Abstract
Background
Recent clinical trials and expert consensus guidelines have typically focused on the issue of systolic blood pressure (SBP) targets for reducing vascular risk. However, little is known about the relationship of diastolic BP (DBP) level with vascular outcomes after a stroke.
Methods
We analyzed a multicenter trial dataset involving 3680 recent (<4 months) noncardioembolic stroke patients followed for 2 years. Subjects were categorized per mean DBP level (mmHg) during follow-up: low-normal (<70), normal (70–<80), high-normal (80–89), and high (≥90). Pulse pressure (PP) was prespecified by three categories of <60; 60–<70; and ≥70 mmHg. Independent associations of mean DBP level with major vascular events (MVEs), and ischemic stroke were assessed.
Results
MVEs occurred in 20.7% of the low-normal, 15.1% of the normal, 16.9% of the high-normal, and 19.2% of the high DBP groups; while stroke occurred in 9.9%, 6.8%, 8.5%, and 10.8%, respectively. Compared with the normal DBP group, risk of MVEs was higher in the low-normal DBP group (adjusted hazard ratio [AHR], 1.33; 95% CI, 1.04–1.71). Among those with SBP 120–<140 mmHg, risk of MVEs (1.89; 1.13–3.15), and stroke (2.87; 1.48–5.53) was higher in subjects with PP ≥70 (mean DBP, 62.4±3.8) than those with the lowest PP (mean DBP, 78.0±5.9); while among those with SBP <120 mmHg, PP 60–<70 (mean DBP, 52.7±2.5) was associated with increased risk of stroke (5.85; 1.25–27.5).
Conclusion
DBP levels in the low-normal range, particularly accompanied by an increased PP of >60, confer increased risk of MVEs and stroke, among patients after recent noncardioembolic stroke.
Keywords: blood pressure, hypertension, stroke, outcomes, vascular events, prognosis
Introduction
Hypertension is the most common and leading risk factor for stroke by ~60% in men and women of all ages [1]. Several published studies have looked at the association of blood pressure (BP) with future risk of vascular events, but reports regarding this relationship in people with a history of stroke, are much fewer, and even less so among those with a recent ischemic stroke [2, 3]. These limited data (one randomized trial and a couple of observational studies) have primarily focused on systolic BP (SBP) level, and have suggested a trend towards benefit for SBP <130 mmHg in patients with lacunar stroke [4], or a J-shaped association for SBP with harm at <120 mmHg for all noncardioembolic stroke types [2, 3].
With regard to diastolic BP (DBP), a post hoc analysis of >22000 hypertensive patients with coronary artery disease showed that lower DBP <70 to 80 mm Hg was associated with an increased risk of all-cause death and myocardial infarction likely due to compromised coronary artery perfusion, thereby causing cardiac ischemia [5]. However little is known about whether such a J-shaped relationship exists for DBP and recurrent vascular risk after a stroke.
Pending the conduct of dedicated clinical trials aimed at addressing the question of the optimal BP target for secondary prevention after stroke, more information about the possible nature of the relationship of achieved DBP and post-stroke vascular risk could be insightful. Therefore, the objective of this study was to evaluate the relationship between DBP and future major vascular events (MVEs) among patients following a recent stroke.
Methods
Study subjects and database
This is a post hoc analysis of data from the Vitamin Intervention for Stroke Prevention (VISP) trial.[6] VISP study was a multinational, double blind, randomized controlled clinical trial performed at 56 centers across the United States, Canada, and Scotland. The study enrolled 3680 subjects aged ≥35 years to determine whether high doses of multivitamin (folic acid, pyridoxine, and cobalamin) given to lower total homocysteine levels would reduce the risk of recurrent stroke and major vascular events in subjects with a non-cardioembolic stroke within 120 days [6]. Demographic, clinical, and laboratory data, including BP recordings, were collected at baseline, with subsequent clinical and laboratory information obtained at follow-up visits of 1, 6, 12, 18, and 24 months [6]. BP was measured twice at five minute interval, and the measurements were averaged. For each patient, presence of hypertension, diabetes, and body mass index (BMI), which was calculated as weight in kilograms divided by square of height in meters, were retrieved at the baseline visit. We further reviewed about secondary prevention medications including antihypertensive, antithrombotic (antiplatelet/anticoagulation), and lipid-modifying therapy (statin mostly), all of them were collected at every 6-month interval follow-up visit. The trial was approved by the ethics committee or institutional review board at each national or local site, and all participants provided written informed consent before enrolment [6].
