Abstract
African lungfishes are ammonotelic in water. They can aestivate for long periods on land during drought. During aestivation, the gills are covered with dried mucus and ammonia excretion ceases. In fishes, ammonia excretion through the gills involves Rhesus glycoproteins (RhGP/Rhgp). This study aimed to obtain the complete cDNA coding sequences of rhgp from the gills of Protopterus annectens, and to determine their branchial mRNA and protein expression levels during the induction, maintenance and arousal phases of aestivation. Three isoforms of rhgp (rhag, rhbg and rhcg) were obtained in the gills of P. annectens. Their complete cDNA coding sequences ranged between 1311 and 1398 bp, coding for 436 to 465 amino acids with estimated molecular masses between 46.8 and 50.9 kDa. Dendrogramic analyses indicated that Rhag was grouped closer to fishes, while Rhbg and Rhcg were grouped closer to tetrapods. During the induction phase, the protein abundance of Rhag, but not its transcript level, was down-regulated in the gills, suggesting that there could be a decrease in the release of ammonia from the erythrocytes to the plasma. Furthermore, the branchial transcript levels of rhbg and rhcg decreased significantly, in preparation for the subsequent shutdown of gill functions. During the maintenance phase, the branchial expression levels of rhag/Rhag, rhbg/Rhbg and rhcg/Rhcg decreased significantly, indicating that their transcription and translation were down-regulated. This could be part of an overall mechanism to shut down branchial functions and save metabolic energy used for transcription and translation. It could also be regarded as an adaptive response to stop ammonia excretion. During the arousal phase, it is essential for the lungfish to regain the ability to excrete ammonia. Indeed, the protein abundance of Rhag, Rhbg and Rhcg recovered to the corresponding control levels after 1 day or 3 days of recovery from 6 months of aestivation.
Introduction
Lungfishes are an archaic group of Sarcopterygian fishes that possess lung opening off the ventral side of the oesophagus. To date, there are six species of extant lungfish worldwide, including Protopterus annectens, P. aethiopicus, P. amphibicus and P. dolloi from Africa. African lungfishes are obligate air-breathers living in fresh water. During drought, they can aestivate in subterranean mud cocoon for up to ~4 years [1–3]. In the laboratory, African lungfishes can be induced to aestivate in completely dried mucus cocoons in plastic boxes [4–6].
Aestivation involves corporal torpor at high environmental temperature without food or water intake for an extended period [1,3]. There are three phases of aestivation: induction, maintenance, and arousal. During the induction phase, the aestivating lungfish detects environmental cues and turns them into internal signals that will initiate the necessary changes to prepare for the maintenance phase. Within 6–8 days, it hyperventilates and secretes mucus which turns into a dry cocoon. During the maintenance phase, the lungfish is completely encased in a cocoon with no feeding and movement. During this period, the lungfish has to prevent cell death, preserve biological structures and sustain a slow rate of waste production to avoid pollution of the internal environment. The lungfish can be aroused from aestivation by the addition of water. Upon arousal, it has to excrete the accumulated waste products and feed for repair and growth. Feeding begins approximately 7–10 days after arousal, and the fish grows and develops as normal thereafter.
Despite being ureogenic [7–9], African lungfishes are ammonotelic in water [10]. They possess a full complement of ornithine-urea cycle enzymes, including glutamine synthetase, carbamoyl phosphate synthetase III (Cps III), ornithine transcarbamylase, argininosuccinate synthase (Ass), argininosuccinate lyase (Asl) and arginase in their livers [6,11,12]. Exposure to terrestrial conditions leads to decreases in ammonia and urea excretion in the African lungfishes, a phenomenon attributable presumably to a lack of water to flush the branchial and cutaneous surfaces [10]. However, ammonia is not just passively retained in the lungfish during emersion; it is converted into urea in the liver and urea is accumulated in the body instead. It was previously reported that aerial exposure led to an increase in the hepatic ornithine-urea cycle capacity, with significant increases in the activities of Cps III (3.8-fold), Ass + Asl (1.8-fold) and glutamine synthetase (2.2-fold) [4]. Furthermore, there were decreases in the rate of ammonia production and increases in the rate of urea synthesis in P. dolloi during 6 days of aerial exposure or 40 days of aestivation in a dried mucus cocoon [4,10]. It has been postulated that the urea accumulated during the induction phase can act as an internal cue for aestivation [13]. Hence, it is possible that African lungfishes down-regulate the transport mechanisms of ammonia and urea in its body surfaces, especially the gills, during the induction phase. During the maintenance phase, urea accumulation continues, indicating the continuous but impeded mobilization of endogenous proteins/amino acids [4,14,15]. The gills become physiologically nonfunctional as they are covered with a thick layer of dried mucus [16], but whether there is a shutdown of branchial function through down-regulation of transporters/channels, including those involved in ammonia and urea excretion, is uncertain. Upon arousal from aestivation, the lungfish rapidly excretes the accumulated urea [1] and revert back to become ammonotelic thereafter. Hence, there must be a restoration of expression of nitrogenous waste transporters in the gills during the arousal phase.
Rhesus glycoproteins (RhGP/Rhgp) are members of the solute transporter family SLC42 that play an important role in transmembrane ammonia transport [17,18]. There are four types of RhGP, three of which, namely Rh-associated glycoprotein (RhAG), Rh family B glycoprotein (RhBG) and Rh family C glycoprotein (RhCG), are glycosylated. The fourth type, Rh30, lacks glycosylation and is found to be associated with RhAG in erythrocytic membranes. For most ammonotelic fishes, ammonia is mainly excreted across the branchial epithelium as NH3 down a favourable blood-to-water diffusion gradient [19–21]. Wright and Wood [22] proposed a model for ammonia excretion in freshwater fishes and its variable connection to Na+ uptake and acid excretion. In this model, Rhag facilitates NH3 flux out of the erythrocyte, Rhbg moves it across the basolateral membrane of the branchial ionocyte, and an apical “Na+/NH4+ exchange complex” consisting of several membrane transporters (Rhcg, vacuolar-type H+-ATPase, Na+/H+ exchanger, Na+ channel) working together as a metabolon to provide an acid trapping mechanism for apical excretion. This model incorporates the premise that Rhgp function as ammonia channels, binding NH4+ but facilitating the diffusion of NH3 [23].
At present, majority of reports in the literature focused predominantly on rhgp/Rhgp in the gills of teleosts and there is no information on rhgp/Rhgp in lungfishes. Therefore, this study was undertaken to sequence the cDNA coding region of rhgp isoforms from the gills of P. annectens. It was hoped that the deduced Rhgp amino acid sequences would shed light on the phylogenetic relationships between P. annectens and other animals. Efforts were made to determine, using quantitative real-time polymerase chain reaction (qPCR), the mRNA expression levels of various rhgp in the gills of P. annectens after 6 days (the induction phase) or 6 months (the maintenance phase) of aestivation, or after 1 day or 3 days of arousal (the arousal phase) from 6 months of aestivation. Based on the deduced Rhgp sequences, custom-made anti-Rhgp antibodies were developed for the determination of the protein abundance of Rhgp in the gills of P. annectens through Western blotting. It was hypothesized that the branchial mRNA and protein expression of rhgp/Rhgp would be down-regulated to suppress ammonia excretion during the induction phase, and also to shut down branchial functions and thwart ammonia excretion during the maintenance phase. Furthermore, it was hypothesized that the branchial mRNA and protein expression of rhgp/Rhgp would be restored in order to regain the ability of ammonia excretion through the gills upon arousal from aestivation.
Note on abbreviations
Two different types of abbreviations were adopted in this report because there are differences between the standard abbreviations of genes/proteins of fishes (http://zfin.org/cgi-bin/webdriver?MIval=aa-ZDB_home.apg) and those of frogs and human/non-human primates (http://www.genenames.org). For fishes, gene symbols are italicized, all in lower case, and protein designations are the same as the gene symbol, but not italicized with the first letter in upper case.
Materials and methods
Animals
This study was performed on the African lungfish P. annectens (Order Lepidosireniformes; Family Protopteridae). Specimens of P. annectens (80–150 g body mass) were collected from Central Africa and imported through a local fish farm in Singapore (Qian Hu Fish Farm Trading Co, Singapore). They were maintained in plastic aquaria filled with dechlorinated tap water at 25°C in the laboratory. Water was changed daily. No attempt was made to separate the sexes. Fish were acclimated to laboratory conditions for at least two weeks. During the acclimatization period, fish were fed with frozen fish meat.
