Abstract
Background
Prostaglandin E2 (PGE2) regulates renin expression in renal juxtaglomerular cells. PGE2 acts through E-prostanoid (EP) receptors in the renal collecting duct (CD) to regulate sodium and water balance. CD cells express EP1 and EP4, which are linked to protein kinase C (PKC) and protein kinase A (PKA) downstream pathways, respectively. Previous studies showed that the presence of renin in the CD, and that PKC, and PKA pathways activate its expression. The (pro)renin receptor (PRR) is also expressed in CD cells and its activation enhances cyclooxygenase-2 (COX-2) through extracellular signal–regulated kinase (ERK). We hypothesized that PGE2 stimulates prorenin and renin synthesis leading to subsequent activation of PRR and upregulation of COX-2.
Methods
We used a mouse M-1 CD cell line that expresses EP1, EP3, and EP4 but not EP2.
Results
PGE2 (10−6 M) treatment increased prorenin and renin protein levels at 4 and 8 h. No differences were found at 12 h post PGE2 treatment. Phospho-ERK was significantly augmented after 12 h. COX-2 expression was decreased after 4 h of PGE2 treatment, but increased after 12 h. Interestingly, the full-length form of the PRR was upregulated only at 12 h. PGE2 mediated phospho-ERK and COX-2 upregulation was suppressed by PRR silencing.
Conclusions
Our results suggest that PGE2 induces biphasic regulation of COX-2 through renin-dependent PRR activation via EP1 and EP4 receptors. PRR-mediated increases in COX-2 expression may enhance PGE2 synthesis in CD cells serving as a buffer mechanism in conditions of activated renin angiotensin system.
Keywords: renin, collecting duct, prostanoid receptor, prostaglandin E2, protein kinase C
INTRODUCTION
Renin is mostly expressed in the juxtaglomerular cells (JG), which is the primary source of plasma renin activity and the limiting step for the activation of the renin-angiotensin system (RAS)1, 2. Cyclooxygenase-2 (COX2)-mediated PGE2 synthesizes in the macula densa (MD) and enhances renin expression in JG cells through the activation of EP4 receptor via cAMP-protein kinase A (PKA) 3,4. After systemic RAS activation, angiotensin II (ANG II) stimulates Ca2+ release5 and protein kinase C (PKC) through type 1 receptor (AT1R) to inhibit renin synthesis in the JG cells6. Renin has also been detected in the CD, and its expression increases after ANG II stimulation7,8 via a mechanism mediated by AT1R and PKC activation9. ANG II also increases the expression of the (pro)renin receptor (PRR), which is abundantly expressed in CD intercalated cells10. PRR activation enhances COX-2 expression through mitogen-activated protein kinase (MAPK) pathways11, which finally leads to the increase in PGE2 synthesis, as suggested previously12, 13. These mechanisms may enhance further formation of PGE2 in the CD.
PGE2 exerts its effects through four types of E-prostanoid (EP) receptors: the EP1, EP2, EP3, and EP414. The EP4 receptors are predominantly expressed in the MD cells, whereas EP3 receptors are more abundant in the thick ascending limb15. The CD segment expresses EP1, EP3, and EP4 receptors16. EP1 activates PKC and increases intracellular Ca2+, whereas the EP3 receptor is coupled to a Gαi protein that inhibits cAMP formation. The EP4 receptor is coupled to a Gαs protein, which enhance cAMP formation16. It has been reported that M-1 cells express EP1, EP3, and EP4, but not EP217.
In ANG II-dependent hypertension, membrane-bound PRR, MAPK signaling, PGE2 levels, and COX-2 expression are augmented in the rat renal medulla mostly composed of CDs12. PGE2 may serve as a buffer against the hypertensinogenic and vasoconstricting effects of ANG II12,13,18. PRR activation by prorenin and renin increases COX-2 expression in conditions of high intrarenal ANG II12,19. However, the role of PGE2 in the regulation of PRR and renin expression and its effects on COX-2 abundance in CD cells has not been elucidated. In this study we tested whether PGE2 stimulates prorenin and renin synthesis leading to subsequent activation of PRR and upregulation of COX-2.
MATERIALS AND METHODS
M-1 cell culture and reagents
The institutional review board of the Faculty of Science of the Pontificia Universidad Católica de Valparaíso approved the experimental design and treatments. M-1 cells (ATCC, VA) is a well characterized CD cell line with phenotypic characteristics of cortical CD cells, including epithelial sodium channel (ENaC) and aquaporin-2 (AQP-2) expression 20. Importantly, M-1 cells are composed of principal and intercalated cells which express COX-221 EP1, EP3, EP417 prorenin-renin, and PRR constitutively 22 which make this cell line a good in vitro model to establish the role of the PRR-COX-2-PGE2 pathway on renin regulation. The M-1 cells were cultured as previously described 9,23. As shown in Figure 1A, M-1 cells express AQP-2 and can respond to vasopressin (AVP, 10 nM, 4 h) increasing AQP-2 abundance in plasma membranes. Dose response curves with different concentrations (10−9 – 10−6 mol/L) of PGE2 (Cayman, Ann Arbor, MI) demonstrated that 10−6 mol/L increased prorenin and renin at 4 h (data not shown). Therefore, we harvested cells after 4, 8, and 12 h of PGE2 treatments. Transfections were performed 32 h before treatments. PGE2 was dissolved in DMSO for stock and ethanol (vehicle) for dilutions. Controls were treated with vehicle with respective subsequent dilutions in cell culture media. Treatments were performed in the presence of indomethacin (1 μmol/L) to block endogenous COX-1 and COX-2 activity and candesartan (1 μmol/L) to rule out the possibility of endogenous ANG II formation and AT1R activation. SC119220 and L161982 were purchased from Cayman (Cayman Ann Arbor, MI, USA) and used according to the company’s recommendations and IC50 described in the literature24.
