Abstract
Valvular heart disease (VHD), particularly aortic valve disease, is prevalent with increasing incidence. When surgery is not possible, or when risks outweigh benefits, percutaneous treatment options may offer effective alternatives. However, procedures may not always go as planned, and frail patients or those whose symptoms are caused by other comorbidities may not benefit from valve intervention at all. Significant effort should be made to assess frailty, comorbidities and patient goals prior to intervention. Palliative care (PC) should play a critical role in the care of patients with severe valve disease. PC is specialised medical care that aims to optimise health-related quality of life by managing symptoms and clarifying patient values and goals of care. It should be implemented at the time of diagnosis and continue throughout the disease course. Because of the paucity of studies dedicated to the provision of PC to patients with advanced VHD, further research is needed.
Burden of Valvular Heart Disease
In previous decades, valve disease was recognised because of its relationship to rheumatic fever, but age-related degenerative valve disease is becoming predominant.1,2 Improvements in imaging have led to earlier diagnosis of valvular heart disease (VHD), and the greater availability of interventions has led to new methods for management.2,3 The cost of the management of VHD has correspondingly increased,3 raising questions about cost-effectiveness of some interventions.1,4
Elderly patients frequently have VHD. The OxValve Study reported a major burden of undiagnosed VHD in the elderly population (defined in that study as 65 years and older). In a large-scale community screening effort, new VHD was found in 51% of the participants. The total population prevalence of moderate and severe disease was 11.3%, with a predicted 122% increase in VHD by 2046.3 The prevalence of at least moderate aortic valve disease is approximately 2% in patients 70–80 years old,1,2 but is as high as 9% in those older than 80 years of age. In comparison, significant aortic regurgitation has a prevalence of 0.5%. At least moderate mitral regurgitation, which can be a structural or functional lesion, has an estimated prevalence of 1.1%–1.6%.1
The age of patients with VHD is increasing, and mortality is high in this population. Advanced age, end organ dysfunction and the presence of comorbidities are associated with reduced survival in VHD. Vahanian and colleagues report that in the decade between 1997 and 2006, the proportion of patients with VHD who were 80 years or older significantly increased from 13% to 20%, and the number of patients with comorbidities such as diabetes, coronary or other vascular disease and renal or pulmonary disease also significantly increased.2 Aortic disease is particularly associated with poor prognosis once symptoms develop. Patients with severe aortic stenosis have been reported to have 40%–50% 1-year mortality, increased to 80% by 3 years.5,6 The decision to intervene on VHD in the elderly is complex due to the common presence of comorbidities, limited life expectancy and the associated increased operative risk.2,5
The cost of VHD is high. In addition to the increased costs from increasing VHD prevalence, Clark and colleagues reported that the mean Hierarchical Condition Categories score for elderly patients with aortic stenosis was 3.4, meaning their expected healthcare costs were over three times those of the average Medicare beneficiary. These patients experienced an average of 4.4 acute hospitalisations per patient over a 5-year period, with a mean length of stay of 26.7 days and 57.4% subsequently required home healthcare, 52% required skilled nursing care and 27.6% enrolled in hospice. Medical costs were estimated between $600 million and $1.3 billion per year, depending on whether half or all were incident cases, respectively.5 Badheka and colleagues reported similar findings. Elderly patients with aortic VHD with higher comorbidity burden had double the hospitalisation rate, increased need for assistance or institutionalisation over time and an annual cost of $2.13 billion in 2011, extrapolated to nearly $3 billion by 2020.7
Emergence of Transcatheter Valve Procedures As Treatment Options
Percutaneous aortic valve replacement (transcatheter aortic valve intervention, TAVI or transcatheter aortic valve replacement, TAVR; henceforth we will use ‘TAVR’), introduced in 2002, was initially designed for use in patients with aortic stenosis who were considered at too high or excessive risk for surgery. Its use has become widespread and is being expanded to lower risk populations.2