Ethics statement
The Institutional Animal Care and Use Committee (IACUC) of the National University of Singapore has specifically approved this study and the procedures used in this study (IACUC 035/09). All efforts were made to minimize the suffering of the lungfish.
Experimental conditions and collection of samples
Food was withdrawn 96 h before experiments (day 0) for both control and aestivating lungfish, which gave sufficient time for the gut to be emptied of all food and waste. Lungfish (N = 5) kept in fresh water served as controls and were killed with an overdose of neutralized 0.05% MS222 for tissue sampling. Following the procedure of Chew et al. [4], some lungfish were induced to aestivate at 27–29°C and 85–90% humidity individually in plastic tanks (L29 cm x W19 cm x H17.5 cm) containing 15 ml dechlorinated tap water (made 0.3‰ with seawater). It took approximately 6 days for the lungfish to be encased in a brown dried mucus cocoon and these 6 days were counted as part of the aestivation period. The lungfish were allowed to aestivate for 6 months. In order to maintain a high humidity (>90%) within the tank, 1–2 ml of water was sprayed onto the side of the tank daily. After 6 months of aestivation, some lungfish were aroused by adding 200 ml of water into the tank and breaking up the cocoon manually. After a few minutes, the lungfish would swim sluggishly in the water; another 800 ml of water was added to cover the lungfish. Some lungfish were pithed after a blow to the head for tissue sampling after 6 days (the induction phase), or after 6 months (the maintenance phase) of aestivation (N = 5 for each group). Some lungfish were killed with an overdose of neutralized 0.05% MS222 after 1 or 3 days of arousal from 6 months of aestivation (the arousal phase) without food (N = 5 for each group). The eye, brain, gills, heart, liver, spleen, pancreas, gut, kidney, lung, muscle and skin were quickly excised and freeze-clamped with aluminum tongs pre-cooled in liquid nitrogen.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from all the samples of P. annectens using Tri Reagent (Sigma-Aldrich Co., St. Louis, MO, USA), and purified using the Qiagen RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). RNA was quantified spectrophotometrically using a BioSpec-nano (Shimadzu, Tokyo, Japan). The integrity of RNA was assessed electrophoretically and gauged by the ratio of 28S/18S rRNA. For polymerase chain reaction (PCR), first strand cDNA synthesis was performed using 4 μg of total RNA, oligo(dT)18 primer and the RevertAid first strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). For rapid amplification of cDNA ends (RACE), 5’-RACE-Ready cDNA and 3’-RACE-Ready cDNA were synthesized from 1 μg of total RNA using SMARTer RACE cDNA Amplification kit (Clontech Laboratories, Mountain View, CA, USA).
For qPCR, Qiagen RNeasy Plus Mini Kit (Qiagen GmbH), which contains gDNA Eliminator spin column (Qiagen GmbH) to remove genomic DNA, was used to purify the RNA from the gills of P. annectens. First strand cDNA synthesis was performed using 4 μg of total RNA, random hexamer primers and RevertAid first strand cDNA synthesis kit (Thermo Fisher Scientific).
PCR and RACE
Partial sequences of rhag, rhbg and rhcg were obtained using gene-specific primers (S1 Table) designed from the highly conserved regions based on multiple alignments of the respective sequences from various fish species available in Genbank (http://www.ncbi.nlm.nih.gov/Genbank/). PCR and RACE was performed, following the procedure of Chng et al. [24], using the respective gene-specific PCR and RACE primers (S1 Table). PCR products were separated by gel electrophoresis. Bands of estimated molecular masses were excised and purified by FavorPrep Gel Purification Mini Kit (Favorgen Biotech Corp., Ping Tung, Taiwan). Sequencing was performed by a 3130XL Genetic Analyzer (Life Technologies Corporation, Carlsbad, California), using BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies Corporation). Multiple sequencing was performed in both 3’ and 5’ directions to obtain the full coding sequence. Sequence assembly and analysis were performed using Bioedit v7.1.3 [25]. The complete coding cDNA sequences of rhag, rhbg and rhcg from P. annectens have been deposited to Genbank with accession numbers KX583645, KX583646 and KX583647, respectively.
In order to detect the mRNA expression levels of each gene in various tissues, PCR was also performed on the cDNAs of eye, brain, gills, heart, liver, spleen, pancreas, gut, kidney, lung, muscle, and skin of P. annectens following the procedure of Chng et al. [24].
Deduced amino acid sequences and dendrogramic analyses
The deduced amino acid sequences of Rhag, Rhbg and Rhcg were translated from the corresponding nucleotide sequences using ExPASy Proteomic server (http://web.expasy.org/translate/). The deduced amino acid sequences were aligned and compared with selected Rhag/RhAG, Rhbg/RhBG and Rhcg/RhCG from various animal species using BioEdit. The transmembrane domains of Rhag, Rhbg and Rhcg of P. annectens were identified using MEMSAT3 and MEMSAT-SVM provided by PSIPRED protein structure prediction server (http://bioinf.cs.ucl.ac.uk/psipred/) [26]. Potential phosphorylation sites were identified using NetPhos 2.0, and potential N-glycosylation sites were identified using NetNGlyc 1.0.
The sequences of Rhag, Rhbg and Rhcg were aligned using ClustalX2 and dendrogramic analyses were performed using neighbor-joining method and 100 bootstrap replicates with Phylip [27]. The accession numbers of selected amino acid sequences of Rhag/RhAG, Rhbg/RhBG and Rhcg/RhCG (from GenBank) used in the dendrogramic analyses are indicated in S2, S3 and S4 Tables, respectively.
qPCR
In this study, the method of relative quantification (2-ΔΔCt) was adopted, whereby the mRNA expression levels of target genes were compared with that of a reference gene within the same sample [28]. The 2-ΔΔCt equation, including the assumptions and validation tests [28], were used to validate the reference gene and analyze the qPCR data. The reference gene adopted for 2-ΔΔCt analysis was sec62 homolog, preprotein translocation factor (sec62). The mRNA expression of rhag, rhbg or rhcg were first normalized to the mRNA expression of sec62 and then compared with the control lungfish and expressed as 2-ΔΔCt. Logarithmic transformation (log2) of the mean fold-change values were used for statistical analyses.
qPCR was performed in duplicates using a StepOnePlus Real-Time PCR System (Life Technologies Corporation), following the procedure of Chng et al. [24]. The mRNA expression levels of rhag, rhbg, rhcg and sec62 were determined using gene-specific qPCR primers (S1 Table). The qPCR reactions contained 5 μl of KAPA SYBR FAST Master Mix (2X) ABI Prism (Kapa Biosystems, Woburn, MA, USA), 0.3 μmol l-1 of forward and reverse qPCR primers each and 1 ng of sample cDNA in a total volume of 10 μl. The PCR efficiencies for rhag, rhbg, rhcg and sec62 were 101.5%, 95.3%, 93.3% and 97.1%, respectively.
SDS-PAGE and Western blotting
A commercial firm (GenScript, Piscataway, NJ, USA) was engaged to raise a rabbit polyclonal antibody against aa 198‒211 (CYRSGLRNGHDNEGS) of Rhag, a rabbit polyclonal antibody against aa 30‒43 (FVRYDKETDPKEWH) of Rhbg and a rabbit polyclonal antibody against aa 29‒42 (IRYSDEADAARWPE) of Rhcg from the gills of P. annectens. Immunoreactive bands of Rhag, Rhbg and Rhcg were visualized at the expected molecular mass of 46.8 kDa, 50.9 kDa and 50.9 kDa, respectively.
Recombinant proteins of Rhag, Rhbg and Rhcg from P. annectens were synthesized from 1 μg of complete coding cDNA fragments of rhag, rhbg and rhcg, respectively, using 1-Step Human Coupled IVT Kit—DNA (Thermo Fisher Scientific) according to manufacturer’s instruction. The recombinant proteins were used to verify the specificity of the anti-Rhag, anti-Rhbg and anti-Rhcg antibodies. The complete coding cDNA fragments of rhag, rhbg and rhcg were generated by PCR (IVT) primers (S1 Table). The reaction mixtures were incubated at 30°C for 6 h and diluted with Laemmli buffer. The recombinant proteins were heated at 70°C for 15 min, and then kept at -80°C until analysis.
