Abstract
Mesenchymal stem cell (MSC) transplantation protects against neonatal severe intraventricular hemorrhage (IVH)-induced brain injury by a paracrine rather than regenerative mechanism; however, the paracrine factors involved and their roles have not yet been delineated. This study aimed to identify the paracrine mediator(s) and to determine their role in mediating the therapeutic effects of MSCs in severe IVH. We first identified significant upregulation of brain-derived neurotrophic factor (BDNF) in MSCs compared with fibroblasts, in both DNA and antibody microarrays, after thrombin exposure. We then knocked down BDNF in MSCs by transfection with small interfering (si)RNA specific for human BDNF. The therapeutic effects of MSCs with or without BDNF knockdown were evaluated in vitro in rat neuronal cells challenged with thrombin, and in vivo in newborn Sprague–Dawley rats by injecting 200 μl of blood on postnatal day 4 (P4), and transplanting MSCs (1 × 105 cells) intraventricularly on P6. siRNA-induced BDNF knockdown abolished the in vitro benefits of MSCs on thrombin-induced neuronal cell death. BDNF knockdown also abolished the in vivo protective effects against severe IVH-induced brain injuries such as the attenuation of posthemorrhagic hydrocephalus, impaired behavioral test performance, increased astrogliosis, increased number of TUNEL cells, ED-1+ cells, and inflammatory cytokines, and reduced myelin basic protein expression. Our data indicate that BDNF secreted by transplanted MSCs is one of the critical paracrine factors that play a seminal role in attenuating severe IVH-induced brain injuries in newborn rats.
Keywords: Brain-derived neurotrophic factor (BDNF); Intraventricular hemorrhage (IVH); Infant, newborn; Infant, premature; Hydrocephalus; Mesenchymal stem cells (MSCs); Cell transplantation
Introduction
Despite recent advances in neonatal intensive care medicine, intraventricular hemorrhage (IVH) remains a major cause of mortality and neurologic disability in premature infants, with few clinically effective treatments1. Therefore, the development of new and effective therapies is urgently needed to improve the prognosis of this serious intractable disease.
Recently, we have shown that intraventricular transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) significantly attenuated the development of posthemorrhagic hydrocephalus and brain injuries after severe IVH in newborn rats2. Moreover, these protective effects of MSCs were time dependent, showing better protection with early rather than late transplantation3, and route dependent, showing better therapeutic efficacy with local intraventricular rather than systemic intravenous transplantation4. We also reported that MSC transplantation significantly attenuated several disorders such as bronchopulmonary dysplasia5 and neonatal stroke6 primarily through paracrine anti-inflammatory and antiapoptotic effects rather than a regenerative mechanism. Several growth factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and interleukins are known to enhance brain repair after hypoxia and/or ischemia7,8. However, the specific paracrine factors responsible for the neuroprotective action of MSC transplantation, and their protective mechanisms after severe IVH, have not yet been elucidated.
In the present study, we tried to identify the specific factors and precise mechanism mediating the neuroprotective effects of MSC transplantation after severe IVH. We first conducted DNA and antibody microarray analyses of MSCs to screen for the genes and proteins upregulated after exposure to thrombin and were able to identify the common elevation of BDNF in both analyses in MSCs but not in fibroblasts. Next, to test the hypothesis that BDNF secreted by MSCs plays a key role in mediating the neuroprotective effects, the neuroprotective effects of MSCs with or without knockdown of BDNF with small interfering (si)RNA were evaluated both in vitro after exposure of neuronal cells to thrombin and in vivo after induction of severe IVH in newborn rats.
Materials and Methods
Cell Preparation
hUCB-MSCs were provided by Medipost Co., Ltd. (Seoul, South Korea)2–4,9. Human fibroblasts (MRC-5; Korean Cell Line Bank No. 10171) were purchased from Korean Cell Line Bank (Seoul, South Korea). The use of hUCB-MSCs was approved by the Institutional Review Board (IRB) of Samsung Medical Center.
DNA and Antibody Microarray Analyses
At transcriptional and translational level, to assess gene expression changes in MSCs responsible for their neuroprotective action, microarray analyses were performed using Agilent Human Oligo Microarray (60 K) chips (Agilent Technologies, Santa Clara, CA, USA) for RNA and using an antibody array chip (RayBiotech, Norcross, GA, USA) for protein from MSCs and fibroblasts after exposure to thrombin for 6 h. For microarray evaluation, RNA was hybridized to Agilent Human Oligo Microarray (60 K) chips, and the hybridized images were scanned using an Agilent DNA microarray scanner and quantified with Feature Extraction Software (Agilent Technologies). Using GeneSpringGX 7.3 (Agilent Technologies), data normalization and selection of fold-changed genes were performed. Changes in gene and protein expression greater than twofold with values of p < 0.05 in each group were considered significant. To assay functional annotation, we used the list of relevant genes in the DAVID (http://david.abcc.ncifcrf.gov/), Medline (http://www.ncbi.nlm.nih.gov/), and KEGG databases (http://www.genome.jp/kegg/).
For antibody array, proteins from thrombin-treated MSCs and fibroblasts were analyzed using a Raybio antibody array system (RayBiotech) according to the manufacturer's instructions.
BDNF siRNA Transfection
BDNF and scrambled siRNAs were purchased from Santa Cruz Biotechnology (sc-42121; sc-37007; Santa Cruz, CA, USA). MSCs were transfected with siRNA oligonucleotides using Oligofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. All assays or transplants were performed 24 h after RNA transfection.
