Abstract
Cardiac cell replacement therapy is a promising therapy to improve cardiac function in heart failure. Persistence, structural and functional maturation, and integration of transplanted cardiomyocytes into recipients' hearts are crucial for a safe and efficient replacement of lost cells. We studied histology, electrophysiology, and quantity of intramyocardially transplanted rat neonatal cardiomyocytes (NCMs) and performed a detailed functional study with repeated invasive (pressure–volume catheter) and noninvasive (echocardiography) analyses of infarcted female rat hearts including pharmacological stress before and 3 weeks after intramyocardial injection of 5 × 106 (low NCM) or 25 × 106 (high NCM) syngeneic male NCMs or medium as placebo (Ctrl). Quantitative real-time polymerase chain reaction (PCR) for Y-chromosome confirmed a fivefold higher persisting male cell number in high NCM versus low NCM after 3 weeks. Sharp electrode measurements within viable slices of recipient hearts demonstrated that transplanted NCMs integrate into host myocardium and mature to an almost adult phenotype, which might be facilitated through gap junctions between host myocardium and transplanted NCMs as indicated by connexin43 in histology. Ejection fraction of recipient hearts was severely impaired after ligation of left anterior descending (LAD; pressure–volume catheter: 39.2 ± 3.6%, echocardiography: 39.9 ± 1.4%). Repeated analyses revealed a significant further decline within 3 weeks in Ctrl and a dose-dependent stabilization in cell-treated groups. Consistently, stabilized cardiac function/morphology in cell-treated groups was seen in stroke volume, cardiac output, ventricle length, and wall thickness. Our findings confirm that cardiac cell replacement is a promising therapy for ischemic heart disease since immature cardiomyocytes persist, integrate, and mature after intramyocardial transplantation, and they dose-dependently stabilize cardiac function after myocardial infarction.
Keywords: Myocardial infarction (MI), Cell replacement therapy, Neonatal cardiomyocytes (NCMs), Echocardiography, Pressure–volume catheterization
Introduction
Because of the very limited regenerative potential of the heart, injured myocardium is replaced by noncontractile scar tissue which leads to loss of systolic function. Cardiac cell replacement therapy aims at direct replacement of lost cardiomyocytes (CMs) with new contractile cells through transplantation of single cells or cell clusters into injured myocardium and is therefore a promising approach to treat left ventricular dysfunction in ischemic heart failure.
In recent years, many research groups investigated several suitable cell types such as various progenitor cells from bone marrow (BM) or peripheral blood (PB)1,2. These cells have a limited significant therapeutic effect on cardiac function, show a very low cardiac persistence and survival after intramyocardial transplantation1,3–5, and are not contractile and cannot (trans)differentiate into contractile cells6. CMs own a priori all necessary properties and have been investigated in cardiac cell replacement therapy as fetal cardiomyocytes (FCMs)7,8, neonatal cardiomyocytes9 (NCMs), adult CMs7, or cardiomyocytes derived from adipose tissue stroma cells10 (ADSC-CMs), embryonic (ESC-CMs)11–13, or induced pluripotent stem cells (iPSC-CMs)14. Although not suitable for clinical application, NCMs appear to show the most reliable engraftment, persistence, and survival after intramyocardial transplantation and were therefore used in this project to assess the true potential of cardiac cell replacement therapy with functional and electrophysiological analyses. To elucidate potentially modest therapeutic effects, it is mandatory to exactly monitor changes in cardiac function of differentially treated individuals over time with repeated functional measurements just like it is standard in clinical trials. Thus, we performed a detailed functional study using repeated invasive (pressure–volume catheter) and noninvasive (echocardiography) analyses of infarcted rat hearts before and after intramyocardial transplantation of syngeneic NCMs in different doses, and we additionally investigated transplanted cell viability, function, maturation, and functional integration into recipient's myocardial syncytium in histology and electrophysiology.