Following the procedure of Chng et al. [24], western blotting was performed on the recombinant proteins of Rhag, Rhbg and Rhcg, and the gill samples obtained from the control fish and fish that had undergone 6 days or 6 months of aestivation, or 1 or 3 days of arousal from 6 months of aestivation. The concentrations of the anti-Rhag, anti-Rhbg and anti-Rhcg antibodies used for Western blotting were 2 μg ml−1. The protein loads of the gill samples for gel separation were 100 μg for Rhag, 100 μg for Rhbg and 50 μg for Rhcg. Aestivation involves a complex interplay between up-regulation and down-regulation of diverse cellular activities [29], whereby many genes and/or proteins clusters are strategically up-regulated or down-regulated to meet the challenges associated with aestivation. At present, we were unable to identify an appropriate reference protein, the expression of which would be unaffected throughout the three phases of aestivation, for Western blotting. Hence, results were expressed as arbitrary densitometric unit per μg protein, i.e. with reference to the total protein abundance, which has been reported previously for several other proteins from P. annectens during aestivation [24,29–34].
Statistical analyses
Results were presented as means ± standard errors of the mean (S.E.M.). Statistical analyses were performed using SPSS version 18 (SPSS Inc, Chicago, USA). Homogeneity of variance was checked using Levene’s Test. Differences between means were tested using one-way analysis of variance followed by multiple comparisons of means by either the Tukey or Dunnett T3 post-hoc test, depending on the homogeneity of variance of the data set. Differences with P<0.05 were reported as statistically significant.
Results
The nucleotide and the deduced amino acid sequences of rhag/Rhag, rhbg/Rhbg and rhcg/Rhcg
The complete coding cDNA sequences of rhag, rhbg and rhcg had been obtained from the gills of P. annectens. The coding sequence of rhag (accession: KX583645) consisted of 1311 bp, coding for 436 amino acids with an estimated molecular mass of 46.8 kDa, and that of rhbg (accession: KX583646) consisted of 1398 bp, coding for 465 amino acids with an estimated molecular mass of 50.9 kDa. For rhcg, the coding sequence (accession: KX583647) consisted of 1386 bp, coding for 461 amino acids with an estimated molecular mass of 50.9 kDa.
A hydropathy analysis demonstrated that the deduced amino acid sequences of Rhag, Rhbg and Rhcg of P. annectens comprised 12 transmembrane regions. Efforts were made to compare the three Rhgp of P. annectens with Escherichia coli ammonia transporter (AmtB), as its high resolution crystal structure had been resolved and based on which theories of NH3/NH4+ by Rhgp had been formulated [35,36].
An alignment of Rhag from P. annectens with E. coli AmtB and Rhag/RhAG from human, mouse, frog and fish revealed highly conserved residues involved in gating of the channel (F148 and F256), NH4+ binding (G198, H204, F256 and N257) and deprotonation of NH4+ for NH3 conduction (D196, S200, H204 and H366, Fig 1). Rhag of P. annectens lacked the potential π cation binding sites of E. coli AmtB (Fig 1). There were 13 potential phosphorylation sites and 2 N-glycosylation sites in Rhag of P. annectens (Fig 1). A comparison of Rhag of P. annectens with Rhag/RhAG of other animal species indicated that it had the highest sequence similarity with amphibian Rhag (70.1–73.1%), followed by those of actinopterygians (69.1–71.7%) and mammals (59.6–64.9%; S5 Table).
Fig 1. Molecular characterization of Rhesus blood group-associated glycoprotein (Rhag) from the gills of Protopterus annectens.
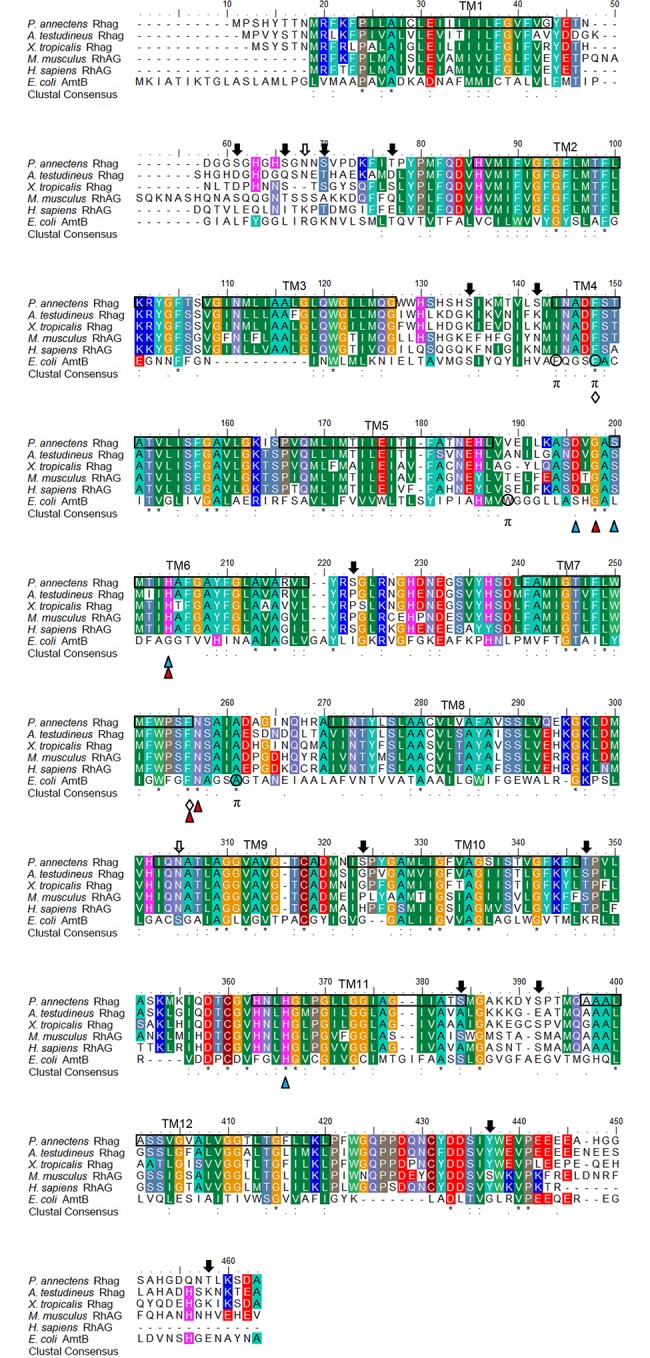
A multiple amino acid alignment of Rhag from Protopterus annectens with Anabas testudineus Rhag (AIC81181.1), Xenopus (Silurana) tropicalis Rhag (XP_002933645.2), Mus musculus RhAG (AAI01942.1), Homo sapiens RhAG (NP_000315.2) and Escherichia coli ammonia transporter (AmtB; NP_414985.1). Identical amino acid residues are indicated by asterisks, strongly similar amino acids are indicated by colons and weakly similar amino acids are indicated by periods. Residues involved in NH4+ binding and deprotonation of NH4+ for NH3 conduction are indicated by red and blue triangles, respectively. The phenylalanine gate is indicated by open diamonds. The π cation binding sites of E. coli AmtB are indicated by “π”. Potential N-glycosylation and phosphorylation sites are indicated by open and shaded arrows, respectively. The predicted transmembrane domains (TM1‒12) of Rhag of P. annectens are indicated by open boxes and were predicted using MEMSATS and MEMSAT-SVA provided by PSIPRED protein structure prediction server.
An alignment of Rhbg of P. annectens with E. coli AmtB and Rhbg/RhBG from human, mouse, frog and two fishes (Squalus acanthias and Anabas testudineus) showed that the residues involved in gating of the channel (F140 and F251), NH4+ binding (G196, H201, F251 and N252) and deprotonation of NH4+ for NH3 conduction (D193, S197, H201 and H360) were highly conserved (Fig 2). Rhbg of P. annectens lacked the potential π cation binding sites of E. coli AmtB (Fig 2). There were 15 potential phosphorylation sites and 2 N-glycosylation sites present in Rhbg of P. annectens (Fig 2). A comparison of Rhbg of P. annectens with Rhbg/RhBG of other animal species indicated that it had the highest sequence similarity with chondrichthyes Rhbg (64.9–69.2%), followed by those of actinopterygians (63.5–69.0%), amphibians (66.4–68.2%) and mammals (49.4–58.1%; S6 Table).
Fig 2. Molecular characterization of Rhesus family B glycoprotein (Rhbg) from the gills of Protopterus annectens.