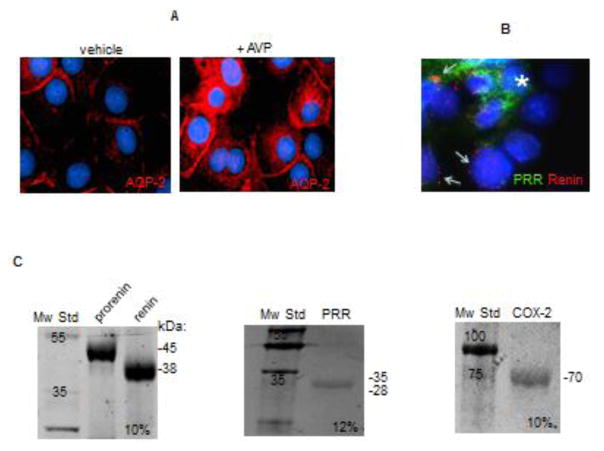
FIGURE 1.
(A) Characterization of M-1 collecting duct cell line. M-1 cells can increase the abundance of aquaporin 2 (AQP-2, red) after vasopressin (AVP) treatment. (B) Co-labeling of (pro)renin receptor (PRR, green) and prorenin-renin (red) showing differential distribution, probably in intercalated cells (asterisks) and principal cells (arrows) respectively. (C) Recombinant proteins used as positive controls to establish antibody specificity and migration.
Immunofluorescence studies
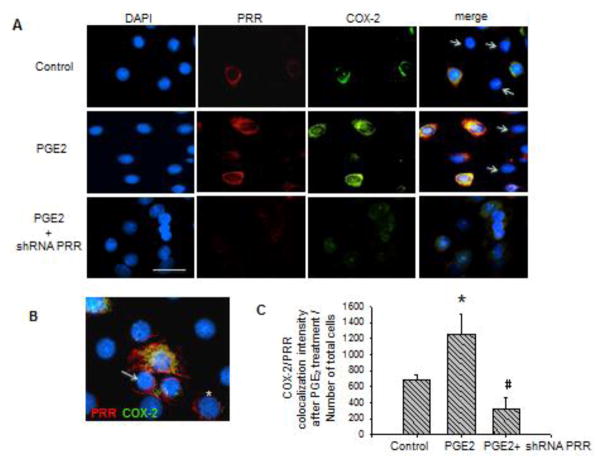
M-1 cells were fixed in cold methanol. Afterward, they were blocked and stained with rabbit anti-ATP6AP2 (Cat. HPA003156, Sigma), mouse anti COX-2 (Cat. sc-1747 Santa Cruz) and detected with Alexa Fluor 594 or 488 conjugated to anti rabbit or anti mouse IgG (Invitrogen, Carlsbad, CA), accordingly. Primary antibodies used for characterization of M-1 cells were: rabbit anti-aquaporin-2 (cat. no. 178612, Calbiochem, San Diego, CA), rabbit anti-renin (sc H-105, Santa Cruz Biotechnology, Santa Cruz, CA). Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen, Carlsbad, CA). Negative controls were obtained by omission of the specific primary antibody. NIS Elements analysis software (Nikon) was used to quantify PRR-COX-2 co-localization intensity by measuring the merging area in 6 fields (40x). Results are expressed as the COX-2-PRR co-localization intensity after PGE2 treatment with or without PRR shRNA versus Number of total cells (Figure 4C).
FIGURE 4.
(A) Co-localization of the (pro)renin receptor (PRR) and the cyclooxygenase-2 (COX-2) indicating the effect of prostaglandin E2 (PGE2) treatment (12 h) and the effect of shRNA PRR on the decreased expression of COX-2 within the cells. Quantification of merge intensity is shown at the bottom. *P < 0.05. Scale bar = 10 μm. Arrow represent negative labeling for PRR and COX-2, probably principal cells. (B) PRR-COX-2 co-localization: PRR (red) is expressed in M-1 cells (probably intercalated cells), which are also positive for COX-2 (green). PRR was observed in the plasma membrane (arrows) and in a peri-nuclear distribution (asterisk) probably associated to the V-ATPase. (C) Quantification of PRR-COX-2 merge intensity after treatments. (D) Temporal protein expression profile of COX-2 in cells at 4, 8, and 12 h post PGE2 treatment in M-1 CD cells transfected with PRR shRNA. *P < 0.05 vs. control. (E). Phosphorylated extracellular signal–regulated kinase (ERK) versus total ERK ratio at 4, 8, and 12 h post PGE2 treatment in cells transfected with PRR shRNA.