Thrombin Exposure In Vitro Cell Culture
Brain neuronal cells were primarily cultured from the embryonic mouse brain at E18.5. Neuronal cells (5 × 103 cells/well) were seeded into 96-well plates in 100 μl of neurobasal medium containing B-27 supplement (Gibco, Gaithersburg, MD, USA) and cultured for 24 h at 37°C. To induce the in vitro neuronal injury after hemorrhagic condition10,11, cells were treated with 40 U of thrombin (Reyon Pharmaceutical Co. Ltd, Seoul, South Korea). Thrombin-treated neuronal cells were then incubated in neurobasal medium (Gibco) alone or neurobasal medium (Gibco) with nontransfected hUCB-MSCs (1 × 103), scrambled siRNA-transfected MSCs, or BDNF siRNA-transfected MSCs in the upper chamber for 24 h. BDNF-blocking antibody (Abcam, Cambridge, MA, USA) or control immunoglobulin (IgG; Dako, Carpinteria, CA, USA) was added to the coculture of thrombin-treated neuronal cells and nontransfected MSCs. Low-dose (100 pg/ml) or high-dose (1 ng/ml) recombinant human (rh) BDNF (R&D Systems, Minneapolis, MN, USA) was added to the thrombin-treated neuronal cells with or with out cocultured BDNF siRNA-transfected MSCs. A colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay (Dojindo Molecular Technologies Inc., Rockville, MD, USA) was used to evaluate cell viability according to the manufacturer's protocol. Relative viabilities were determined by normalizing to 0% (no cells) and 100% (untreated cells) controls.
Animal Model
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Samsung Biomedical Research Institute, and the study followed institutional and National Institutes of Health (NIH) guidelines for laboratory animal care. All animal procedures were performed in an Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited specific pathogen-free facility. Newborn Sprague–Dawley rats (Orient Co., Seoul, South Korea), those reared with their dams in the standard cage, 50-L Plexiglas chamber, were used in this study. Dam rats could access water and laboratory chow freely and were maintained in an alternating 12-h light/dark cycle with constant room humidity and temperature. The experiment began on postnatal day 4 (P4) and continued through to P32. In P4 rat pups, IVH was induced by intracerebroventricular injection of a total of 200 μl of fresh maternal whole blood (100 μl each into the right and left ventricles) as described previously2. On P5 (1 day after IVH), we performed brain magnetic resonance imaging (MRI) to confirm baseline severe IVH, replicating grade III or IV IVH in human infants12, and only severe IVH-induced rats were included. IVH rats with minimal or nonvisible IVH on the brain MRI performed on P5 were excluded, before randomization. On P6, IVH-induced rats were randomly divided into five groups: IVH control (IC, n = 18), IVH with naive MSCs (IM, n = 16), IVH with MSCs transfected with scrambled siRNA (IM-cont, n = 17), and IVH with MSCs transfected with BDNF siRNA (IM-bdnf-kd, n = 17). IM, IM-cont, and IM-bdnf-kd group rats received naive MSCs, MSCs transfected with scrambled siRNA, and MSCs transfected with BDNF siRNA, respectively. IC rats also received an equal volume of normal saline intracerebroventricularly. No rat pups were excluded except expired rats of four in IC, one in IM, two in IM-cont, and five in IM-bdnf-kd. Follow-up brain MRI was performed at 7 (P11) and 28 (P32) days after inducing severe IVH. We assessed and monitored the condition of rat pups twice per day on a daily basis especially for 7 days after modeling. All procedures were performed under inhaled anesthesia using a mixture of isoflurane (Ifran®, Hana Pharm Co., Ltd., Seoul, South Korea) and 2:1 nitrous oxide/oxygen. Animals were euthanized on P32, and brain tissue samples were prepared.
In Vivo MRI and Assessment of the Ventricle to Whole Brain Volume Ratio
One day after IVH modeling (P5), an initial brain MRI was performed to confirm the baseline severity of IVH, and follow-up MRIs were performed on P11 and P32 to monitor the progress of ventricular dilatation and volume ratio of ventricles. MRI examination was performed under anesthesia with 1.5%–2% isoflurane in oxygen-enriched air using a 7.0-Tesla MRI system (Bruker-Biospin, Fällanden, Switzerland). MRI was performed with a 20-cm gradient set capable of providing a rising time of 400 mTm−1. Images were acquired for 12 coronal slices that were 1.0 mm thick with no interslice gap. For T2-weighted images (T2WI), a fast-spin echo sequence was used to acquire images with a repetition time (TR) = 3,000 ms, time to echo (TE) = 60 ms, field of view (FOV) = 25.6 mm × 25.6 mm, matrix size = 256 × 256, and number of excitations (NEX) = 12. One MRI session took 30 min per pup on average. The ventricle-to-whole brain volume ratio was calculated for each pup. A blinded independent examiner calculated the volume ratio by manually outlining the ventricle and the whole brain in 12 MRI slices using ParaVision software (version 2.0.2; Bruker, BioSpin, Karlsruhe, Germany). Volume estimates were then made according to Cavalieri's principle, and the ventricle-to-whole brain volume ratio was calculated to determine the extent of posthemorrhagic hydrocephalus after severe IVH.
Functional Behavioral Test
The negative geotaxis test and rotarod test were performed as described in the previous study2,4. Briefly, a negative geotaxis test, based on the innate reflex rotation to face uphill when placed head down on an inclined wooden platform, was performed on the 21st and 28th day after IVH induction (P25 and 32). Pups were gently held for 3–5 s in a head-downward position on a slanted slope, and the time required for the pups to rotate 180° to face uphill after release was recorded. Rotarod tests were performed consecutively on the 26th to 28th day after IVH induction (P30–32). The rotation speed of the treadmill (Jeung Do Bio & Plant Co. Ltd, Seoul, South Korea) was accelerated from 4 to 40 rpm over 100 s for a maximum of 3 min. The average latency to fall from three trials was used as the final result. Behavior function tests were done and evaluated by investigators blinded to the experimental groups.
