Abstract
Neovasculogenesis induced by stem cell therapy is an innovative approach to improve critical limb ischemia (CLI) in diabetes. Mesenchymal stem cells (MSCs) are ideal candidates due to their angiogenic and immunomodulatory features. The aim of this study is to determine the therapeutic effects of human placenta-derived MSCs (P-MSCs) on diabetic CLI, with or without exogenous insulin administration, and the underlying mechanism of any effect. A series of in vitro experiments were performed to assess the stemness and vasculogenic activity of P-MSCs. P-MSCs were intramuscularly injected at two different doses with and without the administration of insulin. The efficacy of P-MSC transplantation was evaluated by ischemia damage score, ambulatory score, laser Doppler perfusion image (LDPI), capillary, and vascular density. In vivo imaging was applied to track the implanted P-MSCs. In vivo differentiation and in situ secretion of angiogenic cytokines were determined. In vitro experimental outcomes showed the differentiation potential and potent paracrine effect of P-MSCs. P-MSCs survived in vivo for at least 3 weeks and led to the acceleration of ischemia recovery, due to newly formed capillaries, increased arterioles, and secretion of various proangiogenic factors. P-MSCs participate in angiogenesis and vascularization directly through differentiation and cytokine expression.
Keywords: Placenta-derived mesenchymal stem cells (P-MSCs), Critical limb ischemia (CLI), Angiogenesis, Cell therapy
Introduction
Diabetic foot (DF) is a concomitant illness of diabetes mellitus. Neuropathic and neuroischemic foot are two main types, and around half of patients with a DF ulcer have coexisting peripheral artery disease (PAD) (8). DF is associated with high rates of morbidity and mortality. Amputation is inevitable in many cases as blood capillaries cannot be corrected, and restenosis of vessels is very common. Novel approaches have been introduced into the field of CLI including stem cell therapy, which may be a useful approach to restore blood flow and attenuate ischemic disease.
The therapeutic potential of numerous stem or progenitor cells has been investigated in the treatment of ischemic disease. Stem cells investigated include unfractionated bone marrow cells (BMCs) (5), mononuclear cells (MNCs) (10), endothelial progenitor cells (EPCs) (14), and mesenchymal stem cells (MSCs) (20). However the neovascularized capacity of, for example, EPCs derived from the diabetic patients themselves was found to be impaired (36). Therefore, investigation of the viability of allogenic healthy stem cells in the treatment of ischemia is urgently needed. Our group has begun by treating limb ischemic patients with peripheral blood MNCs (PBMNCs) as these cells may significantly promote vascular repair (10).
MSCs are hypoimmunogenic, immunosuppressive, and capable of pluripotent differentiation into three different lineages. These intriguing properties have had a significant impact on experimental and clinical research in regenerative medicine. MSC transplantation has shown potential as a promising treatment of various diseases including graft-versus-host disease (GVHD) (16), hepatic cirrhosis (35), in autoimmune diseases such as systemic lupus erythematosus (30) and Crohn's disease (21), in brain diseases and spinal cord injury, as well as in critical limb ischemia (CLI) (23). MSCs exist in nearly all parts of the body, but were first isolated from the bone marrow. These bone marrow-derived MSCs (BM-MSCs) have been widely investigated. However, the surgical donor procedure can injure the donor and may also result in limited cell numbers harvested, restricting the practical clinical application of BM-MSCs. Umbilical cord and placenta offer rich alternative sources of perinatal MSCs that are easy to obtain, cause no pain to the donor, and could be largely expanded to a clinical scale of MSC use.
Though the therapeutic neovascularization of MSCs from different sources has already been studied in animal model and clinical trials, the utility of placenta-derived MSCs (P-MSCs) is poorly understood, and the fate of MSCs in vivo is unknown. It is also unknown whether the mechanism of action of MSCs in promoting angiogenesis is through the formation of new blood vessels or through the production of multiple cytokines that support angiogenesis or both. We sought to investigate this and to explore the efficacy of combined regular therapy and cell therapy in diabetic hindlimb ischemia.
Materials and Methods
Isolation and Identification of Human P-MSCs
Human placenta was obtained from full-term cesarean section deliveries with written informed consent of the mother. The use of human-derived cells was approved by the Institutional Biomedical Research Ethics Committee of Chinese Academy of Medical Science and Peking Union Medical College. MSCs were isolated based on methods previously described (24). Briefly, tissues were cut into pieces and digested with collagenase II (Gibco, Grand Island, NY, USA) for 1 h at 37°C and further digested with trypsin (Gibco) for 30 min at 37°C. Cells were collected after washing and seeded in T75 flasks (Corning, Corning, NY, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 media (Gibco) with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA). Cells of passage 5 to 10 were used in this study. Once confluence had been reached, adherent cells (passage 0) were detached with 0.125% trypsin-ethylenediaminetetraacetic acid (EDTA) and passaged at a 1:3 split ratio in the T-75 flask.
For surface phenotype analysis, cells were stained with fluorescein isothiocyanate- or phycoerythrin-conjugated monoclonal antibodies (BD Pharmingen, San Diego, CA, USA); cell surface markers include hematopoietic lineage markers (CD34, CD45, CD14), angiogenic markers (CD31, CD133), adhesion integrins (CD44), human leukocyte antigen (HLA)-ABC and HLA-DR, and MSC markers of CD105 (SH2), CD73 (SH3), and CD90. Cells were then analyzed by flow cytometry with a FACScan cytometer.
For differentiation potential analysis, the cells were plated into 24 wells (Corning) in differentiation induction media (Gibco) for 3 weeks. Alizarin red S, Oil red O, and toluidine blue (Sigma-Aldrich, St. Louis, MO, USA) staining were used to confirm the characteristics of osteocytes, adipocytes, and chondrocytes, respectively.
Cell Culture of Human Foreskin Fibroblast and Human Umbilical Vein Endothelial Cells (HUVECs)
Human foreskin tissue was collected from the local children's hospital (Tianjin Children Hospital) with the consent of the parents. Human foreskin fibroblasts were isolated by one-step enzyme incubation. Briefly, the epidermis was mechanically separated and then digested with both dispase (STEMCELL, Vancouver, BC, Canada) and collagenase I (Gibco) for 1.5 h at 37°C. Further digestion was conducted with 0.25% trypsin for 30 min at 37°C. Cells were collected and cultured in DMEM/F12 media (Gibco) with 10% FBS. The cells were passaged at a 1:3 split ratio once confluence was reached.
Human umbilical cord was obtained from full-term cesarean section deliveries with informed consent of the mother. HUVECs were isolated by one-step enzyme incubation. Briefly, the umbilical vein was washed with phosphate-buffered saline (PBS) using a syringe in order to wash out the cord blood. The ends of the umbilical veins were annulated with a glass tube, which was attached to a rubber tube and secured with a nylon tie. The vein was then infused with 10 ml of 0.25% trypsin-EDTA in PBS and clamped at the rubber tube with a hemostat. The umbilical cord was then incubated at 37°C in a water bath for 15 min. Afterward the hemostat was released, and the solution was flushed out of the umbilical vein. This solution was collected, and the cells were pelleted by centrifuging. The cells were cultured in endothelial cell growth medium (EGM)-2 (Lonza, Allendale, NJ, USA). Upon confluence, the cells were detached with 0.125% trypsin-EDTA and passaged at a 1:3 split ratio.