A multiple amino acid alignment of Rhbg from Protopterus annectens with Squalus acanthias Rhbg (AJF44128.1), Anabas testudineus Rhbg (AIC81182.1), Xenopus (Silurana) tropicalis Rhbg (AAU89493.1), Mus musculus RhBG (AAF19371.1), Homo sapiens RhBGA (NP_000315.2) and Escherichia coli ammonia transporter (AmtB; NP_414985.1). Identical amino acid residues are indicated by asterisks, strongly similar amino acids are indicated by colons and weakly similar amino acids are indicated by periods. Residues involved in NH4+ binding and deprotonation of NH4+ for NH3 conduction are indicated by red and blue triangles, respectively. The phenylalanine gate is indicated by open diamonds. The π cation binding sites of E. coli AmtB are indicated by “π”. Potential N-glycosylation and phosphorylation sites are indicated by open and shaded arrows, respectively. The predicted transmembrane domains (TM1‒12) of Rhbg of P. annectens are indicated by open boxes and were predicted using MEMSATS and MEMSAT-SVA provided by PSIPRED protein structure prediction server.
An alignment of Rhcg of P. annectens with E. coli AmtB and Rhcg/RhCG from human, mouse, frog and two fishes (Callorhinchus milii and A. testudineus) revealed that the residues involved in gating of the channel (F147 and F258) and NH4+ binding (G202, H208, F258 and N259) were conserved (Fig 3). The residues involved in the deprotonation of NH4+ for NH3 conduction (D200, H208 and H368) are highly conserved, except for a replacement of T204 in P. annectens Rhcg with S204 in human RhCG (Fig 3). Rhcg of P. annectens lacked the potential π cation binding sites of E. coli AmtB (Fig 3). There were 15 potential phosphorylation sites and 2 N-glycosylation sites in Rhcg of P. annectens (Fig 3). A comparison of Rhcg of P. annectens with Rhcg/RhCG of other animal species indicated that it had the highest sequence similarity with actinopterygian Rhcg (60.2–65.7%), followed by those of C. milii (64.2%), amphibians (63.4–64.1%) and mammals (55.4–61.5%; S7 Table).
Fig 3. Molecular characterization of Rhesus family C glycoprotein (Rhcg) from the gills of Protopterus annectens.
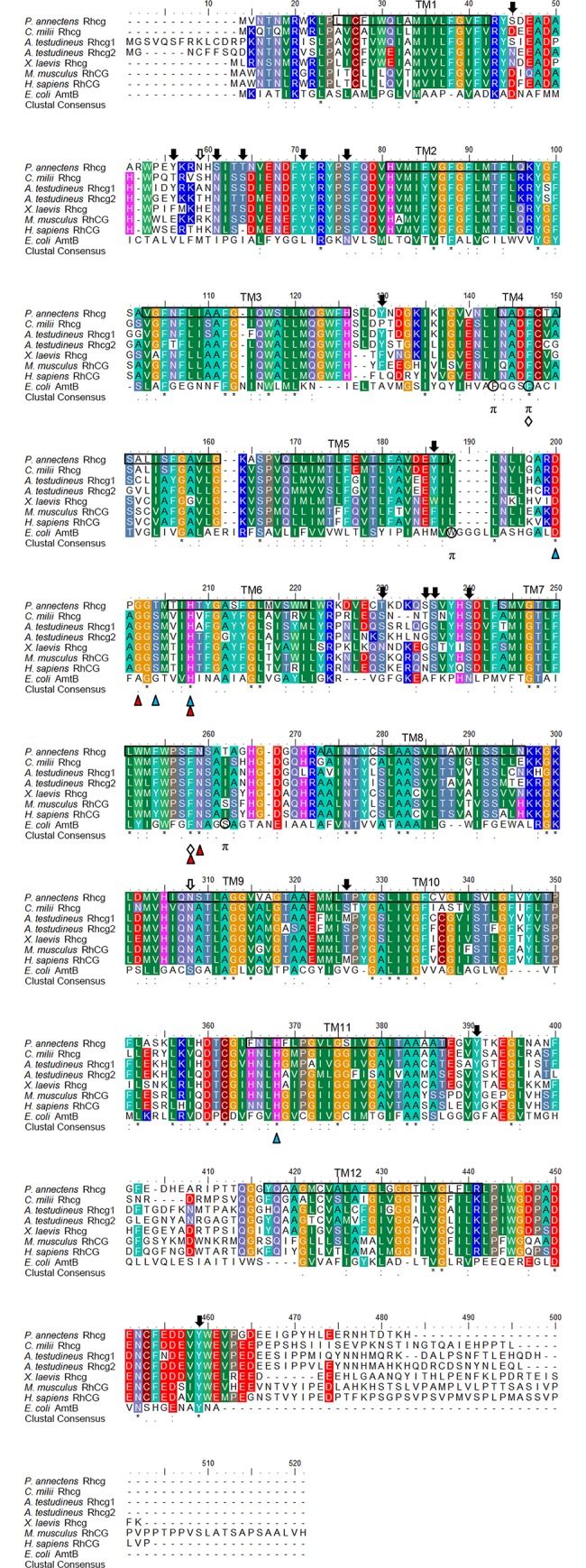
A multiple amino acid alignment of Rhcg from Protopterus annectens with Callorhinchus milii Rhcg (AFO96383.1), Anabas testudineus Rhcg1 (AIC81183.1) and Rhcg2 (AIC81184.1), Xenopus laevis Rhcg (NP_001088553.1), Mus musculus RhCG (AAF19373.1), Homo sapiens RhCG (AAF19372.1) and Escherichia coli ammonia transporter (AmtB; NP_414985.1). Identical amino acid residues are indicated by asterisks, strongly similar amino acids are indicated by colons and weakly similar amino acids are indicated by periods. Residues involved in NH4+ binding and deprotonation of NH4+ for NH3 conduction are indicated by red and blue triangles, respectively. The phenylalanine gate is indicated by open diamonds. The π cation binding sites of E. coli AmtB are indicated by “π”. Potential N-glycosylation and phosphorylation sites are indicated by open and shaded arrows, respectively. The predicted transmembrane domains (TM1‒12) of Rhcg of P. annectens are indicated by open boxes and were predicted using MEMSATS and MEMSAT-SVA provided by PSIPRED protein structure prediction server.
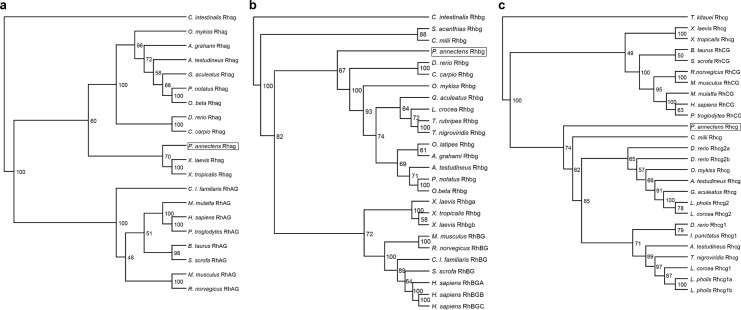
The dendrogramic analyses of Rhag, Rhbg and Rhcg
Rhag of P. annectens was grouped together with amphibian Rhag, separated from the mammal and actinopterygian clades (Fig 4A). By contrast, Rhbg and Rhcg of P. annectens were grouped together with actinopterygian Rhbg (Fig 4B) and Rhcg (Fig 4C), respectively, separated from the tetrapods.
Fig 4.
Dendrograms of (a) Rhesus blood group-associated glycoprotein (Rhag/RhAG), (b) Rhesus family B glycoprotein (Rhbg/RhBG) and (c) Rhesus family C glycoprotein (Rhcg/RhCG) including those of Protopterus annectens. Numbers presented at each branch point represent bootstrap values from 100 replicates. Ciona intestinalis Rhag and Rhbg and Thelohanellus kitauei Rhcg are used as outgroups for their respective dendrogram.
The mRNA expression levels of rhag, rhbg and rhcg in various tissues/organs of P. annectens
For P. annectens kept in fresh water, rhag was expressed in the gills (Fig 5A), rhbg was expressed in the gills, liver and kidney (Fig 5B), and rhcg was expressed in the gills and kidney (Fig 5C).
Fig 5.