TUNEL Assay
We assessed cell death using the immunofluorescent TUNEL technique as described in the previous study2,4. Briefly, the brain slides were incubated with an equilibrium buffer in ApopTag kit (Millipore, Billerica, MA, USA) for 5 min and incubated in reaction buffer with terminal deoxynucleotidyl transferase (Millipore) and 1 nmol of dUTP-digoxigenin (Millipore) for 60 min at 37°C, and then slides were treated by adding stop/wash buffer (Millipore) and washed twice with phosphate-buffered saline (PBS). Antidigoxigenin-fluorescein isothiocyanate (FITC) was added and reacted for 30 min at 37°C. After washing with PBS, nuclei were counterstained with 4′,6-diamidino-2-pheylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA).
Immunohistochemistry
The extent of reactive gliosis, reactive microglia, and myelination, as evidenced by neuronal specific glial fibrillary acidic protein (GFAP), ED-1, and myelin basic protein (MBP), respectively, were immunohistochemically evaluated. Immunohistochemistry was performed on deparaffinized 4-μm-thick brain sections. Brain coronal sections were incubated with the following primary antibodies: GFAP (rabbit polyclonal; 1:1,000 dilution, Dako, Glostrup, Denmark) and MBP (rabbit polyclonal; 1:1,000 dilution; Abcam) or ED-1 (mouse monoclonal; 1:100 dilution; Millipore) as previously reported. Three coronal sections (+0.95 mm to −0.11 mm/bregma) from each brain were stained, and three random nonoverlapping fields in the periventricular area, including the corpus callosum and caudate nucleus, from each section were evaluated. The immunofluorescent intensity of GFAP or MBP staining was measured in the randomly selected fields in the right and left periventricular area using ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA] by an observer blinded to the group assignments. The number of ED-1+ cells was counted in the randomly selected fields by an investigator who was blinded to the group assignments.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of inflammatory cytokines, including interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α, in the periventricular brain tissue homogenates were measured. Frozen samples of brain tissue from the periventricular zone were homogenized and centrifuged at 8,000 × g for 20 min at 4°C. The protein content in the supernatant was measured using the Bradford method with bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) as a standard. The levels of inflammatory cytokines including IL-1α, IL-1β, IL-6, and TNF-α were measured with the Milliplex MAP ELISA Kit in the brain tissue homogenates of periventricular area according to the manufacturer's protocol (Millipore)2. Human-and rat- specific BDNFs were measured with ELISA kit (Quantikine ELISA Kit, R&D Systems) according to the manufacturer's protocol.
Statistical Analyses
Estimation of sample size was based on the intervention-induced differences (10%) of cerebroventricular volumes at P32 according to our previous reports with power 0.8 and type I error probability 0.05. Data are expressed as the mean ± standard deviation of the mean ± standard error of the mean (SEM). Microarray data were analyzed using one-way analysis of variance (ANOVA) with a Benjamini–Hochberg correction for multiple comparisons (GeneSpring, Agilent Technologies). For continuous variables, statistical comparison between groups was performed using one-way ANOVA and Tukey's post hoc analysis. For time-course variables, a univariate general linear model for repeated measures with Tukey's post hoc comparison was performed. All of the data were analyzed using SPSS version 18.0 (IBM, Armonk, NY, USA). A value of p < 0.05 was considered statistically significant.
Results
Knock Down of BDNF Expression From MSCs
To delineate the role of BDNF, which specifically expressed from the donor cells, BDNF-specific siRNA was incorporated into the donor MSCs. At 24 h after transfection, BDNF expression was diminished to 27% of the naive MSCs expression level (Fig. 1).
Figure 1.
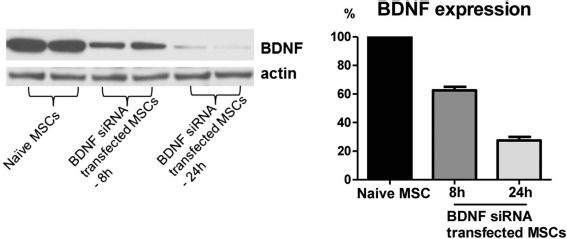
Knock down of brain-derived neurotrophic factor (BDNF) in the mesenchymal stem cells (MSCs). Western blot assay for BDNF expression from the naive MSCs and BDNF small interfering (si)RNA-transfected MSCs after 8 and 24 h of transfection. To confirm the successful knockdown of BDNF, the BDNF level was measured in the culture medium of BDNF siRNA-transfected MSCs. BDNF knockdown MSCs showed substantially reduced BDNF production at 8 h and 24 h after transfection.
Changes in Gene and Protein Expression Profiles of MSCs After Thrombin Exposure
In the DNA and antibody microarray analyses performed to identify gene and protein expression changes in MSCs responsible for their neuroprotective action after thrombin exposure, 46 genes in the DNA microarray and 12 proteins in the antibody microarray were significantly upregulated in MSCs compared with human fibroblasts. Among the top 10 most upregulated genes and proteins in each analysis, BDNF was the only common factor upregulated in both analyses (Fig. 2A).
Figure 2.
