Abstract
Bilirubin has been recognized as a powerful cytoprotectant when used at physiologic doses and was recently shown to have immunomodulatory effects in islet allograft transplantation, conveying donor-specific tolerance in a murine model. We hypothesized that bilirubin, an antioxidant, acts to suppress the innate immune response to islet allografts through two mechanisms: 1) by suppressing graft release of damage-associated molecular patterns (DAMPs) and inflammatory cytokines, and 2) by producing a tolerogenic phenotype in antigen-presenting cells. Bilirubin was administered intraperitoneally before pancreatic procurement or was added to culture media after islet isolation in AJ mice. Islets were exposed to transplant-associated nutrient deprivation and hypoxia. Bilirubin significantly decreased islet cell death after isolation and hypoxic stress. Bilirubin supplementation of islet media also decreased the release of DAMPs (HMGB1), inflammatory cytokines (IL-1β and IL-6), and chemokines (MCP-1). Cytoprotection was mediated by the antioxidant effects of bilirubin. Treatment of macrophages with bilirubin induced a regulatory phenotype, with increased expression of PD-L1. Coculture of these macrophages with splenocytes led to expansion of Foxp3+ Tregs. In conclusion, exogenous bilirubin supplementation showed cytoprotective and antioxidant effects in a relevant model of islet isolation and hypoxic stress. Suppression of DAMP release, alterations in cytokine profiles, and tolerogenic effects on macrophages suggest that the use of this natural antioxidant may provide a method of preconditioning to improve outcomes after allograft transplantation.
Keywords: Islet, Damage-associated molecular patterns (DAMPs), HMGB1, Bilirubin, Innate immune response
Introduction
Pancreatic islet transplantation has the potential to provide a curative, noninvasive treatment for type 1 diabetes mellitus (T1DM). However, mechanical stress, enzymatic injury, and hypoxia cause loss of up to 70% of isolated islet mass in the first 72 h after cellular transplantation, prior to onset of the acquired immune response1,5. Massive cell necrosis and apoptosis cause release of intracellular proteins from the injured islets, triggering an innate, antigen-independent immune response through toll-like receptor (TLR) pathways6,9. The proteins that arise from the donor tissue, termed “damage-associated molecular patterns” (DAMPs), facilitate allorecognition and eventual allograft rejection by sending a danger signal to the host immune system10,11. High-mobility group box protein B1 (HMGB1) is a DAMP that has been shown to play an essential role in early graft loss by altering cytokine gene expression in dendritic cells (DCs) through TLR2-dependent and receptor for advanced glycation end product (RAGE)-dependent pathways8. Consistent with this finding, HMGB1 neutralization in vivo using HMGB1-specific antibodies prevented this graft loss in syngeneic transplants8.
While prevention of early graft loss is of considerable importance, evidence suggests that suppression of the antigen-independent innate immune response can also, in certain circumstances, induce tolerance mediated by regulatory T cells (Tregs)6,7,9,12–14. It is therefore possible that cytoprotective agents have the potential to minimize early islet death and simultaneously contribute to the development of donor-specific tolerance. In support of this notion, islet transplant studies performed by multiple investigators have shown that administration of bilirubin, an endogenous antioxidant and product of the enzyme heme oxygenase (HO), can induce a state of donor-specific tolerance in murine allograft islet transplants without the risks and disadvantages associated with genetic manipulation of the HO system15,21. While the primary mechanism of cytoprotection provided by bilirubin appears to be related to the potent antioxidant effects of this molecule16,22, the mechanism by which a tolerogenic response is achieved has not been completely elucidated. Previous studies in our laboratory and others suggest that bilirubin's therapeutic effects occur at a dose of approximately 10 mg/kg when administered systemically or 10–20 μM when used in organ perfusion solutions, approximating the physiologic range of bilirubin concentrations seen with upregulation of HO, but remaining far below the toxic concentrations that produce kernicterus in infants18,22–25. Our goal in this specific study was to investigate the effects of physiologic doses of bilirubin on early steps in the innate immune response to islet allografts, focusing in particular on the release of DAMPs and inflammatory cytokines by the islet tissue and on the phenotype of antigen-presenting cells (APCs) exposed to bilirubin in vitro. On the basis of the cytoprotective properties of bilirubin and the evidence of tolerogenic activity of this molecule in islet transplantation, we hypothesized that supplemental bilirubin would improve islet viability and would prevent the release of DAMPs and inflammatory cytokines in clinically relevant models of islet stress. Furthermore, we proposed the novel hypothesis that bilirubin would have a direct tolerogenic effect on APCs, conveying a second level for potential immunomodulation in both the graft and the host.
Materials and Methods
Animals
Female AJ mice (aged 6–10 weeks) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Foxp3EGFP (B6.Cg-Foxp3tm2Tch) mice coexpressing enhanced green fluorescent protein (EGFP) and the T-cell-specific transcription factor forkhead box P3 (Foxp3) were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and were bred in-house. Experiments were performed according to the National Institutes of Health (NIH) guidelines for animal care and use and were approved by the institutional animal care and use committee (IACUC) at The Ohio State University.
Bilirubin Preparation
Bilirubin stock solutions were prepared to a final concentration of 2 mM. To make the stock solution, 0.0584 g of bilirubin (bilirubin mixed isomers B4126; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.5 ml of 0.2 N NaOH. The total volume was then increased by the addition of Roswell Park Memorial Institute (RPMI-1640) medium (Invitrogen, Carlsbad, CA, USA) + 10% fetal bovine serum (FBS) to a total volume of 50 ml. Adjustment of pH to 7.4 was achieved by adding hydrochloric (HCl) acid. Aliquots of 2 mM bilirubin in RPMI and 10% FBS were stored at −80°C and were protected from light until being thawed and used for each experiment.
Treatment Groups
Animals were assigned to one of three groups that received either 0.01 ml/g body weight of standard media (vehicle control) as an intraperitoneal (IP) injection 1 h prior to pancreas procurement, 10 mg/kg IP bilirubin 1 h prior to procurement (BR IP), or 20 μM/L bilirubin in the culture media (BR media) after islet isolation. Experiments were performed with 100–300 islets per group and were repeated three times unless otherwise indicated.
