Abstract
Acute lymphoblastic leukemia (ALL) is a malignant hematological disease afflicting hematopoiesis in the bone marrow. While 80-90% of patients diagnosed with ALL will achieve complete remission at some point during treatment, ALL is associated with high relapse rate, with the 5-year overall survival rate of 68%. The initial remission failure and the high rate of relapse can be attributed to intrinsic chemoprotective mechanisms that allow persistence of ALL cells despite therapy. These mechanisms are mediated, at least in part, through the engagement of cell adhesion molecules (CAMs) within the bone marrow microenvironment. This review assembles CAMs implicated in protection of leukemic cells from chemotherapy. Such studies are limited in ALL. Therefore, CAMs that are associated with poor outcomes or are over-expressed in ALL and have been shown to be involved in chemoprotection in other hematological cancers are also included. It is likely that these molecules play parallel roles in ALL because the CAMs identified to be a factor in ALL chemoresistance also work similarly in other hematological malignancies. We review the signaling mechanisms activated by the engagement of CAMs that provide protection from chemotherapy. Development of targeted therapies against CAMs could improve outcome and raise the overall cure rate in ALL.
Keywords: acute lymphoblastic leukemia, cell adhesion molecules, targeted therapy, chemoresistance, bone marrow
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant hematological disease characterized by the accumulation of immature, abnormal white blood cells that replace normal marrow elements within the bone marrow and inhibit the production of functional blood cells. Of those diagnosed, 80–90% will have complete remission at some point during treatment (1). However, about half of these patients will experience a recurrence of the disease, making the overall 5-year survival rate about 40% for adults and 85% for children. Nearly 60% of patients diagnosed are age 20 or younger, but patients older than 20 account for approximately 85% of deaths from ALL (2,3).
Remission is defined as lack of clinical evidence of the disease and restoration of normal hematopoiesis. However, this does not guarantee a cure. Leukemic cells may still be dispersed throughout the body or have resisted the chemotherapy treatments and persisted in the bone marrow. The detectable presence of leukemic cells within the bone marrow following induction therapy is termed minimal residual disease (MRD), and can be considered a measure of drug resistance in vivo. A patient’s prognosis is inversely proportional to their MRD level (Fig. 1) (4,5). These leukemic cells that resist chemotherapy are responsible for the high recurrence and less than optimal long-term survival rates in ALL (6). Therefore, sensitization to chemotherapy is important for disease eradication to prolong survival in ALL patients.
Fig. 1.
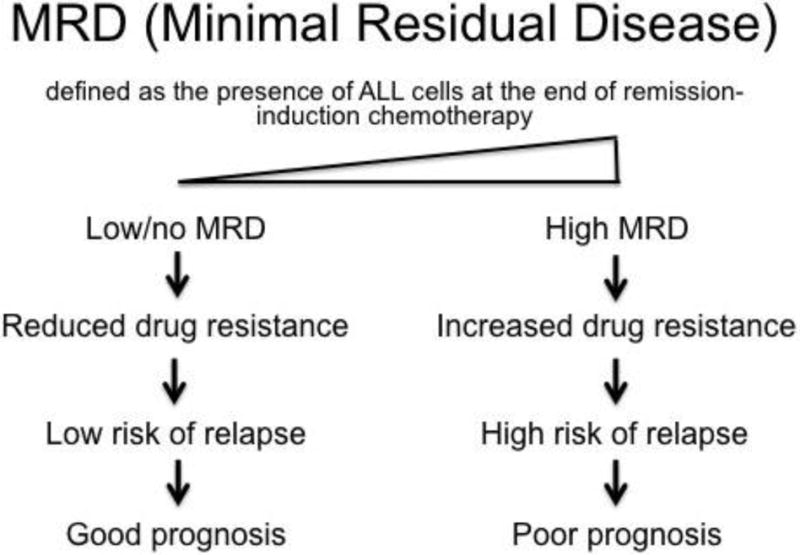
A schematic showing the significance of minimal residual disease in determining prognosis in ALL.
Chemotherapy Resistance Mechanisms in ALL
Chemotherapy resistance in ALL is extensively studied and a variety of mechanisms have been identified as contributing factors (Table 1). An exhaustive examination of all chemotherapy resistance mechanisms in ALL is beyond the scope of this review, which focuses on the microenvironment-induced protection of leukemic cells from the effects of chemotherapeutic drugs. This mechanism of resistance is a major contributor of intrinsic chemoresistance, and pre-exists prior to exposure of the leukemic cells to chemotherapeutics. The intrinsic chemoresistance afforded by the microenvironment is often referred to as ‘chemoprotection’ because it shields neoplastic cells from chemotherapy. The bone marrow microenvironment comprises osteoblasts, endothelial cells, fibroblasts, adipocytes, macrophages and stromal cells (also called bone marrow mesenchymal stem cells) with their associated extracellular matrix (ECM) components and secreted soluble factors (Fig. 2). The bone marrow microenvironment provides a sanctuary for leukemic cells similar to hematopoietic stem cells (HSCs). Leukemic cells modify this environment making it more habitable while leading to HSC dysfunction (7).
Table 1.
Mechanisms of drug resistance in leukemia
| Chemoresistance mechanism | Example protein/pathway | Mode of action and drugs affected | Reference/s |
|---|---|---|---|
| Drug receptor downregulation or inactivation | Glucocorticoid receptor | Reduced expression, decreased activity, internalization or receptor mutations prevents drug activity and induction of apoptosis | (64,65) |
| Drug efflux | ATP-binding cassette (ABC) superfamily of transporters e.g. MRP3 in ALL | Transporters mediate active efflux of a broad spectrum of cytotoxic compounds thereby reducing intracellular drug accumulation and toxicity | (5,66–69) |
| Intracellular drug degradation | NT5C2 cytosolic 5′ Nucleotidase II | Enzyme metabolizes and inactivates nucleoside analogs which constitute chemotherapeutic agents | (70,71) |
| Gene deletion/mutation | DCK/FPGS | Genetic deletions of DCK and FPGS prevent drug activation and lead to resistance against cytarabine and methotrexate respectively | (72) |
| Targeted protein modification | BCR/ABL | BCR/ABL kinase domain mutations confer resistance to imatinib treatments | (73) |
| Upregulation of proliferative proteins | A20 | Overexpression of A20 leads to increased proliferation and anti-apoptotic effects in conjunction with MAPK signaling and p53 to confer chemoresistance | (74) |
| Cellular quiescence | Exit to G0 | Intracellular signaling causes an exit from cell cycle to G0 and resistance to multiple drugs that are effective only on proliferating cells | (75) |
| Overexpression of negative regulators of apoptosis | GSTM1 | Overexpression prevents the activity of apoptotic regulators like Bim | (76) |
| Ion flux | hERG1 | hERG1 channel activity increased pro-survival signaling and conferred multidrug resistance | (11) |
| Redox adaptation | Antioxidant production and MCL-1 | Increased mitochondrial calcium influx increases levels of reactive oxygen species, leading to an adaptation process that increases antioxidant and MCL-1 levels to induce multidrug resistance | (77) |
| Abnormal glucose metabolism | GLUT1 | Increase in transporter expression increases glucose uptake and prevents cells from undergoing metabolic stress and defends against chemotherapy | (78) |
| Unfolded protein response | XBP1 | Expression of XBP1 protects cells from ER stress and leads to chemoresistance | (79) |
| Increased protein expression of DNA repair proteins | Alt-NHEJ pathway | Increased activity of DNA repair pathway allows cells to repair more readily and protect against chemotherapy | (80) |
| Protein stabilization | p73 | p73 stabilization by Kpm/Lats2 phosphorylation of YAP2 protected cells from DNA damaging chemotherapeutics | (81) |
| MicroRNA aberrations | miR125b/100/99a | Dysregulation of miRNAs can alter expression patterns of key proteins and lead to resistance against chemotherapy drugs like vincristine | (82) |
| Cell adhesion mediated drug resistance | Cell-cell/matrix adhesion | Binding of cellular adhesion molecules on the surface of ALL cells to other cells or the ECM in the BM stimulate a chemoprotective effect | (83,84) |
Fig. 2.
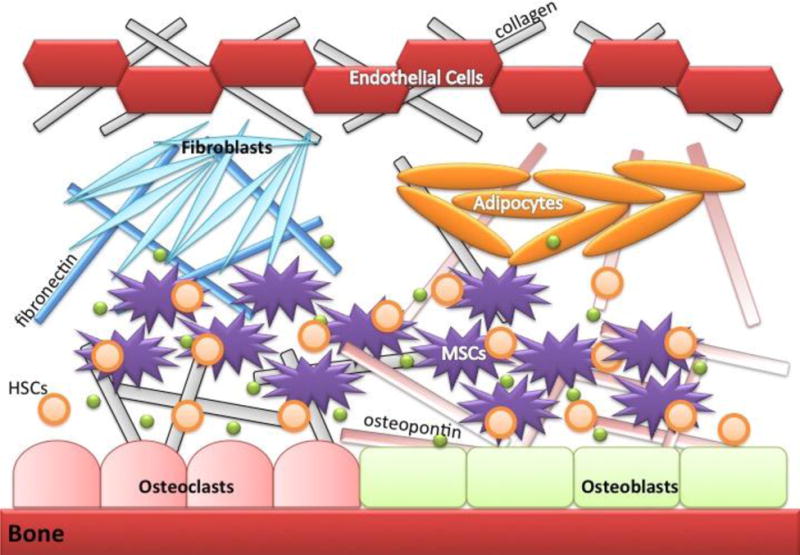
Pictorial representation of elements in the normal bone marrow microenvironment. The bone marrow microenvironment (BM) contains multiple cell types such as osteoblasts, osteoclasts, endothelial cells, mesenchymal stromal cells, fibroblasts and adipocytes that can interact with normal hematopoietic stem cells (HSCs) and control hematopoiesis and quiescence. ECM proteins, specifically fibronectin and osteopontin, and soluble factors such as SDF-1 and galectins (green spheres).