Western blot analysis for phospho-ERK1/2 and total ERK1/2, COX-2 and PRR
After treatments, M-1 cells were homogenized in lysis buffer (mmol/L: NaCl 150, Tris 50, dithiothreitol 100, 1% NP-40, and 1% protease inhibitor cocktail, Sigma) and complete protease inhibitor mixture tablet with 1 mM ethylenediaminetetraacetic acid. Lysates were sonicated for 10 sec on ice and centrifuged at 5,000 rpm for 10 min. The supernatants were measured for protein using the Bradford Protein Assay (Bio-Rad). Twenty μgrams of total protein separated by SDS-PAGE (10 or 12%), transferred to Nitrocellulose membrane (Amersham Biosciences, Freiburg, Germany), probed with renin polyclonal IgG B-12 antibody (sc-133145, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-ATP6AP2 (Cat. HPA003156, Sigma), mouse anti-COX-2 (Cat. sc-1747 Santa Cruz), mouse anti-phospho-p44/42 ERK1/2 (Thr202/Tyr204) antibody (Cat. # 91065, Cell Signaling Technology, Beverly, MA), rabbit anti total ERK (Cat. # 9122, Cell Signaling Technology, Beverly, MA), were revealed using an ECL system. Band identity was confirmed by using mouse recombinant prorenin (AnaSpec, Fremont, CA), renin (Lee Biosolutions, St. Louis, MO), COX-2 (human COX-2 cat. 60122), and a recombinant PRR peptide. Resulting bands were compared with molecular weight standards (M. Biosources, San Diego, CA). Densitometry was performed with ImageJ software and normalized to β-actin and quantified with respect to controls. Results were expressed as percentage vs. control (100%).
PRR knockdown using transient transfections of GFP-shRNA-PRR
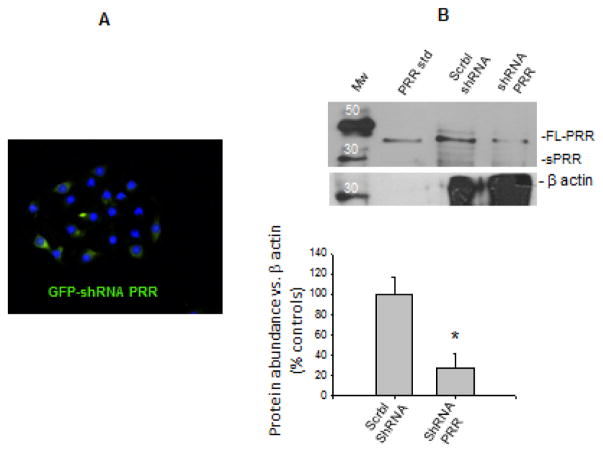
Silencing of PRR expression was performed using SureSilencingTM shRNA plasmid for Mouse Atp6ap2 (Cat. KM26726, SABioscience, Qiagen, Valencia, CA), according to manufacturer’s instructions. Sub-confluent cells (60%) were transfected 32 h prior to treatments. Green fluorescent protein (GFP) positive cells (Figure 3A) confirmed effectiveness of transfections, which reached a rate 70 ± 8% (n = 10). Effectiveness of PRR silencing was also verified by band intensity compared with cells transfected with scramble-shRNA (Figure 3B). Cell viability after PRR knockdown (48 h) was assessed by AlamarBlue® Cell Viability Reagent (ThermoFisher DAL 1025) demonstrating no effects in death cell counting.
FIGURE 3.
(A) Green fluorescent protein (GFP) positive cells confirming effectiveness of GFP-shRNA-PRR transfections, which reached 70 ± 8% (n = 10). (B). Effectiveness of PRR silencing verified by band intensity compared with non-transfected cells and cells transfected with scramble-shRNA (73 ± 14% P < 0.05 vs. scramble-shRNA transfected group, n = 6). Western blot membrane was revealed by quimioluminescent method after incubation with rabbit anti-ATP6AP2 and mouse anti-β-actin. A positive control (recombinant PRR) is also shown. Mw: Molecular weight standard.
Statistical Analyses
For Western blot and mRNA levels, an average of 6 independent observations were performed for each treatment and represented as percentage vs. controls (100%). Data were evaluated by the Grubb test, followed, when appropriate, by paired and unpaired Student t-test or by one-way ANOVA with Tukey post-test. Significance was defined as P < 0.05. No significant differences are expressed as “NS.” Results are expressed as mean ± SEM.
RESULTS
Characterization of the M-1 cells and western blot band identity
M-1 cells are a well characterized CD cell line with phenotypic characteristics of cortical CD cells, including ENaC and aquaporin-2 (AQP-2) expression 20. As shown in Figure 1A, M-1 cells express AQP-2 and can respond to vasopressin (AVP, 10 nM, 4 h), increasing AQP-2 abundance in plasma membrane. M-1 cells also express PRR that has been described in intercalated cells and renin-prorenin staining in principal cells (Figure 1B). To further confirm the specificity and identity of western blot bands, we used recombinant proteins to clarify the expected molecular weight. As shown in Figure 1C, recombinant prorenin was observed near 45 kDa, whereas renin was detected near 38 kDa; this agrees with our previous reports9. Full-length recombinant PRR was detected at 28 kDa. As expected, COX-2 positive control was detected at 70 kDa.