Islet Isolation
Mice were anesthetized using isoflurane (Sigma-Aldrich) and sacrificed by cervical dislocation. Pancreatic digestion was performed by injection of collagenase P (Sigma-Aldrich) into the common bile duct, and islets were isolated with a Ficoll (Histopaque-1077; Sigma-Aldrich) density gradient separation technique as previously described26,28. Islets were manually counted and plated in standard RPMI-1640 media (Invitrogen) with 10% FBS and 1% penicillin–streptomycin (Invitrogen) before incubation at 37°C and 5% CO2.
Islet Viability Assessment
Cell viability was determined on the basis of propidium iodide (PI; Sigma-Aldrich) exclusion. Islets were examined using epifluorescent photomicroscopy after incubating for 15 min with Hoechst 33258 (Sigma-Aldrich) and PI stains. Images were analyzed using the NIH ImageJ software, and the percentage of PI+ cells present in each islet was calculated using a custom islet macro as previously described28.
Nutrient Deprivation and Hypoxic Stress
Nutrient deprivation associated with islet transplantation was simulated by plating islets immediately after isolation and maintenance for 48 h without changing culture media. Aliquots of 100 islets were harvested at 12, 24, and 48 h, and cell viability was estimated using dual staining as described above. Hypoxic stress conditions were imposed by incubation of freshly isolated islets for 3 h at 37°C and 1% O2 in an O2, CO2, and N2 mixed gas incubator (MCO-19M; Sanyo, Osaka, Japan). A previous study by our group showed that intermittent incubation for up to 3 h at 1% O2 produced significant islet loss, while longer periods resulted in nearly complete destruction of murine islets28. Islets were removed from the mixed gas incubator and were allowed to recover for 24 h in a standard 37°C, 21% O2 environment before viability assessment.
Immunoassays
Hypoxic stress experiments were repeated an additional three to five times using 250-300 mouse islets per group in 3 ml of media. After 4 h of recovery from hypoxic stress, islets were removed for gene expression studies, and conditioned media samples were stored at −80°C for subsequent immunoassays. Oxidative stress was measured using an 8-iso-prostaglandin F2α enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY, USA). HMGB1 levels in conditioned media were measured using the HMGBI ELISA Kit II (Shino-Test, Tokyo, Japan). Assay kits for heat shock protein 70 (HSP70), interleukin-1β (IL-1β), and IL-6 were obtained from Enzo Life Sciences. Results for each experiment were normalized to results from non-stressed control islets and were expressed as fold change from baseline.
Flow Cytometry
Flow cytometry was performed on C57BL/6 macrophage cells kept in supplemented RPMI containing 10% FBS, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Invitrogen), 2 mM L-glutamine (Invitrogen), and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich) until confluent in a T75 flask (Thermo Fisher Scientific, Waltham, MA, USA). Macrophages were then plated into a 24-well tissue culture plate (Thermo Fisher Scientific) and cultured in the presence of control media or media + 20 μM bilirubin. An additional population of cells was stimulated with 1 μg/ml lipopolysaccharide (LPS; 055:B5; Sigma-Aldrich) during the culture period. After 48 h of culture, cells were extensively washed with media, blocked with CD16/CD32 (0.5 μg/ml; Fc block; BD Biosciences, San Jose, CA, USA) for 20 min, and washed twice with fluorescence-activated cell sorting (FACS) buffer (BD Biosciences). Cells were labeled (20 min in the dark at 4°C) using programmed death ligand 1 (PD-L1; 0.2 mg/ml; MIH5; BD Biosciences) on allophycocyanin with appropriate isotype controls (BD Biosciences). Cells were washed twice and resuspended in 200 μl of FACs buffer. Cells were then analyzed on a BD Accuri C6 flow cytometer and CFlow Plus software (BD Biosciences).
In Vitro Treg Induction
C57BL/6 macrophage cells were seeded on a 12-well plate (Thermo Fisher Scientific) and allowed 24 h to adhere in supplemented RPMI (as previously described) at a density of 1 × 106 cells/ml. Spleens from mice with reporter Foxp3EGFP (B6.Cg-Foxp3tm2Tch) were harvested, passed through cell strainers (70 μm; BD Falcon; BD Biosciences), collected by centrifugation (200×g for 7 min at 4°C), and subjected to erythrocyte lysis. Responder cells and myeloid cells (MCs) were cultured for 4 days (1:10 ratio of macrophages to responder), and aliquots from cultures were assessed for Foxp3 expression by FACS.
Statistical Analysis
Data were expressed as mean ± SEM. Islet viability data and normalized ELISA data were compared between groups using a one-way analysis of variance (ANOVA), and post hoc pairwise comparisons were made using the Tukey's HSD test. Flow cytometry statistical significance was determined using a Student's t-test. All statistics were calculated using StatView software (SAS, Cary, NC, USA), with p≤0.05 considered statistically significant.
Results
Bilirubin Preserves Islet Viability Following Isolation and Hypoxia-Induced Stress
Isolation-induced cell death was moderate and increased significantly in all groups over the first 48 h of culture as nutrient availability decreased (Fig. 1A). Bilirubin IP (10 mg/kg) administered to the donor 1 h prior to islet harvest caused a significant reduction in islet cell death at all time points compared to control. A similar cytoprotective effect was documented after hypoxic stress, with bilirubin IP to the islet donor causing a significant decrease in cell death at 24 h after hypoxic injury (Fig. 1B). Addition of exogenous bilirubin (20 μM/L) to the cell culture media of control islets 1 h before a 3-h period of hypoxia (1% O2) provided an even more powerful protective effect than donor pretreatment in this model, with almost complete abrogation of the cytotoxic effects of hypoxia (Fig. 1B). Hypoxic stress produced central islet necrosis in larger islets (>2,000 μm2), suggesting decreased oxygen concentrations at the center of large islet cell masses (Fig. 1C). There was a significant interaction between islet size and bilirubin treatment, such that addition of bilirubin to the cell culture media had the greatest effect in reducing hypoxia-induced cell death in the larger islets (p = 0.03) (Fig. 1C).