Vascular endpoints
The primary outcome was a composite of stroke, coronary heart disease (CHD) including myocardial infarction, coronary revascularization, cardiac resuscitation, and fatal CHD, or vascular death defined as MVEs. Secondary outcome was ischemic stroke. Each adjudicated endpoint in VISP was verified through consensus of a review committee [6].
Statistical analysis
On the basis of expert consensus guideline classification of DBP, normal (<80 mm Hg) prehypertension (80 to 89 mm Hg), stage 1 hypertension (90 to 99 mm Hg), and stage 2 hypertension (>100 mm Hg) [7], study participants were categorized into four groups according to their mean DBP level during the follow-up: low-normal (<70 mm Hg), normal (70 to <80 mm Hg), normal to high-normal (80 to 89 mm Hg), and high (≥90 mm Hg). The group with normal DBP was the referent group. Mean follow-up DBP from the baseline to final visit was calculated for each subject. Given that an increased pulse pressure (PP) yielding low DBP reflects increased stiffness in the aorta and other large arteries [8], and the J-shaped relationship between DBP and cardiac outcomes [5], we also evaluated the relationship of PP (by mean SBP – mean DBP) and mean DBP with vascular outcomes. Since low DBP and high pulse pressure (PP) of >60 mm Hg were reported to be risk factors for incident CHD among participants with an SBP of ≥120 mm Hg [9], the PP was prespecified by three categories of <60; 60 to <70; and ≥70 mm Hg. The group with the lowest PP was the referent group. Subjects not having outcome events were censored at last follow-up examination, at last visit until he/she died, or experienced an outcome. Independent associations of systolic BP (SBP) by ordinal category (<120; 120 to <140; 140 to 159; and ≥160 mm Hg) [7] with vascular outcomes were further analyzed with the second SBP level as the referent group. A total of 152 patients had few follow-up BP data since randomization; 109 had one follow-up BP data and 43 had no follow-up. For the latter patients, baseline BP was used as the proxy.
Comparisons across the BP groups and the PP groups were examined using the one-way analysis of variance, followed by the Dunnett test for multiple comparisons, for continuous variables and χ2 test for categorical variables. Multivariate analyses to estimate the risk of outcome events at 2 years were performed using Cox regression models including the following variables: age, sex, race, body mass index, mini-mental state examination score, low-density lipoprotein cholesterol, mean SBP, diabetes mellitus, qualifying stroke severity, history of stroke (before qualifying stroke), history of CHD, history of heart failure, history of carotid endarterectomy, history of alcohol use, lipid modifier use, and antithrombotic use (all P<0.10, model I); and model I plus antihypertensive use (model II). Results are given by hazard ratio (HR) and its 95% confidence interval (CI). The interaction between mean SBP level and each mean DBP category in predicting the risk of vascular outcome event was assessed by including the appropriate interaction terms in the model. Above analyses were conducted using IBM SPSS Version 22.0 (IBM Corp., Armonk, NY) and survival curves were fit by the log-rank tests using MedCalc software version 5.0 (Mariakerke, Belgium). A probability value of <0.05 was considered statistically significant.
Results
Subjects characteristics by mean DBP categories
Mean baseline SBP and DBP of study subjects was 140.5 ± 15.5 mmHg and 77.8 ± 8.2 mmHg, respectively. Distributions of subjects by 10 mm Hg strata of mean DBP are shown in Figure S1. Table 1 presents baseline demographic and clinical characteristics of 3680 participants in the 3 groups of mean DBP. Compared with the low-normal DBP group, high DBP group was younger, had higher levels of body mass index, mean SBP, total cholesterol and low-density lipoprotein cholesterol, had higher frequencies of men, non-white, hypertension, antihypertensive medication, and history of alcohol use, whereas frequencies of diabetes and lipid modifier use, and histories of coronary heart disease, heart failure and carotid endarterectomy were lower. Additionally, qualifying stroke severity was more likely to be greater in the low-normal group. Table 2 displays various BP parameters (ie, mean baseline, mean follow-up, and mean change) by of mean DBP categories. Mean baseline and follow-up SBP/DBP as well as mean SBP/DBP change were highest in the high DBP group. Specifically, baseline DBP was 65.7 ± 7.3 mm Hg in the low-normal DBP group and 92.1 ± 7.1 mm Hg in the high DBP group vs 75.1 ± 7.2 mm Hg in the normal DBP group; follow-up DBP was 67.8 ± 9.7 mm Hg in the low-normal DBP group and 88.6 ± 11.4 mm Hg in the high DBP group vs 74.3 ± 8.6 mm Hg in the normal DBP group; and change in DBP was 1.7 ± 11.9 mm Hg in the low-normal DBP group and −3.5 ± 13.1 mm Hg in the high DBP group vs −0.8 ± 11.0 mm Hg in the normal DBP group.