Micromolar concentrations of PGE2 increases prorenin and renin protein levels at 4 but not at 12 h
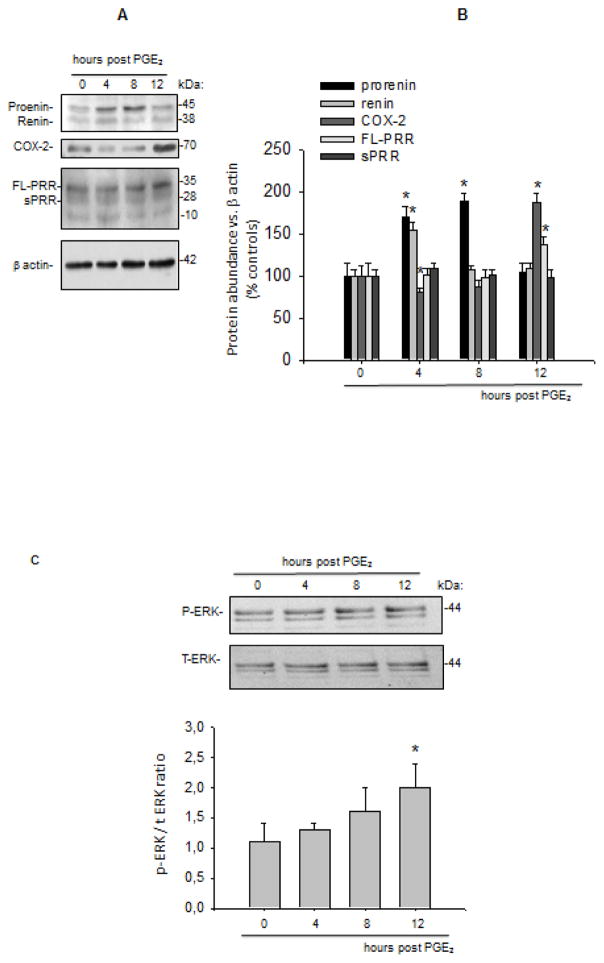
Figure 2A shows a time profile of prorenin-renin protein expression. Prorenin and renin protein levels reached a significant upregulation at 4 h (prorenin: 170 ± 12%, P < 0.05 vs. control; renin 155 ± 9%, P < 0.05 vs. control). The prorenin band was still augmented at 8 h post PGE2 treatment, however the renin band was not significantly different from control levels as shown in the quantification plot (Figure 2B).
FIGURE 2.
(A) Temporal protein expression profile of prorenin, renin, COX-2, and (pro)renin receptor (PRR: full length and soluble forms) at 4, 8, and 12 h post prostaglandin E2 (PGE2) treatment in M-1 cells. Protein levels were analyzed by densitometry and normalized by β-actin. (B) and expressed as percentage of control (100%). *P < 0.05 vs. Vehicle (Veh), n = 6. (C) Phosphorylated-ERK versus total ERK ratio at 4, 8, and 12 h post PGE2 treatment in M-1 CD cells. FL: full length.
PGE2-mediated increases in renin expression are associated with a subsequent upregulation of ERK pathway and COX-2 in a late phase
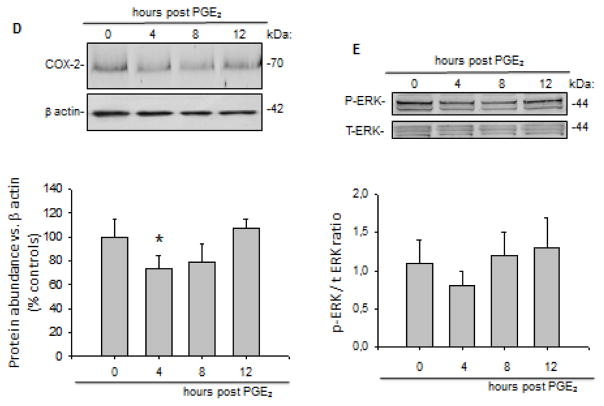
Along with prorenin and renin, protein induction observed after 4 and 8 h post PGE2 treatment, COX-2 protein levels were maximally increased at 12 h (187 ± 11 vs. 100 ± 13%, P < 0.05). A significant reduction in COX-2 protein abundance was observed at 4 h post PGE2 treatment (81 ± 5 vs. 100 ± 11%, P<0.05). Interestingly, PRR expression was augmented at 12 h post PGE2 treatment (137 ± 9 vs. 100 ± 15%, P<0.05, Figure 2A and B). Similarly, ERK1/2 phosphorylation levels were maximally augmented at 12 h (Figure 2C).
PRR knockdown prevented COX-2 upregulation observed after 12 h of PGE2 treatment
To test whether the increased ERK pathway and COX-2 expression was due to PRR stimulation we performed transfections with shRNA-PRR to knockdown PRR expression. Figure 3A shows GFP positive cells transfected with shRNA-PRR. The percentage of GFP positive cells was 70 ± 8. A reduction of 73 ± 14% (n=6) in the expression of PRR as observed by densitometric analysis (Figure 3B). As shown in Figure 4B PRR (red) is expressed in M-1 cells (probably intercalated cells), which are also positive for COX-2 (green). PRR was observed in the plasma membrane (arrows) and in a peri-nuclear distribution (asterisk) probably associated to the V-ATPase; this was previously described in rat primary cultured inner medullary CD cells13. PRR shRNA impairs COX-2 upregulation (Figure 4A and C) at 12 h (107 ± 8 vs. 100 ± 13%, P=NS, Figure 4D) and blunted ERK1/2 phosphorylation (Figure 4E). A transient decrease in COX-2 expression was also observed at 4 h, indicating that PGE2 causes an initial reduction of COX-2 expression as described before 15.