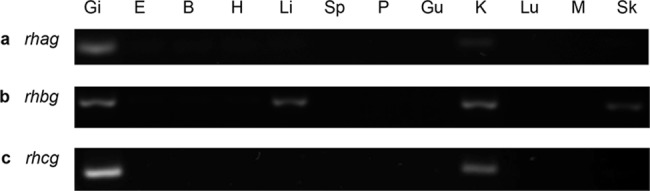
mRNA expression of (a) rhesus blood group-associated glycoprotein (rhag), (b) rhesus family B glycoprotein (rhbg) and (c) rhesus family C glycoprotein (rhcg) in various tissues/organs of Protopterus annectens. The mRNA expression of rhag, rhbg and rhcg were examined in the gills (Gi), eyes (E), brain (B), heart (H), liver (Li), spleen (Sp), pancreas (P), gut (Gu), kidney (K), Lung (Lu), muscle (M) and skin (Sk) of P. annectens kept in fresh water.
Effects of aestivation on the mRNA expression levels of rhag, rhbg and rhcg in the gills of P. annectens
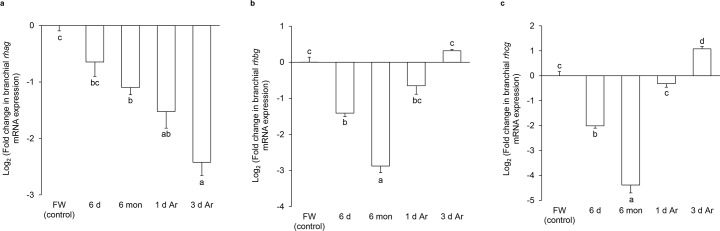
In order to determine the mRNA expression levels of rhag, rhbg and rhcg in the gills of P. annectens during the three phases of aestivation, qPCR based on the ΔΔCt method was performed. The mRNA expression level of rhag remained unchanged in the gills of P. annectens after 6 days of aestivation as compared with the control. However, it decreased significantly after 6 months (by 53%) of aestivation, or after 1 day (by 65%) or 3 days (by 81%) of arousal from 6 months of aestivation (Fig 6A).
Fig 6. mRNA expression levels of rhesus glycoproteins in the gills of Protopterus annectens.
Relative mRNA expression (determined using qPCR and calculated based on 2-ΔΔCT) of (a) rhesus blood group-associated glycoprotein (rhag), (b) rhesus family B glycoprotein (rhbg) and (c) rhesus family C glycoprotein (rhcg) in the gills of Protopterus annectens kept in fresh water on day 0 (FW; control), after 6 days (d; the induction phase) or 6 months (mon; the maintenance phase) of aestivation, or after 1 or 3 d of arousal (Ar; the arousal phase) from 6 mon of aestivation. Results represent means ± S.E.M (N = 5). Means not sharing the same letter are significantly different (P < 0.05).
The branchial mRNA expression level of rhbg decreased significantly after 6 days (by 62%) or 6 months (by 86%) of aestivation, but returned to the control level after 1 day of arousal (Fig 6B).
As for rhcg, its mRNA expression level decreased significantly in the gills of P. annectens after 6 days (by 75%) or 6 months (by 95%) of aestivation as compared to control fish (Fig 6C), but it returned to the control level after 1 day of arousal and became significantly higher (2.1-fold) than the control after 3 days of arousal from 6 months of aestivation (Fig 6C).
Effects of aestivation on the protein abundance of Rhag, Rhbg and Rhcg in the gills of P. annectens
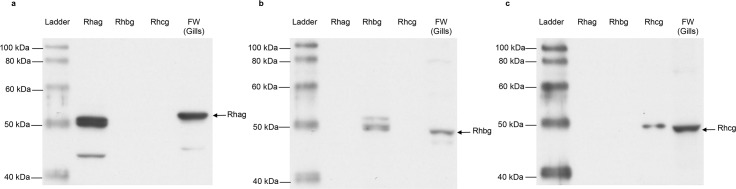
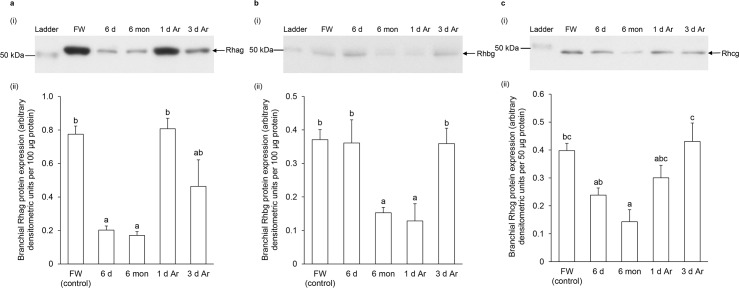
Western blotting using the custom-made anti-Rhag antibody revealed a band at ~50 kDa which is close to the estimated molecular mass of the deduced Rhag sequence of P. annectens (Fig 7A). The anti-Rhag antibody recognized only Rhag recombinant protein and showed no cross-reactivity with Rhbg and Rhcg recombinant proteins (Fig 7A), thus validating the antibody specificity to Rhag. The protein abundance of Rhag decreased significantly in the gills of P. annectens after 6 days (74%) or 6 months (by 78%) of aestivation, but returned to the control level after 1 day or 3 days of arousal from 6 months of aestivation (Fig 8A).
Fig 7. Validation of anti-Rhesus glycoproteins antibodies from Protopterus annectens.
Immunoblots incubated with (a) anti-Rhesus blood group-associated glycoprotein (Rhag), (b) anti-Rhesus family B glycoprotein (Rhbg) and (c) anti-Rhesus family C glycoprotein (Rhcg) antibodies. Recombinant proteins of Rhag, Rhbg, Rhcg and the protein from the gills of Protopterus annectens kept in fresh water on day 0 (FW) were loaded for gel separation.
Fig 8. Protein abundance of Rhesus glycoproteins in the gills of Protopterus annectens.
Protein abundance of (a) Rhesus blood group-associated glycoprotein (Rhag), (b) Rhesus family B glycoprotein (Rhbg) and (c) Rhesus family C glycoprotein (Rhcg) in the gills of Protopterus annectens kept in fresh water on day 0 (FW; control), or after 6 days (d; the induction phase) or 6 months (mon; the maintenance phase) of aestivation, or after 1 or 3 d of arousal (Ar; the arousal phase) from 6 mon of aestivation. (i) Examples of immunoblot of Rhag, Rhbg or Rhcg. (ii) The protein abundance of Rhag, Rhbg or Rhcg expressed as arbitrary densitometric units per 100 μg, 100 μg or 50 μg protein, respectively. Results represent mean ± S.E.M. (N = 4). Means not sharing the same letter are significantly different (P<0.05).
Western blotting using the custom-made anti-Rhbg antibody revealed a band at ~48 kDa which is close to the estimated molecular mass of the deduced Rhbg sequence of P. annectens (Fig 7B). The anti-Rhbg antibody recognized only Rhbg recombinant protein and showed no cross-reactivity with Rhag and Rhcg recombinant proteins (Fig 7B), thus validating the antibody specificity to Rhbg. The protein abundance of Rhbg remained unchanged in the gills of P. annectens after 6 days of aestivation, but decreased significantly after 6 months (by 59%) of aestivation, or after 1 day (by 65%) of arousal from 6 months of aestivation (Fig 8B). The branchial protein abundance of Rhbg returned to the control level on day 3 of arousal (Fig 8B).
Western blotting using the custom-made anti-Rhcg antibody revealed a band at ~48 kDa which is close to the estimated molecular mass of the deduced Rhcg sequence of P. annectens (Fig 7C). The anti-Rhcg antibody recognized only Rhcg recombinant protein and showed no cross-reactivity with Rhag and Rhbg recombinant proteins (Fig 7C), thus validating the antibody specificity to Rhcg. The protein abundance of Rhcg remained unchanged in the gills of P. annectens after 6 days of aestivation (Fig 8C). However, it decreased significantly (by 64%) after 6 months of aestivation as compared to control fish, and returned to the control level after 1 day or 3 days of arousal (Fig 8C).