Table 1.
Baseline demographic and clinical characteristics by mean DBP categories among 3680 participants after a recent noncardioembolic stroke
| Mean DBP categories | P | ||||
|---|---|---|---|---|---|
|
| |||||
| Low-normal (<70 mm Hg) | Normal (70 to <80 mm Hg) | Normal to high-normal (80 to 89 mm Hg) | High (≥90 mm Hg) | ||
| Number of subjects | 527 | 1611 | 1255 | 287 | |
| Age, years | 69.4 ± 10.4 | 67.5 ± 10.4 | 64.7 ± 10.5b | 60.8 ± 11.5a | <0.001 |
| Body mass index, kg/m2 | 27.5 ± 5.6 | 28.0 ± 5.4 | 28.6 ± 5.4a | 30.3 ± 7.6b | <0.001 |
| MMSE, score | 26.5 ± 3.9 | 27.0 ± 3.1 | 26.9 ± 3.3 | 26.7 ± 3.5 | 0.017 |
| Mean SBP, mm Hg | 130.2 ± 14.9 | 137.1 ± 13.6 | 145.7 ± 13.6a | 156.5 ± 14.0b | <0.001 |
| Total cholesterol, mg/dL | 196.9 ± 50.8 | 200.8 ± 47.2a | 203.7 ± 43.4 | 210.2 ± 48.5b | 0.001 |
| LDL-C, mg/dL | 115.8 ± 37.7 | 121.3 ± 41.9a | 124.1 ± 38.9 | 128.1 ± 43.5b | <0.001 |
| Triglycerides, mg/dL | 182.2 ± 308.3 | 172.7 ± 110.0 | 171.6 ± 99.5 | 190.8 ± 155.1 | 0.201 |
| HDL-C, mg/dL | 45.8 ± 16.5 | 45.2 ± 14.8 | 45.6 ± 15.9 | 44.5 ± 14.9 | 0.622 |
| Creatinine, mg/dL | 1.14 ± 0.69 | 1.11 ± 0.55 | 1.12 ± 0.60 | 1.12 ± 0.41 | 0.771 |
| Homocystein, μmol/L | 14.2 ± 5.7 | 14.1 ± 5.4 | 14.1 ± 6.9 | 14.0 ± 4.8 | 0.972 |
|
| |||||
| Male | 277 (52.6) | 997 (61.9) | 824 (65.7) | 203 (70.7) | <0.001 |
| Non-white | 52 (9.9) | 204 (12.7) | 217 (17.3) | 72 (25.1) | <0.001 |
| Hypertension | 415 (78.7) | 1312 (81.4) | 1097 (87.4) | 274 (95.5) | <0.001 |
| Diabetes mellitus | 190 (36.1) | 505 (31.3) | 323 (25.7) | 83 (28.9) | <0.001 |
| Smoker | 79 (15.0) | 269 (16.7) | 212 (16.9) | 62 (21.6) | 0.115 |
| Qualifying stroke NIHSS | 0.031 | ||||
| 0 | 153 (29.0) | 569 (35.3) | 433 (34.5) | 82 (28.6) | |
| 1–4 | 327 (62.0) | 916 (56.9) | 714 (56.9) | 187 (65.2) | |
| ≥5 | 47 (8.9) | 126 (7.8) | 108 (8.6) | 18 (6.3) | |
| History | |||||
| Strokec | 132 (25.0) | 377 (23.4) | 276 (22.0) | 71 (24.7) | 0.486 |
| CHD | 184 (34.9) | 431 (26.8) | 287 (22.9) | 60 (20.9) | <0.001 |
| Heart failure | 44 (8.4) | 88 (5.5) | 48 (3.8) | 13 (4.6) | 0.001 |
| CEA | 48 (9.1) | 118 (7.3) | 75 (6.0) | 6 (2.1) | 0.001 |
| Alcohol use | 264 (51.8) | 944 (60.2) | 746 (60.8) | 173 (62.7) | 0.002 |
| High-dose B vitamin | 249 (47.2) | 804 (49.9) | 632 (50.4) | 142 (49.5) | 0.679 |
| Antihypertensive use | 411 (78.0) | 1277 (79.3) | 1047 (83.4) | 255 (88.9) | <0.001 |
| Lipid modifier use | 283 (53.7) | 928 (57.6) | 675 (53.8) | 123 (42.9) | <0.001 |
| Antithrombotic use | 488 (92.6) | 1516 (94.1) | 1172 (93.4) | 260 (90.6) | 0.140 |
Values provided are number (%) or mean ± SD, as appropriate, unless otherwise stated. BP, blood pressure; DBP, diastolic BP; SBP, systolic BP; MMSE, mini-mental state examination; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; NIHSS, National Institutes of Health Stroke Scale; CHD, coronary heart disease; and CEA, carotid endarterectomy.