Several studies have reported that ALL cells co-cultured with osteoblasts or stromal cells, to mimic the bone marrow microenvironment, have improved survival and reduced sensitivity to chemotherapy (8–14). These effects required direct cell-cell contact and were not replicated in cells contacting ECM or in cells cultured in conditioned medium from stromal cells, indicating the contribution of the ECM and soluble factors was secondary (9). The absence of a change in the expression of drug transporters, has suggested a reliance on adhesion for chemoprotection (15). These adhesive interactions are mediated by cell-cell and cell-matrix contacts via cell adhesion molecules (CAMs) such as integrins, cadherins, selectins, immunoglobulin-like superfamily, and other CAMs on the cell surface (10,14,16) (Fig. 3, 4). The interactions between CAMs on two contacting cells not only serve as glue to bind the two cells together but also activate signaling pathways that regulate a wide array of cellular functions including cell survival, evasion of apoptosis, and cell dormancy resulting in defense against chemotherapy (17). Understanding the role of CAMs in conferring chemoprotection provides the basis for possible development of targeted therapeutics for ALL.
Fig. 3.
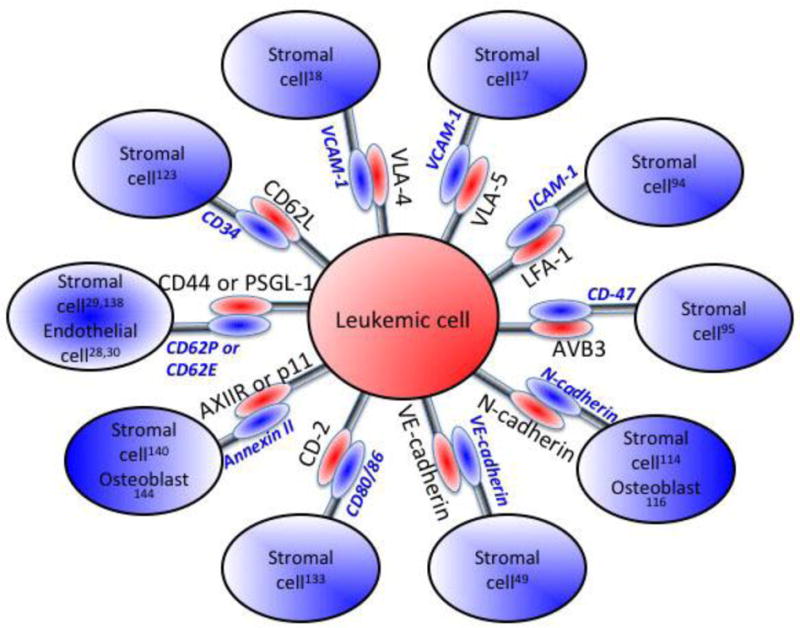
Pictorial representation of CAMs on leukemic cells and their cognate interacting partners on cells within the bone marrow microenvironment. The numbers in superscript correspond to the citation describing the particular interaction.
Fig. 4.
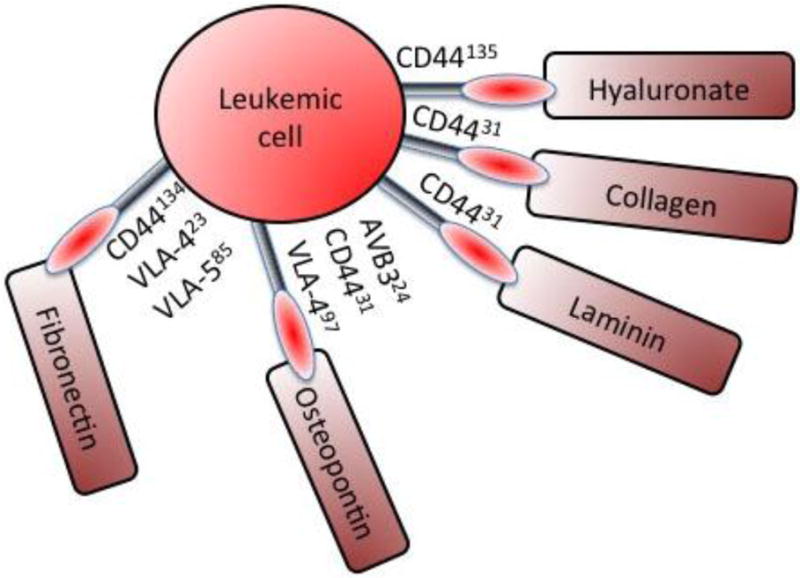
Representation of CAMs mediating ALL cell adhesion to different ECM proteins. The numbers in superscript correspond to the citation describing the particular interaction.
CAMs involved in chemoprotection in ALL
Integrins
Integrins are one of the most extensively studied classes of CAMs in the activation of cell survival pathways and induction of chemoresistance. Integrins are expressed on the cell surface as heterodimers consisting of α and β chains. Different combinations of these subunits as well as alternative splicing allows integrins to bind to a variety of ligands on the cell surface, ligands in the ECM, and even soluble ligands. Different intracellular signaling pathways can be activated upon integrin ligation leading to outcomes such as cell survival, cell migration or cell proliferation and differentiation (18). The physiological role of integrins that play a role in chemoresistance is summarized in Table 2.
Table 2.
Physiological role of integrins with as putative role in chemoresistance
| Integrin | Function in normal cells | Reference/s |
|---|---|---|
| Integrin α4β1 or VLA-4 (very late antigen-4) | Leukocyte attachment, rolling and trans-endothelial migration at sites of inflammation | (85) |
| Integrin α5β1 or VLA-5 (very late antigen-5) | HSC homing and adhesion | (86) |
| Integrin αLβ2 or LFA-1 (leukocyte function- associated antigen-1) | Leukocyte adhesion and recruitment to sites of infection. Promotes interaction between naïve T-cell and antigen-presenting cell for effective T-cell activation | (87) (88) |
Integrin α4β1 or VLA-4 (very late antigen-4) has been widely identified as a key mediator of chemotherapy resistance (Table 3). Therefore, it was not surprising that high VLA-4 expression in children with relapsed B-ALL was associated with unfavorable prognosis (19). Thus, blocking of VLA-4/VCAM-1 binding by a humanized antibody targeting VLA-4 (natalizumab) or a ligand-mimetic small molecule inhibitor of integrin α4 (TBC3486) not only sensitized B-ALL cells to chemotherapy, but also prolonged survival in leukemia bearing mice in preclinical studies (20,21).
Table 3.
Integrins implicated in chemoresistance in ALL
| Integrin | Target Expression | Function in malignant cells | Reference/s |
|---|---|---|---|
| Integrin α4β1 or VLA-4 (very late antigen-4) | Binds to VCAM-1 (vascular cell adhesion molecule-1) expressed on bone marrow stromal cells and osteoblasts |
|
|
| Integrin α5β1 or VLA-5 (very late antigen-5) | Also binds to VCAM-1 (vascular cell adhesion molecule-1) |
|
|
| Integrin αLβ2 or LFA-1 (leukocyte function- associated antigen-1) | Binds to ICAM-1 (intercellular cell adhesion molecule-1) on bone marrow stromal cells |
|
|
Although less studied, integrin α5β1 or VLA-5 (very late antigen-5) also binds to VCAM-1 and mediates chemotherapy resistance (14) (Table 3). Both VLA-4 and VLA-5 have been reported to bind fibronectin, and the data indicate such interactions might be important in cell survival in many hematological malignancies (Table 4). AS101, a nontoxic tellurium compound that targets thiol residues in the exofacial domain of the VLA-4 molecule required for fibronectin ligation, chemosensitized AML cells by triggering intracellular cytoskeletal changes and decreasing PI3K/Akt/Bcl-2 signaling (22) (5). This compound is currently in phase 2 clinical trials to treat AML and myelodysplastic syndrome patients (Table 5).
Table 4.
Consequences of VLA4/5 Binding to fibronectin in normal and malignant cells
| Cell type | Consequences | Reference/s |
|---|---|---|
| Normal cells |
|
|
| B-cell ALL |
|
|
| T-cell ALL |
|
|
| Multiple Myeloma |
|
|
| CLL |
|
|
| AML |
|
|
| CML |
|
|
| T-cell ALL, AML, CML, Lymphoma |
|
|
Table 5.
Summary of clinical trials using compounds to modulate the expression of different CAMs.
| Drug | Target | Disease | Mechanism | ClinicalTrials.gov Identifier | Reference |
|---|---|---|---|---|---|
| Natalizumab | VLA-4 | Multiple myeloma | Monoclonal antibody | NCT00675428 | |
| Natalizumab | VLA-4 | B-ALL | Monoclonal antibody | Pre-clinical | (20) |
| AS101 | VLA-4 | Adult AML | Redox inactivation | NCT01010373 | (22) |
| FNIII14 | VLA-4 | AML | Antagonism | Pre-clinical | (101) |
| TBC3486 | VLA-4 | B-ALL | Ligand-mimetic | Pre-clinical | (21) |
| LFA703 | LFA-1 | Multiple myeloma | Antagonism | Pre-clinical | (25) |
| GMI-1271 | E-selectin | AML | Antagonism | NCT02306291 | (32) |
| GMI-1359 | CXCR4, E- selectin | AML | Antagonism | Pre-clinical | (47) |
| Hu5F9-G4 | CD47 | AML | Antagonism | NCT02216409 | (38) |
| BI-505 | ICAM-1 | Multiple myeloma | Monoclonal antibody | NCT01025206 | (49) |
| Plerixafor | CXCR4 | AML | Antagonism | NCT00906945 | (43) |
| Plerixafor | CXCR4 | Acute leukemia | Antagonism | NCT01068301 | (44) |
| MDX-1338 | CXCR4 | AML | Monoclonal antibody | NCT01120457 | (46) |
| POL5551 | CXCR4 | High-risk ALL | Antagonism | Pre-clinical | (45) |
| Lenalidomide | ICAM-1, VCAM-1 | Multiple myeloma | Downregulation of TNFα | NCT00772915 | (105) |
| Lenalidomide | ICAM-1, VCAM-1 | Acute AML | Downregulation of TNFα | NCT01615042 | (52) |
VLA-4 also binds to other ECM components such as osteopontin, a glycoprotein secreted by osteoblasts. VLA-4 ligation to osteopontin induces dormancy in B-ALL cells by forcing cell cycle exit (23). Since a majority of cytotoxic drugs are thought to target actively proliferating cells, induction of dormancy in ALL cells may result in protection from cytotoxic chemotherapy agents such as cytarabine.
Integrin αLβ2 or LFA-1 (leukocyte function-associated antigen-1) is another key heterodimer expressed on the surface of T-ALL cells that confers chemotherapy resistance (24) (Table 3). Multiple myeloma cells co-cultured with bone stromal cells were successfully sensitized to chemotherapy treatments via the use of monoclonal antibodies or an LFA-1 inhibitor (LFA703) (25) (Table 5).