Cell Culture of UC-MSCs and BM-MSCs
Umbilical cord-derived MSCs (UC-MSCs) and BM-MSCs were prepared as described previously (24). The cells were cultured in DMEM/F12 media (Gibco) with 10% FBS (HyClone). The cells were detached with 0.125% trypsin-EDTA and passaged at a 1:3 split ratio.
Real-Time PCR
The total RNA was extracted following the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA) from human fibroblasts, undifferentiated P-MSCs, and differentiation-induced cells at days 7, 14, 21, and 28. One microgram of total RNA was reversely transcribed into cDNA using random hexamer (Applied Biosystems, Foster City, CA, USA) in 20-μl reactions. Real-time polymerase chain reaction (RT-PCR) analysis was performed in optical eight-tube strips using an ABI 7300 RT-PCR System (Applied Biosystems). PCR primers for peroxisome proliferator-activated receptor g 2(PPARg2), PPARg coactivator 1 a (PPARg-C1a), osterix (OSX), bone sialoprotein (BSP), type I collagen (COL1), core-binding factor a 1 (Cbfa1), and hydroxymethylbilane synthase (HMBS) and proangiogenic factors vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-BB, angiopoietin-1 (Ang-1), endothelium-specific receptor tyrosine kinase (Tie-2), and pigment epithelial-derived factor (PEGF) are shown in Table 1. PCR conditions were 2 min at 95°C, and 40 cycles of 15 s at 95°C and 60 s at 60°C. All reactions were performed in duplicate. Gene expression levels relative to HMBS were calculated using the comparative Ct method.
Table 1.
Oligonucleotide Sequences Used for Real-Time PCR Experiments
| Gene | Upstream Sequence | Downstream Sequence |
|---|---|---|
| PPARγ2 | CAGTGTGAATTACAGCAAACC | ACAGTGTATCAGTGAAGGAAT |
| PPARγ-C1α | GTTCCCGATCACCATATTCCA | GCGGTGTCTGTAGTGGCTTGA |
| OSX | CCCCACCTCTTGCAACCA | GGCTCCACCACTCCCTTCTAG |
| BSP | AAACGAAGAAAGCGAAGCAGAA | GCTGCCGTTGCCGTTTT |
| COL1 | CAGCCGCTTCACCTACAGC | TTTTGTATTCAATCACTGTCTTGCC |
| Cbfa1 | GCAGCACGCTATTAAATCCAAATT | ACAGATTCATCCATTCTGCCACTAG |
| HMBS | GGCAATGCGGCTGCAA | GGGTACCCACGCGAATCAC |
| VEGF | CTACCTCCACCATGCCAAGTG | TGATTCTGCCCTCCTCCTTCT |
| PDGF-BB | GGCCTTCTTAAAGATTGGCTTCT | GCCTCATACCGCACCAAC |
| Ang-1 | GCCATTACCAGTCAGAGGCAG | AATAGGCTCGGTTCCCTTCC |
| Tie-2 | ACTTCACTTACGCGGGCATT | GCCACGTTCTGGCTGGAT |
| PEGF | CAGCTGGAATGGCAGAAACTG | AGGCCCAGAGTCCTGACGGG |
In Vitro Experiment
Differentiations to Vascular Cells
Confluent human P-MSCs were cultured in DMEM/F12 medium supplemented with 2% fetal calf serum (FCS; Hyclone), 100 U/ml penicillin–streptomycin, 50 ng/ml VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and 10 ng/ml basic fibroblast growth factor (bFGF; Sigma-Aldrich) for endothelial cell differentiation. Uptake of DiI-Ac-LDL (Invitrogen) was conducted to verify cellular differentiation toward endothelial phenotype by incubating the postinduced human P-MSCs with 10 μg/ml DiI-Ac-LDL in serum-free medium at 37°C for 4 h.
For smooth muscle cell induction, 10 ng/ml of PDGF-BB (Peprotech, Rocky Hill, NJ, USA) was used. Seven days after induction, immunostaining was performed to detect the expression of endothelial-specific marker von Willebrand Factor (vWF; Abcam, Cambridge, MA, USA) or smooth muscle cell marker smooth muscle α-actin (α-SMA; Chemicon, Billerica, MA, USA).
Tube Formation Assay
Matrigel (Sigma-Aldrich) was thawed on ice to prevent premature polymerization; aliquots of 50 μl were plated into individual wells of 96-well tissue culture plates (Corning) and polymerized at 37°C for 30 min. The cells were washed in serum-containing medium and then resuspended at 106 cells/ml. The cell suspension (100 μl) was added to each well supplemented with 10 ng/ml VEGF (Santa Cruz Biotechnology) and incubated for 2 to 4 h at 37°C before being visualized under phase-contrast microscopy (Olympus, Tokyo, Japan) (22).
Matrigel Plug Assay
Matrigel (500 μl) containing green fluorescent protein (GFP)-labeled P-MSCs (2 × 106) or PBS was injected subcutaneously into the abdomens of nude mice. Three weeks afterward, the Matrigel was removed and frozen for cryosectioning. The blood vessels were stained with vWF in the slides and examined under confocal microscope.
Cytokine Release From Human P-MSCs, BM-MSCs, and UC-MSCs
Cells were cultured in 20% O2 and 5% CO2 during normoxia experiments, or in 3% O2 and 5% CO2 using a hypoxia chamber for hypoxia experiments. Cell-conditioned media (CM) was collected after 24 h of culture, centrifuged at 2,000 rpm for 10 min, and passed through a 0.22-μm filter. The experiments were performed in triplicate. The concentrations of P-MSC, BM-MSC, UC-MSC, and HUVEC CM-containing cytokines, such as VEGF and bFGF, were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits (Biolegend, San Diego, CA, USA).
Cell Proliferation Assay
HUVECs (1 × 104/well) were plated in 24-well plates in DMEM/F12 with 0.1% FCS for 24 h to arrest mitosis. Afterward the media was replaced with CM of P-MSCs, UC-MSCs, or BM-MSCs, recombinant VEGF 4 ng/ml (positive control), DMEM/F12 (normal control), and boiled P-MSC CM (negative control). Cultures were continued for 72 h, after which the cell number was determined by cell counting. The experiments were performed in triplicate. Data are reported as the mean cell number of each group.
Wound Healing Assay
HUVECs were seeded in 35-mm dishes with culture insert (Ibidi, Martinsried, Germany) at a concentration of 6 × 105 cells/ml. After 24 h, the culture inserts were gently removed, and a 500-μm-wide zone was left between two cell patches. After washing with PBS, CM of P-MSCs, UC-MSCs, or BM-MSCs, recombinant VEGF (4 ng/ml) and DMEM/F12 were added to the dishes. The photos of cell migration were taken under invert microscopy at 40× magnification at 0, 6, and 24 h. Wound confluence was measured using Image analysis software (Ibidi).
Cell Migration Assay
The effects of VEGF and PDGF-BB on MSC migration were determined using Transwell assays (Corning). P-MSCs were seeded in the upper chamber at 1 × 105/ml. The bottom chamber contained VEGF (10 ng/ml), PDGF-BB (10 ng/ml), or both in medium with 2% FCS. The medium with 2% FCS only was a negative control. Duplicate wells were set up for each group in this experiment. After 5 h of incubation, the cells in the lower wells were fixed and examined with hematoxylin and eosin (H&E; Sigma-Aldrich) stain. Microscopic photographs were taken to determine the migrating cells.