Pharmacological inhibition of EP1 and EP4 partially prevents prorenin renin upregulation
To look for a PGE2 receptor responsible for starting the pathway leading to increased COX-2 in M-1 cells, we blockaded pharmacological EP1 and EP4 (PKC-Ca2+ and cAMP pathway, respectively) to see if prorenin or renin abundance could be ameliorated. As shown in Figure 5, EP1, and EP4 inhibitors at doses described in the literature suppressed prorenin and renin upregulation, suggesting that PRR stimulation might be reduced leading to an impairment of COX-2 upregulation.
FIGURE 5.
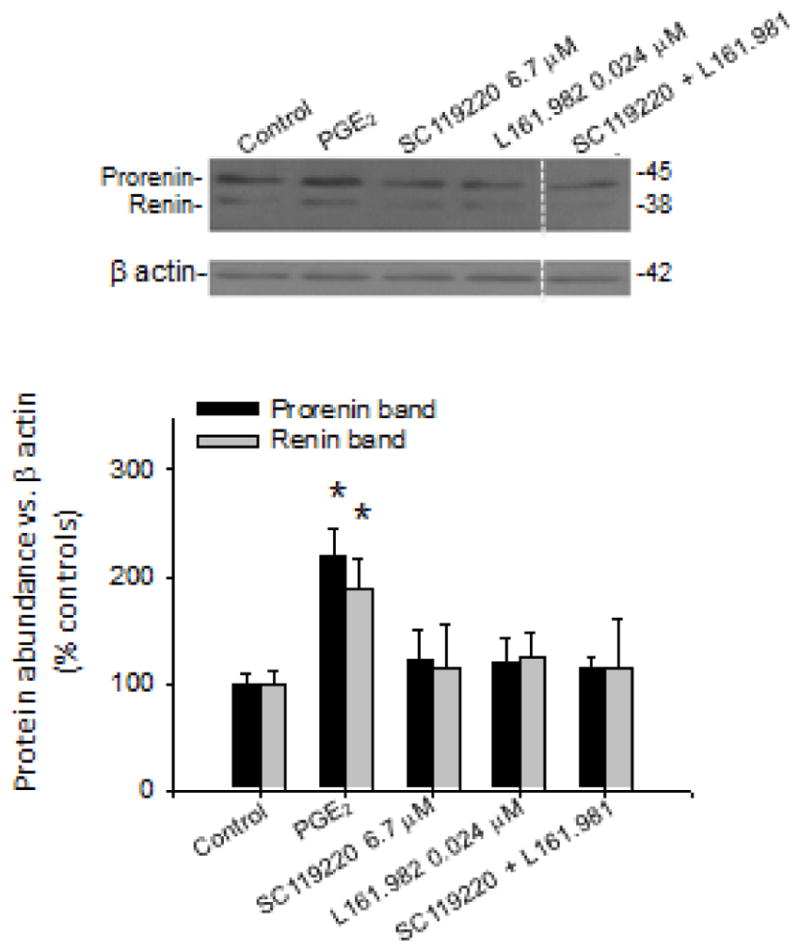
E-prostanoid receptor EP1 and EP4 may be responsible for prorenin-renin upregulation after subsequent stimulation of the (pro)renin receptor (PRR) mediating cyclooxygenase-2 (COX-2) upregulation during the late phase. M-1 cells were incubated with prostaglandin E2 (PGE2) 1 μmol and co-incubated with EP1 and EP4 antagonists at doses shown in the figure. As observed, both antagonists were able to prevent prorenin and renin upregulation. *P < 0.05 vs. vehicle (Veh), n = 6.
DISCUSSION
We have demonstrated that PGE2 stimulates prorenin and renin protein levels at μmolar concentrations in M-1 CD cells. This stimulation was seen at 4 h whereas ERK1/2 phosphorylation and COX-2 protein levels were increased during a late phase (12 h). Moreover, COX-2 was decreased after PGE2 treatment below control levels in agreement with previous reports indicating that EP3 downregulates COX-2 in thick ascending limbs 25. Our findings indicate that PGE2 treatment causes a temporal biphasic expression pattern of COX-2 that depends on PRR activation, since PRR silencing prevented this effect.
Actions of PGE2 in the kidney are related to the stimulation of renin synthesis in JG cells 3,26,27 and the regulation of tubular sodium and water transport 28. EP receptors (EP1–4) are coupled to different signaling pathways. It has been shown that M-1 CD cells express EP1, EP3, and EP4 17. We have previously demonstrated that activation of the AT1 receptor increases prorenin and renin expression via cAMP accumulation, which is dependent on PKC activity 9,23. In the MD, the activation of EP4 receptor enhances cAMP formation and activates the cAMP-CREB pathway and renin synthesis in JG cells29. It is likely that the effect of PGE2 might be also mediated by stimulation of EP1 and EP4 receptors and activation of PKC and cAMP respectively, which finally leads to the upregulation or prorenin and renin protein. More recently we reported that actions of AVP require the intact activity of PKC to enhance prorenin and renin protein expression in CD cells9. Indeed, PKC potentiates PKA-mediated signals responsible for AQP-2 expression in the plasma membrane in CD cells30.
