Abstract
This study investigates manufacturing procedures that affect islet isolation outcomes from donor pancreata standardized by the North American Islet Donor Score (NAIDS). Islet isolations performed at the University of Illinois, Chicago, from pancreata with NAIDS ≥65 were investigated. The research cohort was categorized into two groups based on a postpurification yield either greater than (group A) or less than (group B) 400,000 IEQ. Associations between manufacturing procedures and islet isolation outcomes were analyzed using multivariate logistic or linear regressions. A total of 119 cases were retrieved from 630 islet isolations performed since 2003. Group A is composed of 40 cases with an average postpurified yield of 570,098 IEQ, whereas group B comprised 79 cases with an average yield of 235,987 IEQ. One third of 119 cases were considered successful islet isolations that yielded >400,000 IEQ. The prepurified and postpurified islet product outcome parameters were detailed for future reference. The NAIDS (>80 vs. 65–80) [odds ratio (OR): 2.91, 95% confidence interval (CI): 1.27–6.70], cold ischemic time (≤10 vs. >10 h) (OR: 3.68, 95% CI: 1.61–8.39), and enzyme perfusion method (mechanical vs. manual) (OR: 2.38, 95% CI: 1.01–5.56) were independent determinants for postpurified islet yield ≥400,000 IEQ. The NAIDS (>80, p < 0.001), cold ischemic time (≤10 h, p < 0.05), increased unit of collagenase (p < 0.01), and pancreatic duct cannulation time (<30 min, p < 0.01) all independently correlated with better islet quantity parameters. Furthermore, cold ischemic time (≤10 h, p < 0.05), liberase MTF (p < 0.001), increased unit of collagenase (p < 0.05), duct cannulation time (<30 min, p < 0.05), and mechanical enzyme perfusion (p < 0.05) were independently associated with better islet morphology score. Analysis of islet manufacturing procedures from the pancreata with standardized quality is essential in identifying technical issues within islet isolation. Adequate processing duration in each step of islet isolation, using liberase MTF, and mechanical enzyme perfusion all affect isolation outcomes.
Keywords: Islet of Langerhans, Human islet isolation, North American Islet Donor Score (NAIDS), Good Manufacture Practice, Outcome parameters
Introduction
Allogeneic islet transplantation is a promising treatment for patients with type 1 diabetes mellitus1,2. However, the efficacy of clinical islet transplantation is heavily influenced by islet quantity and quality. The quality of both the donor pancreas and the islet isolation procedure is considered heavily influential factors in achieving an islet product of high quantity and quality.
In the past decade or so, many researchers have investigated donor characteristics that can affect islet isolation outcomes. Numerous factors, including donor age, sex, body mass index (BMI), body height, and usage of vasopressors, have been identified as individual or compound determinants that affect isolation outcomes3–9. In 2005, O'Gorman et al. from the Edmonton group systematically analyzed these determinants and developed a scoring system that has proved useful for predicting the success of islet isolation and transplantations in the Edmonton group10. More recently, based on a similar islet score concept but with different endpoints, we participated in a multicenter study and reported a new multiparametric scoring system, the North American Islet Donor Score (NAIDS), with good diagnostic accuracy11. The NAIDS uses body surface area (BSA), BMI, and body weight to predict the size of the pancreas. Additionally, age, cold ischemic time, own-team procurement, usage of vasopressors, liver enzymes, renal function, sodium, glucose, amylase, and hemoglobin A1c (HbA1c) were used to score the quality of the pancreas. HbA1c is a form of hemoglobin used as a surrogate marker to estimate 3-month average plasma glucose concentration. The NAIDS scale ranges from 0 to 100, with a greater score indicating a greater probability of achieving a postpurified islet yield ≥400,000 islet equivalents (IEQs). IEQ is defined as the number of islets 150 mm in diameter that are equivalent in volume to a given amount of islets.
In addition to the donor pancreata, technical issues related to islet manufacturing procedures are also critical for determining isolation outcomes. Past studies have shown that enzyme brands, enzyme perfusion method, collagenase units per gram of pancreas, and purification procedure (continuous vs. discontinuous) all play significant roles in isolation outcomes2,4,5,12,13. However, there are many limitations with these previous studies. First, with the increasing use of marginal donors, some studies investigating technical factors may be biased by donor pancreata with heterogenic quality. Second, most multicenter studies are less likely to provide comprehensive data regarding the islet manufacturing process, such as density gradient loading time and pancreatic duct cannulation time. Last, another limitation to multicenter studies is the significant variation across centers in islet counting14. Because of these limitations, previous studies may lead to conflicting or skewed results concerning the effect of age, vasopressor use, and enzyme brand on islet isolation outcomes2,3,5,6,15–20. More importantly, to date, no study has investigated the effect of manufacturing procedures on islet isolation outcomes based on donor pancreata with standardized quality.
In this study, we conducted a retrospective analysis of critical technical aspects that occur during the islet isolation process using NAIDS to standardize the quality of donor pancreata. This study was conducted to identify key manufacturing factors that can affect both quality and quantity of islet product.
Materials and Methods
Pancreas Donors and Characterization
Human pancreata were obtained from organ procurement organizations (OPOs) following formal research consent and then transported to the University of Illinois at Chicago (UIC) cell isolation facility for islet isolation. All demographics of the pancreas donors were retrieved from the hard copy of donor information that was collected by the OPOs and sent to UIC along with the procured organs. The donor pancreas quality for islet isolation was evaluated and scored using NAIDS.
To fulfill good manufacturing practice (GMP) regulations requested by the FDA, all islet isolations using liberase HI (Roche Diag nostics Operations, Mannheim, Germany) were excluded from our research cohort (Fig. 1). Islet isolations sponsored by the Clinical Islet Transplant Consortium (CIT) were not allowed to be included in this study because of regulations set by CIT. To reduce heterogeneity, islet products used for autologous islet transplant were also excluded. To reduce the potential confounding effects of poor-quality organs, donor pancreata with insufficient donor information for calculating NAIDS and those with NAIDS <65 were also excluded. Finally, the research cohort was categorized into two groups based on a postpurification yield either greater than (group A) or less than (group B) 400,000 IEQ.
Figure 1.