Figure 1.
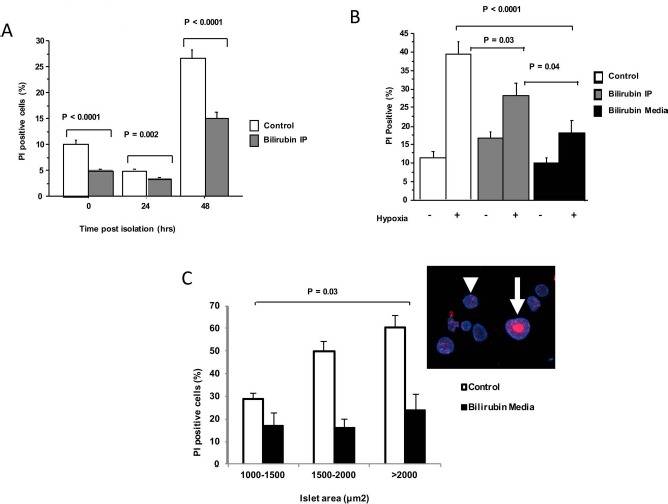
Bilirubin decreases islet cell death in models of transplant-associated stress. AJ mouse islets (100–150 per treatment group) were isolated and maintained in tissue culture (37°C). Dual staining with Hoechst and propidium iodide (PI) was performed, and cell death was estimated based on % PI+ staining. Graphs represent pooled data from three independent experiments in each model. (A) Islets were maintained in culture for 48 h after pancreas digestion and gradient isolation to illustrate the effects of isolation stress. Media were unchanged to cause nutrient deprivation. Progressive cell death occurred, peaking at 48 h after recovery. Bilirubin (10 mg/kg) administered intraperitoneally to the islet donor 1 h before islet harvest (Bilirubin IP) caused significant decreases in cell death. (B) Hypoxic stress was induced by subjecting the islets to 1% O2 for 3 h. Viability was assessed after 24 h of reoxygenation. Hypoxic stress caused marked cell death in the control group. Cell death was significantly decreased by bilirubin IP treatment of the donor prior to islet recovery, but cell death was most effectively prevented by the addition of bilirubin (20 μM/L) to the cell culture media (Bilirubin Media) 1 h before hypoxia. (C) Islets were divided into three size groups based on the measured area of each islet. Percent cell death was greater in large islets exposed to hypoxia, and there was a significant interaction detected between bilirubin treatment and islet size, with bilirubin offering the greatest protection against necrosis of large islets (p = 0.03). A representative image of control islets (inset) shows the large area of central necrosis (arrow) caused by hypoxic injury, while smaller islets were preserved (arrowhead).
Bilirubin Pretreatment Decreases Oxidative Stress in Islets Exposed to Hypoxia and Reoxygenation
Pancreatic islets are highly susceptible to oxidative injury due to low levels of endogenous antioxidant enzymes in β-cells, and the important role of antioxidants in islet allograft transplantation has been reviewed elsewhere29. On the basis of the documented antioxidant activity of the bilirubin molecule30, we suspected that free radical scavenging was a major component of bilirubin's cytoprotective effects in islets subjected to hypoxic stress. Lipid peroxidation was measured using 8-isoprostane, which is considered a stable biomarker of oxidative injury in biological samples31,32. As predicted, we detected increased 8-isoprostane levels in the islet media 24 h after exposure to hypoxic conditions, documenting the occurrence of lipid peroxidation (Fig. 2). Bilirubin pretreatment (only 1 h) of the islet donor significantly decreased lipid peroxidation (p < 0.05) when compared to control islets exposed to hypoxic stress. Interestingly, bilirubin pretreatment of islet donors suppressed oxidative stress in islets exposed to hypoxia such that there was no significant difference from unstressed controls (Fig. 2).
Figure 2.
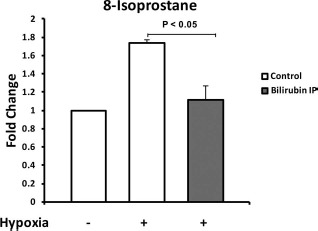
Bilirubin decreases oxidative stress in islets subjected to hypoxic conditions. AJ mice were treated with either vehicle control (Control) or 10 mg/kg of bilirubin intraperitoneally (Bilirubin IP) 1 h before islet harvest. Hypoxic stress conditions were imposed by incubation of mouse islets (250-300 per treatment group) for 3 h at 37°C and 1% O2. Islets were then reoxygenated for 24 h in a standard 37°C, 20% O2 environment. Oxidative stress was determined by measuring 8-iso-prostaglandin F2α levels in the conditioned islet culture media using an enzymatic immunoassay kit. Results of three independent experiments were normalized to values from the unstressed control islets and were expressed as fold change from control. Exposure to hypoxia caused a marked increase in oxidative stress. Donor pretreatment with bilirubin significantly decreased oxidative stress in islets subjected to hypoxia and reoxygenation, maintaining levels of lipid peroxidation similar to unstressed control islets.
Exogenous Bilirubin Decreases Release of the TLR4 Ligands HMGB1 and HSP70
Release of intracellular HMGB1 and HSP70 can be passive (e.g., due to loss of membrane integrity in necrotic cells) or active (secreted in vesicles)33,34. Active secretion of HMGB1 is calcium mediated and is initiated by the generation of reactive oxygen species (ROS) during ischemia33. Suppression of the passive or active release of these DAMPs could provide one explanation for the tolerogenic effects of an antioxidant such as bilirubin, interrupting the innate immune response at the earliest phase of the injury cascade.
We found significant increases in both HMGB1 and HSP70 proteins in the extracellular environment within 4 h after hypoxia when compared to unstressed controls (Fig. 3). Consistent with our hypothesis, supplementation of the islet media with 20 μM/L bilirubin significantly decreased release of HMGB1 in islets exposed to hypoxic stress when compared to control islets (p = 0.003) (Fig. 3). While HSP70 levels also appeared to be decreased by bilirubin treatment, results were not consistent between experiments, and this effect just failed to reach statistical significance (p = 0.05) (Fig. 3) in our studies.