PGE2-mediated increases in renin expression in the CD might be of functional significance in the regulatory buffer system in the CD. We previously revealed that COX-2 expression is upregulated by direct activation of the prorenin receptor (PRR) independently of the activation of AT1 receptors in rat primary cultured renal inner medullary cells 13. We have shown that COX-2 is expressed in intercalated cells of the CD 12,13. The expression of renin and prorenin in the principal cells of the CD is augmented during high ANG II states 7,31,32 and is secreted in the intratubular fluid 33. Renin and prorenin are detected in the cell culture media 9,23 suggesting that augmented prorenin and renin secretion may activate the PRR in the plasma membrane of the neighboring intercalated cell. The activation of the PRR by prorenin increases the expression of COX-2 via activation of MAPK 12,13. Accordingly, increases in prorenin or renin secretion by principal cells activate PRR, which augments COX-2 and PGE2 production in the renal medulla serving as a buffer mechanism against the vasoconstricting and antinatriuretic effects of activated RAS as suggested previously12,18,22,34.
The M-1 CD cell line has cortical origin and expresses ENaC and AQP-2; they are also composed of intercalated cells 20. More importantly, they express all the components common in medullary and cortical CDs, such as prorenin, renin, PRR, COX-2, and EP receptors studied in this report, representing a good in vitro model. Here we showed that a single PGE2 treatment mediates an early upregulation of prorenin and renin (4 h) and the upregulation of COX-2 during later stages (12 h). Then, it is possible that the prorenin and renin bind to PRR and activates ERK1/2 pathway and COX-2. This hypothesis was demonstrated by PRR expression knock down, which dramatically blunted COX-2 upregulation during the late phase (12 h). Interestingly, PRR was also upregulated at 12 h (Figure 1A and B), in agreement with recent studies from Yang’s group, showing that PGE2 upregulates PRR35.
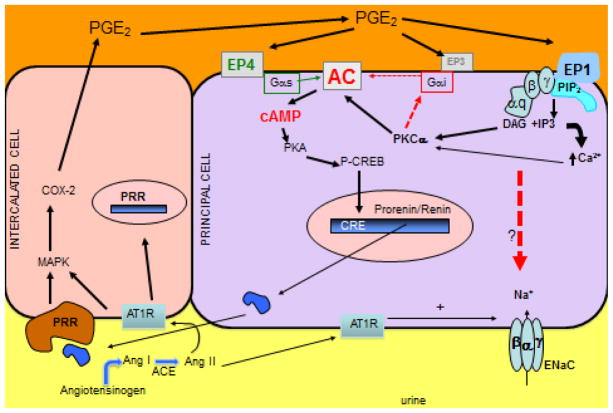
Stimulation of renin synthesis via PGE2 may lead to a positive feedback mechanism by which continuous stimulation of its expression contributes to further activation of EP receptors by COX-2-mediated increases in PGE2. Importantly, downregulation of COX-2 by EP receptors has been described in thick ascending limb15. Our data suggest that PGE2 may be the initial stimuli to promote prorenin-renin mediated stimulation of PRR and COX-2 augmentation in CD cells. Our data suggest that this stimulation is mediated by EP1 and EP4 receptors (Figure 5), which are linked to the stimulation of PKC-Ca2+ and stimulation of cAMP formation within the cell and both mechanisms seem to be involved in renin synthesis in the CD23. We previously showed that during the early phase of ANG II-dependent hypertension, membrane-bound PRR and COX-2 are augmented in the renal medullary tissues12. This increased COX-2 expression forms PGE2 and PGI2, which buffer the hypertensinogenic and vasoconstricting effects of ANG II during this early period by inhibiting ENaC activity and expression 36–39. However, this process is followed by reduced PRR expression associated with reduced COX-2 expression during later stages of ANG II hypertension 12. Recent evidence from Wang et al., showed that increases in systolic blood pressure with ANG II infusion was attenuated by the COX-2 inhibition and was associated with decreases in renal medullary PRR expression35. Thus, feedback interactions between PGE2-mediated increases in renin expression and PRR-COX-2 expression during the early phase of ANG II hypertension may be able to buffer the actions of ANG II (Figure 6).
FIGURE 6.
Hypothetical model of the mechanism of prostaglandin E2 (PGE2)-dependent regulation of renin and subsequent upregulation of cyclooxygenase-2 (COX-2) via (pro)renin receptor (PRR) leading to further PGE2 synthesis as a buffer mechanism against the activation effects of the renin angiotensin system in vivo. Our data suggest that PGE2 enhances prorenin-renin synthesis. The membrane bound PRR promotes signal transduction when activated by prorenin or renin leading to COX-2 upregulation. The activation of EP1 inhibits the activity of the epithelial sodium channel (ENaC) promoting natriuresis, but also might stimulate prorenin-renin synthesis. COX-2 is also greatly augmented in collecting duct cells during the early phase of the activation of the renin angiotensin system. ACE: angiotensin-converting enzyme, ENaC: epithelial sodium channel, AT1R: angiotensin II type 1 receptor, Ang: angiotensin.