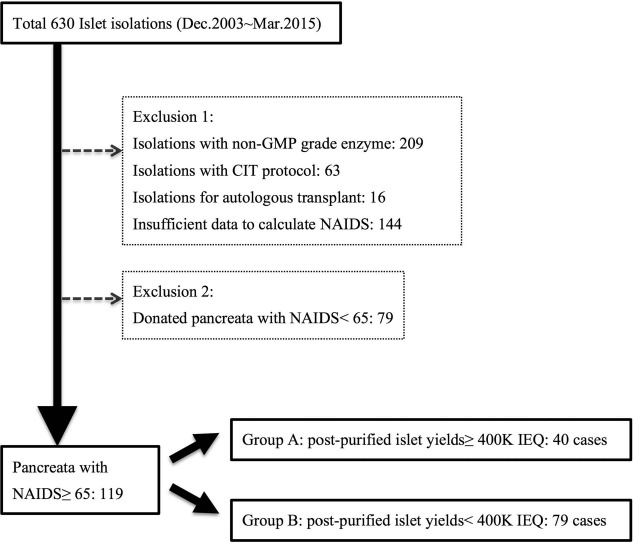
Flowchart of inclusion and exclusion criteria. NAIDS: North American Islet Donor Score; IEQ, islet equivalent number; GMP, good manufacturing practice; CIT, Clinical Islet Transplant Study; 400K, 400,000.
Islet Isolation Using UIC Protocol
The islet isolation procedure has been described in our previously published studies21,22. All operations for islet manufacturing were standardized on the basis of standard operating procedures (SOPs) set by the UIC team, and any deviation during the islet isolation was filed with a deviation report. Briefly, the pancreata were first trimmed, cannulated, and perfused with enzyme solution, and then digested using a modified Ricordi automated method23. The manual perfusion was mainly used before 2014, and mechanical perfusion was used from early 2014 until now. While infusing enzyme into the pancreatic duct via perfusion devices with pressure controlled by the Master Flex Pump (Cole-Parmer, Vernon Hills, IL, USA), the temperature was maintained at around 4°C, and perfusion pressure was set as 80 mmHg for the initial 5 min and then 180 mmHg for the remaining 5 min (total time: 10 min). By contrast, manual perfusion was performed by loading enzyme solution into the pancreatic duct via syringes at a temperature of 4°C and with a pressure subjectively applied by operators. Using mammalian tissue-free liberase (liberase MTF; Roche Diagnostics Corporation, Mannheim, Germany), the enzyme solutions were prepared with collagenase (22–25 U/g of trimmed pancreas tissue; Roche Diagnostics Corporation) and neutral protease thermolysin (800–1,200 U/g of trimmed pancreas weight; Roche Diagnostics Corporation). Serva-NB1 (SERVA Electrophoresis GmbH, Heidelberg, Germany) was reconstituted with variable units of collagenase (1,600–2,057 U) and neutral protease (200–300 U) depending on the trimmed pancreas weight24. Sigma V (Sigma-Aldrich, Milwaukee, WI, USA), with an enzyme activity of FALGPA 1.0–3.0 mg/solid (Sigma-Aldrich), was prepared with 350 ml of perfusion solution (Mediatech, Manassas, VA, USA) to a final concentration of 2.86 mg/ml24. Two ampules of Pulmozyme (2,500 U/amp; Genentech, San Francisco, CA, USA) were added into the enzyme solution before starting circulation of digest circuit. The switch point of digestion depended on the time point when approximately 50% of islets were free from surrounding pancreas acinar tissue. Postdigestion, islets were purified using the UIC UW/Biocoll (UIC-UB) gradient in a COBE 2991 cell processor (TERUMO BCT, Lakewood, CO, USA)25. The major difference between the UIC and CIT islet isolation protocols26 is that the UIC team primarily used the UIC-UB purification gradient. On the other hand, the CIT protocol used the CIT purification gradient as their primary choice for purification and used the UIC-UB gradient as an alternative procedure only if the CIT purification gradient failed. These two gradients differ in composition. The UIC-UB purification gradient consists of UW solution (DuPont Pharma, Bad Homburg, Germany) and Biocoll (Biochrome AG, Berlin, Germany) to create a density gradient ranging from 1.068 to 1.079 g/ml25. Thus, the UIC protocol can purify a maximal postdigestion tissue volume of 45 ml each time, although it may be toxic to the islets if the temperature during purification is not low enough. All details regarding donor information and islet isolation were recorded in the Master Production Batch Record and later entered into the Islet Database with research electronic data capture (REDCap) tools hosted at the UIC27, which is a secure Web-based application designed to support data capture for research studies. The study protocol was reviewed by the institutional review board (IRB) of the Division of Research at UIC (IRB 2007-0330). An exemption for IRB approval was granted, as the study was determined to be a nonhuman subject research based on the Federal regulations (45 CFR 46.102(f)(1), (2)).
Definition of Islet Manufacturing Procedures
Intraparenchymal, superficial fat infiltration and external anatomic injury were grossly inspected by surgeons and recorded before trimming the nonpancreatic tissue. Trimming time in minutes was defined as time spent between placement of the pancreas on the trimming pan and placement of the organ into the decontamination solution. Trimmed pancreas weight in grams was defined as the measured weight of the pancreas after trimming all nonpancreatic tissue. Cannulation time in minutes was defined as the time interval between placement of the pancreas on the cannulation pan and the start of the perfusion of enzyme solution into the pancreatic duct. Enzyme perfusion time in minutes was defined as the total time between the start and completion of enzyme perfusion into the organ. The perfusion method was categorized as either mechanical or manual (use of a handheld syringe). Enzyme brands were categorized into two groups: liberase MTF (GMP grade) and other enzymes, which included either Serva-NB1 or Sigma V collagenase24. Concentration of collagenase was defined as total Wünsch units of collagenase used divided by the trimmed pancreas weight in grams.
Cold ischemic time in minutes was defined as the time interval between cross-clamping the organ and placing the pancreas into the Ricordi digestion chamber (Biorep Technologies, Inc., Miami, FL, USA). Digestion time in minutes was defined as the time interval between the start of digestion and the start of dilution. Dilution time in minutes was defined as the time interval between the start of the dilution solution entering the digestion system and the completion of the collection of digested pancreatic tissue. Purification time in minutes was defined as the time interval between the addition of UW (University of Wisconsin) (Bridge to Life Ltd., Columbia, SC, USA) solution to the digested tissue pellet and the end of purification. A standard duration of 48 min was inferred from a total volume of instilled solution in COBE bag (TERUMO BCT) and an instilling rate of either 20 or 60 ml/min by rolling pump. Adequate time intervals for each step of islet manufacturing were determined on the basis of our previous experience in islet isolations and set as cutoff values for further analysis.