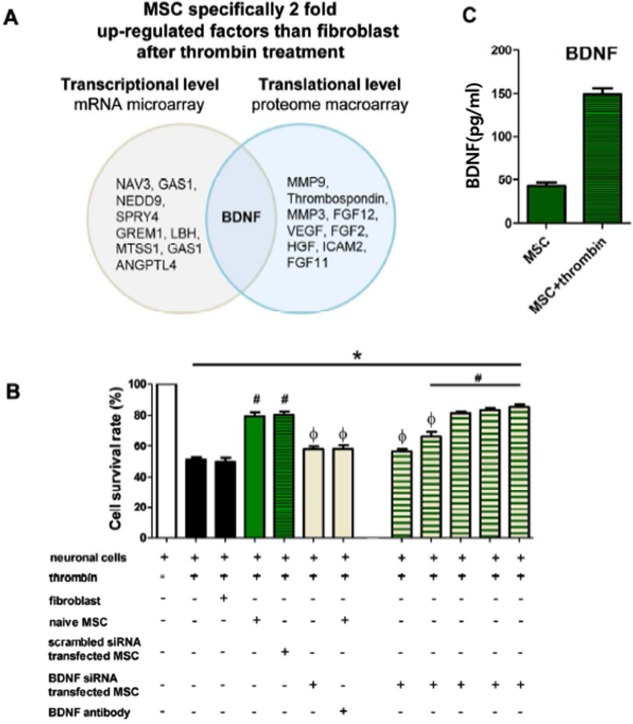
Gene expression analysis of upregulation in MSCs and in vitro protective effects of BDNF secreted from MSCs. (A) Gene expression analysis of upregulation in MSCs compared with fibroblasts in microarray and macroarray. Note that the BDNF gene was commonly upregulated in microarray and macroarray. (B) In vitro expression level of BDNF in MSCs. (C) Cell survival rate in each group was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay. Data are expressed as mean ± standard error of the mean (SEM). *p < 0.05 versus neuronal cells without any intervention, #p < 0.05 versus neuronal cells treated with thrombin, Φp < 0.05 versus neuronal cells treated with thrombin and naive MSCs. Abbreviations: (si)RNA, small interfering; VEGF, vascular endothelial growth factor.
In Vitro Protective Effects of MSCs
In rat primary neuronal cell culture, exposure to 40 U of thrombin and incubation for 24 h significantly reduced the survival rate of neuronal cells. Cotreatment with hUCB-MSCs significantly attenuated neuronal cell death in vitro, but fibroblasts (MRC-5) did not increase the survival rate of neuronal cells (Fig. 2B). These findings indicate that the neuroprotective effects observed in vitro are specific to MSCs. Thrombin-induced neuronal cell death was also attenuated when cocultured with scrambled siRNA-transfected MSCs. However, coculture with BDNF knockdown MSCs did not show clear protective effects.
The protective effects of MSCs against thrombin-induced neuronal cell death were abolished by a BDNF-neutralizing antibody. Overall, these findings suggest that BDNF secreted by the transplanted MSCs might play a seminal role in mediating the neuroprotective effects of MSCs after severe IVH.
In a dose-titration study, supplementation of 100, 150, and 200 pg, but not 25 and 50 pg, of recombinant human BDNF significantly restored the neuroprotective effects of BDNF knockdown MSCs (Fig. 2B). These data suggest the dose-dependent restorative effects along with comparable BDNF levels compared with those of naive MSCs (149 ± 9 pg/ml) (Fig. 2C).
Serial Brain MRI
Figure 3A displays serial brain MRIs from each study group performed at 1, 7, and 28 days after inducing IVH (i.e., P5, P11, and P32, respectively). On P5, 1 day before MSC transplantation, the ventricular dilatation severity, presented as a ratio of ventricle to whole brain volume, did not differ between the study groups (Fig. 3). Progressive ventriculomegaly, observed in the IC on follow-up brain MRIs on P11 and P32, was significantly attenuated in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 3).
Figure 3.
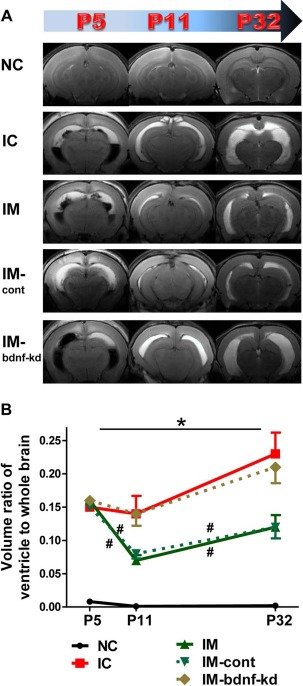
Knock down of BDNF in MSCs abolished the therapeutic effects of MSCs in attenuating ventricular dilatation and its progression after severe intraventricular hemorrhage (IVH). (A) Representative serial brain magnetic resonance images (MRIs) from each group 1, 7, and 28 days after inducing IVH (P5/P11/P32). (B) Ventricle-to-whole brain volume ratio as measured by MRI. Data are expressed as mean ± SEM. Abbreviations: NC, normal control rats; IC, IVH control rats; IM, IVH with transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs); IM-cont, IVH with transplantation of scrambled small interfering (si)RNA-transfected hUCB-MSCs; IM-bdnf-kd, transplantation of BDNF siRNA-transfected hUCB-MSCs. *p < 0.05 versus NC, #p < 0.05 versus IC.
Functional Behavior Tests
To assess sensorimotor function, the negative geotaxis test and rotarod test were performed. In the IVH control group, the negative geotaxis test performed on P25 and P32 displayed significant functional impairment compared with the normal control group. This impaired performance was significantly improved in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 4A).