In Vivo Experiment
Establishment of Ischemic Hindlimb Model in Diabetic Rat
Immunodeficient male nude rats of 6 weeks of age were purchased from Vital River Laboratories (Charles River Laboratories Supplier in China, Beijing, P.R. China). All animals used in this study were treated in accordance with the guidelines of the institutional animal care and use committee (IACUC) of the Chinese Academy of Medical Science and Peking Union Medical College. Diabetes was induced with a single intraperitoneal (IP) injection of streptozotocin (70 mg/kg in citrate buffer solution, only prepared immediately prior to injection) after overnight fasting (27). Fasting plasma glucose levels were measured by blood glucose meter (Accu-Chek, Roche, Basel, Switzerland) every week and rats with plasma glucose between 11 and 15 mM were considered to be diabetic. Age- and weight-matched nude rats receiving an IP citrate buffer injection were used as nondiabetic controls (glycemia between 5.5 and 8 mM).
Two weeks later, diabetic nude rats were anesthetized (IP; 60 mg/kg pentobarbital from Merck, Darmstadt, Germany), and the left femoral artery was occluded by ligating it with 3-0 silk. The ligature was applied 0.5 cm proximally to the bifurcation of the saphenous and popliteal arteries. Lipiodol (Guerbet, Villepinte, France, 1.5 ml/kg) was used to induce an embolism intravascularly before ligation. A sham ligature was applied to the left femoral artery with the left hindlimb remaining nonischemic (32).
Animal Grouping and Human P-MSC Transplant
The nude rats were randomly divided into seven groups, and 10–15 rats per group were enrolled. The groups are as follows: first diabetic ischemic nude rats transplanted with P-MSCs at 2 × 106 (high-dose group) or 0.5 × 106 (low-dose group); diabetic ischemic nude rats treated with insulin (Novo Nordisk) alone (insulin group); the combination therapy groups of diabetic ischemic nude rats treated with insulin and P-MSCs at 2 × 106 (high dose + insulin group) or 0.5 × 106 (low dose + insulin group). Finally the control groups included diabetic nude rats with sham surgery and transplanted with vehicle (Sham group), and diabetic ischemic nude rats transplanted with vehicle (PBS group).
The cell transplant was performed 6 h after the onset of ischemia. Animals were fixed by hand without anesthesia. Cells were suspended in 200 μl of DPBS and injected into the vastus laterials and adductor magnus at four different points. The diabetic ischemic nude rats in the insulin group and the combination therapy groups received long-term intensified insulin (∼2–4 U/day, given subcutaneously daily) to normalize their glycemia.
In Vivo Imaging
In order to track the cells in vivo, the cells were transfected with lentiviral vector-expressing luciferase and GFP. The labeling efficiency was detected under fluorescence microscope (Olympus) or flow cytometry. The distribution and survival of luciferase-expressing cells were assessed at different time points using an IVIS 200 Series Imaging System (PerkinElmer, Waltham, MA, USA). The distribution and fluorescence intensity of labeled cells were monitored weekly after MSC injection until sacrifice. The animal was injected by an IP route with d-luciferin (Gold Biotechnology, St. Louis, MO, USA) prior to anesthesia (60 mg/kg pentobarbital IP). Photons emitted from luciferase-expressing cells within the animal body were quantified using the software program “Living Image” (Xenogen, Caliper Life Sciences, Hopkinton, MA, USA) (19).
Laser Doppler Perfusion Imaging
Laser Doppler perfusion imaging (LDPI; PerimedPIM II, Stockholm, Sweden) was performed to provide functional evidence for ischemia-induced changes in vascularization. The rats were placed on a heating plate at 37°C to minimize temperature variations (29). LDPI is expressed in color-coded images; these represent blood flow distribution. High blood flow is depicted in red, and low or no perfusion is displayed as dark blue. The LDPI index was defined as the ratio between ischemic and nonischemic leg cutaneous blood flows in the same animal.
Microangiography
Microangiographic analysis (Faxitron, Tucson, AZ, USA) was performed by assessment of capillary density. The animals were anesthetized (pentobarbital, 60 mg/kg IP) and contrast medium barium sulfate (1 g/ml; Huber, Atlanta, GA, USA) was injected through a catheter introduced into the abdominal aorta. Images acquired by a digital X-ray transducer were assembled to obtain a total view of the hindlimbs. The angiographic score was expressed as a percentage of pixels occupied by the blood vessels in the quantification area (36). The angiogram was performed 3 weeks after model establishment.
Clinical Scoring
The clinical indications in all groups were scored according to the ischemia and function grading; the scoring was single blinded. Functional grading was performed according to the Tarlov scale and a standardized limb ischemia grading scale as follows:
Tarlov Score Function: 0, no movement; 1, barely perceptible movement, no weight bearing; 2, frequent and vigorous movement, no weight bearing; 3, supports weight, may take 1 or 2 steps; 4, walks with only mild deficit; 5, normal but slow walking; 6, full and fast walking. Ischemia Score Tissue grade: 0, auto amputation > half lower limb; 1, gangrenous tissue > half foot; 2, gangrenous tissue < half foot, with lower limb muscle necrosis; 3, gangrenous tissue < half foot, without lower limb muscle necrosis; 4, pale foot or gait abnormalities; 5, normal.
Immunohistochemical Staining
For cryosectioning, unfixed adductor muscles were removed 21 days after ischemia and immediately frozen in isopentane in liquid N2 for later inclusion in optimal cutting temperature (OCT) compound (Bio Optica, Milan, Italy) and sectioning at 8 μm into a cryostat. For histology, the dissected adductor muscles were embedded in paraffin and sectioned at 5 μm. H&E staining was performed, and capillaries and small vessels were counted in five nonoverlapping fields of each section under light microscopy at 40× magnification by a blinded observer.
To determine the differentiation of human P-MSCs in vivo, the coexpression of GFP and vWF (or α-SMA) was examined in the adductor muscles. To determine the in vivo cytokine secretion of human P-MSCs, dual immunofluorescent staining was performed for GFP and VEGF (PDGF-BB or bFGF) in the adductor muscles. The stained sections were observed under a laser-scanning confocal fluorescence microscope (Leica TCS SP2, Leica, Wetzlar, Germany).
Statistical Analysis
Data are presented as mean ± SD. One-way analysis of variance (ANOVA) was used to make comparisons of parameters among groups with appropriate post hoc testing. Chi-square test was used for counting data. A value of p < 0.05 was considered significant. Graphpad 5.0 software (La Jolla, CA, USA) and SPSS 18.0 software (IBM, Armonk, NY, USA) were used for the statistical tests.
Results
Identification of Human Placenta-Derived MSCs (P-MSCs)
P-MSCs in culture are spindle shaped, are plastic adherent, and have fibroblast-like properties (Fig. 1A). Fluorescence-activated cell sorting (FACS) analyses demonstrated that P-MSCs express significant amounts of MSC markers (CD90, CD105, CD73), adhesion molecules CD44, and HLA-ABC, but not hematopoietic lineage markers (CD34, CD45, CD14), angiogenic markers (CD133, CD31), or HLA-DR (Table 2).
Figure 1.