Discussion
Molecular characterization of Rhgp of P. annectens
Rhag, Rhbg and Rhcg of P. annectens comprise 12 transmembrane domains, which are in agreement with the typical topology of fish Rhgp and mammalian RhGP [37–39]. Four structural motifs involved in (1) the gating of the channel, (2) deprotonation of NH4+ and NH3 conduction, (3) NH4+ binding and (4) π cation binding have been identified in E. coli AmtB and H. sapiens RhCG [35,40,41]. In AmtB of E. coli, F107 and F215 (corresponding to F130 and F235 in human RhCG) are involved in the gating of the channel which supply π-cation interaction for NH4+ [40]. Furthermore, transient movement of side chains of F130 and F235 in human RhCG is necessary for ammonia conductance [35]. For the three Rhgp from P. annectens, both phenylalanine residues (F148 and F256 in Rhag, F140 and F251 in Rhbg, and F147 and F258 in Rhcg) were conserved. In human RhCG, D177, S181, H185 and H344 are involved in the deprotonation of NH4+ for NH3 conduction [40,41]. These residues are conserved in Rhag and Rhbg of P. annectens, but for Rhcg of P. annectens, S181 in human RhCG is replaced with threonine. Baday et al. [41] proposed that S181 was not directly involved in the proton transfer but it played a structural role by maintaining D177 in position, allowing for the formation of a stable water chain between D177 and H185. Therefore, the change of S181 in human RhCG to threonine in Rhcg of P. annectens should not affect the deprotonation of NH4+ for NH3 conduction. Furthermore, based on molecular dynamics simulation, G179, H185, F235 and N236 in human RhCG are involved in NH4+ binding [41], and these four residues are conserved in the three Rhgp of P. annectens. F103, F107 and W148 are the π cation binding sites in E. coli AmtB. F107 in E. coli AmtB is conserved in the three Rhgp of P. annectens, but F103 and W148 in E. coli AmtB are replaced with isoleucine and valine in all the Rhgp of P. annectens, respectively. Notably, E. coli AmtB-W148L mutant increases the flux of NH4+ significantly with dependency on the electrochemical potential gradient across the membrane as compared to wild type AmtB [42]. If indeed Rhgp of P. annectens transport NH4+, the replacement of W148 in E. coli AmtB with smaller non-polar amino acids might potentially increase the flux of NH4+. Taken together, the four structural motifs are conserved in Rhgp of P. annectens, with minor amino acid substitutions at selected positions. The results indicate that Rhgp of P. annectens can deprotonate NH4+ and mediate the transport of NH3 through a hydrophobic pore. However, based on molecular characterization alone, a definite conclusion on the function of the Rhgp cannot be drawn [43,44]. Therefore, experimental testing remains essential to examine the function of the Rhgp of P. annectens and efforts should be made in the future to elucidate whether Rhgp of P. annectens could transport NH3 and/or NH4+.
Dendrogramic analyses of Rhgp
Dendrogramic analyses indicated that Rhag was grouped closer to fish, while Rhbg and Rhcg were grouped closer to tetrapods. When taken together with the phylogenetic information based on the deduced amino acid sequences of other genes of P. annectens, our results are in agreement with the notion that lungfish is the intermediary form between fish and tetrapods. Since lungfishes are the closest living sister group of land vertebrates, they would logically possess some genes/proteins that are closer to those of other fishes (Cps III, [11]; Ass, [12]) and others that have greater similarity to those of tetrapods (Asl, [12]; L-gulono-γ-lactone oxidase, [30]; Na+/K+-ATPase α-subunit isoforms, [31]; Coagulation factor II and Fibrinogen gamma chain [32]; Betaine-homocysteine S-methyltransferase 1, [33]; Myostatin [34]; Aquaporin 1 and 3 [24]; Urea transporter isoforms [29]).
Huang and Peng [38] reported that Rh homologs cluster in subgroups and suggested that natural selection had acted differently on these subgroups. As a whole, Rhgp/RhGP are highly conserved, but its subgroups vary in divergence rate and sites which could be related to functional specification to species adaptation [45]. In invertebrates, there are negative selection on Rh proteins and a similar degree of sequence identity to the individual vertebrate subgroups, RhBG > RhCG > RhAG > Rh30 [45]. While there is negative selection on epithelial Rh homologs (Rhbg and Rhcg) in vertebrates, positive selection occurs to erythroid Rh proteins (Rh30 and Rhag) [46]. Therefore, erythroid Rh proteins diverge more rapidly compared to epithelial Rh proteins [46], accounting why Rhag of P. annectens is grouped closer to the tetrapods, while Rhbg and Rhcg of P. annectens are grouped closer to the fishes.
Transcriptional and translational regulation of branchial rhgp/Rhgp expression during aestivation
After 6 days of aestivation, there was a significant decrease in the protein abundance of Rhag, but not the transcript level of rhag, in the gills of P. annectens. It is possible that there was a decrease in the translation of Rhag or an increase in its removal. Either way, it is logical to postulate that Rhag was regulated mainly at the protein level in the gills of P. annectens. This postulation is supported by the fact that, after 1 day or 3 days of arousal, the transcript level of rhag remained significantly lower than the control, but the protein abundance of Rhag had returned to the control level. By contrast, the regulation of Rhbg and Rhcg expression appears to involve both transcriptional and translational processes. In both cases, changes in protein abundance (translation) were preceded by corresponding changes in transcript levels (transcription). For instance, significant down-regulation in the mRNA expression levels of rhbg and rhcg were detected in the gills of P. annectens during the induction phase, but a significant decrease in the protein abundance of Rhbg and Rhcg occurred only during the maintenance phase of aestivation.
Changes in branchial rhgp/Rhgp expression during the induction phase
In humans, RhAG/RhAG is expressed in erythrocytes and erythropoeitic tissues [22,39]. RhAG is involved in mediating ammonia transport so that erythrocytes are able to transport ammonia in the blood from its site of production to the site of excretion [47]. Since the gills of P. annectens were not perfused before sampling in this study, the gill samples naturally contained erythrocytes. The ammonia produced by the liver (and other organs) may be carried by the erythrocytes (and plasma) to the gills where ammonia is released through Rhag and transferred to the branchial epithelial cells for excretion. Therefore, the decrease in the protein abundance of Rhag in P. annectens during the induction phase might indicate a decrease in the release of ammonia from the erythrocytes to the plasma. It is possible that these erythrocytes carry a sub-normal level of ammonia due to a reduction in ammonia produced in the liver of P. annectens. It has been established that aestivation involves structural and functional modifications in certain organs during the induction phase [48–50]. These modifications involve the recycling of nitrogen between protein degradation and protein synthesis as the aestivating lungfish is undergoing fasting, which would lead to a reduction in the production of ammonia through amino acid catabolism [6,51]. Alternatively, Rhag has been localized to both the apical and basolateral membranes of the pillar cells in Takifugu rubripes [52], membranes that surrounds the lamellar blood spaces in the gills of D. rerio [53], and in the pillar cells, the lamellar epithelial cells and the outer edge of the interlamellar cell mass enriched with Na+/K+-ATPase in Carassius auratus [54]. If indeed Rhag is expressed in the gills of P. annectens, a decrease in the protein abundance of Rhag during the induction phase might lead to a reduction in ammonia excretion through the gills. Despite decreases in the mRNA expression levels of rhbg and rhcg, the protein abundance of Rhbg and Rhcg remained unchanged during the induction phase. These can be regarded as the necessary preparation to shut down gill functions and to stop ammonia excretion as the fish entered into the maintenance phase.
Changes in branchial rhgp/Rhgp expression during the maintenance phase
The gills of P. annectens apparently cease to function during the maintenance phase, as they are covered with a thick layer of dried mucus with a reduction in the surface area of the branchial epithelium [16]. Indeed, ammonia excretion, which occurs mainly through the gills, stops completely in P. annectens during the maintenance phase. Should this be regarded as a passive response to a lack of water to flush the branchial surface and/or a physiological adaptation which involves molecular changes in the gills? Here we demonstrate that arrest of physiological functions (e.g. ammonia excretion) occurs in association with changes in the expression of the related transporters/channels (e.g. rhgp/Rhgp). The decreased branchial transcript levels and protein abundance of rhag/Rhag, rhbg/Rhbg and rhcg/Rhcg during the maintenance phase indicate that the transcription and translation of these rhgp/Rhgp were down-regulated. This can be interpreted as part of an overall mechanism to shut down branchial functions. As it is essential to conserve metabolic fuels during long periods of aestivation without food intake, and as transcription and translation are energy-dependent processes [55], the suppression of transcription and translation of rhgp/Rhgp would certainly reduce the expenditure of metabolic energy. Therefore, these results support the notion that aestivation involves structural and functional modifications in certain organs [48–50], and the complex interplay between up-regulation and down-regulation of diverse cellular activities during different phases of aestivation [1,3].