Figure 4.
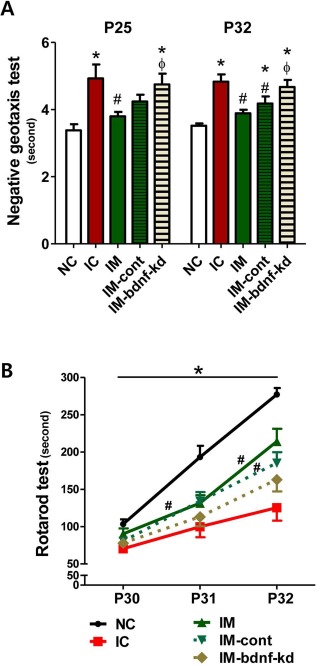
Knock down of BDNF in MSCs abolished the therapeutic effects of MSCs in improving behavioral function after severe IVH. Sensorimotor functional outcomes on the negative geotaxis test on P25 and P32 (A) and rotarod test on P30–32 (B). Data are expressed as mean ± SEM. Abbreviations: NC, normal control rats; IC, IVH control rats; IM, IVH with transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs); IM-cont, IVH with transplantation of scrambled small interfering (si)RNA-transfected hUCB-MSCs; IM-bdnf-kd, transplantation of BDNF siRNA-transfected hUCB-MSCs. *p < 0.05 versus NC, #p < 0.05 versus IC, Fp < 0.05 versus IM.
The rotarod test was performed on P30, P31, and P32. Although initial rotarod test results performed on P30 were not significantly different between the study groups, the normal control group displayed an appropriate learning curve with longer latency to fall on P31 and P32 (Fig. 4B). In contrast, the IVH control group had significantly shorter latency to fall on P31 and P32 compared with the normal control group. This impaired function significantly improved over time in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 4B).
BDNF Levels, Cell Death, Reactive Gliosis, and Myelination
On P7, 1 day after MSC transplantation, human BDNF in brain homogenates was detected in the IVH with naive MSC group and scrambled siRNA-MSC group, but not in the IVH with BDNF siRNA-MSC group nor in the IVH control group. On P7, rat BDNF level was highest in the IVH rats that received naive MSCs and scrambled siRNA-MSCs, and this level was significantly higher in the IVH control and IVH with BDNF siRNA transplantation groups than the normal control group (Fig. 5). On P11, 5 days after MSC transplantation, while no human BDNF was detected in any study group, rat BDNF levels remained significantly increased only in the IVH rats that received naive MSCs and scrambled siRNA-MSCs compared with the normal control rats (Fig. 5).
Figure 5.
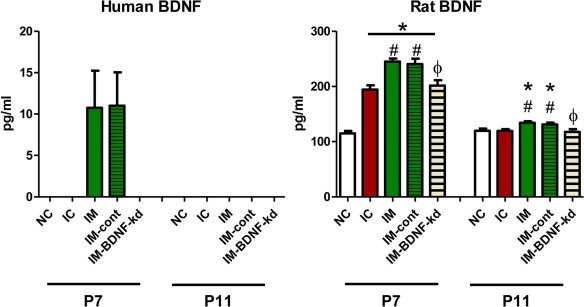
Human and rat BDNF expressions in the brain tissue. On the second and fifth day after human MSC transplantation (P5), human BDNF and rat BDNF were measured, respectively, in rat brains from each group. Abbreviations: NC, normal control rats; IC, IVH control rats; IM, IVH with transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs); IM-cont, IVH with transplantation of scrambled small interfering (si)RNA-transfected hUCB-MSCs; IM-bdnf-kd, transplantation of BDNF siRNA-transfected hUCB-MSCs. *p < 0.05 versus NC, #p < 0.05 versus IC, Φp < 0.05 versus IM.
Myelination in the periventricular brain area was assessed using the optical density of MBP immunostaining and MBP protein expression by Western blot assay and was significantly reduced in the IVH control group compared with the normal control group (Fig. 6A–D). This impaired myelination was significantly improved in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 6A–D).
Figure 6.
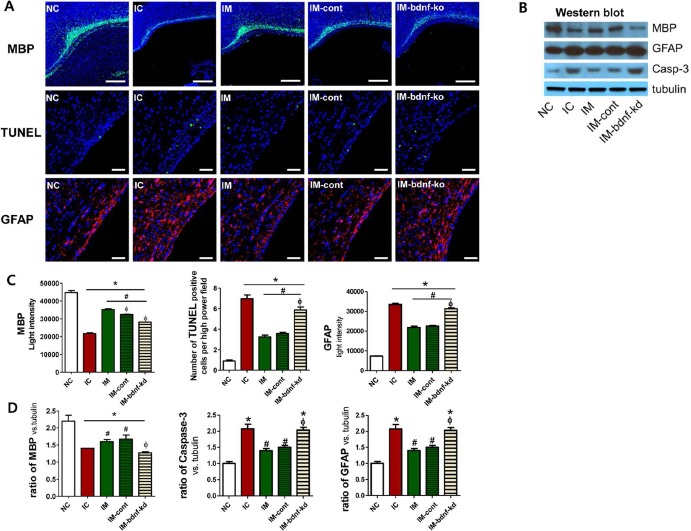
Knock down of BDNF in MSCs abolished the therapeutic effects of MSCs in improving brain myelination and in attenuating cell death and reactive gliosis after severe IVH. (A) Representative immunofluorescence photomicrographs of the periventricular area with staining for myelin basic protein (MBP) (green), TUNEL (green), glial fibrillary acidic protein (GFAP) (red), and 4′,6-diamidino-2-pheylindole (DAPI) (blue) (original magnification: 200×, scale bars: 50 μm for MBP and original magnification: 85×, scale bars: 500 μm for TUNEL and GFAP). (B) Western blot assay of MBP, GFAP, and caspase 3 with tubulin in the brain tissue homogenates on P32. Brain MBP expression, cell death, and reactive gliosis evidenced by light intensity of MBP immunofluorescence, average number of TUNEL+ cells, and light intensity of GFAP staining, respectively, in the periventricular area (C) and those indicated by the ratio of MBP, caspase 3, and GFAP to tubulin with Western assay, respectively, in the brain tissue homogenates (D) on P32. Data are expressed as mean ± SEM. Abbreviations: NC, normal control rats; IC, IVH control rats; IM, IVH with transplantation of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs); IM-cont, IVH with transplantation of scrambled small interfering (si)RNA-transfected hUCB-MSCs; IM-bdnf-kd, transplantation of BDNF siRNA-transfected hUCB-MSCs. *p<0.05 versus NC, #p<0.05 versus IC, Φp <0.05 versus IM.