The most well-known study demonstrating the value of TAVR is the PARTNER trial. In 2010, for those ineligible for surgery, TAVR was shown to significantly reduce rates of death from any cause (30.7% vs 50.7% in those medically managed), significantly reduce the composite endpoint of death from any cause and repeat hospitalisation (43.5% vs 71.6%) and significantly reduce cardiac symptoms compared with medical therapy.8 When compared with surgical aortic valve replacement, TAVR was found to have similar rates of death from any cause and rates of stroke for high-risk patients.9,10
Use of the technology that supports TAVR has been implemented to treat VHD other than aortic stenosis. Valves designed for TAVR have been used to treat aortic regurgitation.11 A transcatheter option for replacement of previously repaired pulmonary valves became available in the mid-2000s.12,13 This valve and those designed for TAVR have been employed in the valve-in-valve technique for treatment of dysfunctional, previously placed prosthetic valves in the aortic and other positions.14–18 Multiple devices have been explored for percutaneous repair or replacement of the mitral valve, but the only currently approved device is the MitraClip,19 which uses a system to percutaneously clip edges of the anterior and posterior mitral valve leaflets together, reducing the effective regurgitant orifice area of the valve. The EVEREST trials showed that repair of mitral regurgitation using the MitraClip technology was feasible, with improvement in clinical symptoms and left ventricular reverse remodelling at 1 year.20–22 There are no devices specifically approved for tricuspid intervention, but percutaneous valves have been placed in the inferior vena cava to reduce the effects of tricuspid regurgitation on abdominal viscera, and edge-to-edge clipping of tricuspid leaflets has been attempted.23,24
‘Can’ does not equal ‘should’
It is increasingly recognised that the primary driver of symptoms, reduced quality of life and shortened longevity, may not be cardiac disease in many patients with severe VHD. In these cases, valve interventions have marginal effects on a patient's overall clinical course.25 Not all patients benefit equally from TAVR. Up to 30% of TAVR survivors in PARTNER either died or experienced persistent NYHA Class III or IV symptoms at 1 year.8,26 For patients who did experience improvements in symptoms and physical function, benefits in psychological dimensions and general health measures were small and inconsistent.27 A persistent mortality benefit at 2 years has been reported, but only for those who survived beyond the first year.28 These findings—of persistent symptoms and/or death despite procedural success—suggest that the existence of comorbid conditions plays a major role in the long-term success of this procedure. This suggestion is echoed by the finding that patients had worse outcomes with the transapical compared with the transfemoral approach, likely as a function of the higher procedural risk in the transapical group.29 Whether TAVR represents a rational use of limited healthcare resources requires a thorough understanding of its long-term outcomes and costs.5 Given the large volume of patients undergoing TAVR and the rapid development of other percutaneous valvular intervention technologies, the potential for inconsistent or insignificant benefit in a large number of patients is concerning but under-studied.
Determining whether a patient will benefit from TAVR is part of the pre-procedure multidisciplinary assessment, which requires consensus from a Heart Team. This Team is composed usually of an interventional cardiologist and a surgeon and, in addition to the patient, includes a multidisciplinary group of caregivers with expertise in VHD such as imaging specialists, anaesthesiologists and nurses.30,31 Assessment of frailty can play an important role. Frailty is defined as ‘a syndrome of decreased physiologic reserve and resistance to stressors, resulting in cumulative declines across multiple organ systems and increased vulnerability to adverse outcomes’. It is characterised by slowness, weakness, poor endurance, and a low activity level. It is distinct from comorbidity and disability and should be assessed independently. Frailty is both a causative and prognostic factor in cardiovascular disease. It is present in 50+% of patients with heart failure (HF) and coronary artery disease, and greatly influences morbidity and mortality. In the PARTNER trial, increased frailty was associated with both a higher mortality and a higher rate of poor functional outcome at 1 year, and lower frailty has been associated with better outcomes.32 Data regarding pre-procedure risk assessment and frailty do not exist for patients undergoing percutaneous interventions other than TAVR.