Based on models of Rhgp in many fishes [22,23,39], Rhbg and Rhcg are localized to the basolateral and apical membrane of branchial epithelial cells, respectively. Nakhoul and Hamm [18] proposed that Rhbg and Rhcg were likely to work in tandem and function as NH3 channel and/or NH4+-specific transporter, facilitating transepithelial ammonia transport. Therefore, the decrease in the protein abundance of Rhbg and Rhcg in the gills of P. annectens during the maintenance phase would lead to a decrease in the transepithelial movement of ammonia. Furthermore, the decrease in the protein abundance of Rhag might imply a reduction in the transfer of ammonia from the erythrocytes to the branchial epithelium. Hence, efforts should be made in the future to elucidate the cellular and/or subcellular localization of Rhag, Rhbg and Rhcg in the gills of P. annectens in order to substantiate their possible functions in transepithelial ammonia transport.
Changes in rhgp/Rhgp expression during the arousal phase
During the arousal phase, it is essential for the lungfish to regain the ability to excrete ammonia. Indeed, the protein abundance of Rhag, Rhbg and Rhcg recovered to the corresponding control levels after 1 day or 3 days of recovery from 6 months of aestivation. This could help to restore the ability of P. annectens to excrete ammonia through the gills upon arousal.
Conclusion
Our results reveal how aestivating P. annectens regulates the gene and protein expression levels of rhag/Rhag, rhbg/Rhbg and rhcg/Rhcg in its gills to impede ammonia excretion during the induction and maintenance phases and to regain the ability of ammonia excretion during the arousal phase. These results reiterate that aestivation involves functional modifications of the gills and the interplay between up-regulation and down-regulation of rhgp/Rhgp during the three phases of aestivation. At present, how the branchial epithelial cells avoided cell death during the maintenance phase and how they initiate the recovery process in response to the availability of water during the arousal phase remain enigmatic and await future studies.
Supporting information
(DOCX)
“*” indicates the outgroup.
(DOCX)
“*” indicates the outgroup.
(DOCX)
“*” indicates the outgroup.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
The nucleotide sequence files of rhag, rhbg, rhcg are available in the Genbank database, with accession numbers KX583645, KX583646 and KX583647, respectively.
Funding Statement
This study was supported in part by the Singapore National Research Foundation administered by the PUB through a grant (1301-IRIS-23) to S. H. Lam and Y. K. Ip, and by the Singapore Ministry of Education through a grant (R154-000-429-112) to Y. K. Ip. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ip YK, Chew SF. Nitrogen metabolism and excretion during aestivation In: Navas CA, Carvalho JE, editors. Aestivation: Molecular and Physiological Aspects, Progress in Molecular and Subcellular Biology. Heidelberg: Springer-Verlag; 2010. pp. 63–93. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JS, Frick NT. Lungfish metabolism In Jorgensen JM, Joss J, editors. The Biology of Lungfishes. Enfield, NH: Science Publishers; 2010. pp. 301–335. [Google Scholar]
- 3.Chew SF, Ching B, Chng YR, Ong JL, Hiong KC, Chen XL, et al. Aestivation in African lungfishes: physiology, biochemistry and molecular biology In: Zaccone G, Dabrowski K, Hedrick MS, Fernandes JMO, Icardo JM, editors. Phylogeny, Anatomy and Physiology of Ancient Fishes. Boca Raton, FL: CRC Press; 2015. pp. 81–132. [Google Scholar]
- 4.Chew SF, Chan NKY, Loong AM, Hiong KC, Tam WL, Ip YK. Nitrogen metabolism in the African lungfish (Protopterus dolloi) aestivating in a mucus cocoon on land. J Exp Biol. 2004;207: 777–786. [DOI] [PubMed] [Google Scholar]
- 5.Ip YK, Yeo PJ, Loong AM, Hiong KC, Wong WP, Chew SF. The interplay of increased urea synthesis and reduced ammonia production in the African lungfish Protopterus aethiopicus during 46 days of aestivation in a mucus cocoon. J Exp Zool. 2005;303: 1054–1065. [DOI] [PubMed] [Google Scholar]
- 6.Loong AM, Hiong KC, Lee SM, Wong WP, Chew SF, Ip YK. Ornithine-urea cycle and urea synthesis in African lungfishes, Protopterus aethiopicus and Protopterus annectens, exposed to terrestrial conditions for six days. J Exp Zool. 2005;303: 354–365. [DOI] [PubMed] [Google Scholar]
- 7.Janssens PA, Cohen PP. Ornithine-urea cycle enzymes in the African lungfish Protopterus aethiopicus. Science. 1966;152: 358–359. doi: 10.1126/science.152.3720.358 [DOI] [PubMed] [Google Scholar]
- 8.Janssens PA, Cohen PP. Biosynthesis of urea in the estivating African lungfish and in Xenopus laevis under conditions of water-shortage. Comp Biochem Physiol. 1968;24: 887–898. [DOI] [PubMed] [Google Scholar]
- 9.Mommsen TP, Walsh PJ. Evolution of urea synthesis in vertebrates: the piscine connection. Science. 1989;243: 72–75. [DOI] [PubMed] [Google Scholar]
- 10.Chew SF, Ong TF, Ho L, Tam WL, Loong AM, Hiong KC, et al. Urea synthesis in the African lungfish Protopterus dolloi: hepatic carbamoyl phosphate synthetase III and glutamine synthetase are up-regulated by 6 days of aerial exposure. J Exp Biol. 2003;206: 3615–3624. [DOI] [PubMed] [Google Scholar]
- 11.Loong AM, Chng YR, Chew SF, Wong WP, Ip YK. Molecular characterization and mRNA expression of carbamoyl phosphate synthetase III in the liver of the African lungfish, Protopterus annectens, during aestivation or exposure to ammonia. J Comp Physiol B. 2012;182: 367–379. doi: 10.1007/s00360-011-0626-7 [DOI] [PubMed] [Google Scholar]
- 12.Chng YR, Ong JL, Ching B, Chen XL, Wong WP, Chew SF, et al. Molecular characterization of argininosuccinate synthase and argininosuccinate lyase from the liver of the African lungfish Protopterus annectens, and their mRNA expression levels in the liver, kidney, brain and skeletal muscle during aestivation. J Comp Physiol B. 2014;184(7): 835–853. doi: 10.1007/s00360-014-0842-z [DOI] [PubMed] [Google Scholar]
- 13.Ip YK, Peh BK, Tam WL, Wong WP, Chew SF. Effects of intra-peritoneal injection with NH4Cl, urea, or NH4Cl+urea on nitrogen excretion and metabolism in the African lungfish Protopterus dolloi. J Exp Zool. 2005;303: 272–282. [DOI] [PubMed] [Google Scholar]
- 14.Janssens PA, Cohen PP. Nitrogen metabolism in the African lungfish. Comp Biochem Physiol. 1968;24: 879–886. [DOI] [PubMed] [Google Scholar]
- 15.Frick NT, Bystriansky JS, Ip YK, Chew SF, Ballantyne JS. Carbohydrate and amino acid metabolism in fasting and aestivating African lungfish (Protopterus dolloi). Comp Biochem Physiol. 2008;151: 85–92. [DOI] [PubMed] [Google Scholar]
- 16.Sturla M, Masini MA, Prato P, Grattarola C, Uva B. Mitochondria-rich cells in gills and skin of an African lungfish, Protopterus annectens. Cell Tissue Res. 2001;303(3): 351–358. [DOI] [PubMed] [Google Scholar]
- 17.Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G. The challenge of understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus Clin Biol. 2006;13: 139–146. doi: 10.1016/j.tracli.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Nakhoul NL, Hamm LL. The challenge of determining the role of Rh glycoproteins in transport of NH3 and NH4+. Wiley Interdiscip Rev Membr Transp Signal. 2014;3(3): 53–61. [Google Scholar]
- 19.Wilkie MP. Mechanisms of ammonia excretion across fish gills. Comp Biochem Physiol A. 1997;118: 39–50. [Google Scholar]
- 20.Wilkie MP. Ammonia excretion and urea handling by fish gills: present understanding and future research challenges. J Exp Zool. 2002;293: 284–301. doi: 10.1002/jez.10123 [DOI] [PubMed] [Google Scholar]
- 21.Evans DH, Piermarini PM, Choe KP. The multifunctional fish gills: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85: 97–177. doi: 10.1152/physrev.00050.2003 [DOI] [PubMed] [Google Scholar]
- 22.Wright PA, Wood CM. A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol. 2009;212: 2303–2312. doi: 10.1242/jeb.023085 [DOI] [PubMed] [Google Scholar]