Cadherins
Cadherins are type-1 transmembrane proteins that mediate Ca2+ ion dependent homophilic cell adhesion, such that adhesion is mediated by cadherin molecules located on contacting cells. The extracellular domains are responsible for mediating tight cell-cell contacts, while the intracellular domains interact with a wide variety of adaptor and signaling proteins (26) to mediate diverse functions such as leukocyte extravasation, HSC maintenance and actin dynamics (Table 6). Like integrins, cadherins such as VE-cadherin (CD144), N-cadherin (CD325) and Fat1 cadherin have been implicated in mediating resistance to chemotherapy in hematological cancers (Table 7).
Table 6.
Physiological role of cadherins involved in chemoresistance in hematological malignancies
| Cell type/cadherin | Consequences | Reference/s |
|---|---|---|
| Endothelial cells/VE-cadherin (CD144) |
|
|
| HSC/N-cadherin (CD325) |
|
|
| Epithelial cells/Fat1-cadherin |
|
|
Table 7.
Role of cadherins in mediating chemoresistance in hematologic malignancies
| Cell type/cadherin | Consequences | Reference/s |
|---|---|---|
| B-cell ALL/VE Cadherin (CD144) |
|
|
| B-cell ALL/N- Cadherin (CD325) |
|
|
| T-cell ALL/N- Cadherin (CD325) |
|
|
| AML/N-cadherin (CD325) |
|
|
| CML/N-cadherin (CD325) |
|
|
| Multiple Myeloma/N-cadherin (CD325) |
|
|
| B-cell ALL/Fat1 cadherin |
|
|
| T-cell ALL/Fat1 cadherin |
|
|
| CLL/Fat1 cadherin |
|
|
Selectins
Selectins are single chain glycoproteins that contain an N-terminal calcium-dependent lectin domain. The selectin family comprises E- (endothelial), P- (platelet) and L- (leukocyte) selectins named CD62E, CD62P, and CD62L respectively (27). Their role in leukocyte homing and recruitment to inflammatory sites is well characterized (Table 8). Recent reports have identified the role of selectins in chemoresistance in a number of hematological malignancies (Table 9). Although there are no studies on the role of selectins in ALL chemoresistance, it is possible that P-selectin glycoprotein ligand 1 (PSGL-1) plays a similar role in ALL because PSGL-1 transcript levels are increased in B-ALL and T-ALL cells compared to normal lymphoblasts (28–30). The pan-selectin inhibitor GMI-1070 was tested in pre-clinical studies for the treatment of multiple myeloma (31). GMI-1271, an E-selectin antagonist selectively sensitized leukemia cells to chemotherapy while avoiding HSC mobilization (32), and is in phase 1/2 clinical trial for treatment of AML (Table 5).
Table 8.
Physiological role of selectins
| Cell type/selectin | Consequences | Reference |
|---|---|---|
| Endothelial cells/E-selectin and P- selectin |
|
(130) |
| T-cell/L-selectin |
|
(27) |
| HSC/E-selectin |
|
(131) |
Table 9.
Role of selectins in mediating chemoresistance in hematologic malignancies
| Cell type/selectin | Consequences | Reference/s |
|---|---|---|
| AML/E-selectin |
|
(132) |
| CLL/L-selectin |
|
(133) |
| CML/L-selectin |
|
(134) |
| Multiple myeloma/P-selectin |
|
(31,135) |
Immunoglobulin superfamily
Members of the immunoglobulin superfamily (IgSF) are transmembrane glycoproteins possessing a structural immunoglobulin domain which perform multiple functions (33) (Table 10). IgSF family members CD28, CD147 (or EMMPRIN – extracellular matrix metalloproteinase inducer) and CD47 have been implicated in inducing chemoresistance in solid tumors or other hematological malignancies (Table 11). These proteins are also highly expressed in ALL, suggesting that further investigation is warranted to determine the role of IgSF family members in chemoresistance in ALL. Targeting CD47 by antibody reduced leukemic burden (34–37). Humanized anti-CD47 antibodies are currently in clinical trial for AML (38) (Table 5).
Table 10.
Physiological role of immunoglobulin superfamily (IgSF) proteins
| Cell type/IgSF | Consequences | Reference |
|---|---|---|
| HSCs/CD47 |
|
(136) |
| Leukocyte CD47 |
|
(137) |
| T-cell/CD28 |
|
(138) |
Table 11.
Role of immunoglobulin superfamily (IgSF) members in mediating chemoresistance in hematologic malignancies
| Cell type/IgSF | Consequences | Reference/s |
|---|---|---|
| T-ALL/CD28 |
|
|
| Multiple myeloma/CD28 |
|
|
| CLL/CD47 |
|
|
| ALL, AML/CD47 |
|
|
| B-ALL/CD147 |
|
|
| Lymphoma/CD147 |
|
Other CAMs
Certain other CAMs, that do not belong to the four CAM families describe above also mediate chemoresistance (Table 12). CXCR4 is the most prominent CAM in this group. Plerixafor, a CXCR4 antagonist, is currently in clinical trials for the treatment of hematological malignancies including AML, CLL, multiple myeloma, and relapsed ALL (Table 5). Plerixafor (or AMD3100) treatment in combination with cytotoxic chemotherapy improved overall survival in pre-clinical studies (39–42) as well as in phase 1/2 clinical trials in acute leukemia (43,44). New drugs that also target CXCR4 are at various stages of development. POL5551, a novel CXCR4 antagonist, was found to be more effective than plerixafor in pre-clinical models of high-risk ALL (45). Ulocuplumab (MDX-1338) is a humanized anti-CXCR4 antibody, which was efficacious as monotherapy in mouse xenografts and is currently in phase 1 trials for treatment of relapsed AML, CLL, non-Hodgkin lymphoma and multiple myeloma (46). GMI-1359, a dual CXCR4/E-selectin inhibitor, showed a 50% increase in survival in combination with cytarabine in pre-clinical mouse models of AML (47). Thus, reverting cell adhesion-mediated drug resistance by targeting CXCR4 has proven to be beneficial and is actively pursued in the clinic (Table 5).
Table 12.
Role of other CAMs in mediating chemoresistance
| Cell type/CAM | Consequences | Reference/s |
|---|---|---|
| B-ALL/CD9 |
|
|
| Lung cancer, small cell/CD9 |
|
|
| HSC/CD44 |
|
|
| Lymphocytes/CD44 |
|
|
| B-ALL and AML/CD44 |
|
|
| Multiple myeloma/CD44 |
|
|
| ALL/CXCR4 |
|
|
| Multiple myeloma/ICAM1 |
|
|
| HSC/Annexin II |
|
|
| Multiple myeloma/Annexin II |
|
|
| B-ALL/Annexin II |
|
|
Human anti-ICAM1 antibody (BI-505) bestowed enhanced survival to multiple myeloma bearing mice (48). A phase 1 dose-escalation study with BI-505 is completed, and it is ready for entering the next phase of clinical trials (49) (Table 5). Thalidomide and its analogue lenalidomide are immunomodulators that function as anti-TNFα agents, via the regulation of cell surface expression of key adhesion molecules like ICAM-1, VCAM-1, E-selectin and L-selectin thereby decreasing cell-cell interaction between T-ALL cells and umbilical vein endothelial cells (50,51). Lenalidomide is being used in clinical trials for the treatment of refractory and relapsed AML (52) (Table 5).
Ectoenzymes
Ectoenzymes are transmembrane proteins bearing their catalytically active sites on the extracellular cell surface (53). However, these proteins are known to have multiple functions including cell adhesion (Table 13). Further studies are warranted to distinguish the contribution of the enzymatic activities and cell adhesion properties of ectoenzymes in imparting chemoresistance.
Table 13.
Role of ectoenzymes in mediating physiological functions and chemoresistance
| Cell type/ectoenzyme | Consequences | Reference/s |
|---|---|---|
| B-ALL/CD10, neutral endopeptidase |
|
|
| Head and neck squamous cell carcinoma/CD10 |
|
|
| CML/CD26, dipeptidyl peptidase 4 |
|
|
| Lymphoma/CD26 |
|
|
| T-ALL/CD26 |
|
|
| T-ALL/CD73, ecto-5′- nucleotidase |
|
|
| CLL/CD73 |
|
|
Co-operation between different CAMs enhances chemoresistance
Association between different CAMs can constitute alternate mechanisms of chemoresistance. Chemokine receptor CXCR4 described above, complexes with integrin β1 (CD29) and enhances adhesion and engraftment of ALL cells in the bone marrow (54). This complex formation also leads to the recruitment of an hERG1 channels in ALL cells co-cultured with mesenchymal stem cells resulting in downstream activation of PI3K/Akt pro-survival pathways and chemotherapy resistance (11). N-cadherin clustering on the cell membrane mediated by tetraspanin CD82 enhanced bone marrow trafficking of AML cells (55). Interaction between VLA-4 and CD44 in AML cells adhering to the stroma regulates chemotherapy efflux via ABC transporters, providing another cell-adhesion mediated drug resistance mechanism (56). Thus, collaboration between CAMs on the leukemic cell surface further strengthens the chemoprotective effect provided by the bone marrow microenvironment.
Soluble factors involved in chemoresistance
Soluble factors within the bone marrow microenvironment which contribute to chemotherapy resistance are listed in brief in the following section because the major focus of this review is CAMs and the intercellular interactions with other CAMs or the ECM components. Stromal cells are known to secrete soluble factors such as galectin-3, which are involved in imparting chemoresistance in hematological cancers (57–59) (Table 14). An example by which a secretory factor from ALL cells influences the bone marrow microenvironment and modulates it to suit its benefit is connective tissue growth factor (CTGF). CTGF interacts with ECM and integrins, and promotes adhesion of B-ALL cells to stromal cells as well as stimulates proliferation of stromal cells and aids in chemoresistance (60). CTGF expression is high in pediatric and adult BALL (61,62) and higher CTGF expression corresponded with reduced overall survival (63).
Table 14.