In vitro differentiation of placenta-derived mesenchymal stem cells (P-MSCs) into osteocytes, adipocytes, and chondrocytes. (A) P-MSCs showed fibroblast-like morphology in the growth medium. (B) Under adipogenic conditions, P-MSCs were induced to differentiate into adipocytes, which were positive for Oil red O staining. (C) P-MSCs differentiated into osteocytes using differentiation induction media and stained positive for Alizarin red. (D) P-MSCs were induced to differentiate into chondrocytes and stained positive for Alcian blue (original magnification: 100× scale bars: 100 μm). (E) Relative gene expression of peroxisome proliferator-activated receptor g (PPARg)2 and PPARg-C1α in adipogenic differentiation-induced cells at different time points. (F) Relative gene expression of osterix (OSX), bone sialoprotein (BSP), type I collagen (COL1), and core binding factor α 1 (Cbfa1) in osteogenic differentiation-induced cells at different time points (*p < 0.05, **p <0.01, ***p < 0.001).
Table 2.
P-MSC Surface Phenotype
| Surface Markers | Positive Percentage |
|---|---|
| Hematopoietic markers | |
| CD34 | 0.25% |
| CD45 | 0.37% |
| CD14 | 1.01% |
| MHC | |
| HLA-ABC | 99.49% |
| HLA-DR | 0.12% |
| Adhesion molecular | |
| CD44 | 98.89% |
| Mesenchymal stem cell markers | |
| CD73 | 96.58% |
| CD105 | 97.78% |
| CD90 | 98.36% |
| Angiogenic markers | |
| CD133 | 0.95% |
| CD31 | 0.91% |
P-MSCs have the potential to differentiate into adipocytes, osteocytes, and chondrocytes after differentiation induction in vitro. P-MSCs formed visible lipid droplets after 14 days of adipogenic induction, which accumulated with continued incubation. After 28 days of induction, lipid droplet presence was confirmed by Oil red O staining (Fig. 1B). P-MSCs were positively stained with Alizarin red after 28 days, which indicates the induction of osteogenic differentiation, confirming the characteristics of osteocytes (Fig. 1C). P-MSCs were positively stained with toluidine blue after 28 days of induction, confirming the characteristics of chondrocytes (Fig. 1D). The adipogenic induced cells expressed PPARγ2, a key regulator of adipogenesis, which initiates adipocyte differentiation (34). PPARγ2 expression peaked at day 7 and then dropped. The induced cells also expressed PPARγ-C1α, another differentiation marker, whose expression increased until achievement of mature adipogenesis (Fig. 1E). The expressions of osteoblast-related genes were upregulated after osteogenic induction. Genes such as OSX (an osteoblast-specific transcription factor essential for osteoblast differentiation and bone formation), COL1 (which is necessary to induce osteoblastic differentiation), and BSP (which is characteristic of late stages of osteoblastic differentiation) reached peak expression at day 35. Expression of Cbfa1 (a transcriptional activator for osteoblast) tripled at day 21 and then subsequently decreased (Fig. 1F). These results indicated that P-MSCs are capable of differentiating into adipocytes, osteocytes, or chondrocytes in response to different combinations of growth factors.
Differentiation Into Vascular-Like Cells and CapillaryLike Structure Formation by P-MSCs
P-MSCs were found to be capable of differentiating into vascular cells under differentiation-inducing culture conditions. P-MSCs were negative for vWF expression or acetylated low-density lipoprotein (ac-LDL) uptake. However in the presence of VEGF and bFGF for 10 days, P-MSCs acquired an endothelial morphology, expressed vWF, and were positive for ac-LDL uptake (Fig. 2A), indicating that the P-MSCs had differentiated into endothelial-like cells. In response to PDGF-BB, P-MSCs were induced to express α-SMA, a typical smooth muscle cell surface marker (Fig. 2B). Vascular smooth muscle cell differentiation was achieved after 10 days in the conditioning medium. This in vitro induction experiment demonstrated that P-MSCs are capable of differentiating across various lineages into both endothelial-like and smooth muscle-like cells.
Figure 2.

In vitro induction of P-MSCs into vascular-like cells, capillary-like structure-forming assay, and Matrigel plug assay. (A) Endothelial differentiation was induced by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) for 10 days. The induced cells expressed endothelial-specific marker von Willebrand factor (vWF, green) and uptake of DiI-Ac-LDL (red); the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; top). P-MSCs in growth medium were negative for vWF staining or DiI-Ac-LDL uptake (bottom). Scale bar: 20 μm. (B) Vascular smooth muscle cells were induced by platelet-derived growth factor (PDGF)-BB for 10 days. The induced cells expressed vascular smooth muscle-specific marker α-smooth muscle actin (α-SMA, green); the nuclei were stained with DAPI (top). P-MSCs in growth medium did not express α-SMA (bottom). Scale bar: 20 μm. (C) The cells were seeded on Matrigel supplemented with VEGF and incubated for 2–4 h. Representative microscopic photos were taken to assess the tube formation ability of P-MSCs (top). Human fibroblast is a negative control (bottom); original magnification: 100×. (D) Assessment of angiogenesis in Matrigel plug was achieved by injection of green fluorescent protein (GFP)-expressing P-MSCs (2 × 106) or phosphate-buffered saline (PBS) subcutaneously into nude mice, 3 weeks afterward, abundant capillary network was formed in Matrigel with P-MSCs, blood vessels were stained with vWF (red), the nuclei were stained with DAPI (top), less blood vessels were shown in Matrigel containing PBS (bottom). Scale bar: 100 μm. (E) GFP-expressing P-MSCs (green) expressed vWF (red) in Matrigel; the nuclei were stained with DAPI. Scale bar: 40 μm. (F) Gene expression of proangiogenic factors in P-MSCs and fibroblasts (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001).
The tube-forming ability of the cells in vitro was tested by culturing the cells on Matrigel in the presence of VEGF. P-MSCs were capable of forming capillary-like structure on Matrigel in 4 h, whereas fibroblasts failed to do so (Fig. 2C). Matrigel plug assays were performed to determine the new blood vessel-forming ability of P-MSCs in vivo. Three weeks after Matrigel implantation subcutaneously, the capillary network was abundant in the Matrigel containing P-MSCs compared to the Matrigel containing fibroblasts. The blood vessels were mainly formed by murine cells, which were attracted by P-MSCs (Fig. 2D). However we also observed that vascular cells differentiated from P-MSCs, which co expressed vWF and GFP (Fig. 2E). These results revealed the potential of P-MSCs in blood vessel formation in vitro and their participation in ischemic neovascularization in vivo by supplying cytokines and/or by directly differentiating into vascular cells.
In addition, P-MSCs express proangiogenic factors VEGF, PDGF-BB, Ang-1, and Tie-2; these expression levels were significantly higher than those in fibroblasts. The expression of PEGF in P-MSCs is very low compared with that in fibroblasts. These results indicated that P-MSCs were able to supply proangiogenic cytokines.