<
, P<0.05 by Dunnett post hoc tests.
Before VISP qualifying stroke.
Table 2.
Baseline, follow-up, and change in BP levels by mean DBP categories
| Mean DBP categories, mm Hg | P | ||||
|---|---|---|---|---|---|
|
| |||||
| Low-normal (<70; n=527) | Normal (70 to 79; n=1611) | Normal to high-normal (80 to 89; n=1255) | High (≥90; n=287) | ||
| BP, mm Hg | |||||
| At baseline | |||||
| Systolic | 130.9 ± 18.6 | 137.9 ± 17.5 | 145.7 ± 17.7a | 154.5 ± 16.1b | <0.001 |
| Diastolic | 65.7 ± 7.3 | 75.1 ± 7.2 | 83.3 ± 7.0a | 92.1 ± 7.1b | <0.001 |
| At follow-up | |||||
| Systolic | 131.5 ± 19.9 | 136.5 ± 18.4 | 142.2 ± 19.4a | 150.2 ± 22.5b | <0.001 |
| Diastolic | 67.8 ± 9.7 | 74.3 ± 8.6 | 80.5 ± 9.1a | 88.6 ± 11.4b | <0.001 |
| Change in level | |||||
| Systolic | −2.6 ± 29.7a | −4.1 ± 28.4 | −9.1 ± 36.5 | −8.9 ± 35.9b | <0.001 |
| Diastolic | 1.7 ± 11.9 | −0.8 ± 11.0a | −2.7 ± 11.8 | −3.5 ± 13.1b | <0.001 |
Values provided are mean ± SD. BP, blood pressure; DBP, diastolic BP. a < b, P<0.05 by Dunnett post hoc tests.
Associations of mean DBP level and vascular outcomes
During 2 years of follow-up, a total of 619 (16.8%) MVEs and 300 (8.2%) strokes were observed. Event of MVEs and stroke was higher in the low-normal DBP group (20.7% and 9.9%, respectively) and high DBP group (19.2% and 10.8%, respectively). Results of the unadjusted and multivariable adjusted associations of mean DBP with vascular outcomes appear in Table 3. Occurrence of MVEs was highest in the low-normal DBP group (HR 1.46; 95% CI, 1.16–1.82; P=0.001), followed by the high DBP group (HR 1.40; 95% CI, 1.05–1.88; P=0.023). Compared with the normal DBP group, occurrence of recurrent stroke was greatest in the high DBP group (HR 1.75; 95% CI, 1.18–2.61; P=0.006), followed by the low-normal DBP group (HR 1.52; 95% CI, 1.09–2.12; P=0.013). In multivariable analyses, compared with the normal DBP group, risk of MVEs was significantly higher in the low-normal DBP group (HR 1.33; 95% CI, 1.04–1.71; P=0.025) and this association remained constant after further adjustment for antihypertensive medication (HR 1.33; 95% CI, 1.04–1.71; P=0.026). High DBP group showed a trend towards higher risk of MVEs (model I and model II).
Table 3.