Role of soluble factors secreted by stromal cells in mediating chemoresistance
| Cell type/soluble factor | Consequence | Reference/s |
|---|---|---|
| Galectin-3, galactose-binding lectin | Galectin-3 implicated in the development of chemoresistance in several solid as well as hematological cancers | (59) |
| B-ALL/Galectin-3 | Galectin-3 protects ALL cells by auto-induction of galectin-3 mRNA and activation of the NFκB pathway and Wnt/β-catenin signaling pathway | (178,179) |
| Ph+ B-ALL/Galectin-3 | Galectin-3 transcript levels upregulated in Ph+ B-ALL cells that had developed resistance to tyrosine kinase inhibitor nilotinib | (180) |
| CML/Galectin-3 | Galectin-3 induced multi-drug resistance by activation of Akt and ERK and preventing apoptosis | (181) |
| Multiple myeloma/Galectin-3 | Galectin-3 implicated in chemoresistance mechanisms | |
| Chemoprotective factor/ALL | This soluble factor induces an increase in calcium influx into mitochondria, which leads to an increase in reactive oxidation species (ROS) in ALL cells. Cells respond by undergoing a redox adaptation to decrease ROS, thereby reducing ALL sensitivity to drug treatment | (77) |
| Chemoprotective factor/AML | This soluble factor promotes chemoresistance by blocking the activity of equilibrative nucleoside transporter (ET1) which regulates cytarabine incorporation inside the cell | (182) |
Conclusion
Resistance to chemotherapeutics still remains a major cause of ALL relapse and poor prognosis. The importance of CAMs in conferring chemotherapy resistance in ALL is slowly becoming evident while the role of cell adhesion as an important mediator of disease pathology is unraveled. Although there are no ongoing clinical trials evaluating CAMs as therapeutic targets for ALL, this approach is being tested in clinical trials in other hematological disorders (Table 5). Activation of pro-survival and quiescence pathways initiated by the binding of CAMs on the leukemia cell surface to targets in the bone marrow microenvironment can be routes for cells to evade chemotherapy. Modulation or blocking of these adhesive interactions provides an opportunity for the design of novel targeted therapies in ALL. Although the majority of CAMs discussed here are also present on HSCs, there are a few CAMs whose expression is restricted to leukemic cells or which can have a greater effect on leukemic cells compared to normal HSCs. Targeting these specific CAMs should retain HSC function, while attacking leukemic cells. Further investigation on novel interactions will furnish opportunities for development of targeted therapy with minimal side effects.
Fig. 5.
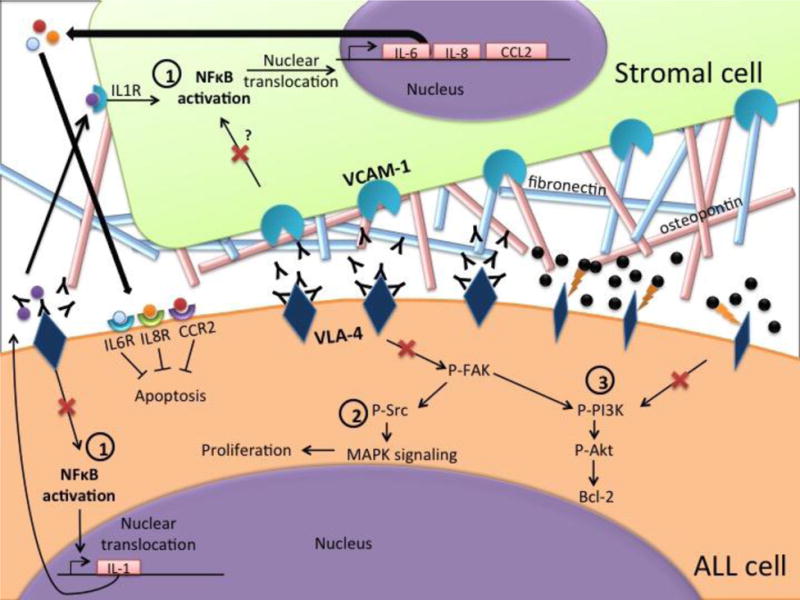
Pictorial representation of the mechanisms of drugs targeting VLA-4. VLA-4 binds to its target VCAM-1 on bone marrow stromal cells or to ECM proteins such as osteopontin and fibronectin. This interaction activates pro-survival signaling pathways such as 1) NF-κb, 2) Src/MAPK and 3) PI3K/Akt. Disruption of these interactions by Natalizumab (Black Ys), a monoclonal antibody that targets VLA-4, or AS101 (black spheres), which oxidizes adjacent thiol residues in the exofacial domain of VLA-4 molecules. This prevents target binding and causes cytoskeletal and conformational changes in the VLA-4 molecule, results in inhibition of these pathways (shown by red crosses).
Fig. 6.
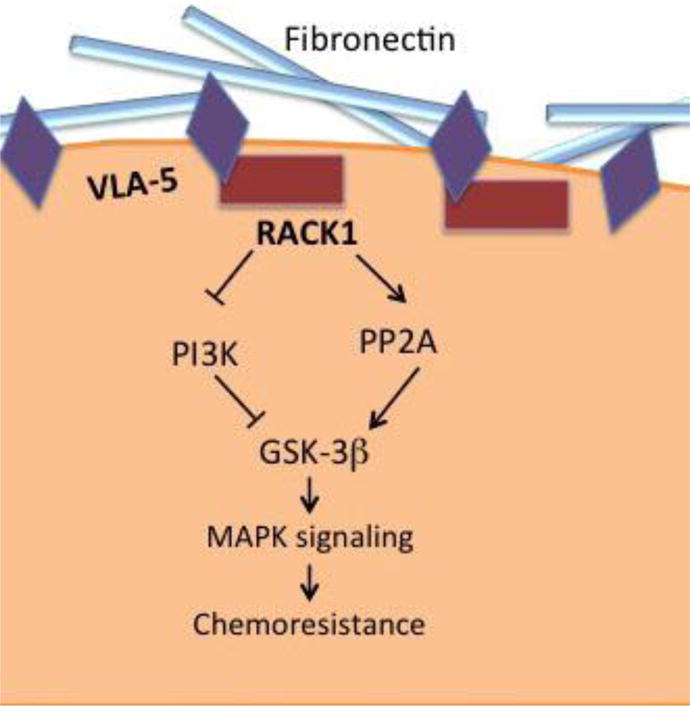
Pictorial representation of the signaling pathway activated by VLA-5 binding to fibronectin. This interaction mediates the binding of scaffolding protein RACK1 to VLA-5 which recruits protein phosphatase PP2A. PP2A dephosphorylates GSK-3β on Ser 9 resulting in its activation. Activated GSK-3β signals via MAPK pathway to induce chemoresistance.
Acknowledgments
Funding for this work was provided by the Delaware-CTR ACCEL (U54GM104941), Delaware INBRE program NIGMS (8P20GM103446), Leukemia Research Foundation of Delaware and the Nemours Foundation. The study sponsors were not involved in the collection, analysis and interpretation of data and in the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, Mullighan CG, Zhou Y, Pui CH, Campana D. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115:4657–4663. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogorzala M, Kubicka M, Rafinska B, Wysocki M, Styczynski J. Drug-resistance Profile in Multiple-relapsed Childhood Acute Lymphoblastic Leukemia. Anticancer Res. 2015;35:5667–5670. [PubMed] [Google Scholar]
- 7.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 8.Fortney JE, Zhao W, Wenger SL, Gibson LF. Bone marrow stromal cells regulate caspase 3 activity in leukemic cells during chemotherapy. Leuk Res. 2001;25:901–907. doi: 10.1016/s0145-2126(01)00051-0. [DOI] [PubMed] [Google Scholar]
- 9.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–2377. [PubMed] [Google Scholar]
- 10.Murti KG, Brown PS, Kumagai M, Campana D. Molecular interactions between human B-cell progenitors and the bone marrow microenvironment. Exp Cell Res. 1996;226:47–58. doi: 10.1006/excr.1996.0201. [DOI] [PubMed] [Google Scholar]
- 11.Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, Amedei A, Veltroni M, D’Amico M, Basso G, Becchetti A, Campana D, Arcangeli A. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood. 2011;117:902–914. doi: 10.1182/blood-2010-01-262691. [DOI] [PubMed] [Google Scholar]
- 12.Moses BS, Slone WL, Thomas P, Evans R, Piktel D, Angel PM, Walsh CM, Cantrell PS, Rellick SL, Martin KH, Simpkins JW, Gibson LF. Bone marrow microenvironment modulation of acute lymphoblastic leukemia phenotype. Exp Hematol. 2016;44:50–59.e51–52. doi: 10.1016/j.exphem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudry RE, Fortney JE, York T, Hall BM, Gibson LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–1932. [PubMed] [Google Scholar]
- 14.Bradstock K, Makrynikola V, Bianchi A, Byth K. Analysis of the mechanism of adhesion of precursor-B acute lymphoblastic leukemia cells to bone marrow fibroblasts. Blood. 1993;82:3437–3444. [PubMed] [Google Scholar]
- 15.Funayama K, Murai F, Shimane M, Nomura H, Asano S. Adhesion-induced drug resistance in leukemia stem cells. Pharmacology. 2010;86:79–84. doi: 10.1159/000305344. [DOI] [PubMed] [Google Scholar]
- 16.Makrynikola V, Bradstock KF. Adhesion of precursor-B acute lymphoblastic leukaemia cells to bone marrow stromal proteins. Leukemia. 1993;7:86–92. [PubMed] [Google Scholar]
- 17.Buckley CD, Rainger GE, Bradfield PF, Nash GB, Simmons DL. Cell adhesion: more than just glue (review) Mol Membr Biol. 1998;15:167–176. doi: 10.3109/09687689709044318. [DOI] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 19.Shalapour S, Hof J, Kirschner-Schwabe R, Bastian L, Eckert C, Prada J, Henze G, von Stackelberg A, Seeger K. High VLA-4 expression is associated with adverse outcome and distinct gene expression changes in childhood B-cell precursor acute lymphoblastic leukemia at first relapse. Haematologica. 2011;96:1627–1635. doi: 10.3324/haematol.2011.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh YT, Gang EJ, Geng H, Park E, Huantes S, Chudziak D, Dauber K, Schaefer P, Scharman C, Shimada H, Shojaee S, Klemm L, Parameswaran R, Loh M, Kang ES, Koo HH, Hofmann WK, Andrade J, Crooks GM, Willman CL, Muschen M, Papayannopoulou T, Heisterkamp N, Bonig H, Kim YM. Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood. 2013;121:1814–1818. doi: 10.1182/blood-2012-01-406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh YT, Gang EJ, Shishido SN, Kim HN, Pham J, Khazal S, Osborne A, Esguerra ZA, Kwok E, Jang J, Bonig H, Biediger RJ, Vanderslice P, Kim YM. Effects of the small-molecule inhibitor of integrin alpha4, TBC3486, on pre-B-ALL cells. Leukemia. 2014;28:2101–2104. doi: 10.1038/leu.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layani-Bazar A, Skornick I, Berrebi A, Pauker MH, Noy E, Silberman A, Albeck M, Longo DL, Kalechman Y, Sredni B. Redox modulation of adjacent thiols in VLA-4 by AS101 converts myeloid leukemia cells from a drug-resistant to drug-sensitive state. Cancer Res. 2014;74:3092–3103. doi: 10.1158/0008-5472.CAN-13-2159. [DOI] [PubMed] [Google Scholar]
- 23.Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, Sipkins DA. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121:4821–4831. doi: 10.1182/blood-2012-12-475483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phongpradist R, Chittasupho C, Okonogi S, Siahaan T, Anuchapreeda S, Ampasavate C, Berkland C. LFA-1 on leukemic cells as a target for therapy or drug delivery. Curr Pharm Des. 2010;16:2321–2330. doi: 10.2174/138161210791920450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidmaier R, Baumann P, Simsek M, Dayyani F, Emmerich B, Meinhardt G. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood. 2004;104:1825–1832. doi: 10.1182/blood-2003-12-4218. [DOI] [PubMed] [Google Scholar]
- 26.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 27.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 28.Andersson A, Ritz C, Lindgren D, Eden P, Lassen C, Heldrup J, Olofsson T, Rade J, Fontes M, Porwit-Macdonald A, Behrendtz M, Hoglund M, Johansson B, Fioretos T. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia. 2007;21:1198–1203. doi: 10.1038/sj.leu.2404688. [DOI] [PubMed] [Google Scholar]