TAVR benefits are also negatively impacted by comorbidities such as renal dysfunction, significant lung disease, coronary artery disease and, possibly, reduced ejection fraction (EF). Most patients in the PARTNER trial had normal EF, and those with EF <20% were excluded. Those with low-flow low-gradient AS had worse outcomes. Nonetheless, the extent to which functional recovery following TAVR is related to patient characteristics is poorly understood.26 Even the definition of ‘benefit’ in this setting is up for debate, especially for patients who place a higher value on functional status and quality of life than longevity.27 Traditional outcome parameters used in predictive models may fail to appreciate the full effects on emotional, physical, functional and mental well-being.26 The European System for Cardiac Operative Risk Evaluation (EUROSCORE) and Society of Thoracic Surgeons (STS) scores are poorly calibrated for TAVR,25 and they are not able to predict symptom resolution, quality-of-life improvement, or return to independent living.26 EUROSCORE II and ACEF (acronym for age, preoperative creatinine and EF) perform reasonably, but were not developed in TAVR populations.25 Recommended tools for use include the Charlson Comorbidity Index, Kansas City Cardiomyopathy Questionnaire, Minnesota Living with Heart Failure Questionnaire, SF-12/36 PCS and MCS scores, EuroQoL-5D and 6-metre walk test.2,27 Use of the Vancouver Functional Assessment (which includes 5-metre gait speed score, grip strength, activities of daily living (ADL) and instrumental activities of daily living (IADL) assessment, Mini-Mental State Exam and the Canadian Study on Health and Aging frailty score) and the ‘look test’ (a full-body photograph in street clothes, with mobility aids) have also been reported.33
Following evaluation, 30%–40% of those referred to TAVR are turned down because they are thought too sick or frail to benefit.2 Some describe these as ‘Cohort C,’ characterised by STS >20, FEV >40%, long-term O2 dependence with history of smoking, pulmonary hypertension, chronic kidney or liver disease, extreme obesity, neuromuscular disease, extreme frailty and dementia—factors linked to high 1-year mortality in PARTNER and similar studies. The Centers for Medicare & Medicaid Services (CMS) coverage policy precludes payment for TAVR for patients whose comorbidities would impede the expected benefit from correction of the aortic stenosis.34 How to counsel and manage patients in this group as they return to their referring providers facing the natural history of their valve disease and their comorbidities has not been well investigated.
High Surgical Risk Implies The Need for Advance Care and Valve Preparedness Planning
VHD is often a life-limiting illness.5,6 It is associated with reduced physical capacity which leads to the inability to participate in daily activities that make life meaningful.26 Medically vulnerable patients with advanced illness fit the mould of those who benefit from advance care planning (ACP) and palliative care (PC) discussions. There exists a significant unmet need to bridge the procedural management of VHD with the integration of high quality, patient centred care as these patients approach end-of-life.33 It is important to recognise that nothing in cardiology is curative; even successful interventions simply change the course of disease. Patients will still die, and elderly patients with multiple co-morbidities tend to die sooner, even after successful intervention.
PC is specialised medical care of people with serious illnesses. It is ‘patient and family centred care that optimises health related quality of life by anticipating, preventing and treating suffering’.35 PC does not mean withdrawal of care, and it is not equivalent to hospice. PC can and should be instituted alongside life-prolonging and disease-modifying interventions.36,37 Its focus is on symptom management, defining goals of care and facilitating appropriate discussions about what to expect to help patients live fully despite illness. It can help reduce anxiety and increase a patient's sense of control as patients can more fully understand their illness and put its effect on their lives into perspective. PC affords patients the opportunity to think about challenging decisions before they need to be made,38 while their thinking is not impaired by pain, panic or delirium.36,37 In cancer, PC has been shown to improve quality of life and, in some cases, prolong it.39–41 A recent systematic review of PC in HF cited improved quality of life and symptoms, with decreased hospitalisations and in-hospital deaths.42 Although there is not comparable literature in the population of patients with severe valve disease, 29% of the patients included in the EuroHeart study had concomitant valve disease.43
PC discussions including description of expected prognosis and initial end-of-life planning should begin at the time of diagnosis of a life-limiting condition.35,44 PC clinicians are trained to expertly facilitate communication between patients, family members and medical providers. This is particularly useful when there exists uncertainty in goals of care or when conversations have led to psychosocial distress.36 It is also key in situations when ‘aggressive’ treatment options may not offer outcomes consistent with patient values. In addition to the services available to patients, PC may also provide support and guidance for caregivers, both to family members and to providers, and it offers bereavement and grief counselling to families after a patient's death. Boxes 1 and 2 summarise the aims of PC in VHD and the benefits to ACP in this group.