Cytokine Secretion by P-MSCs Enhance HUVEC Proliferation and Migration, and P-MSCs Had the Strongest Capacity Among Different MSCs
Proangiogenic factors VEGF and bFGF were detected by ELISA in CM of P-MSCs, UC-MSCs, and BM-MSCs. VEGF secretion by P-MSCs per 106 cells was 302 ± 25.79 pg/ml after 24 h of culture, which was similar to the levels in HUVECs. VEGF levels in BM-MSCs and P-MSCs are significantly higher than that in UC-MSCs; BM-MSCs secreted the highest level of VEGF among them (Fig. 3A). The bFGF expression in the P-MSC CM was about 50 ± 30.72 pg/ml after 24 h in culture, which was similar to that in UC-MSCs and less than that in HUVECs. BM-MSCs secreted the lowest level of bFGF among them (Fig. 3B). In summary, different MSCs have different cytokine profiles.
Figure 3.
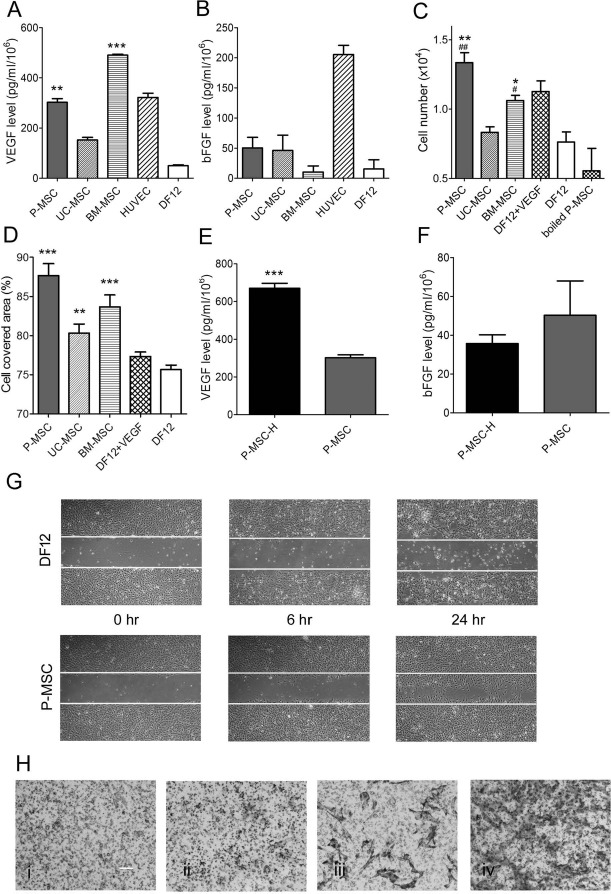
Different mesenchymal stem cells (MSCs) have different cytokine profiles, and P-MSCs possess the strongest capacity on promoting human umbilical vein endothelial cell (HUVEC) proliferation and migration. Cell-conditioned medium (CM) of P-MSCs, umbilical cord-derived MSCs (UC-MSCs), and bone marrow-derived MSCs (BM-MSCs) was collected after 24 h of culture. The concentrations of cytokines VEGF (A) and bFGF (B) were measured in the conditioned media using enzyme-linked immunosorbent assay (ELISA) kits. Growth medium served as negative control, and HUVEC-conditioned medium served as positive control. (C) Cell viability of HUVECs cultured in conditioned medium for 72 h was determined by cell counting. DF12 containing 4 ng/ml of VEGF was positive control; growth medium and boiled P-MSC CM were negative control. The experiments were done in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 versus Dulbecco's modified Eagle's medium (DMEM)/F12 (DF12 in figure), #p < 0.05, ##p < 0.01 versus UC-MSCs. (D) Cell-covered area of HUVECs stimulated by CM of P-MSCs, UC-MSCs, and BM-MSCs at 24 h. The experiments were done in triplicate. **p < 0.01, ***p < 0.001 versus DF12. (E) VEGF expression of P-MSC under normal and hypoxia conditions (n = 3, ***p < 0.001). (F) bFGF expression of P-MSC under normal and hypoxia conditions (n = 3). (G) HUVEC migration in CM of P-MSCs, UC-MSCs, and BM-MSCs at 0, 6, and 24 h. (H) Cell migration of 1 × 105 of P-MSCs in response to VEGF and PDGF-BB separately or simultaneously was determined using Transwell system. The migrating cells in the lower wells were fixed and stained with H&E staining after 5 h of incubation. The experiments were performed in duplicate. Representative microscopic photographs were taken to determine the migrating cell response to medium with 2% fetal calf serum (FCS) (i), containing VEGF (ii), PDGF (iii), and both (iv) (original magnification in pictures: 100×; scale bar: 100 μm).
To explore the effect of MSCs on vascular cell, HUVECs were cultured in CM of P-MSCs, UC-MSCs, and BM-MSCs collected after 24 h of culture. The proliferation of HUVECs was significantly increased in P-MSC and BM-MSC CM, but not in UC-MSC CM compared to normal control (the cells were cultured in DMEM/F12 medium) (Fig. 3C). The migration of HUVECs was significantly promoted in P-MSC, UC-MSC, and BM-MSC CM compared to normal control (Fig. 3D and G). The results showed that P-MSC CM had the strongest capacity to promote HUVEC proliferation and migration compared with other MSCs, even higher than the positive control (in the presence of 4 ng/ml VEGF).
Hypoxia stimulated VEGF secretion in P-MSCs. P-MSCs at the number of 106 expressed VEGF levels of 670 ± 45.46 pg/ml, which is double that of the normoxia culture (p < 0.001) (Fig. 3E). The bFGF level in P-MSC CM did not respond to hypoxia (Fig. 3F).
The effects of VEGF and PDGF-BB on P-MSC migration were determined using Transwell assays. After 5 h of culture, the cells migrated through Transwell in response to VEGF and PDGF-BB, respectively (Fig. 3G); this effect was more efficient when the two cytokines were added simultaneously. These results manifested the chemotactic effects of VEGF and PDGF-BB on P-MSC migration.
P-MSC Transplants Improve Ischemia and Functional Recovery, and Combination Therapy of P-MSCs and Insulin Did Not Show Increased Recovery
P-MSCs failed to regulate plasma glucose level through intramuscular injection. The fasting plasma glucose level of the high-dose group and low-dose group was about 15 mmol/L after 3 weeks of treatment, which was similar to that in the PBS group (13 mmol/L, p > 0.05) (Fig. 4A). Body weight was lost after surgery, but subsequently it increased over time. Locally cell transplant had no effort to body weight gain. Neither the combination therapy group nor insulin treatment group made significant improvements in body weight gain (p > 0.05) (Fig. 4B).
Figure 4.

Evaluation of clinical indicators. Fasting blood glucose levels (A) and body weights (B) were measured weekly. Ischemia damage (C) and ambulatory impairment (D) were evaluated by clinical scoring from the establishment of the diabetic hindlimb ischemic model. n = 10–15, *p < 0.05, **p < 0.01 versus PBS group. (E) Representative photographs of ischemia grading are shown.
P-MSCs improved ischemia damage and functional recovery in diabetic rats; however, the combination therapy of cell treatment and insulin injection did not show increased improvement. After the ischemia model was established, the surgical limb deficiency was obvious as the limb could no longer support any weight, and the paw was swollen and red. Over time the ischemic damage improved to different degrees in all the groups. The recovery of ischemic damage was significantly improved by cell therapy at high dose at days 17 and 28, but not by insulin injection alone or by combination therapy (Fig. 4C). The recovery of limb function was significantly increased in both the high- and low-dose groups, but not by insulin injection alone or combination therapy (Fig. 4D).