- 29.Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, Stow P, Su X, Shurtleff S, Pui CH, Downing JR, Campana D. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC, De Vos J, Hernandez JM, Hofmann WK, Mills KI, Gilkes A, Chiaretti S, Shurtleff SA, Kipps TJ, Rassenti LZ, Yeoh AE, Papenhausen PR, Liu WM, Williams PM, Foa R. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28:2529–2537. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, Patton JT, Maiso P, Monrose V, Sacco A, Ngo HT, Flores LM, Lin CP, Magnani JL, Kung AL, Letai A, Carrasco R, Roccaro AM, Ghobrial IM. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119:1468–1478. doi: 10.1182/blood-2011-07-368050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devata S, Sood SL, Henmer MV, H F, Kramer W, Nietubicz C, Hawley A, Angelini DE, Myers DD, Blackburn S, Froehlich J, Wakefield TW, Magnani JL, HL T. First in Human Phase 1 Single Dose Escalation Studies of the E-Selectin Antagonist GMI-1271 Show a Favorable Safety, Pharmacokinetic, and Biomarker Profile. 57th Annual Meeting and Exposition, American Society for Hematology 2015 [Google Scholar]
- 33.Barclay AN. Membrane proteins with immunoglobulin-like domains–a master superfamily of interaction molecules. Semin Immunol. 2003;15:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 34.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uno S, Kinoshita Y, Azuma Y, Tsunenari T, Yoshimura Y, Iida S, Kikuchi Y, Yamada-Okabe H, Fukushima N. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncol Rep. 2007;17:1189–1194. [PubMed] [Google Scholar]
- 37.Kong F, Gao F, Li H, Liu H, Zhang Y, Zheng R, Zhang Y, Chen J, Li X, Liu G, Jia Y. CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol. 2016;18:1051–1055. doi: 10.1007/s12094-016-1489-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, Chao M, Weissman IL, Majeti R. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, DiPersio JF. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sison EA, Magoon D, Li L, Annesley CE, Rau RE, Small D, Brown P. Plerixafor as a chemosensitizing agent in pediatric acute lymphoblastic leukemia: efficacy and potential mechanisms of resistance to CXCR4 inhibition. Oncotarget. 2014;5:8947–8958. doi: 10.18632/oncotarget.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welschinger R, Liedtke F, Basnett J, Dela Pena A, Juarez JG, Bradstock KF, Bendall LJ. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol. 2013;41:293–302 e291. doi: 10.1016/j.exphem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011;25:1314–1323. doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF, Stockerl-Goldstein KE, Vij R, Westervelt P, DiPersio JF. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan A, Panetta JC, Cross SJ, Pillai A, Triplett BM, Shook DR, Dallas MH, Hartford C, Sunkara A, Kang G, Jacobsen J, Choi J, Leung W. Phase I study of the safety and pharmacokinetics of plerixafor in children undergoing a second allogeneic hematopoietic stem cell transplantation for relapsed or refractory leukemia. Biol Blood Marrow Transplant. 2014;20:1224–1228. doi: 10.1016/j.bbmt.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sison EA, Magoon D, Li L, Annesley CE, Romagnoli B, Douglas GJ, Tuffin G, Zimmermann J, Brown P. POL5551, a novel and potent CXCR4 antagonist, enhances sensitivity to chemotherapy in pediatric ALL. Oncotarget. 2015;6:30902–30918. doi: 10.18632/oncotarget.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, Cardarelli PM. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19:357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Patel N, Fogler WE, Magnani JL, Andreeff M. The Dual E-Selectin/CXCR4 Inhibitor, GMI-1359, Enhances Efficacy of Anti-Leukemia Chemotherapy in FLT3-ITD Mutated Acute Myeloid Leukemia. 57th Annual Meeting and Exposition, American Society for Hematology 2015 [Google Scholar]
- 48.Veitonmaki N, Hansson M, Zhan F, Sundberg A, Lofstedt T, Ljungars A, Li ZC, Martinsson-Niskanen T, Zeng M, Yang Y, Danielsson L, Kovacek M, Lundqvist A, Martensson L, Teige I, Tricot G, Frendeus B. A human ICAM-1 antibody isolated by a function-first approach has potent macrophage-dependent antimyeloma activity in vivo. Cancer Cell. 2013;23:502–515. doi: 10.1016/j.ccr.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Hansson M, Gimsing P, Badros A, Niskanen TM, Nahi H, Offner F, Salomo M, Sonesson E, Mau-Sorensen M, Stenberg Y, Sundberg A, Teige I, Van Droogenbroeck J, Wichert S, Zangari M, Frendeus B, Korsgren M, Poelman M, Tricot G. A Phase I Dose-Escalation Study of Antibody BI-505 in Relapsed/Refractory Multiple Myeloma. Clin Cancer Res. 2015;21:2730–2736. doi: 10.1158/1078-0432.CCR-14-3090. [DOI] [PubMed] [Google Scholar]
- 50.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 51.Settles B, Stevenson A, Wilson K, Mack C, Ezell T, Davis MF, Taylor LD. Down-regulation of cell adhesion molecules LFA-1 and ICAM-1 after in vitro treatment with the anti-TNF-alpha agent thalidomide. Cell Mol Biol (Noisy-le-grand) 2001;47:1105–1114. [PubMed] [Google Scholar]
- 52.Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, Garzon R, Walker A, Chandler JC, Whitman SP, Curfman J, Liu S, Schaaf L, Mickle J, Kefauver C, Devine SM, Grever MR, Marcucci G, Byrd JC. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010;28:4919–4925. doi: 10.1200/JCO.2010.30.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goding JW, Howard MC. Ecto-enzymes of lymphoid cells. Immunol Rev. 1998;161:5–10. doi: 10.1111/j.1600-065x.1998.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 54.Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Exp Hematol. 2001;29:1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- 55.Marjon KD, Termini CM, Karlen KL, Saito-Reis C, Soria CE, Lidke KA, Gillette JM. Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin. Oncogene. 2016;35:4132–4140. doi: 10.1038/onc.2015.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malfuson JV, Boutin L, Clay D, Thepenier C, Desterke C, Torossian F, Guerton B, Anginot A, de Revel T, Lataillade JJ, Le Bousse-Kerdiles MC. SP/drug efflux functionality of hematopoietic progenitors is controlled by mesenchymal niche through VLA-4/CD44 axis. Leukemia. 2014;28:853–864. doi: 10.1038/leu.2013.256. [DOI] [PubMed] [Google Scholar]
- 57.Pena C, Mirandola L, Figueroa JA, Hosiriluck N, Suvorava N, Trotter K, Reidy A, Rakhshanda R, Payne D, Jenkins M, Grizzi F, Littlefield L, Chiriva-Internati M, Cobos E. Galectins as therapeutic targets for hematological malignancies: a hopeful sweetness. Ann Transl Med. 2014;2:87. doi: 10.3978/j.issn.2305-5839.2014.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giordano M, Croci DO, Rabinovich GA. Galectins in hematological malignancies. Curr Opin Hematol. 2013;20:327–335. doi: 10.1097/MOH.0b013e328362370f. [DOI] [PubMed] [Google Scholar]
- 59.Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863:427–437. doi: 10.1016/j.bbamcr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Lu H, Kojima K, Battula VL, Korchin B, Shi Y, Chen Y, Spong S, Thomas DA, Kantarjian H, Lock RB, Andreeff M, Konopleva M. Targeting connective tissue growth factor (CTGF) in acute lymphoblastic leukemia preclinical models: anti-CTGF monoclonal antibody attenuates leukemia growth. Ann Hematol. 2014;93:485–492. doi: 10.1007/s00277-013-1939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Brigstock DR, de Klerk NH, Kees UR. High expression of connective tissue growth factor in pre-B acute lymphoblastic leukaemia. Br J Haematol. 2007;138:740–748. doi: 10.1111/j.1365-2141.2007.06739.x. [DOI] [PubMed] [Google Scholar]
- 62.Tesfai Y, Ford J, Carter KW, Firth MJ, O’Leary RA, Gottardo NG, Cole C, Kees UR. Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res. 2012;36:299–306. doi: 10.1016/j.leukres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Wells JE, Howlett M, Halse HM, Heng J, Ford J, Cheung LC, Samuels AL, Crook M, Charles AK, Cole CH, Kees UR. High expression of connective tissue growth factor accelerates dissemination of leukaemia. Oncogene. 2016;35:4591–4600. doi: 10.1038/onc.2015.525. [DOI] [PubMed] [Google Scholar]
- 64.Geley S, Hartmann BL, Hala M, Strasser-Wozak EM, Kapelari K, Kofler R. Resistance to glucocorticoid-induced apoptosis in human T-cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression. Cancer Res. 1996;56:5033–5038. [PubMed] [Google Scholar]
- 65.Catts VS, Farnsworth ML, Haber M, Norris MD, Lutze-Mann LH, Lock RB. High level resistance to glucocorticoids, associated with a dysfunctional glucocorticoid receptor, in childhood acute lymphoblastic leukemia cells selected for methotrexate resistance. Leukemia. 2001;15:929–935. doi: 10.1038/sj.leu.2402128. [DOI] [PubMed] [Google Scholar]
- 66.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinbach D, Legrand O. ABC transporters and drug resistance in leukemia: was P-gp nothing but the first head of the Hydra? Leukemia. 2007;21:1172–1176. doi: 10.1038/sj.leu.2404692. [DOI] [PubMed] [Google Scholar]