To determine the extent of cell death after severe IVH, the number of TUNEL+ cells was assessed by immunohistochemistry and caspase 3 expression using Western blot analysis in the periventricular brain tissue of animals on P32 (Fig. 6A–D). The number of TUNEL+ cells and protein expression of caspase 3 were significantly increased in the IVH control group compared with the normal control group. These increases were significantly attenuated in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 6A–D).
Reactive gliosis in periventricular brain tissue, as sessed using the optical density of GFAP+ cells via immunohistochemistry and GFAP protein expression using Western blot assay, was significantly increased in the IVH control group compared with the normal control group (Fig. 6A–D). These increases were significantly attenuated in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 6A–D).
Inflammation in the Periventricular Brain
To determine whether transplanted MSCs attenuated brain inflammation induced by severe IVH, the levels of inflammatory cytokines, such as IL-1α, IL-1β, IL-6, and TNF-α, in periventricular brain tissue homogenates, and the number of ED-1+ cells in brain coronal sections were analyzed on P32. ED-1+ cells and the levels of inflammatory cytokines in the periventricular brain tissue were significantly higher in the IVH control group than the normal control group (Fig. 7). These increased inflammatory responses were significantly attenuated in IM and IM-cont, but not in the IVH-bdnf-kd (Fig. 7).
Figure 7.
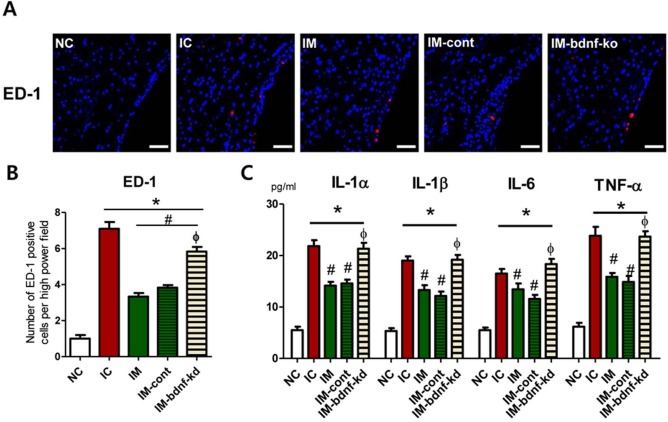
Knock down of BDNF in MSCs abolished the therapeutic effects of MSCs in downregulating brain inflammation after severe IVH. (A) Representative immunofluorescence photomicrographs of the periventricular area with staining for active macrophages (ED-1, red) and DAPI (blue) (original magnification: 200×, scale bars: 25 μm). (B) Average number of ED-1+ cells, which indicate active macrophages in the periventricular area on P32. (C) Levels of inflammatory cytokines including interleukin (IL)-1α, IL-β, IL-6, and tumor necrosis factor (TNF)-α in the brain tissue homogenates on P32. Data are expressed as mean ± SEM. NC, normal control rats; IC, IVH control rats; IM, IVH with transplantation of hUCB-MSCs; IM-cont, IVH with transplantation of scrambled small interfering (si)RNA-transfected hUCB-MSCs; IM-bdnf-kd, transplantation of BDNF siRNA-transfected hUCB-MSCs. *p < 0.05 versus NC, #p < 0.05 versus IC, Φp < 0.05 versus IM.
Discussion
In the present study, MSCs, but not fibroblasts, significantly attenuated neuronal cell death after thrombin exposure in vitro. However, little is currently known about the precise mechanism underlying the neuroprotective effects specific to MSCs. Our DNA and antibody microarray analyses enabled us to simultaneously investigate both the transcriptional and translational responses of the entire MSC genome and proteins after exposure to thrombin, independent of any prior knowledge about the molecular mechanisms of the neuroprotective effects of MSCs. Our data showing the common significant upregulation of BDNF in both the DNA and antibody micro-array analyses suggest that BDNF produced by MSCs might be the key paracrine factor responsible for their neuroprotective effects. However, as even small changes in gene and/or protein expression often result in biologically important differences13, the failure of our data to achieve significant upregulation above our arbitrarily set standards in both the gene and protein expression profiles of other paracrine factors does not exclude their potential role in mediating the neuroprotective effects of MSCs. Therefore, as our data do not guarantee that BDNF is the exclusive neuroprotective mechanism of MSCs, further studies will be necessary that take account of the potentially diverse neuroprotective mechanisms of MSCs, including other potentially important paracrine factors such as VEGF14.
