cohn DE. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecologic oncology. 2009;114:128–34. doi: 10.1016/j.ygyno.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. Journal of the National Cancer Institute. 1997;89:1758–62. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 13.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic oncology. 2007;107:159–62. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Bruegl AS, Djordjevic B, Batte B, et al. Evaluation of clinical criteria for the identification of Lynch syndrome among unselected patients with endometrial cancer. Cancer Prev Res (Phila) 2014;7:686–97. doi: 10.1158/1940-6207.CAPR-13-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodfellow PJ, Billingsley CC, Lankes HA, et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33:4301–8. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419–28. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks YM, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 19.Grover S, Syngal S. Risk assessment, genetic testing, and management of Lynch syndrome. J Natl Compr Canc Netw. 2010;8:98–105. doi: 10.6004/jnccn.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila) 2012;5:320–7. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130:665–71. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks Y, Franken P, Dierssen JW, et al. Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol. 2003;162:469–77. doi: 10.1016/S0002-9440(10)63841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17:2413–7. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 25.Salvesen HB, MacDonald N, Ryan A, et al. Methylation of hMLH1 in a population-based series of endometrial carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:3607–13. [PubMed] [Google Scholar]
- 26.Simpkins SB, Bocker T, Swisher EM, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Human molecular genetics. 1999;8:661–6. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 27.Bruegl AS, Djordjevic B, Urbauer DL, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch Syndrome in women with endometrial cancer. Curr Pharm Des. 2014;20:1655–63. doi: 10.2174/13816128113199990538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCourt CK, Mutch DG, Gibb RK, et al. Body mass index: relationship to clinical, pathologic and features of microsatellite instability in endometrial cancer. Gynecologic oncology. 2007;104:535–9. doi: 10.1016/j.ygyno.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Peterson LM, Kipp BR, Halling KC, et al. Molecular characterization of endometrial cancer: a correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2012;31:195–205. doi: 10.1097/PGP.0b013e318231fc51. [DOI] [PubMed] [Google Scholar]
- 30.Whelan AJ, Babb S, Mutch DG, et al. MSI in endometrial carcinoma: absence of MLH1 promoter methylation is associated with increased familial risk for cancers. International journal of cancer Journal international du cancer. 2002;99:697–704. doi: 10.1002/ijc.10429. [DOI] [PubMed] [Google Scholar]
- 31.Zauber NP, Denehy TR, Taylor RR, et al. Microsatellite instability and DNA methylation of endometrial tumors and clinical features in young women compared with older women. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20:1549–56. [PubMed] [Google Scholar]
- 32.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon JS, Scott JL, Gilks CB, Daniels MS, Sun CC, Lu KH. Testing women with endometrial cancer to detect Lynch syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2247–52. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leenen CH, van Lier MG, van Doorn HC, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer </= 70 years. Gynecologic oncology. 2012;125:414–20. doi: 10.1016/j.ygyno.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 35.Resnick K, Straughn JM, Jr, Backes F, Hampel H, Matthews KS, Cohn DE. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstetrics and gynecology. 2009;114:530–6. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]
- 36.Resnick KE, Hampel H, Fishel R, Cohn DE. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecologic oncology. 2009;114:128–34. doi: 10.1016/j.ygyno.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989;17:6463–71. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuismanen SA, Moisio AL, Schweizer P, et al. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Pathol. 2002;160:1953–8. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaeger R, Saltz L. BRAF mutations in colorectal cancer: clinical relevance and role in targeted therapy. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:1456–8. doi: 10.6004/jnccn.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutch DG, Powell MA, Mallon MA, Goodfellow PJ. RAS/RAF mutation and defective DNA mismatch repair in endometrial cancers. American journal of obstetrics and gynecology. 2004;190:935–42. doi: 10.1016/j.ajog.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Broaddus RR, Lynch PM, Lu KH, Luthra R, Michelson SJ. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17:981–9. doi: 10.1038/modpathol.3800150. [DOI] [PubMed] [Google Scholar]
- 42.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 43.Moline J, Mahdi H, Yang B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecologic oncology. 2013;130:121–6. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Resnick K, Straughn JM, Jr, Backes F, Hampel H, Matthews KS, Cohn DE. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet Gynecol. 2009;114:530–6. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]
- 45.Ring KL, Bruegl AS, Allen BA, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batte BA, Bruegl AS, Daniels MS, et al. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecologic oncology. 2014;134:319–25. doi: 10.1016/j.ygyno.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhees J, Arnold M, Boland CR. Inversion of exons 1–7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam Cancer. 2014;13:219–25. doi: 10.1007/s10689-013-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147:1308–16 e1. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jansen A, van Wezel T, van den Akker B, et al. Combined mismatch repair and POLE/POLD1 defects explain unresolved suspected Lynch syndrome cancers. European Journal of Human Genetics. 2016;24:1089–92. doi: 10.1038/ejhg.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geurts-Giele WR, Leenen CH, Dubbink HJ, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. The Journal of pathology. 2014;234:548–59. doi: 10.1002/path.4419. [DOI] [PubMed] [Google Scholar]
- 51.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146:643–6 e8. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Westin SN, Lacour RA, Urbauer DL, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26:5965–71. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–7. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]