Materials and Methods
Animal Model
Wild-type Fischer344 rats were purchased from Charles River Laboratories (Sulzfeld, Germany) and were housed in our institution's Animal Care Facility. All experiments were approved by the local animal welfare committee (Bezirksregierung Köln, Nos. 9.93.2.10.31.07.177 and 576.1.36.6.A35/10. Be) and conformed to the “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 85-23; revised 2011; National Academy Press, Washington, DC, USA) as well as EU Directive 2010/63/EU for animal experiments. The study design is depicted in Figure 1.
Figure 1.
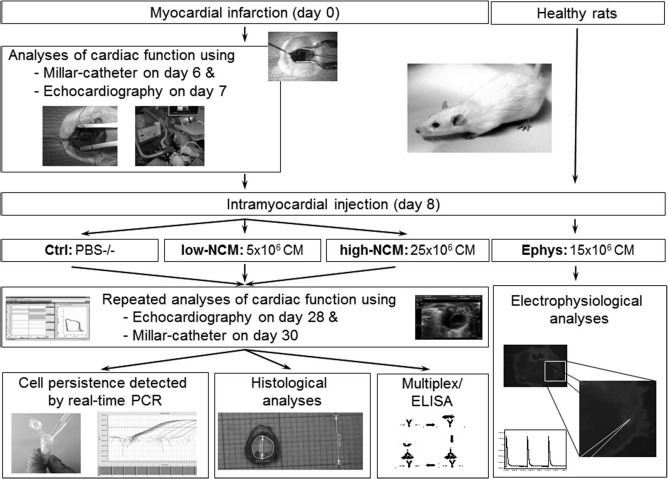
Study design.
Isolation and Cultivation of Neonatal Rat CMs
After decapitation, hearts from 3-day-old male wildtype Fischer344 rats were excised, and ventricles were harvested. Cells were dissociated with a collagenase–trypsin suspension (Biochrom AG, Berlin, Germany; PAA Laboratories GmbH, Pasching, Austria) and resuspended in Dulbecco's modified Eagle's medium (DMEM) HAMs F12 (PAA Laboratories GmbH) supplemented with 15% horse serum (Invitrogen, Darmstadt, Germany), 5% fetal bovine serum (FCS; PAA Laboratories GmbH), and 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA). Subsequently, the cell suspension was preplated for 30 min at 37°C and 5% CO2 on 10-cm cell culture dishes to allow attachment of fibroblasts and there with purification of CMs. Finally, the supernatant was transferred to new cell culture plates and cultivated overnight. For transplantation, NCMs were detached with trypsin/ethylenediaminetetraacetic acid (EDTA; PAA Laboratories GmbH), washed, and resuspended in phosphate-buffered saline (PBS)-/- (Lonza, Basel, Switzerland) at a concentration of 5 × 106 or 25 × 106 cells/500 μl.
Additional staining with Hoechst33342 (1:500; Sigma-Aldrich, Munich, Germany) for 1 h at 37°C and 5% CO2 before preplating was performed only for transplantations followed by electrophysiological analyses. Previous in vitro testing did not reveal any morphological or functional evidence of cellular damage by this staining compared to unlabeled cells.
Myocardial Infarction and Cell Transplantation
Adult female wild-type Fischer 344 rats (2–3 months old) were used as syngeneic recipients in order to avoid any need for immunosuppression after transplantation. Rats were anesthetized by inhalation of a mixture of N2O and O2 (1:1) and isoflurane (1.5%; Baxter, Unterschleissheim, Germany), intubated, and placed on a heating table. Left thoracotomy was performed, and myocardial infarction (MI) was induced by ligation of left coronary artery as described previously9,15. An intraoperative [tramadol 15–20 mg/kg subcutaneously (SC); ratiopharm GmbH, Ulm, Deutschland] and postoperative (tramadol 40 mg/day in drinking water and caprofen 5 mg/kg/day SC for 4 days) analgesic was used to mitigate pain.
In a second, similar surgery 8 days after MI, 500 μl PBS-/- without (Ctrl) or with NCMs [5 × 106 (low NCM) or 25 × 106 (high NCM)] was injected into five sites of infarction border zone using a Hamilton syringe (H. Faust GmbH, Rheinbach, Germany) attached to a 29-gauge needle.