P-MSCs Promote Vessel Reconstruction and Improve Blood Supply in the Surgical Hindlimb
In order to look for functional evidence of ischemia-induced changes in vascularization, LDPI was performed. The image in Figure 5A shows that blood flow was completely blocked in the left hindlimb on the day of surgery; this is indicated by the deep dark blue color. Three weeks later blood flow perfusion was restored to some degree in all the groups but did not return to normal in the PBS group where the ischemia/nonischemia perfusion ratio only achieved 57%. The perfusion recovery in the cell transplant groups was significantly higher. The ratio was 89% in the high-dose group (p < 0.01 vs. PBS group) and 83% in the low-dose group (p < 0.05 vs. PBS group) as determined by quantitative analysis. Insulin was found to have a positive but not statistically significant effect on perfusion recovery; however, the perfusion restoration was lower in the combination therapy groups (74% in high dose + insulin group and 55% in low dose + insulin group), and when compared to the PBS group the difference is not statistically significant (Fig. 5A and B). The results revealed that P-MSCs improve blood flow perfusion and that the higher dose of cells is more effective in doing so. In fact high-dose P-MSCs showed significant improvement in the restoration of blood flow when compared to the PBS groups.
Figure 5.

Pathological examination by laser Doppler perfusion imaging (LDPI), angiography, and immunohistology. (A) Representative LDPI images obtained on postoperative day 0 and day 21. In color-coded images, high blood flow is depicted in red, while low perfusion is displayed as blue to dark blue. (B) Quantitative analysis of perfusion recovery measured by ischemia/nonischemia perfusion ratio at postoperative day 21. (C) Representative angiograms obtained on postoperative day 21. (D) The angiographic score measured as a percentage of pixels occupied by the blood vessels in the quantification area at postoperative day 21. (E) Histological analysis of long adductor muscle of surgical limbs in each group was shown (original magnification in pictures: 100×; scale bar: 100 μm). (F) Quantitative analysis of capillary number in histological sections. (G) Immunofluorescence analysis of cytokines VEGF and PDGF in the high-dose group, high-dose + insulin group, and PBS group; nuclear staining with DAPI (scale bar: 40 μm). n = 6–11, *p < 0.05, **p < 0.01, ***p < 0.001 versus PBS group.
The arteriole density in the ischemic hindlimb of nude rats was assessed by microangiography. The arteriole density was 45% in the PBS group, which is significantly less than that in the sham group (p < 0.01). The arteriole density in the cell transplant groups was obviously augmented compared to that in the PBS groups (p < 0.001 vs. high-dose group or low-dose group) (Fig. 5C and D). Insulin injection alone did not enhance arteriole and capillary density significantly, but the combination therapy significantly improved the vessel formation (p < 0.05 vs. high-dose + insulin group, p < 0.05 vs. low dose + insulin group). These data revealed an augmentation of arteriole density in the ischemic hindlimb after P-MSC transplantation.
The histological data showed that the capillary density was increased in the cell transplant groups compared to the PBS group or sham group (Fig. 5E). More capillary numbers were observed in the cell transplant groups in comparison to the PBS group (p < 0.05 and p < 0.01) (Fig. 5F). Immunofluorescent staining results suggested that there was a large amount of VEGF and PDGF-BB expressed in the high-dose group, compared to the PBS group, and high-dose + insulin group expressed less amount of VEGF and PDGF-BB compared to the high-dose group (Fig. 5G).
Dynamic Change of P-MSCs In Vivo
To study the distribution and survival of P-MSCs in vivo, in vivo imaging was performed. The results indicated that P-MSCs could not propagate in vivo after local transplantation. The cell number decreased over time; 45% of cells were lost by day 7, and 98% of cells were lost by day 14. Cells still remained in the ischemic site by day 21, but the cell number had decreased to only 1.5% of the original number (Fig. 6A and B).
Figure 6.

In vivo imaging of P-MSC and in vivo differentiation and secretion of P-MSCs. (A) The luciferase-expressing cells were tracked in vivo using an IVIS 200 series imaging system. Distribution of P-MSCs in the injured region was determined from postoperative day 0 to day 21. (B) Quantitative analysis of photo intensity at the ischemic hindlimb. (C) Representative confocal microscopic photographs obtained on day 21 of cell treatment group. GFP-labeled P-MSC coexpressed GFP (green) and α-SMA (red, top). GFP-labeled P-MSC also coexpressed GFP (green) and vWF (red, bottom). Nuclear staining was performed using DAPI (scale bar: 40 μm). (D) GFP-labeled P-MSC expressed VEGF (top), PDGF (middle), and b-FGF (bottom) in vivo. Nuclear staining was performed using DAPI (scale bar: 40 μm).
Immunofluorescent staining data showing GFP-expressing P-MSCs presented in the ischemic adductor muscle of the rats (Fig. 6C and D). These results confirmed that the cells still existed at the ischemic site after at least 21 days, although the cell number had decreased sharply.
P-MSCs Participate in Angiogenesis and Vascularization Through Direct Differentiation and Cytokine Secretion
To investigate if P-MSCs differentiate in vivo, double stains of GFP and SMA (or vWF) were analyzed in frozen sections of ischemic adductor muscle. GFP+ P-MSCs expressed SMA and vWF (Fig. 6C), demonstrating that the cells participate in blood vessel formation by differentiating directly into vascular cells.
The coexpression of bGFP and cytokines such as VEGF, PDGF-BB, and bFGF was observed in the ischemic adductor muscle (Fig. 6D). Thus, the cells indirectly participated in blood vessel formation by secreting VEGF, PDGF-BB, and bFGF.
Discussion
This study focuses on the therapeutic neovascularization effect of placental MSCs and provides insights into their potential for clinical use as a cell-based therapy with a combination of insulin injection for critical hindlimb ischemia in diabetes. The cells participate in angiogenesis and therapeutic vascularization to improve ischemia and restore blood flow perfusion by directly differentiating into vascular cells. They also accelerate collateral vessel formation via secretion of proangiogenic cytokines. Though insulin has a positive effect on angiogenesis, it did not cooperate with the therapeutic effect of P-MSCs. This study indicates that cell therapy may be a promising new approach for diabetic CLI.
Both of our in vitro and in vivo experimental data imply that P-MSCs improved ischemia recovery in diabetic rats via direct de novo differentiation and paracrine mechanisms. Although two mechanisms exist simultaneously according to our observations, the paracrine mechanisms are more important than direct differentiation. The cells need 10 days to convert to vascular cells based on our in vitro experiments, while the cell number decrease to 55% 7 days posttransplant, and only 2% of cells exist by day 14. Thus, only a few of the cells successfully differentiated into vascular cells in vivo, while most of the cells are lost before in vivo differentiation. Instead the infused cells secreted a number of proangiogenic cytokines within a short time posttransplant, and the levels of these cytokines were largely enhanced under the pressure of hypoxia in vivo. Therefore, we believe that these cytokines secreted by MSCs attract endothelial cells from circulation in order to participate in neovascularization and inhibit the inflammation at the injured site. The MSCs may exert their therapeutic potential less through their multidifferentiation potential but more through their paracrine secretion, homing, and immunomodulation, which have previously been suggested (1). In addition, the exosomes released from MSCs may also participate in neovascularization and anti-inflammation after MSC transplantation (7,9).