Box 1. Key aims of palliative care in severe valvular heart disease.
Optimise quality of life
Aggressively treat non-cardiac symptoms
Improve communication about prognosis, treatment options and outcomes, particularly eliciting patients' and families' understanding
Clarify patient values, goals and preferences
Provide psychosocial and spiritual support
Provide bereavement support
Box 2. Benefits of advance care planning in valvular heart disease.
Reduces patient and caregiver/family anxiety
Increases patient's sense of control
Opportunity to more fully explore treatment options before crises moments arise
Helps surrogate decision-makers to follow patient's wishes
Potentially avoids conflict among caregivers/family members/care team
PC can be described in terms of ‘primary’ and ‘specialty’.45 There are too few specialist PC clinicians to meet the need in cardiovascular disease. ‘Primary PC’ is performed by clinicians who may not have advanced training or expertise in PC but have the advantage of drawing on the resources of an already established physician–patient relationship and understanding the prognosis and available treatments for a patient's disease state. PC in this context includes assessment for physical and emotional distress, clear discussion of prognosis and what to expect, exploration of a patient's values, goals and preferences to guide treatment decisions and identification of a surrogate decision-maker. Refractory symptoms, conditions outside of the clinician's expertise, difficult discussions about complex decision making and conflicts over ACP may prompt referral for specialty PC (box 3).
Box 3. Reasons to consider specialty palliative care consult for advance care planning (ACP) in valvular heart disease.
Patient/family fear of facing issues related to illness and death
Patient difficulty defining values, goals and preferences
Assistance in eliciting patient understanding of prognosis/treatment of VHD in the larger context of multimorbidity
Disagreement among stakeholders (patient, caregiver/family, Heart Team members) regarding goals of care
Limited Heart Team time/availability to address complex ACP
Expected ‘rocky course’ following procedure
The time of diagnosis of severe VHD is the perfect time to engage patients in discussions of ACP and to introduce the concept of PC. The goal of valvular interventions is not just the prolongation of life, but restoration of a satisfactory quality of life.26 Decisions about valvular interventions should be guided by the principles of shared decision-making, using simple and honest language.46 However, things do not always work out as planned. Though rare, a patient may experience a severe complication from the procedure, leaving them worse off than before, or may undergo a successful procedure but remain limited, with a poor quality of life, by symptoms unrelated to the valvular lesion. Or a patient may benefit initially from the intervention, only to experience progression of a non-valvular disease that limits longevity and/or quality of life. The patient and structural heart team can work together to construct a ‘valve preparedness plan,’ akin to that developed by Swetz et al for patients undergoing ventricular assist device (VAD) placement.47 A guide for incorporating PC into the VHD treatment plan is offered in figure 1.
Figure 1.