For electrophysiological analyses, an intermediate number of 15 × 106 stained NCMs were injected into healthy rat hearts in a single surgery since all extensive attempts of these measurements in infarcted rat hearts had failed.
Invasive and Noninvasive Analyses of Cardiac Function
After MI but before cell injection, cardiac function was analyzed both invasively (pressure–volume catheterization 6 days post-MI) and noninvasively (trans-thoracic echocardiography 7 days post-MI) at rest and with dobutamine stress (Fresenius Kabi, Bad Homburg v. D. H., Germany), and both measurements were repeated 3 weeks after cell transplantation (4 weeks post-MI). All functional measurements and analysis were performed by a blinded investigator.
For echocardiography, rats (n = 37) were lightly anesthetized with inhalation of O2 and 1.5% isoflurane through a nose cap, their chests were epilated, and animals were placed on a heating table to prevent hypothermia and cardiodepressive effects. A commercial echocardiography system (Philips iE33 ultrasonic system; Qlab Cardiac Analysis-Software; Philips Medical Systems, Hamburg, Germany) equipped with a 15-MHz linear array transducer (L15-io7) allowing frame rates of 270 Hz to be used in these experiments. Transducer was moved along the parasternal long and short axis of left ventricle, and loops of 3-s duration were recorded in one-dimensional (M-mode) and two-dimensional plane. An electrocardiogram was used to monitor the animal's heart rate during measurements. For reconstructive three-dimensional echocardiography, multiple short-axis slices were recorded every 500 μm using a millimeter screw tripod16,17. Furthermore, all measurements were repeated under pharmacologically induced stress by intraperitoneal dobutamine injection [5 μg/g body weight (BW)].
Catheterization of the left ventricle was performed as described previously in closed chest method18. Rats (n = 33) were anesthetized by inhalation of a mixture of N2O and O2 (1:1) and 1.5% isoflurane, intubated, and placed on a heating table. Right carotid artery was isolated, ligated, and cannulated with a pressure–volume catheter (Millar PV-system MPVS Ultra; Mikro-Tip® Catheter Transducers; Model SPR-869; Size 2F; Length 12 cm; Millar Inc., Houston, TX, USA). The catheter was advanced retrogradely across the aortic valve into the left ventricle under control of pressure–volume loops. All detailed measurements were then performed in short phases of apnea for approximately 5 s to prevent any changes due to mechanical ventilation. Parallel conductance, which is necessary to determine intracavital volume of blood for calibration, was measured by repeated intravenous injection of hypertonic NaCl2 solution (30%; 5 × 10 μl; Merck, Darmstadt, Germany). Finally, all measurements were repeated under stress induced by dobutamine similar to respective procedures in echocardiography. An intraoperative (tramadol 15–20 mg/kg SC; Ratiopharm GmbH, Ulm, Germany) and postoperative (tramadol 40 mg/day in drinking water and caprofen 5 mg/kg/day SC for 4 days) analgesic was used to mitigate pain. All data were recorded with LabChart 7 (V7.3.7; AD Instruments, Spechbach, Germany) and analyzed with PVAN Ultra 1.1 (Millar Inc.).
Second catheterization (22 days after cell transplantation) was performed similarly, with cannulation of right carotid artery proximal to the first cannulation site. Afterward, animals were sacrificed under deep anesthesia by cervical dislocation and hearts, lungs, and blood were harvested for histological and molecular biological analysis.