Endpoint
Successful islet isolation was defined as a postpurified islet yield equal to or greater than 400,000 IEQ and was set as the primary endpoint. This number was used because it fulfills the criteria, using a single donor's pancreatic islets, for clinical islet transplantation of 5,000 IEQ/kg in a recipient whose body weight is equal to or less than 80 kg.
Islet Product Outcome Parameters
Islet product quantity and quality parameters after islet purification were analyzed. Quantity parameters included total islet yield, islet yield per gram of digested pancreas, and ratio of IEQ to islet particle number (IEQ/IPN). Quality parameters were assessed using an islet morphology score based on five subscores, including size, degree of fragmentation, density of islet stained by dithizone (Sigma-Aldrich), border, and shape28. The five individual subscores ranged from 0 to 2; the total islet score was the sum of the five subscores and ranged from 0 to 10.
Statistical Analysis
Correlation between NAIDS and postpurified islet yield was calculated using Spearman's correlation coefficient. The cumulative incidence of donor pancreata with different NAIDS was calculated. Normality of data was examined by the Kolmogorov–Smirnov test or the Shapiro–Wilk test. Continuous variables were expressed as median with interquartile range (IQR), and Mann–Whitney U-tests were performed to detect statistical differences. For categorical variables, a Fisher exact test or chi-square test was used to analyze differences between the groups. Multivariate logistic regressions were conducted to determine the odds ratio of postpurified islet yield ≥400,000 IEQ among different manufacturing procedures. In addition, univariate and multivariate linear regressions were conducted to investigate the association between manufacturing procedures and various postpurified quantity- and quality-related parameters of final islet product. All analyses used SPSS Statistics for Macintosh, Version 22.0 (IBM Corp., Armonk, NY, USA). The level of statistical significance was set at 0.05 by a two-tailed test.
Results
A total of 630 islet isolations were performed at UIC between 2003 and 2015. Inclusion and exclusion criteria for this study are shown in Figure 1. Donor pancreata with NAIDS ≥65 accounted for 60% of the total UIC islet products; by contrast, those with NAIDS ≥80 accounted for 20% of all isolations (Fig. 2A). A significant positive correlation (Spearman's rank correlation coefficient ρ = 0.379, p < 0.001) between NAIDS of the donor pancreata and the postpurified islet yield was observed (Fig. 2B). Throughout the study period, there were no significant temporal trends in islet yield and NAIDS that suggested consistency of manufacturing methods (Fig. 2C).
Figure 2.
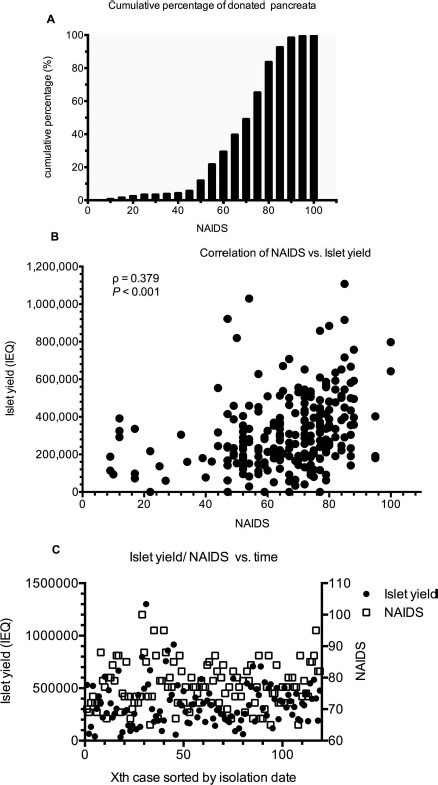
(A) Cumulative percentage of donated pancreata. The donated pancreata with NAIDS ≥65 consisted of 60% of all GMP-grade islet isolation products (n = 198); those with NAIDS ≥80 only consisted of 20% of all isolations. (B) Correlation of NAIDS and islet yield. A significant positive correlation appears between NAIDS and islet yield (n = 198, Spearman's rank correlation coefficient = 0.379, p < 0.001). (C) Temporal trend in either islet yield or NAIDS. Islet yield is not in a significant linear association with isolation date, neither is NAIDS (n = 119, p > 0.05). Xth case means the number of isolation batch categorized by isolation date (e.g., fifth case of islet isolation sorted by isolation date). NAIDS, North American Islet Donor Score; IEQ, islet equivalent number; GMP, good manufacturing practice.
All of the demographic characteristics between group A (n = 40) and group B (n = 79) were not significantly different, including age, gender, body height, body weight, BMI, BSA, types of vasopressors used, volume of perfused organ storage solution, type of organ procurement team, in-hospital days, cause of death, history of cardiac massage, liver function test, amylase, lipase, and highest blood glucose level (Table 1).
Table 1.
Demographics of Pancreas Donors
| ≥400 K IEQ (n = 40) | <400K IEQ (n = 79) | p | |||
|---|---|---|---|---|---|
| Organ procurement team | ns | ||||
| Local, n (%) | 14 | (35.0) | 21 | (26.6) | |
| Distant, n (%) | 26 | (65.0) | 58 | (73.4) | |
| Age (years)† | 47.5 | (15.5) | 46.0 | (18.0) | ns |
| Sex | |||||
| Male, n (%) | 23 | (57.5) | 55 | (69.6) | ns |
| Female, n (%) | 17 | (42.5) | 24 | (30.4) | |
| Height (cm)† | 174.0 | (16.1) | 177.8 | (12.7) | ns |
| Weight (kg)† | 102.6 | (13.7) | 99.0 | (22.3) | ns |
| BMI (kg/m2)† | 34.3 | (8.2) | 32.3 | (8.9) | ns |
| BSA (m2)† | 2.2 | (0.1) | 2.2 | (0.2) | ns |
| Types of vasopressors† | 2 | (1) | 2 | (1) | ns |
| Hospital days before procurement (days)† | 3 | (2) | 3 | (2) | ns |
| Used perfusion solution (L)† | 4.0 | (1.4) | 4.0 | (1.8) | ns |
| AST (IU/L)† | 49.5 | (65.8) | 48.0 | (62.0) | ns |
| ALT (IU/L)† | 39.5 | (43) | 37.0 | (55.0) | ns |
| Peak sodium level (mmol/L)† | 156.0 | (14.0) | 155.0 | (11.0) | ns |
| Amylase (IU/L)† | 63.0 | (72.0) | 70.5 | (130.3) | ns |
| Lipase (IU/L)† | 40.0 | (80.0) | 29.0 | (55.0) | ns |
| Peak glucose level (mmol/L)† | 14.2 | (4.7) | 12.6 | (5.9) | ns |
| History of cardiac massage | |||||
| Yes, n (%) | 6 | (15.0) | 15 | (19.0) | ns |
| No, n (%) | 34 | (85.0) | 64 | (81.0) | |
| Cause of death | |||||
| Anoxia, n (%) | 0 | (0) | 7 | (8.9) | ns |
| Stroke, n (%) | 26 | (65.0) | 43 | (54.4) | |
| Head injury, n (%) | 14 | (35.0) | 29 | (36.7) | |
Categorical variables were analyzed by Fisher exact test; continuous variables were analyzed by Mann–Whitney U-test.