PRR activation upregulates COX-2 via ERK 1/2 pathways 11, which contribute to inflammatory processes in the kidney. Therefore, it is important to evaluate the effects of PRR activation in the distal nephron because the synthesis of prorenin is augmented in the CD during diabetic nephropathy40, which may contribute to the activation of PRR and signaling pathways that promote tubular damage. Ohashi et al. reported that plasma soluble PRR reflects renal damage 41. In agreement with our previous studies, plasma soluble PRR might have a role in intratubular ANG II formation leading to further stimulation of renin synthesis in the CD.18 Another study from Huang et al. described the enhanced intrarenal receptor-mediated prorenin activation in chronic progressive nephritis in rats 42. On the other hand, the role of PGE2 during early activation of prorenin-dependent PRR activation may represent a non-physiopathological role, which may help to buffer hypertensinogenic responses.
In summary, these results demonstrated that PGE2 increases prorenin and renin protein levels in M-1 CD cells, and the increased prorenin and renin may subsequently increase intracellular signaling pathways mediated by PRR, stimulating COX-2 and further PGE2 synthesis. Distal intraluminal secretion of prorenin or renin can stimulate the PRR-mediated upregulation of COX-2 and PGE2 synthesis in vivo. Increases in prorenin, renin, PRR, and COX-2 in the CD during the early phase of hypertension support this concept and reflect the complex regulation of natriuretic and pro-hypertensive effects of the interplay among COX-2, PRR, and renin.
Acknowledgments
Source of Funding
This work was supported by NIH-NIDDK (R01DK104375) to M.C.P., DREAMS 039.317/2016 Pontificia Universidad Católica de Valparaíso and by FONDECYT-Chile 11121217 grant to A.A.G.
We thank to Nancy B. Busija, M.A., CCC-SLP, Senior Editor, Department of Pharmacology, Tulane University School of Medicine for final edition of the manuscript.
Abbreviations
- ACE
angiotensin-converting enzyme
- Ang
angiotensin
- ANG II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- CD
collecting duct
- cAMP
cyclic adenosine monophosphate
- ENaC
epithelial sodium channel
- EP
E prostanoid receptor
- ERK
extracellular signal–regulated kinase
- MAPK
mitogen-activated protein kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PRR
(pro)renin receptor
Footnotes
Conflict of Interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castrop H, Hocherl K, Kurtz A, et al. Physiology of Kidney Renin. Physiol Rev. 2010;90:607–73. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 2.Celio MR, Inagami T. Renin in the Human-Kidney - Immunohistochemical Localization. Histochemistry. 1981;72:1–10. doi: 10.1007/BF00496773. [DOI] [PubMed] [Google Scholar]
- 3.Harris RC, Mckanna JA, Homma T, et al. Cycloxygenase-2 Is Localized to the Macula Densa and Papillary Interstitial-Cells and Increases with Salt Restriction. J Am Soc Nephrol. 1994;5:680. [Google Scholar]
- 4.Traynor TR, Smart A, Briggs JP, et al. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol-Renal. 1999;277:F706–F10. doi: 10.1152/ajprenal.1999.277.5.F706. [DOI] [PubMed] [Google Scholar]
- 5.Klar J, Sandner P, Muller MWH, et al. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflug Arch Eur J Phy. 2002;444:335–44. doi: 10.1007/s00424-002-0818-9. [DOI] [PubMed] [Google Scholar]
- 6.Muller MWH, Todorov V, Kramer BK, et al. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflug Arch Eur J Phy. 2002;444:499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 7.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–9. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Carrasquero MC, Kobori H, Ozawa Y, et al. AT(1) receptor- mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol-Renal. 2005;289:F632–F7. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez AA, Liu L, Lara LS, et al. PKC-alpha-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am J Physiol-Renal. 2015;309:E880–E8. doi: 10.1152/ajprenal.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advani A, Kelly DJ, Cox AJ, et al. The (Pro) Renin Receptor Site-Specific and Functional Linkage to the Vacuolar H(+)-ATPase in the Kidney. Hypertension. 2009;54:261–U129. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 11.Kaneshiro Y, Ichihara A, Takemitsu T, et al. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–6. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Green T, Luffman C, et al. Renal medullary cyclooxygenase-2 and (pro)renin receptor expression during angiotensin II-dependent hypertension. Am J Physiol-Renal. 2014;307:F962–F70. doi: 10.1152/ajprenal.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez AA, Luffman C, Bourgeois CRT, et al. Angiotensin II-Independent Upregulation of Cyclooxygenase-2 by Activation of the (Pro) Renin Receptor in Rat Renal Inner Medullary Cells. Hypertension. 2013;61:443-+. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol. 1996;7:8–17. doi: 10.1681/ASN.V718. [DOI] [PubMed] [Google Scholar]
- 15.Hao SJ, Hernandez A, Quiroz-Munoz M, et al. PGE(2) EP3 receptor downregulates COX-2 expression in the medullary thick ascending limb induced by hypertonic NaCl. Am J Physiol-Renal. 2014;307:F736–F46. doi: 10.1152/ajprenal.00204.2014. [DOI] [PubMed] [Google Scholar]
- 16.Hebert RL, Breyer RM, Jacobson HR, et al. Functional and Molecular Aspects of Prostaglandin-E Receptors in the Cortical Collecting Duct. Can J Physiol Pharm. 1995;73:172–9. doi: 10.1139/y95-026. [DOI] [PubMed] [Google Scholar]