- 23.Wright PA, Wood CM. Seven things fish know about ammonia and we don’t. Resp Physiol Neurobiol. 2012;184(3): 231–240. [DOI] [PubMed] [Google Scholar]
- 24.Chng YR, Ong JL, Ching B, Chen XL, Hiong KC, Wong WP, et al. Molecular characterization of aquaporin 1 and aquaporin 3 from the gills of the African lungfish, Protopterus annectens, and changes in their branchial mRNA expression levels and protein abundance during three phases of aestivation. Front Physiol. 2016;7: 532. doi: doi: 10.3389/fphys.2016.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall TA. BioEdit: a user-friendly biological sequence editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41: 95–98. [Google Scholar]
- 26.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16(4): 404–405. [DOI] [PubMed] [Google Scholar]
- 27.Felsenstein J. PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics. 1989;5: 164–166. [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4): 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29.Chng YR, Ong JL, Ching B, Chen XL, Hiong KC, Wong WP, et al. Aestivation induces changes in the mRNA expression levels and protein abundance of two isoforms of urea transporters in the gills of the African lungfish, Protopterus annectens. Front Physiol. 2017;8: 71. doi: doi: 10.3389/fphys.2017.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ching B, Ong JL, Chng YR, Chen XL, Wong WP, Chew SF, et al. L-gulono-γ-lactone oxidase expression and vitamin C synthesis in the brain and kidney of the African lungfish, Protopterus annectens. FASEB J. 2014;28(8): 3506–3517. doi: 10.1096/fj.14-249508 [DOI] [PubMed] [Google Scholar]
- 31.Hiong KC, Ip YK, Wong WP, Chew SF. Brain Na+/K+-ATPase α-subunit isoforms and aestivation in the African lungfish, Protopterus annectens. J Comp Biol B. 2014;184(5): 571–587. [DOI] [PubMed] [Google Scholar]
- 32.Hiong KC, Tan XR, Boo MV, Wong WP, Chew SF, Ip YK. Aestivation induces changes in transcription and translation of coagulation factor II and fibrinogen gamma chain in the liver of the African lungfish Protopterus annectens. J Exp Biol. 2015;218(23): 3717–3728. [DOI] [PubMed] [Google Scholar]
- 33.Ong JL, Woo JM, Hiong KC, Ching B, Wong WP, Chew SF, et al. (2015). Molecular characterization of betaine-homocysteine methyltransferase 1 from the liver, and effects of aestivation on its expressions and homocysteine concentrations in the liver, kidney and muscle, of the African lungfish, Protopterus annectens. Comp Biochem Physiol B Biochem Mol Biol. 2015;183: 30–41. doi: 10.1016/j.cbpb.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 34.Ong JL, Chng YR, Ching B, Chen XL, Hiong KC, Wong WP, et al. (2017). Molecular characterization of myostatin from the skeletal muscle of the African lungfish, Protopterus annectens, and changes in its mRNA and protein expression levels during three phases of aestivation. J Comp Physiol B. 2017;187(4): 575–589. doi: 10.1007/s00360-017-1057-x [DOI] [PubMed] [Google Scholar]
- 35.Khademi S, O’Connell III J, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science. 2004;305: 1587–1594. doi: 10.1126/science.1101952 [DOI] [PubMed] [Google Scholar]
- 36.Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci. 2004;101: 17090–17095. doi: 10.1073/pnas.0406475101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Chen Y, Mo R, Hui CC, Cheng JF, Mohandas N, et al. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem. 2000;275(33): 25641–25651. doi: 10.1074/jbc.M003353200 [DOI] [PubMed] [Google Scholar]
- 38.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci. 2005;102: 15512–15517. doi: 10.1073/pnas.0507886102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weihrauch D, Wilkie MP, Walsh PJ. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J Exp Biol. 2009;212: 1716–1730. doi: 10.1242/jeb.024851 [DOI] [PubMed] [Google Scholar]
- 40.Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, et al. Function of human Rh based on structure of RhCG at 2.1 Å. Proc Natl Acad Sci. 2010;107(21): 9638–9643. doi: 10.1073/pnas.1003587107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baday S, Orabi EA, Wang S, Lamoureux G, Bernèche S. Mechanism of NH4+ recruitment and NH3 transport in Rh proteins. Structure. 2015:23(8): 1550–1557. doi: 10.1016/j.str.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 42.Fong RN, Kim KS, Yoshihara C, Inwood WB, Kustu S. The W148L substitution in the Escherichia coli ammonium channel AmtB increases flux and indicates that the substrate is an ion. Proc Natl Acad Sci. 2007;104(47): 18706–18711. doi: 10.1073/pnas.0709267104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D, Redfern O, Orengo C. Predicting protein function from sequence and structure. Nat Rev Mol Cell Biol. 2007;8(12): 995–1005. doi: 10.1038/nrm2281 [DOI] [PubMed] [Google Scholar]
- 44.Jacobson MP, Kalyanaraman C, Zhao S, Tian B. Leveraging structure for enzyme function prediction: methods, opportunities, and challenges. Trends Biol Sci. 2014;39(8): 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang CH. Molecular origin and variability of the Rh gene family: an overview of evolution, genetics and function (The 13th Congress of the European Haematology Association, Copenhagen, Denmark). Haematologica. 2008;2: 149–157. [Google Scholar]
- 46.Huang CH, Ye M. The Rh protein family: gene evolution, membrane biology, and disease association. Cell Mol Life Sci. 2010; 67(8): 1203–1218. doi: 10.1007/s00018-009-0217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem. 2002;277: 12499–12502. doi: 10.1074/jbc.C200060200 [DOI] [PubMed] [Google Scholar]
- 48.Ojeda JL, Wong WP, Ip YK, Icardo JM. Renal corpuscle of the African lungfish Protopterus dolloi: structural and histochemical modifications during aestivation. Anat Rec (Hoboken). 2008;291: 1156–1172. [DOI] [PubMed] [Google Scholar]
- 49.Icardo JM, Amelio D, Garofalo F, Colvee E, Cerra MC, Wong WP, et al. The structural characteristics of the heart ventricle of the African lungfish Protopterus dolloi: freshwater and aestivation. J Anat. 2008;213: 106–119. doi: 10.1111/j.1469-7580.2008.00901.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Icardo JM, Loong AM, Colvee E, Wong WP, Ip YK. The alimentary canal of the African lungfish Protopterus annectens during aestivation and after arousal. Anat Rec. 2012;295: 60–72. [DOI] [PubMed] [Google Scholar]
- 51.Loong AM, Pang CY, Hiong KC, Wong WP, Chew SF, Ip YK. Increased urea synthesis and/or suppressed ammonia production in the African lungfish, Protopterus annectens, during aestivation in air or mud. J Comp Physiol B. 2008;178: 351–363. doi: 10.1007/s00360-007-0228-6 [DOI] [PubMed] [Google Scholar]
- 52.Nakada T, Westhoff CM, Kato A, Hirose S. Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J. 2007;21(4): 1067–1074. doi: 10.1096/fj.06-6834com [DOI] [PubMed] [Google Scholar]
- 53.Braun MH, Steele SL, Perry SF. The responses of zebrafish (Danio rerio) to high external ammonia and urea transporter inhibition: nitrogen excretion and expression of rhesus glycoproteins and urea transporter proteins. J Exp Biol. 2009;212: 3846–3856. doi: 10.1242/jeb.034157 [DOI] [PubMed] [Google Scholar]
- 54.Perry SF, Schwaiger T, Kumai Y, Tzaneva B, Braun MH. The consequences of reversible gill remodeling on ammonia excretion in goldfish (Carassius auratus). J Exp Biol 2010;213: 3656–3665. doi: 10.1242/jeb.045955 [DOI] [PubMed] [Google Scholar]
- 55.Storey KB, Storey JM. Metabolic regulation and gene expression during aestivation In: Navas CA, Carvalho JE, editors. Aestivation: Molecular and Physiological Aspects, Progress in Molecular and Subcellular Biology. Heidelberg: Springer-Verlag; 2010. pp. 25–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
“*” indicates the outgroup.
(DOCX)
“*” indicates the outgroup.
(DOCX)
“*” indicates the outgroup.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The nucleotide sequence files of rhag, rhbg, rhcg are available in the Genbank database, with accession numbers KX583645, KX583646 and KX583647, respectively.