Median (IQR). IQR, interquartile range; BMI, body mass index; BSA, body surface area; AST, aspartate aminotransferase; ALT, alanine transaminase; IEQ, islet equivalent number; 400K, 400,000; ns, nonsignificant.
Using donor pancreas with the NAIDS equal to or greater than 65, group A was remarkably superior to group B in prepurified islet product outcome parameters (Table 2), including final digested pancreas weight [90.1 g (31.5) in group A vs. 83.9 g (32.9) in group B, p = 0.029]; packed tissue volume [49.0 ml (28.8) vs. 35.0 ml (12.0), p < 0.001]; and prepurified islet yield [605,700 IEQ (238,700) vs. 335,000 IEQ (199,300), p < 0.001]. However, percentage of digestion was comparable between both groups [81.8% (8.8) in group A vs. 78.9% (12.5) in group B]. For postpurified islet product outcome parameters (Table 2), group A was also better than group B in total islet yield [524,800 IEQ (149,700) in group A vs. 239,800 IEQ (138,300) in group B, p < 0.001]; islet yield per gram of digested pancreas [5,800 IEQ/g (2,500) vs. 2,800 IEQ/g (1,700), p < 0.001]; IEQ/IPN [1.5 (0.6) vs. 1.2 (0.8), p = 0.010]; islet yield of high-purity fraction [387,500 IEQ (151,000) vs. 160,000 IEQ (167,500), p < 0.001]; high-purity islet yield per gram of digested pancreas [4,500 IEQ (1,900) vs. 2,100 IEQ (1,900), p < 0.001]; recovery rate of high-purity fraction [71.4% (36.6) vs. 53.7% (43.1), p < 0.001]; and viability assay [94.6% (2.9) vs. 93.3% (4.4), p = 0.004].
Table 2.
Difference in Islet Product Outcome Parameters Between Isolations With Versus Without Postpurified Yield ≥400,000 IEQ
| ≥400 K IEQ (n = 40) |
<400K IEQ (n = 79) |
p | |||
|---|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | ||
| Prepurified | |||||
| Percentage of digestion (%) | 81.8 | (8.8) | 78.9 | (12.5) | ns |
| Final digested pancreas weight (g) | 90.1 | (31.5) | 83.9 | (32.9) | † |
| Packed tissue volume (ml) | 49.0 | (28.8) | 35.0 | (12.0) | ‡ |
| IEQ (×103) | 605.7 | (238.7) | 335.0 | (199.3) | ‡ |
| IEQ/g of digested pancreas (×103) | 6.2 | (2.5) | 4.0 | (2.8) | ‡ |
| Islet morphology score | 7.5 | (1.0) | 6.5 | (2.0) | ‡ |
| Postpurified | |||||
| IEQ (×103) | 524.8 | (149.7) | 239.8 | (138.3) | ‡ |
| IEQ/g of digested pancreas (×103) | 5.8 | (2.5) | 2.8 | (1.7) | ‡ |
| IEQ/IPN ratio* | 1.5 | (0.6) | 1.2 | (0.8) | † |
| Packed cell volume of high purity fraction (ml) | 1.5 | (0.9) | 0.9 | (0.7) | ‡ |
| IEQ(×103) in high purity fraction | 387.5 | (151.0) | 160.0 | (167.5) | ‡ |
| IEQ(×103) in high purity fraction/g of digested pancreas tissue | 4.5 | (1.9) | 2.1 | (1.9) | ‡ |
| Islet morphology score of high purity fraction | 7.0 | (1.5) | 6.0 | (2.0) | § |
| Recovery rate of high purity fraction (%) | 71.4 | (36.6) | 53.7 | (43.1) | ‡ |
| Viability assay (%) | 94.6 | (2.9) | 93.3 | (4.4) | § |
| GSIR index | 2.1 | (0.6) | 1.9 | (1.7) | ns |
Continuous variables were analyzed by Mann–Whitney U test.
IEQ/IPN ratio defined as total yield at IEQ divided by total yield at IPN;
p < 0.05;
p < 0.001;
p < 0.01. IQR, interquartile range; IEQ, islet equivalent number; IPN, islet particle number; GSIR index, glucose stimulated insulin release index; 400K, 400,000; ns: nonsignificant.
In multivariate logistic regression, NAIDS >80 [odds ratio (OR): 2.91, 95% confidence interval (CI): 1.27– 6.70], cold ischemic time ≤10 h (OR: 3.68, 95% CI: 1.61–8.39), and mechanical enzyme perfusion (OR: 2.38, 95% CI: 1.01–5.56) were all independent determinants for postpurified islet yield equal to or greater than 400,000 IEQ (Table 3). In addition, 6 of 119 isolations used Sigma V as digestion enzyme: two cases in group A and four cases in group B.
Table 3.