HRs for vascular outcomes by mean DBP categories at 2 years
| Mean DBP categories, mm Hg | ||||
|---|---|---|---|---|
|
| ||||
| Low-normal (<70; n=527) | Normal (70 to <80; n=1611) | Normal to high-normal (80 to 89; n=1255) | High (≥90; n=287) | |
|
| ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Stroke, CHD, or vascular death | ||||
| Unadjusted | 1.46 (1.16–1.82)a | 1 [Reference] | 1.14 (0.95–1.37) | 1.40 (1.05–1.88)b |
| Adjusted | ||||
| Model Ic | 1.33 (1.04–1.71)b | 1 [Reference] | 1.11 (0.90–1.38) | 1.37 (0.96–1.96) |
| Model IId | 1.33 (1.04–1.71)b | 1 [Reference] | 1.11 (0.90–1.38) | 1.37 (0.96–1.96) |
|
| ||||
| n (% rate) | 109 (20.7) | 243 (15.1) | 212 (16.9) | 55 (19.2) |
|
| ||||
| Stroke | ||||
| Unadjusted | 1.52 (1.09–2.12)b | 1 [Reference] | 1.27 (0.97–1.66) | 1.75 (1.18–2.61)a |
| Adjusted | ||||
| Model Ic | 1.43 (0.99–2.05) | 1 [Reference] | 1.09 (0.81–1.49) | 1.30 (0.79–2.14) |
| Model IId | 1.43 (0.99–2.04) | 1 [Reference] | 1.09 (0.81–1.48) | 1.30 (0.79–2.13) |
|
| ||||
| n (% rate) | 52 (9.9) | 110 (6.8) | 107 (8.5) | 31 (10.8) |
DBP, diastolic blood pressure; CHD, coronary heart disease; HR, hazard ratio; CI, confidence interval.
P<0.01;
P<0.05.
Adjusted for age, sex, ethnicity, body mass index, mini-mental state examination score, low-density lipoprotein cholesterol, mean systolic BP, diabetes mellitus, qualifying stroke severity, history of stroke (before qualifying stroke), history of CHD, history of heart failure, history of carotid endarterectomy, history of alcohol use, lipid modifier use, and antithrombotic use.
Adjusted for model I plus antihypertensive use.
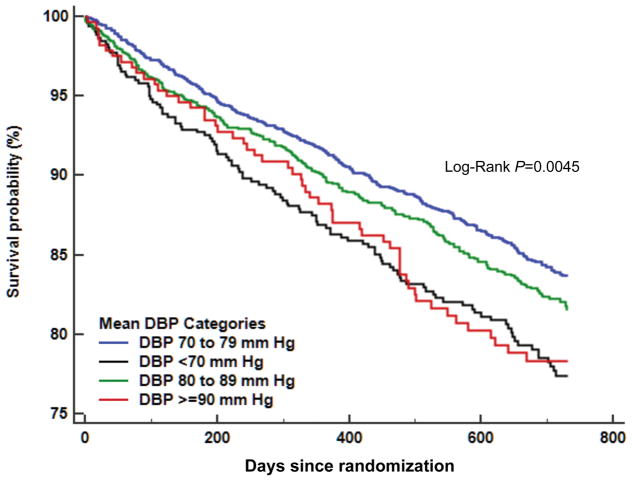
For the secondary outcome, compared with the normal DBP group, significant association of low-normal DBP or high DBP with risk of recurrent stroke disappeared after multivariable adjustment (model I and model II). Figure 1 displays the Kaplan-Meier curves for the endpoints of MVEs among subjects over 2 years after a recent noncardioembolic stroke by DBP categories, which revealed a higher risk in the low-normal DBP group (P=0.0045 by log-rank test). Figures S2 and S3 depict percentages of MVEs and recurrent stroke, respectively by mean DBP category, that reveal near-similar J-curve patterns to these relationships with vascular outcomes. The adjusted HRs of covariates included in the multivariable model appears in Table S1. Lipid modifier use was linked to a lesser risk of both MVEs and stroke, while diabetes was a potent predictor of both vascular outcomes. History of CHD and history of heart failure were predictors of MVEs. Table S2 provides the unadjusted and adjusted HRs for vascular outcomes by mean SBP categories. For MVEs, compared with the SBP level (120 to <140 mm Hg), SBP level (≥160 mm Hg) had a greater risk of MVEs (HR 1.54), but SBP level (<120 mm Hg) showed a trend towards higher risk after multivariable adjustment. For stroke, compared with the SBP level (120 to <140 mm Hg), both SBP level (<120 mm Hg) and SBP level (≥160 mm Hg) were associated with increased risk of ischemic stroke after multivariable adjustment (HR 1.71 and HR 1.57, respectively). The interaction effect between variable and mean DBP categories on the risk of MVEs is shown in Table S3. There was a significant interaction of age with the MVEs (ie, more events in the low-normal DBP group; P=0.044), but the interaction effect between mean SBP level and mean DBP category was not significant.