Figure 3.
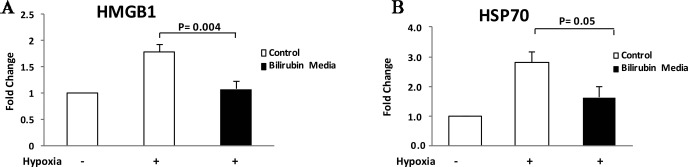
Bilirubin decreases release of HMGB1 and HSP70 at early time points after hypoxic stress. Hypoxic stress conditions were imposed as described in Figure 2, and protein levels of high-mobility group box protein B1 (HMGB1) (A) and heat shock protein 70 (HSP70) (B) were measured in islet culture media after 24 h of recovery. Results were normalized to values from the unstressed control islets and were expressed as fold change from control. Hypoxic stress caused 1.8- and 2.8-fold increases in HMGB1 HSP70 levels in control islets, respectively. Addition of bilirubin (20 μM/L) to islet culture media 1 h before hypoxic stress caused a significant reduction in HMGBI release from treated islets (p = 0.004). While HSP70 release was decreased from a mean of 2.8-fold in the control hypoxia group to 1.6-fold in the bilirubin media group, results were variable and this effect failed to reach statistical significance over four repetitions of the experiment (p = 0.054).
Bilirubin Suppresses Release of Proinflammatory Cytokines in Islets Exposed to Hypoxic Stress
Analysis of islet-conditioned media demonstrated that hypoxic stress caused approximately twofold increases in concentrations of the inflammatory cytokines IL-6 and IL-1β and in the chemokine monocyte chemoattractant protein-1 (MCP-1) when compared to unstressed control islets (Fig. 4). Media concentrations of IL-6 and IL-1β were significantly decreased by the addition of 20 μM/L bilirubin 1 h before hypoxic stress (p = 0.004 and p < 0.001, respectively) (Fig. 4). A similar trend was seen for the release of the chemokine MCP-1, with statistically significant decreases in the media in bilirubin-treated samples (p = 0.004, control hypoxia vs. bilirubin media) (Fig. 4).
Figure 4.
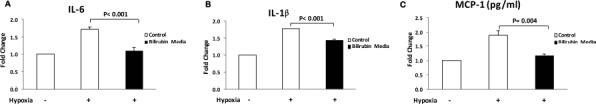
Bilirubin decreases release of inflammatory cytokines and chemokines from stressed islets. Hypoxic stress conditions were imposed as described in Figure 2, and concentrations of the inflammatory cytokines (IL-1β and IL-6) and the chemokine macrophage chemoattractant protein 1 (MCP-1) were measured in islet culture media after 24 h of recovery. Results were normalized to values from the unstressed control islets and were expressed as fold change from control. Hypoxic stress caused increases in IL-6 (A), IL-1β (B), and MCP-1 (C) in the islet-conditioned media. Addition of bilirubin (20 μM/L) to islet culture media 1 h before hypoxic stress significantly reduced the release of IL-1β (p<0.001), IL-6 (p<0.001), and MCP-1 (p = 0.004) after hypoxia and reoxygenation.
Bilirubin Has a Direct Tolerogenic Effect on LPS-Stimulated Macrophages
We investigated the effects of direct exposure to bilirubin in LPS-stimulated C57BL/6 macrophages using FACS. Quantification of PD-L1 expression was used as an initial marker of a tolerogenic phenotype. Bilirubin supplementation caused a significant (p < 0.05) increase in PD-L1 expression when macrophages were incubated in the presence of LPS (a TLR4 ligand) and bilirubin (Fig. 5A), compared to media control cells. To confirm tolerogenic phenotype, we then evaluated whether bilirubin-exposed macrophages could increase Treg numbers in coculture experiments. Using reporter Foxp3EGFP mice, we found that macrophages exposed to 20 μM bilirubin induced the formation of Foxp3+ T cells over a 4-day culture with naive immune cells (Fig. 5B).
Figure 5.
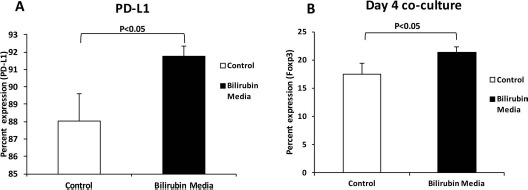
Bilirubin increases the expression of PD-L1 on macrophages and increased the capacity of Treg induction. C57BL/6 macrophages were seeded in a 12-well tissue culture plate and cultured in the presence of media or 20 μM bilirubin. Additional groups were incubated with 1 μg/ml LPS during the culture period. (A) After 48 h of culture, cells were washed twice and stained for programmed death ligand 1 (PD-L1) for analysis by fluorescence-activated cell sorting (FACS). (B) The ability of these cells (after a 4 day coculture) to induce forkhead box p3 positive (Foxp3+) cells from naive Foxp3-enhanced green fluorescent green (EGFP) reporter immune cells was also determined by FACS. Experiments were performed three times to confirm results.
Discussion
Bilirubin is a powerful endogenous cytoprotectant that has been shown to improve outcomes in models of sepsis, in ischemia reperfusion injury, and in solid organ transplantation18,22–25. However, our study is the first to specifically dissect the mechanism of bilirubin on the innate immune response to islet allografts, providing insight into the immunomodulation that has been demonstrated in previous islet allograft transplant models18,35,36. Our results show that the use of this cytoprotectant would provide a means to address the two most pressing problems in islet allograft transplantation by decreasing early cell death and providing an immunomodulatory therapy that is nontoxic to β-cells. In particular, the ability to minimize antigen presentation and apoptosis in coordination with influencing a tolerogenic phenotype in APCs has been associated with the development of tolerogenic response37.