Multivariate Logistic Regression: The Association Between Manufacturing Procedures and Postpurified Islet Yield More Than 400,000 IEQ
| Manufacturing Factors | ≥400K IEQ (n = 40) |
<400K IEQ (n = 79) |
Islet yield ≥400K IEQ |
||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | OR | (95% CI)* | ||
| NAIDS | 65–80 | 23 | (57.5) | 63 | (79.7) | 1.00 | (reference) |
| >80 | 17 | (42.5) | 16 | (20.3) | 2.91 | (1.27-6.70)¶ | |
| Cold ischemic time (h) | >10 | 11 | (27.5) | 46 | (58.2) | 1.00 | (reference) |
| ≤10 | 29 | (72.5) | 33 | (41.8) | 3.68 | (1.61-8.39)¶ | |
| Collagenase†‡ | (U/g of tissue) | 25.2 | (6.0) | 24.7 | (5.9) | 0.01 | (0.04–0.17) |
| Enzyme brand | Serva-NB1 or | 6 | (15.0) | 24 | (30.3) | 1.00 | (reference) |
| Sigma V | 2 | (5.0) | 4 | (5.1) | |||
| Liberase MTF | 32 | (80.0) | 51 | (64.6) | 2.20 | (0.89–5.41) | |
| Pancreas injury | No injury | 19 | (47.5) | 38 | (48.1) | 1.00 | (reference) |
| Any injury | 21 | (52.5) | 41 | (51.9) | 0.88 | (0.40–1.90) | |
| Pancreas fat | Mild/moderate | 23 | (57.5) | 44 | (55.7) | 1.00 | (reference) |
| Severe | 16 | (42.5) | 35 | (44.3) | 0.87 | (0.40–1.90) | |
| Trimming time | <20 min | 12 | (30.0) | 24 | (30.4) | 1.00 | (reference) |
| ≥20 min | 28 | (70.0) | 55 | (69.6) | 1.02 | (0.44–2.33) | |
| Cannulation time | ≥30 min | 3 | (7.5) | 18 | (22.8) | 1.00 | (reference) |
| <30 min | 37 | (92.5) | 61 | (77.2) | 3.57 | (1.01–12.5) | |
| Perfusion time | <12 or >15 min | 20 | (50.0) | 48 | (60.8) | 1.00 | (reference) |
| 12–15 min | 20 | (50.0) | 31 | (39.2) | 1.55 | (0.72–3.33) | |
| Perfusion method | Manual | 25 | (62.5) | 64 | (81.0) | 1.00 | (reference) |
| Mechanical | 15 | (37.5) | 15 | (19.0) | 2.38 | (1.01-5.56)¶ | |
| Pancreas pieces§ | <15 pieces | 27 | (67.5) | 50 | (63.3) | 1.00 | (reference) |
| ≥15 pieces | 11 | (32.5) | 25 | (36.7) | 0.82 | (0.35–1.91) | |
| Digestion time | <10 min | 8 | (20) | 20 | (25.3) | 1.00 | (reference) |
| ≥10 min | 32 | (80) | 59 | (74.7) | 1.36 | (0.54–3.42) | |
| Dilution time | <30 min | 3 | (7.5) | 10 | (12.7) | 1.00 | (reference) |
| ≥30 min | 37 | (92.5) | 69 | (87.3) | 1.79 | (0.46–6.90) | |
| Purification time | <48 min | 6 | (15.0) | 20 | (25.3) | 1.00 | (reference) |
| ≥48 min | 33 | (85.0) | 58 | (74.7) | 1.90 | (0.69–5.19) | |
Adjusted odds ratio and 95% confidence intervals;
mean (SD);
collagenase defined as concentration of used collagenase per gram of digested pancreas tissue;
Tpancreas pieces defined as number of cut pancreatic pieces.
p < 0.05. NAIDS, North American Islet Donor Score; SD, standard deviation; IEQ, islet equivalent number; 400K, 400,000.
In linear regression, NAIDS >80 (p < 0.01), cold ischemic time ≤10 h (p < 0.05), and cannulation time <30 min (p < 0.01) independently correlated with higher postpurified total islet yield (Table 4a). Cold ischemic time ≤10 h (p < 0.05), increased unit of collagenase (p < 0.01), and cannulation time less than 30 min (p < 0.01) were independently associated with increased islet yield per gram of digested pancreas. Cannulation time of less than 30 min (p < 0.01) independently correlated with a greater IEQ/IPN. By contrast, digestion time of greater than 10 min was associated with a lower IEQ/IPN (p < 0.05).
Table 4a.
Linear Regression: Associations Between Manufacturing Factors and “Postpurified” Quantity Parameters in Final Islet Product
| Manufacturing Factors | IEQ (×1,000) |
IEQ/g*
|
IEQ/IPN |
|
|---|---|---|---|---|
| Coef.† | Coef.† | Coef.† | ||
| NAIDS | [> 80 vs. 65–80 (reference)] | 121.3‡§ | 0.3¶ | |
| Cold ischemic time (h) | (≤10 vs. >10) | 87.7§¶ | 893.2§¶ | |
| Enzyme brand | (liberase MTF vs. Serve-NB1 or Sigma V) | 106.6‡ | 1,013.4¶ | |
| Collagenase# | (U/g of digested pancreas) | 102.1ठ| ||
| Cannulation time (min) | (<30 vs. ≥30) | 131.5‡§ | 1,643.3‡§ | 0.4‡§ |
| Digestion time (min) | (≥10 vs. <10) | -0.3§¶ | ||
| Dilution time (min) | (≥30 vs. <30) | 0.3¶ |
Each cell description: regression coefficient in either multivariate (marked by §) or univariate linear regressions.
Total islet yield per gram of digested pancreas tissue;
coefficient from regression models;
collagenase defined as concentration of used collagenase per gram of digested pancreas tissue;
statistics calculated from multivariate regressions;
p < 0.01;
p < 0.05. NAIDS, North American Islet Donor Score; IEQ, islet equivalent; IPN, islet particle number.
As for islet product quality parameters, cold ischemic time ≤10 h was independently associated with islet morphology subscore of density (p < 0.05). Use of liberase MTF was independently associated with greater total islet score and islet morphology subscores of size, fragment, and border (all p < 0.05) (Table 4b). Cannulation time less than 30 min was significantly associated with greater total islet score and islet morphology subscores of size, border, and shape (all p < 0.05). Mechanical enzyme perfusion significantly correlated with a higher total islet score and subscores of size, fragment, border, and shape (all p < 0.05).
Table 4b.
Linear Regression: Associations Between Manufacturing Factors and “Postpurified” Quality Parameters in High-Purity Fraction of Islet Product
| Manufacturing Factors | Islet Score*
|
Size†
|
Fragment†
|
Density†
|
Border†
|
Shape†
|
|
|---|---|---|---|---|---|---|---|
| Coef.‡ | Coef.‡ | Coef.‡ | Coef.‡ | Coef.‡ | Coef.‡ | ||
| Cold ischemic time (h) | (≤10 vs. >10) | 0.1§ | 0.1§¶ | 0.1§ | |||
| Enzyme brand | (liberase MTF vs. Serve-NB1 or Sigma V) | 1.1¶# | 0.4¶** | 0.3¶** | 0.1§ | 0.2§¶ | 0.2# |
| Collagenase†† | (U/g of digested pancreas) | 0.01¶# | |||||
| Trimming time (min) | (≥20 vs. <20) | 0.9# | |||||
| Cannulation time (min) | (<30 vs. ≥30) | 1.0¶# | 0.3§¶ | 0.2§ | 0.2§ | ||
| Perfusion method | (mechanical vs. manual) | 1.0# | 0.3# | 0.2§ | 0.2§¶ | 0.2# | |
| No. of cut pancreatic pieces | (≥15 vs. <15) | -0.2§ | |||||
| Digestion time (min) | (≥10 vs. <10) | -0.2§ | |||||
| Dilution time (min) | (≥30 vs. <30) | 0.3§ |
Each cell description: regression coefficient in either multivariate (marked by ¶) or univariate linear regressions.