- 68.Winter SS, Ricci J, Luo L, Lovato DM, Khawaja HM, Serna-Gallegos T, Debassige N, Larson RS. ATP Binding Cassette C1 (ABCC1/MRP1)-mediated drug efflux contributes to disease progression in T-lineage acute lymphoblastic leukemia. Health (Irvine Calif) 2013;5 doi: 10.4236/health.2013.55A005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, Haefer R, Zintl F, Sauerbrey A. The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood. 2003;102:4493–4498. doi: 10.1182/blood-2002-11-3461. [DOI] [PubMed] [Google Scholar]
- 70.Tzoneva G, Perez-Garcia A, Carpenter Z, Khiabanian H, Tosello V, Allegretta M, Paietta E, Racevskis J, Rowe JM, Tallman MS, Paganin M, Basso G, Hof J, Kirschner-Schwabe R, Palomero T, Rabadan R, Ferrando A. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med. 2013;19:368–371. doi: 10.1038/nm.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunsucker SA, Mitchell BS, Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107:1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Nowak D, Liem NL, Mossner M, Klaumunzer M, Papa RA, Nowak V, Jann JC, Akagi T, Kawamata N, Okamoto R, Thoennissen NH, Kato M, Sanada M, Hofmann WK, Ogawa S, Marshall GM, Lock RB, Koeffler HP. Variegated clonality and rapid emergence of new molecular lesions in xenografts of acute lymphoblastic leukemia are associated with drug resistance. Exp Hematol. 2015;43:32–43 e31–35. doi: 10.1016/j.exphem.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeifer H, Wassmann B, Pavlova A, Wunderle L, Oldenburg J, Binckebanck A, Lange T, Hochhaus A, Wystub S, Bruck P, Hoelzer D, Ottmann OG. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2007;110:727–734. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 74.Chen S, Xing H, Li S, Yu J, Li H, Liu S, Tian Z, Tang K, Rao Q, Wang M, Wang J. Up-regulated A20 promotes proliferation, regulates cell cycle progression and induces chemotherapy resistance of acute lymphoblastic leukemia cells. Leuk Res. 2015;39:976–983. doi: 10.1016/j.leukres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Redner A, Hegewisch S, Haimi J, Steinherz P, Jhanwar S, Andreeff M. A study of multidrug resistance and cell kinetics in a child with near-haploid acute lymphoblastic leukemia. Leuk Res. 1990;14:771–777. doi: 10.1016/0145-2126(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 76.Hosono N, Kishi S, Iho S, Urasaki Y, Yoshida A, Kurooka H, Yokota Y, Ueda T. Glutathione S-transferase M1 inhibits dexamethasone-induced apoptosis in association with the suppression of Bim through dual mechanisms in a lymphoblastic leukemia cell line. Cancer Sci. 2010;101:767–773. doi: 10.1111/j.1349-7006.2009.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, Masurekar A, Johnson S, Chakraborty S, Griffiths J, Smith D, Alexander S, Dempsey C, Parker C, Harrison S, Li Y, Miller C, Di Y, Ghosh Z, Krishnan S, Saha V. Stromal cell-mediated mitochondrial redox adaptation regulates drug resistance in childhood acute lymphoblastic leukemia. Oncotarget. 2015;6:43048–43064. doi: 10.18632/oncotarget.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu T, Kishton RJ, Macintyre AN, Gerriets VA, Xiang H, Liu X, Abel ED, Rizzieri D, Locasale JW, Rathmell JC. Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis. 2014;5:e1470. doi: 10.1038/cddis.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kharabi Masouleh B, Geng H, Hurtz C, Chan LN, Logan AC, Chang MS, Huang C, Swaminathan S, Sun H, Paietta E, Melnick AM, Koeffler P, Muschen M. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2014;111:E2219–2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hahnel PS, Enders B, Sasca D, Roos WP, Kaina B, Bullinger L, Theobald M, Kindler T. Targeting components of the alternative NHEJ pathway sensitizes KRAS mutant leukemic cells to chemotherapy. Blood. 2014;123:2355–2366. doi: 10.1182/blood-2013-01-477620. [DOI] [PubMed] [Google Scholar]
- 81.Kawahara M, Hori T, Chonabayashi K, Oka T, Sudol M, Uchiyama T. Kpm/Lats2 is linked to chemosensitivity of leukemic cells through the stabilization of p73. Blood. 2008;112:3856–3866. doi: 10.1182/blood-2007-09-111773. [DOI] [PubMed] [Google Scholar]
- 82.Akbari Moqadam F, Lange-Turenhout EA, Aries IM, Pieters R, den Boer ML. MiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemia. Leuk Res. 2013;37:1315–1321. doi: 10.1016/j.leukres.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 83.Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20:43–50. doi: 10.1023/a:1013156407224. [DOI] [PubMed] [Google Scholar]
- 84.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Schlesinger M, Bendas G. Contribution of very late antigen-4 (VLA-4) integrin to cancer progression and metastasis. Cancer Metastasis Rev. 2015;34:575–591. doi: 10.1007/s10555-014-9545-x. [DOI] [PubMed] [Google Scholar]
- 86.van der Loo JC, Xiao X, McMillin D, Hashino K, Kato I, Williams DA. VLA-5 is expressed by mouse and human long-term repopulating hematopoietic cells and mediates adhesion to extracellular matrix protein fibronectin. J Clin Invest. 1998;102:1051–1061. doi: 10.1172/JCI3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giblin PA, Lemieux RM. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des. 2006;12:2771–2795. doi: 10.2174/138161206777947731. [DOI] [PubMed] [Google Scholar]
- 88.Perez OD, Mitchell D, Jager GC, South S, Murriel C, McBride J, Herzenberg LA, Kinoshita S, Nolan GP. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4:1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 89.Filshie R, Gottlieb D, Bradstock K. VLA-4 is involved in the engraftment of the human pre-B acute lymphoblastic leukaemia cell line NALM-6 in SCID mice. Br J Haematol. 1998;102:1292–1300. doi: 10.1046/j.1365-2141.1998.00899.x. [DOI] [PubMed] [Google Scholar]
- 90.Jacamo R, Chen Y, Wang Z, Ma W, Zhang M, Spaeth EL, Wang Y, Battula VL, Mak PY, Schallmoser K, Ruvolo P, Schober WD, Shpall EJ, Nguyen MH, Strunk D, Bueso-Ramos CE, Konoplev S, Davis RE, Konopleva M, Andreeff M. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood. 2014;123:2691–2702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weekes CD, Kuszynski CA, Sharp JG. VLA-4 mediated adhesion to bone marrow stromal cells confers chemoresistance to adherent lymphoma cells. Leuk Lymphoma. 2001;40:631–645. doi: 10.3109/10428190109097661. [DOI] [PubMed] [Google Scholar]
- 92.Noborio-Hatano K, Kikuchi J, Takatoku M, Shimizu R, Wada T, Ueda M, Nobuyoshi M, Oh I, Sato K, Suzuki T, Ozaki K, Mori M, Nagai T, Muroi K, Kano Y, Furukawa Y, Ozawa K. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene. 2009;28:231–242. doi: 10.1038/onc.2008.385. [DOI] [PubMed] [Google Scholar]
- 93.Bazzoni G, Carlesso N, Griffin JD, Hemler ME. Bcr/Abl expression stimulates integrin function in hematopoietic cell lines. J Clin Invest. 1996;98:521–528. doi: 10.1172/JCI118820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, Slayton WB. Integrin VLA-5 and FAK are Good Targets to Improve Treatment Response in the Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Front Oncol. 2014;4:112. doi: 10.3389/fonc.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winter SS, Sweatman JJ, Lawrence MB, Rhoades TH, Hart AL, Larson RS. Enhanced T-lineage acute lymphoblastic leukaemia cell survival on bone marrow stroma requires involvement of LFA-1 and ICAM-1. Br J Haematol. 2001;115:862–871. doi: 10.1046/j.1365-2141.2001.03182.x. [DOI] [PubMed] [Google Scholar]
- 96.Harima A, Nakaseko C, Yokota A, Kitagawa M, Morimoto C, Harigaya K, Saito Y. Fibronectin promotes cell proliferation of human pre-B cell line via its interactions with VLA-4 and VLA-5. Hematology. 2008;13:236–243. doi: 10.1179/102453308X348315. [DOI] [PubMed] [Google Scholar]
- 97.Liu CC, Leclair P, Yap SQ, Lim CJ. The membrane-proximal KXGFFKR motif of alpha-integrin mediates chemoresistance. Mol Cell Biol. 2013;33:4334–4345. doi: 10.1128/MCB.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 99.de la Fuente MT, Casanova B, Moyano JV, Garcia-Gila M, Sanz L, Garcia-Marco J, Silva A, Garcia-Pardo A. Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol. 2002;71:495–502. [PubMed] [Google Scholar]
- 100.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, Kato J, Hirayama Y, Sakamaki S, Kohda K, Miyake K, Niitsu Y. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 101.Matsunaga T, Fukai F, Miura S, Nakane Y, Owaki T, Kodama H, Tanaka M, Nagaya T, Takimoto R, Takayama T, Niitsu Y. Combination therapy of an anticancer drug with the FNIII14 peptide of fibronectin effectively overcomes cell adhesion-mediated drug resistance of acute myelogenous leukemia. Leukemia. 2008;22:353–360. doi: 10.1038/sj.leu.2405017. [DOI] [PubMed] [Google Scholar]
- 102.De Toni-Costes F, Despeaux M, Bertrand J, Bourogaa E, Ysebaert L, Payrastre B, Racaud-Sultan C. A New alpha5beta1 integrin-dependent survival pathway through GSK3beta activation in leukemic cells. PLoS One. 2010;5:e9807. doi: 10.1371/journal.pone.0009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 104.Saito Y, Owaki T, Matsunaga T, Saze M, Miura S, Maeda M, Eguchi M, Tanaka R, Taira J, Kodama H, Goto S, Niitsu Y, Terada H, Fukai F. Apoptotic death of hematopoietic tumor cells through potentiated and sustained adhesion to fibronectin via VLA-4. J Biol Chem. 2010;285:7006–7015. doi: 10.1074/jbc.M109.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Gertz MA. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014;15:223–230. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 107.Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120:303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012;120:295–302. doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 110.Tanoue T, Takeichi M. New insights into Fat cadherins. J Cell Sci. 2005;118:2347–2353. doi: 10.1242/jcs.02398. [DOI] [PubMed] [Google Scholar]