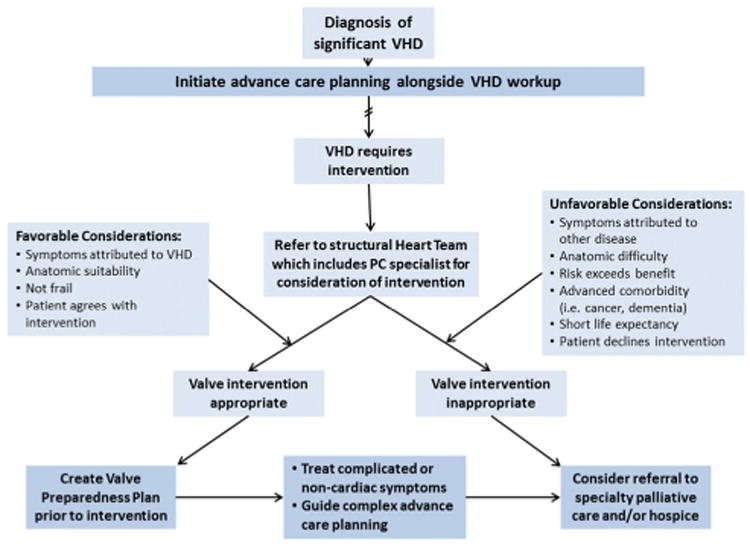
Guide for incorporating palliative care into VHD treatment plan, both early and later in disease course. VHD, valvular heart disease.
Perhaps most important are PC conversations for the ‘cohort C’ patients not eligible for procedural intervention. Being turned down for life-saving intervention can easily leave patients feeling scared and abandoned as they return to their referring physicians, and they would benefit most immediately from ongoing PC.
Requiring Palliative Care as A Component of The Heart Team
For advanced HF therapies such as VAD and transplant, the CMS developed a national coverage determination for destination therapy VAD, and the Joint Commission (JC) created certification criteria, both requiring the inclusion of a PC specialist on the care team.48,49 With the rapid expansion of the application of transcatheter procedures to treat advanced valve disease in patients with serious comorbidities, it may be beneficial to enact similar measures in the end-stage valve population as in the end-stage HF population. Though the morbidity of MCS placement in general exceeds that of TAVR, as does the level of necessary ongoing medical care involving device management, there are substantial arguments in favour of involving PC specialists prior to transcatheter procedures, particularly in elderly patients with advanced cardiac disease. PC specialists can formally assist with clarification of goals of the procedure and to ensure that the expected outcome and the risk burden are in line with patient values. PC involvement in the care team can formalise advance directives and identification of surrogate decision makers. Symptoms unlikely to improve with the valve intervention can be addressed and if appropriate, PC specialists can facilitate referrals to hospice. Geriatricians often have expertise in PC and can assist in assessing and addressing the needs of frail patients with severe VHD and, as such, can contribute much to the Heart Team.
The large and growing number of percutaneous valve procedures that occur each year may require creative solutions to providing adequate PC to patients. Developing and strengthening primary PC skills, specifically around communication and ability to assess global symptoms, should be a priority for members of the Heart Team taking care of these patients. An interdisciplinary team that may include nurses, advanced care practitioners and social workers can provide additional support in meeting patients' PC needs. Standardised tools that incorporate PC principles can be used to guide discussions, and specialty PC providers may be present at larger team meetings.50
Conclusion
The application of VHD procedures to an increasingly large group of patients, particularly those who are elderly with multiple comorbidities, and the recognition that many of these patients will not be optimally served by VHD interventions argue for increased PC involvement. There is a pressing need for investigation into the application of PC for patients with VHD. Studies are needed examining the outcomes of pre-procedural involvement of PC specialists on VHD teams, post-procedural PC needs in the VHD population, application of primary PC principles, especially related to ACP, application of PC to patients in ‘Cohort C’ and patient-centred outcomes of PC involvement, including quality of life, satisfaction, readmissions and cost.