Figure 1.
Kaplan-Meier curves for the endpoints of major vascular events among subjects over 2 years after a recent noncardioembolic stroke based on diastolic blood pressure categories.
Associations of PP with vascular outcomes by mean SBP categories
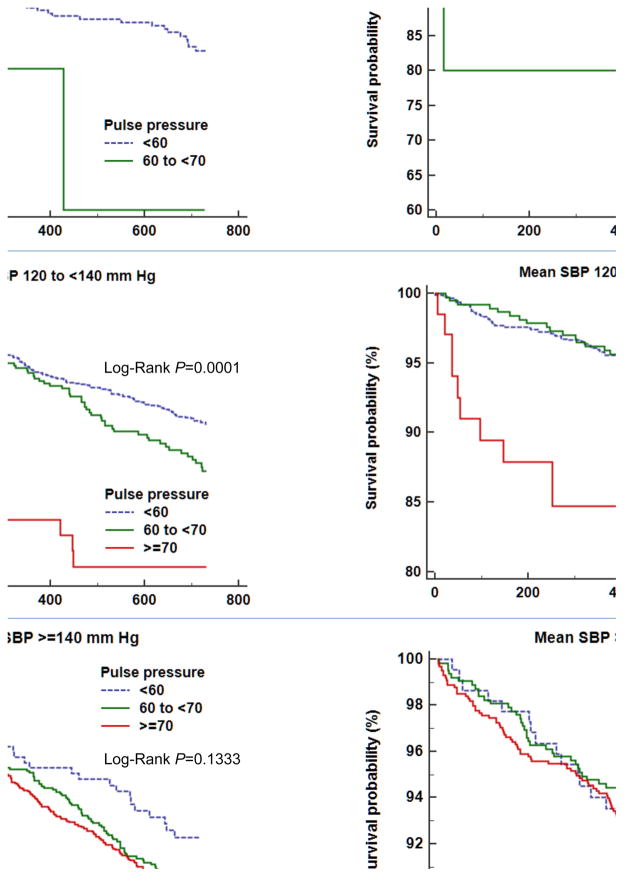
Results of the adjusted associations of PP with vascular outcomes stratified by mean SBP categories are shown in Table 4. The SBP categories were compressed to preserve power (<120; 120 to <140; and ≥140 mm Hg) due to no stroke events in patients with an SBP of ≥160 mm Hg and a PP of <60. Compared with the PP of <60 group, the mean DBP levels of subjects with the highest PP (>70 mm Hg) were significantly lower in each of the SBP categories (P<0.001 for all). Among those with an SBP 120 to <140 mm Hg, compared with the lowest PP group, low mean DBP and a PP of ≥70 were linked to an increased risk of MVEs (HR 1.89; 95% CI, 1.13–3.15; P=0.016). Consistent with this, the association of low mean DBP and the highest PP with stroke was also evident among those with an SBP 120 to <140 mm Hg (HR 2.87; 95% CI, 1.48–5.53; P=0.002). Among those with an SBP <120 mm Hg, PP 60 to <70 mm Hg (mean DBP, 52.7 ± 2.5 mm Hg) showed a pattern towards higher risk of MVEs (HR 3.68; 95% CI, 0.84–16.15) and was associated with increased risk of stroke (HR 5.85; 95% CI, 1.25–27.5; P=0.025). However, these associations were not observed in those with an SBP ≥140 mm Hg. The Kaplan-Meier curves for the vascular endpoints in each of mean SBP categories over the 2-year follow-up period depict higher probabilities of MVEs and stroke in subjects with an SBP 120 to <140 mm Hg (P=0.0001 for all) and greater probability of stroke in those with an SBP <120 mm Hg (P=0.0111, Figure 2).
Table 4.