- 17.Sandrasagra S, Cuffe JE, Regardsoe EL, et al. PGE(2) stimulates Cl- secretion in murine M-1 cortical collecting duct cells in an autocrine manner. Pflug Arch Eur J Phy. 2004;448:411–21. doi: 10.1007/s00424-004-1260-y. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez AA, Lara LS, Luffman C, et al. Soluble Form of the (Pro) Renin Receptor Is Augmented in the Collecting Duct and Urine of Chronic Angiotensin II-Dependent Hypertensive Rats. Hypertension. 2011;57:859–64. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi ZH, Hao CM, Langenbach RI, et al. Opposite effects of cyclooxygenase-1 and-2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–9. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoos BA, Narayfejestoth A, Carretero OA, et al. Characterization of a Mouse Cortical Collecting Duct Cell-Line. Kidney Int. 1991;39:1168–75. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- 21.Nasrallah R, Laneuville O, Ferguson S, et al. Effect of COX-2 inhibitor NS-398 on expression of PGE(2) receptor subtypes in M-1 mouse CCD cells. Am J Physiol-Renal. 2001;281:F123–F32. doi: 10.1152/ajprenal.2001.281.1.F123. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez AA, Prieto MC. Renin and the (pro)renin receptor in the renal collecting duct: Role in the pathogenesis of hypertension. Clin Exp Pharmacol P. 2015;42:14–21. doi: 10.1111/1440-1681.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez AA, Cifuentes-Araneda F, Ibaceta-Gonzalez C, et al. Vasopressin/V2 receptor stimulates renin synthesis in the collecting duct. Am J Physiol-Renal. 2016;310:F284–F93. doi: 10.1152/ajprenal.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiriyama M, Ushikubi F, Kobayashi T, et al. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Brit J Pharmacol. 1997;122:217–24. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao SJ, DelliPizzi A, Jiang HL, et al. Activation of EP3 receptors suppresses COX-2 in thick ascending limb (TAL) and inhibits water excretion. Faseb J. 2015:29. [Google Scholar]
- 26.Peti-Peterdi J, Harris RC. Macula Densa Sensing and Signaling Mechanisms of Renin Release. J Am Soc Nephrol. 2010;21:1093–6. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Komlosi P, Fuson AL, et al. Luminal NaCl delivery regulates basolateral PGE(2) release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breyer MD. Regulation of Water and Salt Transport in Collecting Duct through Calcium-Dependent Signaling Mechanisms. Am J Physiol. 1991;260:F1–F11. doi: 10.1152/ajprenal.1991.260.1.F1. [DOI] [PubMed] [Google Scholar]
- 29.Schweda F, Klar J, Narumiya S, et al. Stimulation of renin release by prostaglandin E(2) is mediated by EP(2) and EP(4) receptors in mouse kidneys. Am J Physiol-Renal. 2004;287:F427–F33. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 30.Lee YJ, Song IK, Jang KJ, et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT(1) receptor. Am J Physiol-Renal. 2007;292:F340–F50. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 31.Prieto MC, Williams DE, Liu L, et al. Enhancement of renin and prorenin receptor in collecting duct of Cyp1a1-Ren2 rats may contribute to development and progression of malignant hypertension. Am J Physiol-Renal. 2011;300:F581–F8. doi: 10.1152/ajprenal.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prieto-Carrasquero MC, Botros FT, Cabrera MA, et al. Collecting duct renin expression in Goldblatt hypertensive rats. Faseb J. 2005;19:A1588-A. [Google Scholar]
- 33.Liu L, Gonzalez AA, McCormack M, et al. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol-Renal. 2011;301:F1195–F201. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez AA, Womack JP, Liu L, et al. Angiotensin II Increases the Expression of (Pro)Renin Receptor During Low-Salt Conditions. Am J Med Sci. 2014;348:416–22. doi: 10.1097/MAJ.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Lu XH, Peng KX, et al. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol-Renal. 2014;307:F25–F32. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert RL, Jacobson HR, Breyer MD. Prostaglandin-E2 Inhibits Sodium-Transport in Rabbit Cortical Collecting Duct by Increasing Intracellular Calcium. J Clin Invest. 1991;87:1992–8. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breyer MD, Breyer RM, Fowler BJ, et al. EP1 receptor antagonists block PGE2 dependent inhibition of Na+ absorption in the cortical collecting duct. J Am Soc Nephrol. 1996;7:A1982-A. [Google Scholar]
- 38.Hebert RL, Fredin D, Jacobson HR, et al. Pge2 Inhibits Sodium-Transport in the Rabbit Cortical Collecting Duct (Ccd) by Increasing Intracellular Calcium. Clin Res. 1990;38:A313-A. [Google Scholar]
- 39.Gonzalez AA, Cespedes C, Villanueva S, et al. E Prostanoid-1 receptor regulates renal medullary alpha ENaC in rats infused with angiotensin II. Biochem Bioph Res Co. 2009;389:372–7. doi: 10.1016/j.bbrc.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 40.Kang JJ, Toma I, Sipos A, et al. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohashi N, Isobe S, Ishigaki S, et al. Plasma Soluble (Pro) Renin Receptor Reflects Intrarenal Renin Angiotensin System Activity and Renal Damage. Nephrol Dial Transpl. 2016;31:180. [Google Scholar]
- 42.Huang YJ, Yamamoto T, Misaki T, et al. Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol-Renal. 2012;303:F130–F8. doi: 10.1152/ajprenal.00275.2011. [DOI] [PubMed] [Google Scholar]