In the present study, the protective effects of MSCs observed in vitro in the primary culture of rat neuronal cells exposed to thrombin were abolished by BDNF-knockdown or BDNF-neutralizing antibody and were restored by rhBDNF supplementation. Furthermore, the in vivo beneficial effects of MSCs in the newborn Sprague–Dawley rat model of severe IVH2, such as significant attenuation of posthemorrhagic hydrocephalus, impaired behavioral test performance and myelination, and increased cell death and inflammation, were also abolished by BDNF knockdown with BDNF siRNA but not by scrambled siRNA. Taken together, these findings suggest that BDNF secreted by transplanted MSCs might be a critical paracrine factor mediating their neuroprotective effects after severe IVH in the newborn rats. These findings also raise the possibility that the BDNF level secreted by MSCs could be used as a biomarker of the potency of stem cell transplants in severe IVH.
Understanding the mechanism by which human BDNF secreted by exogenous MSCs protects newborn rat brain tissue against severe IVH-induced injury is of particular interest. BDNF directly influences downstream cell signaling involved in cell survival and function via tyrosine kinase receptor B15 and significantly reduced neuronal death both in vitro after excitotoxic insults16 and in vivo after focal ischemia and bacterial meningitis17,18. Increased endogenous BDNF levels were observed in various diseases such as focal ischemia, hypoglycemia, and meningitis and showed positive correlation with neuronal survival19,21. These findings support the critical role of BDNF in neuronal survival. In the present study, while rat BDNF levels were significantly elevated in the IVH control group compared with the normal control group, human BDNF was detected, and rat BDNF was most significantly elevated in both the naive MSCs and the MSCs with scrambled siRNA transfection groups, but not in the MSCs with BDNF siRNA transfection group on 1 day after MSC transplantation. On 5 days after transplantation, while no human BDNF was detected, rat BDNF levels remained significantly elevated in the naive MSCs and MSCs with scrambled siRNA transfection groups but not in the MSCs with BDNF siRNA transfection group compared with the IVH and normal control groups. This may suggest that the brain damage by IVH in this study might be too severe to overwhelm the presumed neuroprotective effects of increased endogenous rat BDNF. Furthermore, the exogenous human BDNF secreted by the transplanted MSCs might play a pivotal role in neuroprotection not only by directly enhancing neuronal survival but also by further augmenting the increased production of endogenous rat BDNF to overcome the relative deficiency of endogenous BDNF production in severe IVH. Moreover, despite the gradual loss of exogenous human BDNF, the intact host neural tissue protected by MSC transplantation might be able to sustain the significant upregulation of endogenous rat BDNF expression up to 5 days after MSC transplantation19,20.
Anti-inflammatory and antiapoptotic effects might be other potential neuroprotective mechanisms of BDNF18,22. In the present study, the significant attenuation of severe IVH-induced brain injury with MSC transplantation was abolished by BDNF siRNA. Our data support the assumption that the neuroprotective effects of BDNF secreted by MSCs might be mediated by their antiapoptotic and anti-inflammatory effects.
Given the neuroprotective effects of BDNF observed in this study, the administration of exogenous BDNF might be a new therapy to improve the outcome of various neurologic disorders19. However, due to its short half-life, difficulty in crossing the blood–brain barrier after peripheral administration, limited availability in sufficiently large quantities, and lack of effective and safe dosing data as hurdles for clinical translation, developing a practical and effective means of delivering BDNF to the brain remains a challenge. Therefore, MSCs overexpressing BDNF might be a more advantageous alternative therapeutic approach for BDNF delivery and more promising than simple MSC or BDNF treatment alone. Kurozumi et al.23 reported that intracerebral transplantation of BDNF gene-modified MSCs 1 day after middle cerebral artery occlusion (MCAO) promoted functional recovery and reduced infarct size in rats. In contrast, van Velthoven et al.24 reported that intranasal transplantation of BDNF overexpressing MSCs at 3 days after neonatal stroke did not further enhance the recovery of MCAO-induced brain injury. These findings suggest several details including timing, route, and dose might determine the therapeutic efficacy and thus the success or failure of transplanting BDNF-overexpressing MSCs. Therefore, these issues have to be addressed in future studies before clinical use could be considered. Apart from efficacy, safety is another key issue for future clinical translation. The major concern for the clinical translation of BDNF-modified MSC transplantation is tumorigenicity. Although the risk of developing neoplasms could be reduced using an adenovirus that does not integrate into the host genome instead of a lenti- or retrovirus, the fate of transplanted BDNF-modified MSCs needs to be closely monitored to attenuate safety concerns related to the use of gene-modified MSCs in future studies.
In the present study, as behavior function tests, rotarod test and negative geotaxis test were performed to assess sensorimotor learning in the rats after severe IVH. However, in the clinical point of view, problems of cognition, learning, and memory would be the most concerned to the parents of infants with IVH. In the next step translational research, well-designed behavior test for cognition, learning, and memory would be required. In the present study, human origin MSCs were xenotransplanted into the rats, and thus potential negative side effects related with adverse immune reaction in the recipient brain might be possible. However, we have transplanted human cells that are already used for clinical trials (NCT02274428) in this animal model to discriminate the role of BDNF secreted by human cells from that secreted by the animal for neuroprotection.
In conclusion, BDNF secreted by transplanted MSCs plays a critical role in mediating their beneficial effects such as the attenuation of cell death, inflammation, astrogliosis, and the development of posthemorrhagic hydrocephalus and improved myelination after severe IVH in newborn rats. As the paracrine potency of MSCs might be quite variable according to their origin9, our data also suggest that BDNF could be used as potency markers for MSCs in IVH therapy to select MSCs with the best neuroprotective therapeutic efficacy.