Given the massive cell loss that occurs during clinical islet transplantation and the subsequent need for multiple donors for each recipient, islet transplant research has focused intently on discovering methods to prevent early graft loss during islet hypoxia1,5,29,38–44. Isolation of pancreatic islets leads to complete disruption of the microvascular supply, leaving transplanted islets dependent on simple diffusion for days to months after islet transplantation40,43,45,46. Massive apoptosis and necrosis occur in the first 48 h after islet transplantation in both syngeneic and immunocompromised hosts, supporting the assertion that hypoxic injury is the largest contributor to islet loss41. Cell death is maximized when hypoxia is enacted in combination with nutrient deprivation, producing a phenotype of central islet necrosis that is consistent with the effects of a diffusion gradient47. On the basis of this information, we elected to focus our in vitro model on nutrient deprivation and hypoxia-induced cell injury in an attempt to mirror the clinical transplant environment. Our results showed that supplementation of islet culture media with physiologic doses (20 μM/L or 1.1 mg/dl) of bilirubin significantly decreased islet cell death in clinically relevant models of isolation and hypoxic stress. The 20 μM/L dose was selected for these studies because preliminary studies in our laboratory showed that higher doses of bilirubin caused cytotoxicity in both islets and macrophages—an effect that would be consistent with the toxicity associated with supraphysiologic concentrations of bilirubin in patients with severe icterus. It is important to note that ex vivo therapy (adding bilirubin to the islet media) was superior to pretreatment of the islet donor (IP bilirubin administration 1 h before pancreas procurement). Ex vivo supplementation of preservation solutions with natural or synthetic bilirubin would provide a safe and practical means of preconditioning islets prior to the hypoxic stress that is expected to occur after intraportal islet transplantation in clinical patients.
Aside from the cytoprotective effects of bilirubin, this molecule has been shown to have additional immunomodulatory effects in the context of islet allograft transplantation, inducing a state of donor-specific tolerance in mice receiving major histocompatibility complex (MHC) mismatched allografts18,35,36. Because of a lack of known direct immunosuppressive qualities associated with the bilirubin molecule, previous investigators have postulated that bilirubin acted by decreasing the danger signals released by islets during cellular transplantation35. While these investigators sought to analyze these effects in an allograft model that included recipient T cells and DCs, it is difficult to isolate and study the danger signaling from the islet graft in vivo. The experimental models that we used in the current study were designed to allow examination of the extracellular danger signals and cytokine expression by isolated islets when subjected to clinically relevant models of cellular stress. For our mechanistic studies, we focused on the hypoxic model of cell stress, as hypoxia is believed to be the major contributor to islet cell death during clinical intraportal islet transplantation1,38,48. Our findings suggest that at the earliest time points after hypoxic injury, injured islets release large quantities of the DAMP HMGB1 and HSP70 into the extracellular environment. Simultaneously, islets were shown to secrete increased quantities of IL-6, IL-1β, and MCP-1, an effect that was significantly blunted by the administration of bilirubin to the islet culture medium. The combined effect of massive release of TLR4 ligands in the milieu of these inflammatory cytokines and chemokines would be infiltration and activation of both islet donor (passenger) and recipient APCs6-8,14,34,49. Activation of the TLR pathway in infiltrating immune cells through binding of islet-derived DAMPs leads to the production of inflammatory cytokines and generation of a TH1 response toward the islet graft9. In contrast, blockade of the TLR pathway using TLR2- and TLR4-deficient mice has been shown to significantly prolong islet allograft survival through a Foxp3+ Treg-mediated effect7,14, similar to the Foxp3+ Treg-mediated mechanism noted in the development of bilirubin-induced tolerance in a murine model of allograft transplantation18,35,36. Our current findings show clear reduction in the release of known TLR4 ligands during islet stress, providing a key mechanistic explanation of the tolerogenic effects of bilirubin in islet transplantation (Fig. 6).
Figure 6.
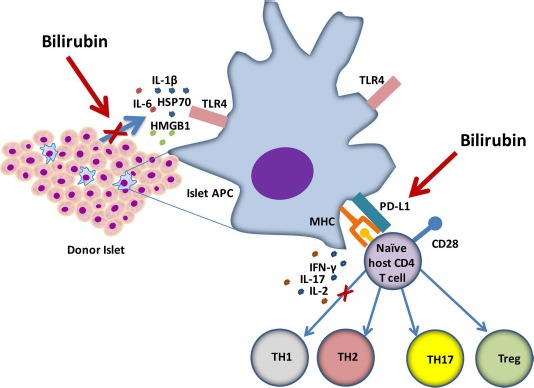
Schematic illustration of the mechanisms for the tolerogenic effects of bilirubin on islet transplantation. Bilirubin improved islet viability and significantly decreased release of the damage-associated molecular pattern (DAMP) molecule HMGB1, as well as suppressed the release of proinflammatory cytokines IL-1β, IL-2, and the macrophage chemoattractant protein MCP-1. These effects would decrease toll-like receptor 4 (TLR4) stimulation and suppress the innate immune response in both passenger and host APCs. Furthermore, bilirubin was shown to induce expression of the regulatory marker PD-L1 and the formation of Tregs after coculture with naive immune cells. The combined cytoprotective and immunomodulatory effects of bilirubin may lead to the presentation of a smaller dose of antigen by tolerogenic antigen-presenting cells, inducing a state of Treg-mediated tolerance.
Our studies also revealed the novel finding that bilirubin has a direct effect on the phenotype of APCs, specifically macrophages. APCs have numerous methods of regulating the immune response including inhibitory or regulatory cell surface markers. PD-L1 is an extracellular marker constitutively expressed on T cell, B cells, macrophages, and DCs50. PD-L1 is considered a regulatory cell marker because of its ability to inhibit lymphocyte activation when bound to its receptor, PD-151,53. Specifically, stimulation of the PD-1 receptor has been shown to induce tolerance in islet allograft models54. In addition, APCs expressing PD-L1 have been shown to enhance islet engraftment by induction of Tregs55. In our studies, we showed that C57BL/6 macrophages exposed to 20 μM bilirubin for 48 h had a statistically significant increase in extracellular expression of PD-L1. Our data suggest that physiologic doses of bilirubin have the capacity to induce a regulatory phenotype in macrophages. In addition to documenting changes in macrophage phenotype, we also show that macrophages exposed to bilirubin for 48 h, washed, and then cocultured with naive responder immune cells for 4 days cause a significant increase in the induction of Tregs. Further studies will be required to document the efficacy of bilirubin-treated macrophages in suppressing the antigen-specific T-cell response.