Preparation of Viable Ventricular Slices and Electrophysiological Measurements
To study electrical integration and electrophysiological properties of transplanted NCMs, noninfarcted recipients were sacrificed 3 or 6 days after intramyocardial transplantation. Hearts were harvested and separated from large vessels and atria. Ventricular slices were prepared as described previously19,20 to allow electrophysiological measurements of transplanted NCMs within intact host myocardium21. Briefly, ventricles were embedded in 4% low-melt agarose (Carl Roth, Karlsruhe, Germany) suspended in Tyrode's solution (composition in mmol/L: NaCl 136, KCl 5.4, NaH2PO4 0.33, MgCl2 1, d-glucose 10, HEPES 5, 2,3-butanedione monoxime 30; pH 7.4 adjusted with NaOH; Sigma-Aldrich), and 200-μm-thick short-axis slices were cut with a microtome (Leica VT1000S; Leica Microsystems, Wetzlar, Germany). Slices were incubated in DMEM at 37°C with 95% O2 and 5% CO2 for another 30 min.
Electrophysiological measurements were performed at 37°C and pH 7.4 in culture medium aerated with carbogen. Slices were stimulated with defined beating frequency using unipolar custom-made stimulation electrode placed into host tissue and SD9 square pulse stimulator (Grass Technologies, West Warwick, RI, USA). Intracellular action potential (AP) recordings in host tissue and Hoechst dye (Sigma-Aldrich)-stained transplanted NCMs were performed using glass microelectrodes (World Precision Instruments, Sarasota, FL, USA) with resistances of 20–50 MW. Signals were amplified with SEC-10LX amplifier (npi electronic, Tamm, Germany) and acquired with Pulse software (HEKA, Lambrecht/Pfalz, Germany). Data were analyzed offline with Mini Analysis program (Synaptosoft, Fort Lee, NJ, USA).
Because electrical excitation originated from host tissue, we used temporal interdependency of stimulation artifacts, and APs were recorded intracellularly in transplanted CMs as indicator of electrical integration. NCMs were considered not to be electrically integrated, if there was no temporal interdependency of stimulation artifacts and (spontaneous) APs or if there were no APs but a stable resting membrane potential. Quality of electrical integration was assessed by maximal stimulation frequency without conduction blocks (i.e., maximal stimulation frequency leading to a 1:1 generation of APs after each stimulus) and by delay between stimulation artifacts and APs.
To elucidate AP morphology prior to transplantation, additional sharp-electrode measurements were performed in vitro in Hoechst dye-stained NCMs after 7 days in culture at 37°C and 5% CO2 in DMEM HAMs F12.
DNA Preparation and Quantitative Analysis of Cell Persistence and Survival by Real-Time PCR
Harvested hearts (n = 23) were minced and lysed weight specifically with lysis buffer (ATL-buffer; Qiagen GmbH, Hilden, Germany) and proteinase K (Sigma-Aldrich). Subsequently, DNA was isolated using DNeasy Blood & Tissue Kit (Qiagen GmbH). Cell persistence and survival were determined by quantitative real-time TaqMan-PCR (7300 Real time PCR System; Applied Biosystems, Carlsbad, CA, USA) as described previously22 with primers against the sex-determining region of Y-chromosome (SRY; RatSRY Primer: RSRY+: 5′-GGA-GAG-AGG-CAC-AAG-TTG-GC-3′, RSRY-: 5′-TCC-CAG-CTG-CTT-GCT-GAT-C-3′, RatSRY Probe: 6-Fam-CAA-CAG-AAT-CCC-AGC-ATG-MGB) and osteopontin (OPN; RatOPN Primer: ROPN+: 5′CAC-CAG-CAG-CAG-GAC-TGA-AG-3′, ROPN-: 5′-ATG-CTT-GCC-GCA-GGA-GAC-T-3′, RatOPN Probe: 6-Fam-AGC-TAA-GCC-TCA-GCA-TC-MGB) as housekeeping gene (Custom TaqMan® Gene Expression Assay für RatOPN or RatSRY; Applied Biosystems, Life Technologies Corporation). Samples with known dilutions of male with female DNA were used to derive a calibration curve for the calculation of male/total DNA ratio in experimental samples.