Islet score equal to sum of islet morphology subscores.
Islet morphology subscores include morphologic evaluation on size, fragment, density, border, and shape; each subscore ranges from 0 to 2, and total islet score ranges from 0 to 10.
Coefficient from regression models.
Collagenase defined as concentration of used collagenase per gram of digested pancreas tissue.
Statistics calculated from multivariate regressions.
p
p < 0.01;
p < 0.001.
Discussion
This study is the first to report the impact of manufacturing procedures on islet product outcome parameters from donor pancreata standardized by NAIDS and to provide valuable reference data for islet product outcome parameters in successful islet isolations (Table 2). This study reveals that manufacturing procedures, such as enzyme brand, enzyme perfusion method, and time intervals of manufacturing processes of islet isolation, are all critical in affecting the quantity and quality of islet products.
Donor pancreata with NAIDS equal to or greater than 65 were chosen as the study cohort for three reasons. First, in the UIC dataset, 33% of organs with NAIDS ≥65 achieved a postpurified yield ≥400,000 IEQ; in contrast, only 10% of organs with NAIDS <65 achieved a comparable islet yield (Fig. 2B). This suggests that it is challenging to obtain a high islet yield from donor pancreata with poor quality, although current trends encourage increased utilization of organs from marginal donors. Second, high-quality pancreata (NAIDS ≥80) comprised only 20% of total received organs, although 46% of these organs resulted in an islet yield of more than 400,000 IEQ (data not shown). Third, the average postpurified islet yield of pancreata with NAIDS between 65 and 80 was 306,000 IEQ. With respect to cost per islet, this yield is still considered a good number for islet research in cases where clinical transplant cannot be performed. Thus, considering both acceptable quality of donor pancreas and shortage of organ resources, NAIDS ≥65 was set as the cutoff for selection of pancreata of appropriate quality in this study.
To accurately investigate the relationship between manufacturing factors and islet isolation outcomes, it was necessary to control and reduce confounding factors from poor-quality organs. Thus we used NAIDS as a screening tool to exclude pancreata with unacceptable quality. Despite this, it was unavoidable that organs with slightly heterogeneous quality were enrolled. This was suggested by the results in multivariate logistic analysis that demonstrated that pancreata with NAIDS more than 80 were at significantly greater probability (2.9-fold) than that of NAIDS between 65 and 80 to achieve a postpurified yield greater than 400,000 IEQ. In addition, NAIDS is listed as one of the manufacturing factors because cold ischemic time, which is a component of NAIDS, is defined as the time interval between cross-clamping on donors and placing the donor pancreata into the Ricordi digestion chamber in UIC. However, considering the significant effect of cold ischemic time on islet isolation outcomes, cold ischemic time was still a covariate used in statistic models.
Accuracy in recording time points of the different islet processing steps is essential and critical for retrospectively reviewing the quality of the islet isolation process. This study highlights that appropriate time controls at each isolation step, including trimming time, cannulation time, enzyme perfusion time, cold ischemic time, digestion time, and dilution time, are critical for optimal islet isolation outcomes. For example, reduced cannulation time was independently associated with a higher islet yield and larger islet particle (IEQ/IPN). This may be attributed to shorter cold ischemic time and the reduced risk of pancreata exposed to a non-ice-cold environment during cannulation. Thus, shortening the time period of this manufacturing step, using experienced operators, and keeping pancreata in an ice-cold environment as long as possible during the procedure all may improve the outcome. These principles can be applied to all steps of islet isolation that significantly correlate with quantity- and quality-related parameters of the final islet products.
Enzyme brand is another important factor that affects both the quantity and the quality of postpurified islet product outcomes. The use of liberase MTF in this study may be associated with better islet product quantity and quality outcomes. Previous studies that compared the efficacy of different enzymes in the islet isolation procedure mainly focused on liberase HI, a product of non-GMP-grade manufacturing2,16–18. Several studies indicated that Serva-NB1 is superior to liberase HI in yield and endocrine function of postpurified islet products2,16–18. However, in following GMP guidelines requested by the FDA, as of 2007, liberase HI cannot be used in islet isolation for clinical transplant anymore. Hence, all research findings regarding liberase HI cannot be directly applied in islet isolations for clinical islet transplantation2,16–18. Very few studies have been designed to compare liberase MTF with other enzymes20,29. Our finding corresponds to a very recently published study by Qi et al., in which liberase MTF was associated with better digestion efficacy and postpurified endocrine function20. However, a large-scale comparison of the various enzyme types should be performed to obtain a more definite conclusion. As for the use of protease in the islet isolation process, this study did not investigate its impact because of different manufacturers' use of differing units of enzymes.
Examination of islet product parameters in the high-purity fraction is a key step to evaluate islet isolation quality. Group A had a remarkably higher islet yield, islet morphology score, and islet recovery rate (postpurified IEQ/prepurified total IEQ) in the high-purity fraction than group B did (Table 2). The difference in high-purity islet yield accounted for a major difference of total islet yield between groups A and B, which reiterates the importance of having a high-purity fraction for the final islet product. In addition, the IEQ/IPN ratio, which provides an indication of general islet size and distribution30, was remarkably higher in group A than in group B (1.5 vs. 1.2, p < 0.05) (Table 2). Both findings imply that better islet isolation comes from not only increased total islet yield and islet morphology score but also relatively larger islets and greater recovery rate in the high-purity fraction of islet product.
The difference of islet yield between both groups may have partially resulted from the quality of donated pancreas rather than the size of pancreas, given that BMI and BSA were comparable between both groups (Table 1). Longer cold ischemic time in group B may have increased risk of islet apoptosis31 and release of DNA string, and subsequently led to more embedded islets. This idea is supported by a significantly lower recovery rate in the high-purity fraction of group B (Table 2). In addition, consistent with the previous study13, mechanical enzyme perfusion was associated with larger islet yield and better islet morphology score. Thus, we consider mechanical enzyme perfusion under well-controlled pressure and temperature to be superior to manual perfusion and believe that this may account for better outcomes in islet isolations.