Acknowledgment
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3051220), the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (HR14C0008), and by grants 20 by 20 Project (Best No. 3, GFO2160091) and from Samsung Medical Center and by Samsung Biomedical Research Institute grant (SMX1161301). Won Soon Park and Yun Sil Chang declare potential conflicts of interest arising from a filed or issued patent titled “Composition for treating intraventricular hemorrhage in preterm infants comprising mesenchymal stem cells” as coinventors, not as patentees.
References
- 1.Vohr B.R., Wright L.L., Dusick A.M., Mele L., Verter J., Steichen J.J., Simon N.P., Wilson D.C., Broyles S., Bauer C.R., Delaney-Black V., Yolton K.A., Fleisher B.E., Papile L.A., Kaplan M.D.. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 2000;105(6): 1216–26. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S.Y., Chang Y.S., Sung D.K., Sung S.I., Yoo H.S., Lee J.H., Oh W.I., Park W.S.. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 2013; 44(2): 497–504. [DOI] [PubMed] [Google Scholar]
- 3.Park W.S., Sung S.I., Ahn S.Y., Sung D.K., Im G.H., Yoo H.S., Choi S.J., Chang Y.S.. Optimal timing of mesenchymal stem cells therapy for neonatal intraventricular hemorrhage. Cell Transplant. 2016; 25(6): 1131–44. [DOI] [PubMed] [Google Scholar]
- 4.Ahn S.Y., Chang Y.S., Sung D.K., Sung S.I., Yoo H.S., Im G.H., Choi S.J., Park W.S.. Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS One 2015; 10(7): e0132919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y.S., Ahn S.Y., Jeon H.B., Sung D.K., Kim E.S., Sung S.I., Yoo H.S., Choi S.J., Oh W.I., Park W.S.. Critical role of VEGF secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014; 51(3): 391–9. [DOI] [PubMed] [Google Scholar]
- 6.Kim E.S., Ahn S.Y., Im G.H., Sung D.K., Park Y.R., Choi S.H., Choi S.J., Chang Y.S., Oh W., Lee J.H., Park W.S.. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012; 72(3): 277–84. [DOI] [PubMed] [Google Scholar]
- 7.van Velthoven C.T., Kavelaars A., van Bel F., Heijnen C.J.. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011; 25(7): 1342–8. [DOI] [PubMed] [Google Scholar]
- 8.Qu R., Li Y., Gao Q., Shen L., Zhang J., Liu Z., Chen X., Chopp M.. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology 2007; 27(4): 355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn S.Y., Chang Y.S., Sung D.K., Yoo H.S., Sung S.I., Choi S.J., Park W.S.. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 2015; 17(8): 1025–35. [DOI] [PubMed] [Google Scholar]
- 10.Xi G., Keep R.F., Hoff J.T.. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006; 5(1): 53–63. [DOI] [PubMed] [Google Scholar]
- 11.Hua Y., Wu J., Keep R.F., Nakamura T., Hoff J.T., Xi G.. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery 2006; 58(3): 542–50; discussion 542–50. [DOI] [PubMed] [Google Scholar]
- 12.Papile L.A., Burstein J., Burstein R., Koffler H.. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92(4): 529–34. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7(5): 393–5. [DOI] [PubMed] [Google Scholar]
- 14.Horie N., Pereira M.P., Niizuma K., Sun G., Keren-Gill H., Encarnacion A., Shamloo M., Hamilton S.A., Jiang K., Huhn S., Palmer T.D., Bliss T.M., Steinberg G.K.. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 2011; 29(2): 274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaratnasingam S., Janca A.. Brain derived neurotrophic factor: A novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol Ther. 2012; 134(1): 116–24. [DOI] [PubMed] [Google Scholar]
- 16.Cheng B., Mattson M.P.. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994; 640(1–2): 56–67. [DOI] [PubMed] [Google Scholar]
- 17.Schabitz W.R., Schwab S., Spranger M., Hacke W.. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997; 17(5): 500–6. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Shui Q.X., Liang K., Ren H.. Brain-derived neurotrophic factor rescues neurons from bacterial meningitis. Pediatr Neurol. 2007; 36(5): 324–9. [DOI] [PubMed] [Google Scholar]
- 19.Kokaia Z., Andsberg G., Yan Q., Lindvall O.. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: Evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998; 154(2): 289–301. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall O., Ernfors P., Bengzon J., Kokaia Z., Smith M.L., Siesjo B.K., Persson H.. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci USA 1992; 89(2): 648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L., Shui Q.X., Zhao Z.Y.. Regulation of brain-derived neurotrophic factor (BDNF) expression following antibiotic treatment of experimental bacterial meningitis. J Child Neurol. 2003; 18(12): 828–34. [DOI] [PubMed] [Google Scholar]
- 22.Makar T.K., Trisler D., Sura K.T., Sultana S., Patel N., Bever C.T.. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. 2008; 270(1–2): 70–6. [DOI] [PubMed] [Google Scholar]
- 23.Kurozumi K., Nakamura K., Tamiya T., Kawano Y., Ishii K., Kobune M., Hirai S., Uchida H., Sasaki K., Ito Y., Kato K., Honmou O., Houkin K., Date I., Hamada H.. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005; 11(1): 96–104. [DOI] [PubMed] [Google Scholar]
- 24.van Velthoven C.T., Sheldon R.A., Kavelaars A., Derugin N., Vexler Z.S., Willemen H.L., Maas M., Heijnen C.J., Ferriero D.M.. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013; 44(5): 1426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