Hazard ratios (HRs) for vascular outcomes after stratifying subjects by pulse pressure in each of mean SBP categories
| Mean SBP categories | PP (mm Hg) | Mean DBP (mean ± SD) | Stroke, CHD, or vascular death | Stroke | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n/N (% rate) | HR (95% CI)b | P | n/N (% rate) | HR (95% CI)b | P | |||
|
|
|
|||||||
| <120 mm Hg | <60 | 70.2 ± 6.1 | 43/269 (16.0) | 1 [Reference] | — | 23/269 (8.6) | 1 [Reference] | — |
| 60 to <70 | 52.7 ± 2.5a | 2/5 (40.0) | 3.68 (0.84–16.15) | 0.084 | 2/5 (40.0) | 5.85 (1.25–27.5) | 0.025 | |
| ≥70 | — | 0/0 (0.0) | — | — | 0/0 (0.0) | — | — | |
|
|
|
|||||||
| 120 to <140 mm Hg | <60 | 78.0 ± 5.9 | 136/1097 (12.4) | 1 [Reference] | — | 60/1097 (5.5) | 1 [Reference] | — |
| 60 to <70 | 70.5 ± 4.8 | 64/375 (17.1) | 1.12 (0.81–1.53) | 0.501 | 27/375 (7.2) | 1.20 (0.74–1.94) | 0.457 | |
| ≥70 | 62.4 ± 3.8a | 19/68 (27.9) | 1.89 (1.13–3.15) | 0.016 | 12/68 (17.6) | 2.87 (1.48–5.53) | 0.002 | |
|
|
|
|||||||
| ≥140 mm Hg | <60 | 88.8 ± 5.1 | 32/223 (14.3) | 1 [Reference] | — | 18/223 (8.1) | 1 [Reference] | — |
| 60 to <70 | 82.3 ± 6.2 | 119/636 (18.7) | 1.10 (0.74–1.65) | 0.639 | 57/636 (9.0) | 1.00 (0.58–1.73) | 0.992 | |
| ≥70 | 78.2 ± 8.1a | 204/1007 (20.3) | 1.12 (0.75–1.66) | 0.589 | 101/1007 (10.0) | 1.08 (0.63–1.86) | 0.769 | |
BP, systolic blood pressure; SBP, systolic BP; DBP, diastolic BP; PP, pulse pressure; CHD, coronary heart disease; HR, hazard ratio; CI, confidence interval.
P<0.001 by one-way analysis of variance.
Adjusted for age, sex, mini-mental state examination score, diabetes mellitus, smoking, qualifying stroke severity, history of stroke (before qualifying stroke), history of coronary heart disease, history of heart failure, history of carotid endarterectomy, history of alcohol use, lipid modifier use, antithrombotic use, and antihypertensive use.
Figure 2.
Kaplan-Meier curves for the endpoints of major vascular events (A) and stroke (B) among subjects with an SBP <120 mm Hg, an SBP 120 to <140 mm Hg, and an SBP ≥140 mm Hg over 2 years after a recent noncardioembolic stroke based on pulse pressure (<60, 60 to <70, and ≥70). SBP, systolic blood pressure.
Discussion
This is the first study as far as we are aware that sought to examine the independent association of DBP with vascular outcomes after a recent stroke over a 2 year follow up period. We observed that compared with the normal DBP group, risk of MVEs was significantly higher by over 30% for the low-normal DBP group (<70 mmHg) than the normal DBP group after multivariable adjustment. Since the low-normal DBP group had higher comorbidities of diabetes, CHD, heart failure and carotid artery disease, and had more severe qualifying strokes, it is conceivable that the relationship between low-normal DBP and adverse vascular outcomes after stroke might reflect underlying poor health condition per se that predisposes to negative vascular outcomes. However, our finding was independent of mean SBP levels, vascular comorbidities, treatment strategy including antithrombotic therapies, lipid modifiers, and antihypertensive medications. Also, mean SBP showed no interaction with mean DBP. High DBP level (>90 mm Hg) was linked to an increased risk of MVEs, but this association was attenuated after adjusting for covariates. For the secondary outcome of stroke alone, when compared with the normal DBP group, low-normal DBP showed a pattern of greater risk of recurrent stroke after multivariable adjustment.
With regard to the PP, risk of MVEs was significantly greater by approximately 2-fold for subjects with a PP of ≥70 (mean DBP, 62.4 ± 3.8 mm Hg) than those with the lowest PP (mean DBP, 78.0 ± 5.9 mm Hg) among those with an SBP 120 to <140 mm Hg. which is a similar pattern to findings from a recent study [9] that low DBP of <70 mm Hg was associated with subclinical myocardial damage and CHD events in the Atherosclerosis Risk In Communities cohort comprising >11,000 adults. Risk of coronary events in patients with CHD is more related to lower DBP than lower SBP, since low DBP could compromise coronary perfusion, yielding cardiac ischemia [5]. Given that a history of CHD was a predictor of MVEs in our study, subjects with low DBP and history of CHD would have been susceptible to MVEs. However, the finding that occurrence of incident stroke was also significantly higher (HR 2.87) in that category (SBP 120 to <140 mm Hg) conflicts with the result [9] that low DBP was not associated with incident stroke. Factors that may contribute to the conflicting findings of our sub-analysis of VISP trial [6] vs. the other study [9] include study population (stroke survivors vs. healthy community adults), mean age (66 years vs. 57 years), and different rates of male sex (63% vs. 43%).