Only 33% of the total number of islets processed in this single-center study were considered successful cases (islet yield ≥400,000 IEQ). Two factors may account for ineffectiveness of the prediction model. The first includes unpredicted donor factors that may not be scored out by NAIDS, such as the length of warm ischemic time during pancreas procurement or unclear length of cardiac down-time11. The other factor is the interaction between multiple islet manufacturing factors. In this study, we know that in addition to the quality of donated organs, multiple islet manufacturing factors may also play critical roles in the results of islet isolations. Thus, we believe that improving success rates in our center necessitates accepting organs of good quality (NAIDS >80), using liberase MTF, using mechanical perfusion for delivering enzymes, and experienced processing to reduce cold ischemic time as much as possible.
The findings of this study should be interpreted cautiously because of the following limitations. First, because of differences in definitions of time periods for many manufacturing procedures between different islet isolation centers, a direct comparison of the findings in this study with other centers may be challenging. Second, unlike multicenter studies2,5, the case number in this single-center study is limited. However, this study still has advantages including more consistent and comprehensive data collection as well as reduced interobserver variability in islet counting, which was reported in our previous study14. Furthermore, though limited in sample size, our findings cannot be ignored but should be verified with a larger number of cases in the future. Third, the influence of proteinase in islet yield and quality was not thoroughly investigated in this study because of varying units between different brands of enzyme and insufficient remaining enzyme specimen for analysis of activity. However, even in our subpopulation analysis for subjects using liberase MTF, we could not find a significant effect of used units of proteinase per gram of trimmed pancreas weight on islet quantity and quality parameters (data not shown). The role of proteinase in islet isolation outcomes still remains controversial32,33 and needs to be clarified by future studies.
Islet manufacturing is a complicated and technically demanding task. While islet isolation outcome is closely associated with donor pancreas quality, it is also strongly related to manufacturing techniques. Thus, it is essential to use donor pancreata with standardized quality in order to identify specific technical problems in islet isolations. Using islet product outcome parameters (Table 2) as references could be helpful to recognize critical steps of less-effective islet isolations in the future. Our research concludes that appropriate time controls for each independent isolation step, the specific enzyme brand, and the use of mechanical enzyme perfusion are all parameters that, if optimized, can be of great importance in improving islet isolation outcomes.
Acknowledgments
This study was supported by a research grant provided by the Gift of Hope Organ and Tissue Donor Network, Illinois, and partially supported by the Chicago Diabetes Project (CDP). The REDCap Database, which is supported by the Institute for Health Research and Policy grant [Center for Clinical and Translational Science (CCTS); UL1RR029879], was used in this study. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to show gratitude to the organ coordinators, Gail Skolek, Gayle Blake, Mary Polk, and the Illinois organ procurement organization, Gift of Hope, for coordinating and providing high-quality valuable donated organs for islet isolation in UIC. The authors declare no conflicts of interest.
References
- 1.Alejandro R., Barton F.B., Hering B.J., Wease S.. 2008. Update from the Collaborative Islet Transplant Registry. Transplantation 2008; 86: 1783–8. [DOI] [PubMed] [Google Scholar]
- 2.Balamurugan A.N., Naziruddin B., Lockridge A., Tiwari M., Loganathan G., Takita M., Matsumoto S., Papas K., Trieger M., Rainis H., Kin T., Kay T.W., Wease S., Messinger S., Ricordi C., Alejandro R., Markmann J., Kerr-Conti J., Rickels M.R., Liu C., Zhang X., Witkowski P., Posselt A., Maffi P., Secchi A., Berney T., O'Connell P.J., Hering B.J., Barton F.B.. Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am J Transplant. 2014;14: 2595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakey J.R., Warnock G.L., Rajotte R.V., Suarez-Alamazor M.E., Ao Z., Shapiro A.M., Kneteman N.M.. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation 1996; 61: 1047–53. [DOI] [PubMed] [Google Scholar]
- 4.Nano R., Clissi B., Melzi R., Calori G., Maffi P., Antonioli B., Marzorati S., Aldrighetti L., Freschi M., Grochowiecki T., Socci C., Secchi A., Di Carlo V., Bonifacio E., Bertuzzi F.. Islet isolation for allotransplantation: Variables associated with successful islet yield and graft function. Diabetologia 2005; 48: 906–12. [DOI] [PubMed] [Google Scholar]
- 5.Kaddis J.S., Danobeitia J.S., Niland J.C., Stiller T., Fernandez L.A.. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am J Transplant. 2010; 10: 646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponte G.M., Pileggi A., Messinger S., Alejandro A., Ichii H., Baidal D.A., Khan A., Ricordi C., Goss J.A., Alejandro R.. Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell Transplant. 2007; 16: 595–607. [DOI] [PubMed] [Google Scholar]
- 7.Brandhorst H., Brandhorst D., Hering B.J., Federlin K., Bretzel R.G.. Body mass index of pancreatic donors: A decisive factor for human islet isolation. Exp Clin Endocrinol Diabetes 1995; 103(Suppl 2): 23–6. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto I., Sawada T., Nakano M., Sakai T., Liu B., Ansite J.D., Zhang H.J., Kandaswamy R., Sutherland D.E., Hering B.J.. Improvement in islet yield from obese donors for human islet transplants. Transplantation 2004; 78: 880–5. [DOI] [PubMed] [Google Scholar]
- 9.Wang L.J., Cochet O., Wang X.J., Krzystyniak A., Misawa R., Golab K., Tibudan M., Grose R., Savari O., Millis J.M., Witkowski P.. Donor height in combination with islet donor score improves pancreas donor selection for pancreatic islet isolation and transplantation. Transplant Proc. 2014; 46: 1972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Gorman D., Kin T., Murdoch T., Richer B., McGhee-Wilson D., Ryan E.A., Shapiro J.A., Lakey J.R.. The standardization of pancreatic donors for islet isolations. Transplantation 2005; 80: 801–6. [DOI] [PubMed] [Google Scholar]