According to our observation, P-MSCs have little impact on plasma glucose level regulation in vivo, although the in vitro differentiation of MSCs into insulin-producing cells is well documented (6), and a variety of animal models have shown a beneficial effect of MSC transplantation on glycemic control (4,18,25). We suspect that this property of MSCs is dependent on the route of administration and the microenvironment surrounding them. The ischemic environment triggers the cells to differentiate into vascular cells and secrete proangiogenic cytokines rather than triggering them to act in glycemic regulation.
Systemic transplant is presumed to be an effective way for plasma glucose regulation. The mechanism of action might be due to the direct effect of the MSCs differentiating into cells capable of producing insulin (4) or an indirect effect of cell secretion of immunomodulators, which prevent endogenous T cells from eliciting pancreatic β-cell destruction (25). Intramuscular injection was found to be efficient for neovascularization in situ and represents a safer method of clinical application. However, systemic injection may also benefit neovascularization and is more effective for glycemic control.
Insulin has previously been shown to have positive effects on perfusion recovery in experimental clinical trials (31). Insulin may mobilize circulating progenitor cells and increase clonogenic and angiogenic potential of EPCs (11). However, in this study we observed that insulin treatment alone did not significantly augment the vessel formation, though it has a positive trend toward vessel formation. Combined therapy of cell transplant and insulin injection was found to affect the beneficial effect of cell therapy. These data indicate that the efficacy of combined insulin administration and cell therapy on vascularization may not be synergistic. Some investigators showed that cell transplant reduced insulin requirement (2), but the impact of combined therapy on vascularization requires further study.
The origin of tissue influences the plasticity of MSCs. According to our observation, P-MSCs are efficient at differentiating into adipocytes but are less efficient at differentiating into osteocytes than UC-MSCs (data not shown), although they showed similar morphology. P-MSCs expressed a number of proangiogenic factors, which play a key role in the neovascularization. P-MSCs possess a more powerful paracrine effect on endothelial cell proliferation compared to UC-MSCs and BM-MSCs, and P-MSCs have the strongest capacity to promote HUVEC proliferation compared with other MSCs. However, P-MSCs do not express the highest level of cytokines. In our study, we found that P-MSCs are prone to generate endothelial-like cells. These results are in agreement with previous reports that P-MSCs are predominantly directed toward the endothelial lineage, which could make P-MSCs an ideal biological source for cell therapy of ischemic disease (26). Other investigators have observed opposite results due to heterogeneity of the cells or different culture conditions (28). P-MSCs also express the highest level of CD106 compared with other MSCs according to our previous investigations, which is associated with the immuno regulatory properties of the cells (33).
A growing body of studies focusing on the therapeutic effect of MSCs in diabetes or its complication have been performed in the last 10 years. A clinical trial involving 30 patients investigating the treatment of diabetic ulcers using bone marrow-derived CD90+ MSCs has been completed. Improvement of the microcirculation was observed, and MSC therapy potentially supported wound healing in DF patients (13). A pilot study using human term P-MSCs on type 2 diabetes has been carried out by our group; a total of 10 patients were included in this study. Cell transplant rescued patients from pancreatic islet cell dysfunction and improved renal and cardiac functions to varying degrees; no side effects were observed (12). Das et al. observed significant pain relief, ulcer healing, and blood flow restoration in CLI patients after intra-arterial allogenic MSC transplantation (3). Adipose-derived MSCs (AD-MSCs) have been reported to improve the formation of numerous collateral vessels after multiple intramuscular transplants in CLI patients (17). A phase II study using a combination of MSCs and EPCs for the treatment of CLI patients was considered to be efficacious as the walking function and blood perfusion were largely improved (15).
So far MSC therapy represents a simple, safe, and effective therapeutic approach for diabetes and its complications. These studies lay the groundwork for the transition from the experimental bench to the clinical bedside.
Acknowledgments
The authors thank Maura Hannon from Cork, Ireland, for her English editing of the manuscript. This work was supported by Servier Phamaceutical Co., Ltd. France. The authors declare no conflicts of interest.
References
- 1.Bernardo M. E.; Pagliara D.; Locatelli F.. Mesenchymal stromal cell therapy: A revolution in Regenerative Medicine? Bone Marrow Transplant. 47: 164–171; 2012. [DOI] [PubMed] [Google Scholar]
- 2.Cai J.; Wu Z.; Xu X.; Liao L.; Chen J.; Huang L.; Wu W.; Luo F.; Wu C.; Pugliese A.; Pileggi A.; Ricordi C.; Tan J.. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: A pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 39(1): 149–157; 2016. [DOI] [PubMed] [Google Scholar]
- 3.Das A. K.; Bin Abdullah B. J.; Dhillon S. S.; Vijanari A.; Anoop C. H.; Gupta P.K.. Intra-arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: Report of a phase I study. World J. Surg. 37(4): 915–922; 2013. [DOI] [PubMed] [Google Scholar]
- 4.Ezquer F. E.; Ezquer M. E.; Parrau D. B.; Carpio D.; Yañez A. J.; Conget P. A.. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transplant. 14(6): 631–640; 2008. [DOI] [PubMed] [Google Scholar]
- 5.Gabr H.; Hedayet A.; Imam U.; Nasser M.. Limb salvage using intramuscular injection of unfractionated autologous bone marrow mononuclear cells in critical limb ischemia: A prospective pilot clinical trial. Exp. Clin. Transplant. 9(3): 197–202; 2011. [PubMed] [Google Scholar]
- 6.Guo Q. S.; Zhu M. Y.; Wang L.; Fan X. J.; Lu Y. H.; Wang Z. W.; Zhu S. J.; Wang Y.; Huang Y.. Combined transfection of the three transcriptional factors, PDX-1, NeuroD1, and MafA, causes differentiation of bone marrow mesenchymal stem cells into insulin-producing cells. Exp. Diabetes Res. 2012: 672013; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hang H. C.; Liu X. B.; Huang S.; Bi X. Y.; Wang H. X.; Xie L. X.; Wang Y. Q.; Cao X. F.; Lv J.; Xiao F. J.; Yang Y.; Guo Z. K.. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cell Dev. 21(18): 3289–3297; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinchliffe R. J.; Brownrigg J. R.; Apelqvist J.; Boyko E. J.; Fitridge R.; Mills J. L.; Reekers J.; Shearman C. P.; Zierler R. E.; Schaper N. C..; International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab. Res. Rev. 32(Suppl. 1): 37–44; 2016. [DOI] [PubMed] [Google Scholar]