HMGB1 has been well recognized as a biomarker that indicates cellular injury, but the importance of HMGB1 as a chemokine and mediator of transplant immune response has only recently been reported. Matsuoka et al. demonstrated that treatment with an HMGB1-specific antibody prevented early graft loss in a mouse model of islet allograft transplantation8. In that study, deficiency of the known HMGB1 receptors TLR2 and RAGE protected against islet loss, while TLR4 deficiency had no effect. Effects of HMGB1 were thought to be mediated by stimulation of host DCs, causing increased CD40 expression and release of the proinflammatory cytokine IL-12. Evidence suggests that active secretion of HMGB1 into the extracellular environment is calcium mediated and is initiated by the generation of ROS33. These findings concur with the results of our study and suggest that the demonstrated antioxidant properties of bilirubin may inhibit both passive release through preventing cell necrosis during hypoxic stress and active release of HMGB1 through ROS-mediated secretion in vesicles.
HSP70 is a mediator with seemingly contradictory effects on islet transplantation. Intracytoplasmic HSP70 has a cytoprotective role in the stabilization and folding of cytosolic proteins during oxidative stress. In several in vitro studies, induction of intracellular HSP70 has been associated with improved islet viability and function56,61. However, like HMGB1, HSP70 is also a known ligand of TLR2 and TLR4 that is involved in early pattern recognition by the innate immune system. Once HSP70 is released into the extracellular environment by injured cells, HSP70 ceases to have a cytoprotective function, instead becoming a “danger signal” that contributes to the TLR-mediated innate immune response to injured islets. Numerous studies have demonstrated that extracellular HSP70 contributes to the innate immune response through the MyD88/IRAK/NF-κB signal transduction pathway (reviewed in Calderwood et al.34). Data in our experiments showed that hypoxia caused measurable increases in the release of HSP70 from islet tissues. While bilirubin appeared to suppress this release, results were highly variable between independent experiments and failed to reach statistical significance.
Although bilirubin was initially believed to be a potentially toxic by-product of heme catabolism, subsequent research has identified that at physiologic doses, bilirubin serves a crucial role as an endogenous antioxidant22. Bilirubin has been shown to convey a variety of beneficial effects on vascular endothelial health, smooth muscle cell proliferation, and immunomodulatory effects in both health and disease, but the primary mechanism of action appears to be through the powerful antioxidant effects of the bilirubin molecule22. In the current study, the effect of bilirubin on oxidative stress was determined by measuring 8-isoprostane F2α levels in the islet-conditioned media 4 h after exposure to hypoxic conditions. Isoprostanes are formed as a by-product of arachidonic acid during periods of oxidative stress and are considered to be extremely reliable biomarkers due to their stability and their specificity for lipid peroxidation in biological fluids and islet media31,32. ROS play a key role in several aspects of islet transplantation, which may explain the profound effects of bilirubin in these experiments. β-Cells are particularly sensitive to reactive oxygen and nitrogen species (ROS/RNS) due to the low levels of antioxidant enzymes expressed in these cells62. Mechanistically, generation of ROS is central to islet cell death during exposure to inflammatory cytokines or hypoxia/reoxygenation62.
There are a several practical considerations that must be taken into account before clinical application of bilirubin therapy in the islet transplant arena. Commercially available bilirubin is derived from animal sources (pigs) and is not approved for use in human subjects, leading some to suggest that upregulation of the endogenous bilirubin levels by inducing the heme oxygenase system may be a simpler method of therapy63. However, pretreatment of organ donors with inducers of heme oxygenase would cause both logistical and ethical problems that would further complicate the organ procurement process. On the basis of these considerations and our data that suggest direct supplementation of islet media can provide even more powerful protective effects than those conferred by pretreatment of the donor, we are currently focusing on identifying methods to produce a safe human-derived or synthetic form of bilirubin that may be added directly to organ perfusion solutions immediately after organ procurement. After practical methods of delivery of this natural antioxidant are developed, bilirubin supplementation will offer several distinct advantages in the unique and challenging field of islet transplantation, improving islet viability and suppressing the innate immune response to islet grafts at several levels in the pathway.
Acknowledgments
The authors would like to acknowledge Dr. Mariano Viapiano for the use of the islet macro described in the Materials and Methods section. The project described was supported by Award No. KL2 RR025754 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not represent the official views of the National Center for Research Resources or the NIH. The authors declare no conflicts of interest.