- 11.Wang L.J., Kin T., O'Gorman D., Shapiro A.M., Naziruddin B., Takita M., Levy M.F., Posselt A.M., Szot G.L., Savari O., Barbaro B., McGarrigle J., Yeh C.C., Oberholzer J., Lei J., Chen T., Lian M., Markmann J.F., Alvarez A., Linetsky E., Ricordi C., Balamurugan A.N., Loganathan G., Wilhelm J.J., Hering B.J., Bottino R., Trucco M., Liu C., Min Z., Li Y., Naji A., Fernandez L.A., Ziemelis M., Danobeitia J.S., Millis J.M., Witkowski P.. A multicenter study: North American Islet Donor Score in donor pancreas selection for human islet isolation for transplantation. Cell Transplant. 2016; 25(8): 1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rheinheimer J., Ziegelmann P.K., Carlessi R., Reck L.R., Bauer A.C., Leitao C.B., Crispim D.. Different digestion enzymes used for human pancreatic islet isolation: A mixed treatment comparison (MTC) meta-analysis. Islets 2014; 6: e977118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakey J.R., Warnock G.L., Shapiro A.M., Korbutt G.S., Ao Z., Kneteman N.M., Rajotte R.V.. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999; 8: 285–92. [DOI] [PubMed] [Google Scholar]
- 14.Kissler H.J., Niland J.C., Olack B., Ricordi C., Hering B.J., Naji A., Kandeel F., Oberholzer J., Fernandez L., Contreras J., Stiller T., Sowinski J., Kaufman D.B.. Validation of methodologies for quantifying isolated human islets: An islet cell resources study. Clin Transplant. 2010; 24: 236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S.C., Han D.J., Kang C.H., We Y.M., Back J.H., Kim Y.H., Kim J.H., Lim D.G.. Analysis on donor and isolation-related factors of successful isolation of human islet of Langerhans from human cadaveric donors. Transplant Proc. 2005; 37: 3402–3. [DOI] [PubMed] [Google Scholar]
- 16.Hilling D.E., Bouwman E., Terpstra O.T., Marang-van de Mheen P.J.. Effects of donor-, pancreas-, and isolation-related variables on human islet isolation outcome: A systematic review. Cell Transplant. 2014; 23: 921–8. [DOI] [PubMed] [Google Scholar]
- 17.Bucher P., Mathe Z., Bosco D., Andres A., Kurfuerst M., Ramsch-Gunther N., Buhler L., Morel P., Berney T.. Serva collagenase NB1: A new enzyme preparation for human islet isolation. Transplant Proc. 2004; 36: 1143–4. [DOI] [PubMed] [Google Scholar]
- 18.Brandhorst H., Friberg A., Nilsson B., Andersson H.H., Felldin M., Foss A., Salmela K., Tibell A., Tufveson G., Korsgren O., Brandhorst D.. Large-scale comparison of Liberase HI and collagenase NB1 utilized for human islet isolation. Cell Transplant. 2010; 19: 3–8. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias I., Valiente L., Shiang K.D., Ichii H., Kandeel F., Al-Abdullah I.H.. The effects of digestion enzymes on islet viability and cellular composition. Cell Transplant. 2012; 21: 649–55. [DOI] [PubMed] [Google Scholar]
- 20.Qi M., Valiente L., McFadden B., Omori K., Bilbao S., Juan J., Rawson J., Scott S., Ferreri K., Mullen Y., El-Shahawy M., Dafoe D., Kandeel F., Al-Abdullah I.H.. The choice of enzyme for human pancreas digestion is a critical factor for increasing the success of islet isolation. Transplant Direct 2015; 1(4)1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi M., Barbaro B., Wang S., Wang Y., Hansen M., Oberholzer J.. Human pancreatic islet isolation: Part II: Purification and culture of human islets. J Vis Exp. 2009; 27: e1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi M., Barbaro B., Wang S., Wang Y., Hansen M., Oberholzer J.. Human pancreatic islet isolation: Part I: Digestion and collection of pancreatic tissue. J Vis Exp 2009; 27: e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricordi C., Lacy P.E., Finke E.H., Olack B.J., Scharp D.W.. Automated method for isolation of human pancreatic islets. Diabetes 1988; 37: 413–20. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Paushter D., Wang S., Barbaro B., Harvat T., Danielson K.K., Kinzer K., Zhang L., Qi M., Oberholzer J.. Highly purified versus filtered crude collagenase: Comparable human islet isolation outcomes. Cell Transplant. 2011; 20: 1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbaro B., Salehi P., Wang Y., Qi M., Gangemi A., Kuechle J., Hansen M.A., Romagnoli T., Avila J., Benedetti E., Mage R., Oberholzer J.. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation 2007; 84: 1200–3. [DOI] [PubMed] [Google Scholar]
- 26.The NIH CIT Consortium Chemistry Manufacturing Controls Monitoring Committee. Purified human pancreatic islets (PHPI) Master Production Batch Record—A standard operating procedure of the NIH Clinical Islet Transplantation Consortium. CellR4; 2014. [accessed 2016 Jun 16]. http://www.cellr4.org/article/891. [PMC free article] [PubMed]
- 27.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G.. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto S., Qualley S.A., Goel S., Hagman D.K., Sweet I.R., Poitout V., Strong D.M., Robertson R.P., Reems J.A.. Effect of the two-layer (University of Wisconsin solution-perfluorochemical plus O2) method of pancreas preservation on human islet isolation, as assessed by the Edmonton Isolation Protocol. Transplantation 2002; 74: 1414–9. [DOI] [PubMed] [Google Scholar]
- 29.O'Gorman D., Kin T., Imes S., Pawlick R., Senior P., Shapiro A.M.. Comparison of human islet isolation outcomes using a new mammalian tissue-free enzyme versus collagenase NB-1. Transplantation 2010; 90: 255–9. [DOI] [PubMed] [Google Scholar]
- 30.Wang L.J., Kissler H.J., Wang X., Cochet O., Krzystyniak A., Misawa R., Golab K., Tibudan M., Grzanka J., Savari O., Kaufman D.B., Millis M., Witkowski P.. Application of digital image analysis to determine pancreatic islet mass and purity in clinical islet isolation and transplantation. Cell Transplant. 2015; 24: 1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pileggi A., Ribeiro M.M., Hogan A.R., Molano R.D., Cobianchi L., Ichii H., Embury J., Inverardi L., Fornoni A., Ricordi C., Pastori R.L.. Impact of pancreatic cold preservation on rat islet recovery and function. Transplantation 2009; 87: 1442–50. [DOI] [PubMed] [Google Scholar]
- 32.O'Gorman D., Kin T., Pawlick R., Imes S., Senior P.A., Shapiro A.M.. Clinical islet isolation outcomes with a highly purified neutral protease for pancreas dissociation. Islets 2013; 5: 111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kin T., O'Gorman D., Senior P., Shapiro A.M.. Experience of islet isolation without neutral protease supplementation. Islets 2010; 2: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