In this study, mean SBP <120 mm Hg was associated with higher risk of stroke (HR 1.71 in Table S2), which is a roughly similar pattern to finding (HR 1.29) from a previous study [3]. Of note, that association was evident in subjects whose PP was over 60 with low mean DBP of <60 mm Hg, the finding of which likely reflects more advanced vascular morbidity in those with a PP ≥ 60 vs. <60. However, since the sample size was very small (n=5) and the CI was wide in subjects with an SBP <120 mm Hg with a PP >60, our finding needs to be validated in a much larger study.
Contrary to the finding among subjects with an SBP <140 mm Hg, elevated PP was not a powerful determinant of the risk of MVEs or stroke in those with an SBP ≥140 mm Hg. This null finding in those with an SBP ≥140 mm Hg may be because all the mean DBP levels were around 80 mm Hg or more, irrespective of PP category. An increase in SBP might have outweighed a decrease in DBP.
Also, no association of high DBP (>90 mm Hg) with the risk of MVEs or stroke might have been due to the relatively small number of patients with DBP of >90 mm Hg (n=239, 6.5%), particularly in those with DBP of >100 mm Hg (n=14, 0.4%), which is shown in Figure S1. Taking the aforementioned findings into account, our findings suggest that low DBP (vs. high DBP) along with an increased PP may confer higher risk of recurrent vascular events among patients following a noncardioembolic stroke.
Across mean DBP categories, there was a significant interaction between age and mean DBP on outcome in this study such that older age was associated with more MVEs in the low-normal DBP group. Given that the low-normal DBP group was older and more likely to be lean across the DBP categories, this finding may concur with a previous study, which implied that lean stroke patients may be more prone to risk of MVEs [10], in an “obesity paradox” [11].
This study has some limitations. This is a post hoc exploratory analysis, thus our findings showing associations of low DBP and increased PP with vascular outcomes do not prove that low DBP to <70 mmHg and increased PP will definitely lead to adverse vascular outcomes. These results should be seen simply as hypothesis-generating. Moreover, all study participants experienced noncardioembolic strokes thereby limiting generalizability to all stroke types (ie, including cardioembolic and hemorrhagic strokes). There was a relatively modest number of subjects with DBP >90 mm Hg, particularly those with DBP of >100 mm Hg (0.4%), which might have influenced the statistical power for observing vascular events in the category of high DBP. Also the very small sample size (n=5) in subjects with an SBP <120 mm Hg with a PP >60 jeopardizes the precision of estimates for vascular event. Finally, we could not adjust for beta-blockers and some calcium antagonists (ie, diltiazem, verapamil) that may aggravate the diastolic hypotension by widening PP [8] because there was no specific information about these particular drugs in the VISP database, which might have otherwise, influenced the current results.
In conclusion, among patients with a recent noncardioembolic stroke, low DBP (<70 mm Hg), particularly along with increased PP of >60 to 70 was linked to a significantly greater risk of MVEs and stroke over a 2-year follow-up period. However, high DBP level by itself did not have significant impact on the risk of vascular event over the same time period, which underscores stronger impact of high SBP on vascular risk. This study suggests that among patients being treated with antihypertensive therapy for secondary stroke prevention, closer surveillance may need to be paid not only to SBP, but also to achieved DBP and PP to avoid the occurrence of untoward vascular events. Our findings need to be validated through prospective studies with higher numbers of patients and minimization of potential confounders including underlying cerebrovascular status.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Ovbiagele is supported by Awards NS079179 and NS094033 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Disclosure of conflicts of interest
None declared.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Diener HC, Yusuf S, et al. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011;306:2137–2144. doi: 10.1001/jama.2011.1650. [DOI] [PubMed] [Google Scholar]
- 3.Ovbiagele B. Low-normal systolic blood pressure and secondary stroke risk. J Stroke Cerebrovasc Dis. 2013;22:633–638. doi: 10.1016/j.jstrokecerebrovasdis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Group SPSS, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. doi: 10.1016/j.jacc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy JW, Chen Y, Rawlings A, et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener HC. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- 11.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.