- 9.Hu G. W.; Li Q.; Niu X.; Hu B.; Liu J.; Zhou S. M.; Guo S. C.; Lang H. L.; Zhang C. Q.; Wang Y.; Deng Z. F.. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 6(1): 10; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang P.; Li S.; Han M.; Xiao Z.; Yang R.; Han Z. C.. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 28(9): 2155–2160; 2005. [DOI] [PubMed] [Google Scholar]
- 11.Humpert P. M.; Djuric Z.; Zeuge U.; Oikonomou D.; Seregin Y.; Laine K.; Eckstein V.; Nawroth P. P.; Bierhaus A.. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol. Med. 14(5–6): 301–308; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang R.; Han Z.; Zhuo G.; Qu X.; Li X.; Wang X.; Shao Y.; Yang S.; Han Z. C.. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: A pilot study. Front. Med. 5(1): 94–100; 2011. [DOI] [PubMed] [Google Scholar]
- 13.Kirana S.; Stratmann B.; Prante C.; Prohaska W.; Koerperich H.; Lammers D.; Gastens M. H.; Quast T.; Negrean M.; Stirban O. A.; Nandrean S. G.; Götting C.; Minartz P.; Kleesiek K.; Tschoepe D.. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 66(4): 384–393; 2012. [DOI] [PubMed] [Google Scholar]
- 14.Lara-Hernandez R.; Lozano-Vilardell P.; Blanes P.; Torreguitart-Mirada N.; Galmés A.; Besalduch J.. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann. Vasc. Surg. 24(2): 287–294; 2010. [DOI] [PubMed] [Google Scholar]
- 15.Lasala G. P.; Silva J. A.; Minguell J. J.. Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac. Cardiovasc. Surg. 144(2): 377–382; 2012. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K.; Frassoni F.; Ball L.; Locatelli F.; Roelofs H.; Lewis I.; Lanino E.; Sundberg B.; Bernardo M. E.; Remberger M.; Dini G.; Egeler R. M.; Bacigalupo A.; Fibbe W.; Ringdén O.. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 371(9624): 1579–1586; 2008. [DOI] [PubMed] [Google Scholar]
- 17.Lee H. C.; An S. G.; Lee H. W.; Park J. S.; Cha K. S.; Hong T. J.; Park J. H.; Lee S. Y.; Kim S. P.; Kim Y. D.; Chung S. W.; Bae Y. C.; Shin Y. B.; Kim J. I.; Jung J. S.. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 76(7): 1750–1760; 2012. [DOI] [PubMed] [Google Scholar]
- 18.Li Y. Y.; Liu H. H.; Chen H., L; Li Y. P.. Adipose-derived mesenchymal stem cells ameliorate STZ-induced pancreas damage in type 1 diabetes. Biomed. Mater. Eng. 22: 97–103; 2012. [DOI] [PubMed] [Google Scholar]
- 19.Li Z.; Wu J. C.; Sheikh A. Y.; Kraft D.; Cao F.; Xie X.; Patel M.; Gambhir S. S.; Robbins R. C.; Cooke J. P.; Wu J. C.. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116(11 Suppl): 146–54; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian Q.; Zhang Y.; Zhang J.; Zhang H. K.; Wu X.; Zhang Y.; Lam F. F.; Kang S.; Xia J. C.; Lai W. H.; Au K. W.; Chow Y. Y.; Siu C. W.; Lee C. N.; Tse H. F.. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121 (9): 1113–1123; 2010. [DOI] [PubMed] [Google Scholar]
- 21.Liang L.; Dong C.; Chen X.; Fang Z.; Xu J.; Liu M.; Zhang X.; Gu D. S.; Wang D.; Du W.; Zhu D.; Han Z. C.. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 20(9): 1395–1408; 2011. [DOI] [PubMed] [Google Scholar]
- 22.Liao W.; Xie J.; Zhong J.; Liu Y.; Du L.; Zhou B.; Xu J.; Liu P.; Yang S.; Wang J.; Han Z.; Han Z. C.. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 87(3): 350–359; 2009. [DOI] [PubMed] [Google Scholar]
- 23.Liew A.; O'Brien T.. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res. Ther. 3(4): 28; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L. L.; Liu Y. J.; Yang S. G.; Zhao Q. J.; Wang X.; Gong W.; Han Z. B.; Xu Z. S.; Lu Y. X.; Liu D.; Chen Z. Z.; Han Z. C.. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91: 1017–1026; 2006. [PubMed] [Google Scholar]
- 25.Madec A. M.; Mallone R.; Afonso G.; Abou Mrad E.; Mesnier A.; Eljaafari A.; Thivolet C.. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52(7): 1391–1399; 2009. [DOI] [PubMed] [Google Scholar]
- 26.Meraviglia V.; Vecellio M.; Grasselli A.; Baccarin M.; Farsetti A.; Capogrossi M. C.; Pompilio G.; Coviello D. A.; Gaetano C.; Di Segni M.; Rossini A.. Human chorionic villus mesenchymal stromal cells reveal strong endothelial conversion properties. Differentiation 83(5): 260–270; 2012. [DOI] [PubMed] [Google Scholar]
- 27.Naruse K.; Hamada Y.; Nakashima E.; Kato K.; Mizubayashi R.; Kamiya H.; Yuzawa Y.; Matsuo S.; Murohara T.; Matsubara T.; Oiso Y.; Nakamura J.. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 54: 1823–1828; 2005. [DOI] [PubMed] [Google Scholar]
- 28.Roobrouck V. D.; Clavel C.; Jacobs S. A.; Ulloa-Montoya F.; Crippa S.; Sohni A.; Roberts S. J.; Luyten F. P.; Van Gool S. W.; Sampaolesi M.; Delforge M.; Luttun A.; Verfaillie C. M.. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells 29(5): 871–82; 2011. [DOI] [PubMed] [Google Scholar]
- 29.Silveste J. S.; Mallat Z.; Duriez M.; Tamarat R.; Bureau M. F.; Scherman D.; Duverger N.; Branellec D.; Tedgui A.; Levy B. I.. Angiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 87: 448–452; 2002. [DOI] [PubMed] [Google Scholar]
- 30.Sun L.; Akiyama K.; Zhang H.; Yamaza T.; Hou Y.; Zhao S.; Xu T.; Le A.; Shi S.. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27(6): 1421–1432; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundell J.; Nuutila P.; Laine H.; Luotolahti M.; Kalliokoski K.; Raitakari O.; Knuuti J.. Dose-dependent vasodilating effects of insulin on adenosine-stimulated myocardial blood flow. Diabetes 51: 1125–1130; 2002. [DOI] [PubMed] [Google Scholar]
- 32.Westvik T. S.; Fitzgerald T. N.; Muto A.; Maloney S. P.; Pimiento J. M.; Fancher T. T.; Magri D.; Westvik H. H.; Nishibe T.; Velazquez O. C.; Dardik A.. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J. Vasc. Surg. 49(2): 464–473; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z. X.; Han Z. B.; Ji Y. R.; Wang Y. W.; Liang L.; Chi Y.; Yang S. G.; Li L. N.; Luo W. F.; Li J. P.; Chen D. D.; Du W. J.; Cao X. C.; Zhuo G. S.; Wang T.; Han Z. C.. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One 8(3): e59354; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu S.; Matsusue K.; Kashireddy P.; Cao W. Q.; Yeldandi V.; Yeldandi A. V.; Rao M. S.; Gonzalez F. J.; Reddy J. K.. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 278(1): 498–505; 2003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z.; Lin H.; Shi M.; Xu R.; Fu J.; Lv J.; Chen L.; Lv S.; Li Y.; Yu S.; Geng H.; Jin L.; Lau G. K.; Wang F. S.. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. Suppl. 2: 112–120; 2012. [DOI] [PubMed] [Google Scholar]
- 36.Zhou B.; Bi Y. Y.; Han Z. B.; Ren H.; Fang Z. H.; Yu X. F.; Poon M. C.; Han Z. C.. G-CSF-mobilized peripheral blood mononuclear cells from diabetic patients augment neovascularization in ischemic limbs but with impaired capability. J. Thromb. Haemost. 4: 993–1002; 2006. [DOI] [PubMed] [Google Scholar]