Footnotes
Competing interests: Dr Cooper has contributed to UptoDate.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–72. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Iung B, Himbert D, et al. Changing demographics of valvular heart disease and impact on surgical and transcatheter valve therapies. Int J Cardiovasc Imaging. 2011;27:1115–22. doi: 10.1007/s10554-011-9804-7. [DOI] [PubMed] [Google Scholar]
- 3.d'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–22. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezzoug N, Vaes B, de Meester C, et al. The clinical impact of valvular heart disease in a population-based cohort of subjects aged 80 and older. BMC Cardiovasc Disord. 2016;16:1–7. doi: 10.1186/s12872-016-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MA, Arnold SV, Duhay FG, et al. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis: results from a medicare claims analysis. Circ Cardiovasc Qual Outcomes. 2012;5:697–704. doi: 10.1161/CIRCOUTCOMES.112.966002. [DOI] [PubMed] [Google Scholar]
- 6.Varadarajan P, Kapoor N, Bansal RC, et al. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111–5. doi: 10.1016/j.athoracsur.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Badheka AO, Singh V, Patel NJ, et al. Trends of hospitalizations in the United States from 2000 to 2012 of patients >60 years with aortic valve disease. Am J Cardiol. 2015;116:132–41. doi: 10.1016/j.amjcard.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 9.Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–8. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 11.Franzone A, Piccolo R, Siontis GC, et al. Transcatheter aortic valve replacement for the treatment of pure native aortic valve regurgitation: a systematic review. JACC Cardiovasc Interv. 2016;9:2308–17. doi: 10.1016/j.jcin.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 12.McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122:507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElhinney DB, Hennesen JT. The Melody® valve and ensemble® delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci. 2013;1291:77–85. doi: 10.1111/nyas.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez GJ, Ng BH, Wilson MK, et al. Transcatheter valve-in-valve replacement in complex cyanotic congenital heart disease with a single ventricle. JACC Cardiovasc Interv. 2014;7:e133–5. doi: 10.1016/j.jcin.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Hoendermis ES, Douglas YL, van den Heuvel AF. Percutaneous edwards SAPIEN valve implantation in the tricuspid position: case report and review of literature. EuroIntervention. 2012;8:628–33. doi: 10.4244/EIJV8I5A95. [DOI] [PubMed] [Google Scholar]
- 16.Cullen MW, Cabalka AK, Alli OO, et al. Transvenous, antegrade melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv. 2013;6:598–605. doi: 10.1016/j.jcin.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Huber C, Praz F, O'Sullivan CJ, et al. Transcarotid aortic valve-in-valve implantation for degenerated stentless aortic root conduits with severe regurgitation: a case series. Interact Cardiovasc Thorac Surg. 2015;20:694–700. doi: 10.1093/icvts/ivv053. [DOI] [PubMed] [Google Scholar]
- 18.Coylewright M, Cabalka AK, Malouf JA, et al. Percutaneous mitral valve replacement using a transvenous, transseptal approach: transvenous mitral valve replacement. JACC Cardiovasc Interv. 2015;8:850–7. doi: 10.1016/j.jcin.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Ramlawi B, Gammie JS. Mitral valve surgery: current minimally invasive and transcatheter options. Methodist Debakey Cardiovasc J. 2016;12:20–6. doi: 10.14797/mdcj-12-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol. 2005;46:2134–40. doi: 10.1016/j.jacc.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 21.Glower D, Ailawadi G, Argenziano M, et al. EVEREST II randomized clinical trial: predictors of mitral valve replacement in de novo surgery or after the MitraClip procedure. J Thorac Cardiovasc Surg. 2012;143:S60–3. doi: 10.1016/j.jtcvs.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leafet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol. 2012;59:130–9. doi: 10.1016/j.jacc.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill BP, Wheatley G, Bashir R, et al. Study design and rationale of the heterotopic implantation of the Edwards-Sapien XT transcatheter valve in the inferior VEna cava for the treatment of severe tricuspid regurgitation (HOVER) trial. Catheter Cardiovasc Interv. 2016;88:287–93. doi: 10.1002/ccd.26530. [DOI] [PubMed] [Google Scholar]
- 24.Braun D, Nabauer M, Orban M, et al. Transcatheter treatment of severe tricuspid regurgitation using the edge-to-edge repair technique. EuroIntervention. 2017;12:e1837–44. doi: 10.4244/EIJ-D-16-00949. [DOI] [PubMed] [Google Scholar]