- 111.O’Leary H, Akers SM, Piktel D, Walton C, Fortney JE, Martin KH, Craig M, Coad J, Gibson LF. VE-cadherin Regulates Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Sensitivity to Apoptosis. Cancer Microenviron. 2010;3:67–81. doi: 10.1007/s12307-010-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen C, Zhang HX, Wang M, Song XG, Cao J, Wang L, Qiao JL, Lu XY, Han ZX, Zhu P, Pan B, Wu QY, Zhao K, Yan ZL, Li ZY, Zeng LY, Xu KL. Stromal cells attenuate the cytotoxicity of imatinib on Philadelphia chromosome-positive leukemia cells by up-regulating the VE-cadherin/beta-catenin signal. Leuk Res. 2014;38:1460–1468. doi: 10.1016/j.leukres.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, O’Leary H, Fortney J, Gibson LF. Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood. 2007;110:3334–3344. doi: 10.1182/blood-2007-01-068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dandekar S, Romanos-Sirakis E, Pais F, Bhatla T, Jones C, Bourgeois W, Hunger SP, Raetz EA, Hermiston ML, Dasgupta R, Morrison DJ, Carroll WL. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014;167:87–99. doi: 10.1111/bjh.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Y, Mallampati S, Sun B, Zhang J, Kim SB, Lee JS, Gong Y, Cai Z, Sun X. Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett. 2013;333:9–17. doi: 10.1016/j.canlet.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu Y, Chen Y, Douglas L, Li S. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 117.Nygren MK, Dosen-Dahl G, Stubberud H, Walchli S, Munthe E, Rian E. beta-catenin is involved in N-cadherin-dependent adhesion, but not in canonical Wnt signaling in E2A-PBX1-positive B acute lymphoblastic leukemia cells. Exp Hematol. 2009;37:225–233. doi: 10.1016/j.exphem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 118.Zhang B, Groffen J, Heisterkamp N. Increased resistance to a farnesyltransferase inhibitor by N-cadherin expression in Bcr/Abl-P190 lymphoblastic leukemia cells. Leukemia. 2007;21:1189–1197. doi: 10.1038/sj.leu.2404667. [DOI] [PubMed] [Google Scholar]
- 119.Zhi L, Wang M, Rao Q, Yu F, Mi Y, Wang J. Enrichment of N-Cadherin and Tie2-bearing CD34+/CD38-/CD123+ leukemic stem cells by chemotherapy-resistance. Cancer Lett. 2010;296:65–73. doi: 10.1016/j.canlet.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 120.Qiu S, Jia Y, Xing H, Yu T, Yu J, Yu P, Tang K, Tian Z, Wang H, Mi Y, Rao Q, Wang M, Wang J. N-Cadherin and Tie2 positive CD34(+)CD38(−)CD123(+) leukemic stem cell populations can develop acute myeloid leukemia more effectively in NOD/SCID mice. Leuk Res. 2014;38:632–637. doi: 10.1016/j.leukres.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–1838. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadler NM, Harris BR, Metzger BA, Kirshner J. N-cadherin impedes proliferation of the multiple myeloma cancer stem cells. Am J Blood Res. 2013;3:271–285. [PMC free article] [PubMed] [Google Scholar]
- 123.de Bock CE, Ardjmand A, Molloy TJ, Bone SM, Johnstone D, Campbell DM, Shipman KL, Yeadon TM, Holst J, Spanevello MD, Nelmes G, Catchpoole DR, Lincz LF, Boyd AW, Burns GF, Thorne RF. The Fat1 cadherin is overexpressed and an independent prognostic factor for survival in paired diagnosis-relapse samples of precursor B-cell acute lymphoblastic leukemia. Leukemia. 2012;26:918–926. doi: 10.1038/leu.2011.319. [DOI] [PubMed] [Google Scholar]
- 124.Neumann M, Seehawer M, Schlee C, Vosberg S, Heesch S, von der Heide EK, Graf A, Krebs S, Blum H, Gokbuget N, Schwartz S, Hoelzer D, Greif PA, Baldus CD. FAT1 expression and mutations in adult acute lymphoblastic leukemia. Blood Cancer J. 2014;4:e224. doi: 10.1038/bcj.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ardjmand A, de Bock CE, Shahrokhi S, Lincz LF, Boyd AW, Burns GF, Thorne RF. Fat1 cadherin provides a novel minimal residual disease marker in acute lymphoblastic leukemia. Hematology. 2013;18:315–322. doi: 10.1179/1607845413Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 126.Neumann M, Heesch S, Schlee C, Schwartz S, Gokbuget N, Hoelzer D, Konstandin NP, Ksienzyk B, Vosberg S, Graf A, Krebs S, Blum H, Raff T, Bruggemann M, Hofmann WK, Hecht J, Bohlander SK, Greif PA, Baldus CD. Whole-exome sequencing in adult ETP-ALL reveals a high rate of DNMT3A mutations. Blood. 2013;121:4749–4752. doi: 10.1182/blood-2012-11-465138. [DOI] [PubMed] [Google Scholar]
- 127.Neumann M, Vosberg S, Schlee C, Heesch S, Schwartz S, Gokbuget N, Hoelzer D, Graf A, Krebs S, Bartram I, Blum H, Bruggemann M, Hecht J, Bohlander SK, Greif PA, Baldus CD. Mutational spectrum of adult T-ALL. Oncotarget. 2015;6:2754–2766. doi: 10.18632/oncotarget.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S, Eng S, Kannan K, Zou Y, Peng L, Banuchi VE, Paty P, Zeng Z, Vakiani E, Solit D, Singh B, Ganly I, Liau L, Cloughesy TC, Mischel PS, Mellinghoff IK, Chan TA. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45:253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Messina M, Del Giudice I, Khiabanian H, Rossi D, Chiaretti S, Rasi S, Spina V, Holmes AB, Marinelli M, Fabbri G, Piciocchi A, Mauro FR, Guarini A, Gaidano G, Dalla-Favera R, Pasqualucci L, Rabadan R, Foa R. Genetic lesions associated with chronic lymphocytic leukemia chemo-refractoriness. Blood. 2014;123:2378–2388. doi: 10.1182/blood-2013-10-534271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Postigo AA, Marazuela M, Sanchez-Madrid F, de Landazuri MO. B lymphocyte binding to E- and P-selectins is mediated through the de novo expression of carbohydrates on in vitro and in vivo activated human B cells. J Clin Invest. 1994;94:1585–1596. doi: 10.1172/JCI117500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 132.Pezeshkian B, Donnelly C, Tamburo K, Geddes T, Madlambayan GJ. Leukemia Mediated Endothelial Cell Activation Modulates Leukemia Cell Susceptibility to Chemotherapy through a Positive Feedback Loop Mechanism. PLoS One. 2013;8:e60823. doi: 10.1371/journal.pone.0060823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burgess M, Gill D, Singhania R, Cheung C, Chambers L, Renyolds BA, Smith L, Mollee P, Saunders N, McMillan NA. CD62L as a therapeutic target in chronic lymphocytic leukemia. Clin Cancer Res. 2013;19:5675–5685. doi: 10.1158/1078-0432.CCR-13-1037. [DOI] [PubMed] [Google Scholar]
- 134.Krause DS, Lazarides K, Lewis JB, von Andrian UH, Van Etten RA. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood. 2014;123:1361–1371. doi: 10.1182/blood-2013-11-538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, Wang Z, Liu Z, Li H, He J, Lin P, Weber D, Davis RE, Kwak L, Cai Z, Yi Q. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia. 2013;27:702–710. doi: 10.1038/leu.2012.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Porter JC, Hogg N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- 138.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan Y, Lu X, Chen X, Shao H, Huang S. Jagged1 contributes to the drug resistance of Jurkat cells in contact with human umbilical cord-derived mesenchymal stem cells. Oncol Lett. 2013;6:1000–1006. doi: 10.3892/ol.2013.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chadwick N, Zeef L, Portillo V, Fennessy C, Warrander F, Hoyle S, Buckle AM. Identification of novel Notch target genes in T cell leukaemia. Mol Cancer. 2009;8:35. doi: 10.1186/1476-4598-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murray ME, Gavile CM, Nair JR, Koorella C, Carlson LM, Buac D, Utley A, Chesi M, Bergsagel PL, Boise LH, Lee KP. CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma. Blood. 2014;123:3770–3779. doi: 10.1182/blood-2013-10-530964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 143.Martinez-Torres AC, Quiney C, Attout T, Boullet H, Herbi L, Vela L, Barbier S, Chateau D, Chapiro E, Nguyen-Khac F, Davi F, Le Garff-Tavernier M, Moumne R, Sarfati M, Karoyan P, Merle-Beral H, Launay P, Susin SA. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCgamma1 activation: evidence from mice and humans. PLoS Med. 2015;12:e1001796. doi: 10.1371/journal.pmed.1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lanciotti M, D’Apolito M, Paolucci P, Dufour C. Chromosomal locus 19p13 as potential hotspot for aberrant gene expression in relapsed paediatric acute lymphoblastic leukaemia. Br J Haematol. 2006;135:274–275. doi: 10.1111/j.1365-2141.2006.06303.x. [DOI] [PubMed] [Google Scholar]
- 145.Beesley AH, Cummings AJ, Freitas JR, Hoffmann K, Firth MJ, Ford J, de Klerk NH, Kees UR. The gene expression signature of relapse in paediatric acute lymphoblastic leukaemia: implications for mechanisms of therapy failure. Br J Haematol. 2005;131:447–456. doi: 10.1111/j.1365-2141.2005.05785.x. [DOI] [PubMed] [Google Scholar]