References
- 1.Harlan D.M., Kenyon N.S., Korsgren O., Roep B.O., Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes 2009; 58: 2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng G., Wood K.J., Bushell A.. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: Potential avenues for cellular therapy. Transplantation 2008; 86: 578–89. [DOI] [PubMed] [Google Scholar]
- 3.Davalli A.M., Ogawa Y., Ricordi C., Scharp D.W., Bonner-Weir S., Weir G.C.. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995; 59: 817–20. [PubMed] [Google Scholar]
- 4.Davalli A.M., Ogawa Y., Scaglia L., Wu Y.J., Hollister J., Bonner-Weir S., Weir G.C.. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes 1995; 44: 104–11. [DOI] [PubMed] [Google Scholar]
- 5.Korsgren O., Nilsson B., Berne C., Felldin M., Foss A., Kallen R., Lundgren T., Salmela K., Tibell A., Tufveson G.. Current status of clinical islet transplantation. Transplantation 2005; 79: 1289–93. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q., Ma L.L., Gao X., Yan W., Williams P., Yin D.P.. TLR4 mediates early graft failure after intraportal islet transplantation. Am J Transplant. 2010; 10: 1588–96. [DOI] [PubMed] [Google Scholar]
- 7.Kruger B., Yin N., Zhang N., Yadav A., Coward W., Lal G., Zang W.S., Heeger P., Bromberg J.S., Murphy B., Schroppel B.. Islet-expressed TLR2 and TLR4 sense injury and mediate early graft failure after transplantation. Eur J Immunol. 2010; 40: 2914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka N., Itoh T., Watarai H., Sekine-Kondo E., Nagata N., Okamoto K., Mera T., Yamamoto H., Yamada S., Maruyama I., Taniguchi M., Yasunami Y.. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010; 120: 735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alegre M.L., Goldstein D.R., Chong A.S.. Toll-like receptor signaling in transplantation. Curr Opin Organ Transplant. 2008; 13: 358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzinger P.. Friendly and dangerous signals: Is the tissue in control? Nat Immunol. 2007; 8: 11–13. [DOI] [PubMed] [Google Scholar]
- 11.Andrade C.F., Waddell T.K., Keshavjee S., Liu M.. Innate immunity and organ transplantation: The potential role of toll-like receptors. Am J Transplant. 2005; 5: 969–75. [DOI] [PubMed] [Google Scholar]
- 12.Wild C.A., Brandau S., Lindemann M., Lotfi R., Hoffmann T.K., Lang S., Bergmann C.. Toll-like receptors in regulatory T cells of patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2010; 136: 1253–9. [DOI] [PubMed] [Google Scholar]
- 13.Dai J., Liu B., Ngoi S.M., Sun S., Vella A.T., Li Z.. TLR4 hyper-responsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J Immunol. 2007; 178: 3219–25. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N., Kruger B., Lal G., Luan Y., Yadav A., Zang W., Grimm M., Waaga-Gasser A.M., Murphy B., Bromberg J.S., Schroppel B.. Inhibition of TLR4 signaling prolongs Treg-dependent murine islet allograft survival. Immunol Lett. 2010; 127: 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D.Y., Lee S., Nam J.H., Byun Y.. Minimization of immunosuppressive therapy after islet transplantation: Combined action of heme oxygenase-1 and PEGylation to islet. Am J Transplant. 2006; 6: 1820–8. [DOI] [PubMed] [Google Scholar]
- 16.Ollinger R., Wang H., Yamashita K., Wegiel B., Thomas M., Margreiter R., Bach F.H.. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007; 9: 2175–85. [DOI] [PubMed] [Google Scholar]
- 17.Rocuts F., Zhang X., Yan J., Yue Y., Thomas M., Bach F.H., Czismadia E., Wang H.. Bilirubin promotes de novo generation of T regulatory cells. Cell Transplant. 2010; 19: 443–51. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Lee S.S., Dell'Agnello C., Tchipashvili V., D'Avila J.C., Czismadia E., Chin B.Y., Bach F.H.. Bilirubin can induce tolerance to islet allografts. Endocrinology 2006; 147: 762–8. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H., Wang J., Jiang H., Ma Y., Pan S., Reddy S., Sun X.. Bilirubin protects grafts against nonspecific inflammation-induced injury in syngeneic intraportal islet transplantation. Exp Mol Med. 2010; 42: 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pileggi A., Molano R.D., Berney T., Cattan P., Vizzardelli C., Oliver R., Fraker C., Ricordi C., Pastori R.L., Bach F.H., Inverardi L.. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 2001; 50: 1983–91. [DOI] [PubMed] [Google Scholar]
- 21.Pileggi A., Molano R.D., Berney T., Ichii H., San Jose S., Zahr E., Poggioli R., Linetsky E., Ricordi C., Inverardi L.. Prolonged allogeneic islet graft survival by protoporphyrins. Cell Transplant. 2005; 14: 85–96. [PubMed] [Google Scholar]
- 22.Kirkby K.A., Adin C.A.. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006; 290: F563–71. [DOI] [PubMed] [Google Scholar]
- 23.Adin C.A., Croker B.P., Agarwal A.. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005; 288: F778–84. [DOI] [PubMed] [Google Scholar]
- 24.Barabas K., Milner R., Farese J., Baylis C., Croker B., Archer L., Adin C.. Hyperbilirubinemia's protective effect against cisplatin nephrotoxicity in the Gunn rat. Anticancer Drugs 2008; 19: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Amotz R., Bonagura J., Velayutham M., Hamlin R., Burns P., Adin C.. Intraperitoneal bilirubin administration decreases infarct area in a rat coronary ischemia/reperfusion model. Front Physiol. 2014; 5: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman M.G., Lu D., Kim M.L., Kociba G.J., Shukri T., Buteau J., Wang X., Frankel W.L., Guttridge D., Prentki M., Grey S.T., Ron D., Hai T.. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004; 24: 5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zmuda E.J., Powell C.A., Hai T.. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp. 2011; 50: 2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zmuda E.J., Viapiano M., Grey S.T., Hadley G., Garcia-Ocana A., Hai T.. Deficiency of Atf3, an adaptive-response gene, protects islets and ameliorates inflammation in a syngeneic mouse transplantation model. Diabetologia 2010; 53: 1438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohseni Salehi Monfared S.S., Larijani B., Abdollahi M.. Islet transplantation and antioxidant management: A comprehensive review. World J Gastroenterol. 2009; 15: 1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N.. Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043–6. [DOI] [PubMed] [Google Scholar]
- 31.Carpagnano G.E., Kharitonov S.A., Resta O., Foschino-Barbaro M.P., Gramiccioni E., Barnes P.J.. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003; 124: 1386–92. [DOI] [PubMed] [Google Scholar]