- 25.Sintek M, Zajarias A. Patient evaluation and selection for transcatheter aortic valve replacement: the heart team approach. Prog Cardiovasc Dis. 2014;56:572–82. doi: 10.1016/j.pcad.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Deutsch MA, Bleiziffer S, Elhmidi Y, et al. Beyond adding years to life: health-related quality-of-life and functional outcomes in patients with severe aortic valve stenosis at high surgical risk undergoing transcatheter aortic valve replacement. Curr Cardiol Rev. 2013;9:281–94. doi: 10.2174/1573403X09666131202121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CA, Rasania SP, Aflalo J, et al. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160:243–54. doi: 10.7326/M13-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 29.Lefèvre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–57. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkey MC, Lauck SB, Perpetua EM, et al. Transcatheter aortic valve replacement program development: recommendations for best practice. Catheter Cardiovasc Interv. 2014;84:859–67. doi: 10.1002/ccd.25529. [DOI] [PubMed] [Google Scholar]
- 31.Lauck SB, Gibson JA, Baumbusch J, et al. Transition to palliative care when transcatheter aortic valve implantation is not an option: opportunities and recommendations. Curr Opin Support Palliat Care. 2016;10:18–23. doi: 10.1097/SPC.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial) Am J Cardiol. 2015;116:264–9. doi: 10.1016/j.amjcard.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauck S, Garland E, Achtem L, et al. Integrating a palliative approach in a transcatheter heart valve program: bridging innovations in the management of severe aortic stenosis and best end-of-life practice. Eur J Cardiovasc Nurs. 2014;13:177–84. doi: 10.1177/1474515114520770. [DOI] [PubMed] [Google Scholar]
- 34.Miller R. Some valve patients are too sick for TAVI or surgery. Heartwire. 2012 http://www.medscape.com/viewarticle/763271.
- 35.Braun LT, Grady KL, Kutner JS, et al. Palliative care and cardiovascular disease and stroke: a policy statement from the American Heart Association/American Stroke Association. Circulation. 2016;134:e198–225. doi: 10.1161/CIR.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 36.Sagin A, Kirkpatrick JN, Pisani BA, et al. Emerging collaboration between palliative care specialists and mechanical circulatory support teams: a qualitative study. J Pain Symptom Manage. 2016;52:491–7. doi: 10.1016/j.jpainsymman.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Lindvall C, Hultman TD, Jackson VA. Overcoming the barriers to palliative care referral for patients with advanced heart failure. J Am Heart Assoc. 2014;3:e000742. doi: 10.1161/JAHA.113.000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connor SR, Pyenson B, Fitch K, et al. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33:238–46. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 40.Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J. 2010;16:423–35. doi: 10.1097/PPO.0b013e3181f684e5. [DOI] [PubMed] [Google Scholar]
- 41.Jordhøy MS, Fayers P, Loge JH, et al. Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol. 2001;19:3884–94. doi: 10.1200/JCO.2001.19.18.3884. [DOI] [PubMed] [Google Scholar]
- 42.Diop MS, Rudolph JL, Zimmerman KM, et al. Palliative Care Interventions for patients with Heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20:84–92. doi: 10.1089/jpm.2016.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleland JG, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 44.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–5. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 46.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–52. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swetz KM, Freeman MR, AbouEzzeddine OF, et al. Palliative medicine consultation for preparedness planning in patients receiving left ventricular assist devices as destination therapy. Mayo Clin Proc. 2011;86:493–500. doi: 10.4065/mcp.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Medicare and Medicaid Services. National Coverage determination (NCD) for Ventricular Assist Devices. [accessed 12 Dec 2016];2015 www.cms.gov/Regulationsand-AvailableGuidance/Guidance/Manuals/downloads/ncd103c1_part1.pdf.
- 49.The Joint Commission. Certification for ventricular assist devices. [accessed 12 Dec 2016];2016 https://www.jointcommission.org/certifcation/ventricular_assist_device.aspx.
- 50.O'Connor NR, Moyer ME, Kirkpatrick JN. Scripted nurse visits: a resource-efficient palliative care model for ventricular assist devices. J Palliat Med. 2016;19:1312–5. doi: 10.1089/jpm.2016.0065. [DOI] [PubMed] [Google Scholar]