- 146.Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016;159:481–490. doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu XL, Hu T, Du JM, Ding JP, Yang XM, Zhang J, Yang B, Shen X, Zhang Z, Zhong WD, Wen N, Jiang H, Zhu P, Chen ZN. Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem. 2008;283:18056–18065. doi: 10.1074/jbc.M802694200. [DOI] [PubMed] [Google Scholar]
- 148.Li ZJ, Chen ZX, Cen JN, He J, Qiu QC. Direct contact with bone marrow stromal cells promotes the invasions of SHI-1 leukemia cells. Chin Med J (Engl) 2013;126:2731–2735. [PubMed] [Google Scholar]
- 149.Jia L, Wei W, Cao J, Xu H, Miao X, Zhang J. Silencing CD147 inhibits tumor progression and increases chemosensitivity in murine lymphoid neoplasm P388D1 cells. Ann Hematol. 2009;88:753–760. doi: 10.1007/s00277-008-0678-2. [DOI] [PubMed] [Google Scholar]
- 150.Pan Y, He B, Song G, Bao Q, Tang Z, Tian F, Wang S. CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep. 2012;27:2003–2009. doi: 10.3892/or.2012.1729. [DOI] [PubMed] [Google Scholar]
- 151.Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, Qu LL, Wang SK. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61. doi: 10.1186/1756-9966-29-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arnaud MP, Vallee A, Robert G, Bonneau J, Leroy C, Varin-Blank N, Rio AG, Troadec MB, Galibert MD, Gandemer V. CD9, a key actor in the dissemination of lymphoblastic leukemia, modulating CXCR4-mediated migration via RAC1 signaling. Blood. 2015;126:1802–1812. doi: 10.1182/blood-2015-02-628560. [DOI] [PubMed] [Google Scholar]
- 153.Yamazaki H, Xu CW, Naito M, Nishida H, Okamoto T, Ghani FI, Iwata S, Inukai T, Sugita K, Morimoto C. Regulation of cancer stem cell properties by CD9 in human B-acute lymphoblastic leukemia. Biochem Biophys Res Commun. 2011;409:14–21. doi: 10.1016/j.bbrc.2011.04.098. [DOI] [PubMed] [Google Scholar]
- 154.Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S, Yoshida M, Kumagai T, Tachibana I, Yokota S, Kawase I. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010;70:8025–8035. doi: 10.1158/0008-5472.CAN-10-0996. [DOI] [PubMed] [Google Scholar]
- 155.Zoller M. CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Front Immunol. 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 157.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khan NI, Cisterne A, Devidas M, Shuster J, Hunger SP, Shaw PJ, Bradstock KF, Bendall LJ. Expression of CD44, but not CD44v6, predicts relapse in children with B cell progenitor acute lymphoblastic leukemia lacking adverse or favorable genetics. Leuk Lymphoma. 2008;49:710–718. doi: 10.1080/10428190701861660. [DOI] [PubMed] [Google Scholar]
- 159.La Starza R, Borga C, Barba G, Pierini V, Schwab C, Matteucci C, Lema Fernandez AG, Leszl A, Cazzaniga G, Chiaretti S, Basso G, Harrison CJ, Te Kronnie G, Mecucci C. Genetic profile of T-cell acute lymphoblastic leukemias with MYC translocations. Blood. 2014;124:3577–3582. doi: 10.1182/blood-2014-06-578856. [DOI] [PubMed] [Google Scholar]
- 160.Quere R, Andradottir S, Brun AC, Zubarev RA, Karlsson G, Olsson K, Magnusson M, Cammenga J, Karlsson S. High levels of the adhesion molecule CD44 on leukemic cells generate acute myeloid leukemia relapse after withdrawal of the initial transforming event. Leukemia. 2011;25:515–526. doi: 10.1038/leu.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van Driel M, Gunthert U, van Kessel AC, Joling P, Stauder R, Lokhorst HM, Bloem AC. CD44 variant isoforms are involved in plasma cell adhesion to bone marrow stromal cells. Leukemia. 2002;16:135–143. doi: 10.1038/sj.leu.2402336. [DOI] [PubMed] [Google Scholar]
- 162.Juarez J, Bendall L. SDF-1 and CXCR4 in normal and malignant hematopoiesis. Histol Histopathol. 2004;19:299–309. doi: 10.14670/HH-19.299. [DOI] [PubMed] [Google Scholar]
- 163.de Lourdes Perim A, Amarante MK, Guembarovski RL, de Oliveira CE, Watanabe MA. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell Mol Life Sci. 2015;72:1715–1723. doi: 10.1007/s00018-014-1830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Passaro D, Irigoyen M, Catherinet C, Gachet S, Da Costa De Jesus C, Lasgi C, Tran Quang C, Ghysdael J. CXCR4 Is Required for Leukemia-Initiating Cell Activity in T Cell Acute Lymphoblastic Leukemia. Cancer Cell. 2015;27:769–779. doi: 10.1016/j.ccell.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 165.Juarez J, Dela Pena A, Baraz R, Hewson J, Khoo M, Cisterne A, Fricker S, Fujii N, Bradstock KF, Bendall LJ. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007;21:1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 166.Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.D’Souza S, Kurihara N, Shiozawa Y, Joseph J, Taichman R, Galson DL, Roodman GD. Annexin II interactions with the annexin II receptor enhance multiple myeloma cell adhesion and growth in the bone marrow microenvironment. Blood. 2012;119:1888–1896. doi: 10.1182/blood-2011-11-393348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gopalakrishnapillai A, Kolb EA, Dhanan P, Mason RW, Napper A, Barwe SP. Disruption of Annexin II/p11 Interaction Suppresses Leukemia Cell Binding, Homing and Engraftment, and Sensitizes the Leukemia Cells to Chemotherapy. PLoS One. 2015;10:e0140564. doi: 10.1371/journal.pone.0140564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lavabre-Bertrand T, Janossy G, Ivory K, Peters R, Secker-Walker L, Porwit-MacDonald A. Leukemia-associated changes identified by quantitative flow cytometry: I. CD10 expression. Cytometry. 1994;18:209–217. doi: 10.1002/cyto.990180404. [DOI] [PubMed] [Google Scholar]
- 170.Sumitomo M, Shen R, Walburg M, Dai J, Geng Y, Navarro D, Boileau G, Papandreou CN, Giancotti FG, Knudsen B, Nanus DM. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fukusumi T, Ishii H, Konno M, Yasui T, Nakahara S, Takenaka Y, Yamamoto Y, Nishikawa S, Kano Y, Ogawa H, Hasegawa S, Hamabe A, Haraguchi N, Doki Y, Mori M, Inohara H. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer. 2014;111:506–514. doi: 10.1038/bjc.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davies S, Beckenkamp A, Buffon A. CD26 a cancer stem cell marker and therapeutic target. Biomed Pharmacother. 2015;71:135–138. doi: 10.1016/j.biopha.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 173.Sato T, Yamochi T, Yamochi T, Aytac U, Ohnuma K, McKee KS, Morimoto C, Dang NH. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta1, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res. 2005;65:6950–6956. doi: 10.1158/0008-5472.CAN-05-0647. [DOI] [PubMed] [Google Scholar]
- 174.Carbone A, Gloghini A, Zagonel V, Aldinucci D, Gattei V, Degan M, Improta S, Sorio R, Monfardini S, Pinto A. The expression of CD26 and CD40 ligand is mutually exclusive in human T-cell non-Hodgkin’s lymphomas/leukemias. Blood. 1995;86:4617–4626. [PubMed] [Google Scholar]
- 175.Mikhailov A, Sokolovskaya A, Yegutkin GG, Amdahl H, West A, Yagita H, Lahesmaa R, Thompson LF, Jalkanen S, Blokhin D, Eriksson JE. CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J Immunol. 2008;181:464–475. doi: 10.4049/jimmunol.181.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang W, Gao L, Li Y, Li ZL, Gong M, Huang FZ, Chen YR, Zhang CX, Gao YY, Ma YG. The application of CD73 in minimal residual disease monitoring using flow cytometry in B-cell acute lymphoblastic leukemia. Leuk Lymphoma. 2015:1–8. doi: 10.3109/10428194.2015.1070153. [DOI] [PubMed] [Google Scholar]
- 177.Serra S, Horenstein AL, Vaisitti T, Brusa D, Rossi D, Laurenti L, D’Arena G, Coscia M, Tripodo C, Inghirami G, Robson SC, Gaidano G, Malavasi F, Deaglio S. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fei F, Joo EJ, Tarighat SS, Schiffer I, Paz H, Fabbri M, Abdel-Azim H, Groffen J, Heisterkamp N. B-cell precursor acute lymphoblastic leukemia and stromal cells communicate through Galectin-3. Oncotarget. 2015;6:11378–11394. doi: 10.18632/oncotarget.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu K, Gu Y, Lou L, Liu L, Hu Y, Wang B, Luo Y, Shi J, Yu X, Huang H. Galectin-3 mediates bone marrow microenvironment-induced drug resistance in acute leukemia cells via Wnt/beta-catenin signaling pathway. J Hematol Oncol. 2015;8:1. doi: 10.1186/s13045-014-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fei F, Abdel-Azim H, Lim M, Arutyunyan A, von Itzstein M, Groffen J, Heisterkamp N. Galectin-3 in pre-B acute lymphoblastic leukemia. Leukemia. 2013;27:2385–2388. doi: 10.1038/leu.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamamoto-Sugitani M, Kuroda J, Ashihara E, Nagoshi H, Kobayashi T, Matsumoto Y, Sasaki N, Shimura Y, Kiyota M, Nakayama R, Akaji K, Taki T, Uoshima N, Kobayashi Y, Horiike S, Maekawa T, Taniwaki M. Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2011;108:17468–17473. doi: 10.1073/pnas.1111138108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Macanas-Pirard P, Leisewitz A, Broekhuizen R, Cautivo K, Barriga FM, Leisewitz F, Gidi V, Riquelme E, Montecinos VP, Swett P, Besa P, Ramirez P, Ocqueteau M, Kalergis AM, Holt M, Rettig M, DiPersio JF, Nervi B. Bone marrow stromal cells modulate mouse ENT1 activity and protect leukemia cells from cytarabine induced apoptosis. PLoS One. 2012;7:e37203. doi: 10.1371/journal.pone.0037203. [DOI] [PMC free article] [PubMed] [Google Scholar]