- 32.Stiegler P., Schaffellner S., Hackl F., Iberer F., Aigner R., Christine B., Tscheliessnigg K., Stadlbauer V.. Isoprostanes as markers of oxidative stress-induced cell damage in porcine islet cell isolation. Transplant Proc. 2010; 42: 1618–20. [DOI] [PubMed] [Google Scholar]
- 33.Tsung A., Klune J.R., Zhang X., Jeyabalan G., Cao Z., Peng X., Stolz D.B., Geller D.A., Rosengart M.R., Billiar T.R.. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007; 204: 2913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderwood S.K., Mambula S.S., Gray P.J. Jr, Theriault J.R.. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007; 581: 3689–94. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.S., Gao W., Mazzola S., Thomas M.N., Csizmadia E., Otterbein L.E., Bach F.H., Wang H.. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007; 21: 3450–7. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Lee S.S., Gao W., Czismadia E., McDaid J., Ollinger R., Soares M.P., Yamashita K., Bach F.H.. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes 2005; 54: 1400–6. [DOI] [PubMed] [Google Scholar]
- 37.Turner M.S., Kane L.P., Morel P.A.. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009; 183: 4895–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Windt D.J., Bottino R., Casu A., Campanile N., Cooper D.K.. Rapid loss of intraportally transplanted islets: An overview of pathophysiology and preventive strategies. Xenotransplantation 2007; 14: 288–97. [DOI] [PubMed] [Google Scholar]
- 39.Biarnes M., Montolio M., Nacher V., Raurell M., Soler J., Montanya E.. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002; 51: 66–72. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson P.O., Palm F., Andersson A., Liss P.. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 2001; 50: 489–95. [DOI] [PubMed] [Google Scholar]
- 41.Emamaullee J.A., Shapiro A.M.. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes 2006; 55: 1907–14. [DOI] [PubMed] [Google Scholar]
- 42.Gunther L., Berberat P.O., Haga M., Brouard S., Smith R.N., Soares M.P., Bach F.H., Tobiasch E.. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes 2002; 51: 994–9. [DOI] [PubMed] [Google Scholar]
- 43.Menger M.D., Yamauchi J., Vollmar B.. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001; 25: 509–15. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi H.. Pancreatic islet transplantation. World J Gastro-intest Surg. 2009; 1: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsson P.O., Jansson L., Palm F.. Unaltered oxygen tension in rat pancreatic islets despite dissociation of insulin release and islet blood flow. Acta Physiol Scand. 2002; 176: 275–81. [DOI] [PubMed] [Google Scholar]
- 46.Linn T., Schmitz J., Hauck-Schmalenberger I., Lai Y., Bretzel R.G., Brandhorst H., Brandhorst D.. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006; 144: 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giuliani M., Moritz W., Bodmer E., Dindo D., Kugelmeier P., Lehmann R., Gassmann M., Groscurth P., Weber M.. Central necrosis in isolated hypoxic human pancreatic islets: Evidence for postisolation ischemia. Cell Transplant. 2005; 14: 67–76. [DOI] [PubMed] [Google Scholar]
- 48.Korsgren O., Nilsson B., Berne C., Felldin M., Foss A., Kallen R., Salmela K., Tibell A., Tufveson G., Ekdahl K.N., Elgue G., Korsgren O., Nilsson B.. Current status of clinical islet transplantation. Transplantation 2005; 79: 1289–93. [DOI] [PubMed] [Google Scholar]
- 49.Chen X.B., Li Y.X., Jiao Y., Dong W.P., Li G., Chen J., Tan J.M.. Influence of heme oxygenase-1 gene transfer on the viability and function of rat islets in in vitro culture. World J Gastroenterol. 2007; 13: 1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazaki T., Akiba H., Iwai H., Matsuda H., Aoki M., Tanno Y., Shin T., Tsuchiya H., Pardoll D.M., Okumura K., Azuma M., Yagita H.. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002; 169: 5538–45. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki T., Akiba H., Koyanagi A., Azuma M., Yagita H., Okumura K.. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J Immunol. 2005; 175: 1586–92. [DOI] [PubMed] [Google Scholar]
- 52.Tseng S.Y., Otsuji M., Gorski K., Huang X., Slansky J.E., Pai S.I., Shalabi A., Shin T., Pardoll D.M., Tsuchiya H.. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001; 193: 839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin T., Yoshimura K., Shin T., Crafton E.B., Tsuchiya H., Housseau F., Koseki H., Schulick R.D., Chen L., Pardoll D.M.. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005; 201: 1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao W., Demirci G., Strom Tb, Li X.. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation 2003; 76: 994–9. [DOI] [PubMed] [Google Scholar]
- 55.Wu C., Zhang Y., Jiang Y., Wang Q., Long Y., Wang C., Cao X., Chen G.. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cell Mol Immunol. 2013; 10: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chien V., Aitken J.F., Zhang S., Buchanan C.M., Hickey A., Brittain T., Cooper G.J., Loomes K.M.. The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of beta-sheet-containing aggregates by human amylin: A potential role for defective chaperone biology in Type 2 diabetes. Biochem J. 2010; 432: 113–21. [DOI] [PubMed] [Google Scholar]
- 57.Jang H.J., Kwak J.H., Cho E.Y., We Y.M., Lee Y.H., Kim S.C., Han D.J.. Glutamine induces heat-shock protein-70 and glutathione expression and attenuates ischemic damage in rat islets. Transplant Proc. 2008; 40: 2581–4. [DOI] [PubMed] [Google Scholar]
- 58.Kanitkar M., Bhonde R.R.. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme oxygenase-1, during cryopreservation. Life Sci. 2008; 82: 182–9. [DOI] [PubMed] [Google Scholar]
- 59.Burkart V., Liu H., Bellmann K., Wissing D., Jaattela M., Cavallo M.G., Pozzilli P., Briviba K., Kolb H.. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000; 275: 19521–8. [DOI] [PubMed] [Google Scholar]
- 60.Scarim A.L., Heitmeier M.R., Corbett J.A.. Heat shock inhibits cytokine-induced nitric oxide synthase expression by rat and human islets. Endocrinology 1998; 139: 5050–7. [DOI] [PubMed] [Google Scholar]
- 61.Bellmann K., Jaattela M., Wissing D., Burkart V., Kolb H.. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996; 391: 185–8. [DOI] [PubMed] [Google Scholar]
- 62.Lenzen S.. Oxidative stress: The vulnerable beta-cell. Biochem Soc Trans. 2008; 36: 343–7. [DOI] [PubMed] [Google Scholar]
- 63.McCarty M.F.. “Iatrogenic Gilbert syndrome”—A strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses 2007; 69: 974–94. [DOI] [PubMed] [Google Scholar]