Multiplex and ELISA Analyses of Blood Serum
Blood was collected from some animals (n = 17) at day 30 after final pressure–volume (P-V) catheter and centrifuged at 200 × g for 10 min. Concentration of brain natriuretic peptide (BNP), tumor necrosis factor (TNF)-α, interleukin (IL)-6, troponin T, and troponin I in serum was determined with Rat CVD Panel1 Kit (Milliplex® MAP; Merck Millipore, Temecula, CA, USA) following kit-specific protocols. Analytes were quantified using Luminex® technology (Bio-Plex200 systems, Bio-Rad, Munich, Germany) and Milliplex Analyst Software Version 5.1 (Merck Millipore, Darmstadt, Germany).
Quantikine ELISA kit specific for rat vascular endothelial growth factor (VEGF; R&D Systems, Minneapolis, MN, USA) was used to determine VEGF concentrations in serum according to manufacturer's instructions with measurements at 450 nm with Glomax Multi Detection System spectrophotometer (Promega, Madison, WI, USA).
Histology and Immunohistochemistryt
For histological and immunohistochemical analyses, some exemplary hearts (n = 15) were fixed in diastolic pressure of approx. 10 mmHg for 180 min in 4% CH2O (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and then embedded in paraffin (McCormick Scientific, LLC, St. Louis, MO, USA). Short-axis slices were prepared in thickness of 7 μm. Preparations were stained routinely with hematoxylin and eosin (H&E; Merck, Darmstadt, Germany; Eosin Y·Na-salt; SERVA Electrophoresis GmbH, Heidelberg, Germany) and additionally with Masson's trichrome (Carl Roth GmbH + Co. KG; Sigma-Aldrich) to visualize scar tissue. For immunohistochemical analyses, primary antibodies were anti-connexin (CXN-43 mouse-IgM C8093; 1:500; Sigma-Aldrich) and anti-a-smoothmuscle (anti-α-Smooth Muscle-Cy3 clone 1A4; 1:250; Sigma-Aldrich), and secondary detection was performed with anti-mouse-IgM Alexa Fluor647 (A21238; 1:1,000; Invitrogen). Nuclei were stained with Hoechst33342 (Sigma-Aldrich). Images were done with Axiovert 200M microscope and Axiovision 4.3 software (Carl Zeiss, Jena, Germany).
Statistics
All data are presented as mean ± standard error (SEM). Statistical significance was analyzed by one-way analysis of variance (ANOVA) with posttest for intergroup comparisons, or by paired Student's t-test for intragroup comparisons. Values of p < 0.05 were considered statistically significant. Microsoft Office (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) were used for calculations and visualizations.
Results
Cardiac Persistence and Morphology of Transplanted NCMs
Absolute number of detected NMCs 3 weeks after transplantation was approximately fivefold higher in high NCM (2,401,741 ± 603,317 cells per heart, n = 13) than in low NCM (407,255 ± 180,704 cells per heart, p < 0.01 vs. high NCM, n = 10) (Fig. 2A). Relative cell persistence after 3 weeks was similar in both groups with 9.6 ± 2.4% of injected cells detected in high NCM and 8.2 ± 3.6% in low NCM (Fig. 2B).
Figure 2.
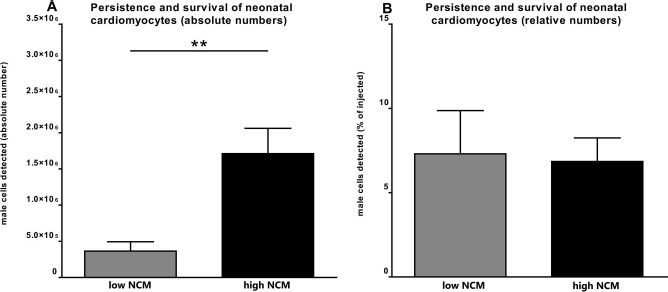
Persistence and survival of transplanted neonatal cardiomyocytes (NCMs). Quantity of male cells detected 3 weeks after cell transplantation by quantitative real-time PCR in absolute (A) or relative numbers (B). Absolute number of NCMs was approximately fivefold higher in high NCM (n = 13) than in low NCM (n = 10; **p < 0.01), whereas relative cell persistence was similar in both groups.
Heart morphology, infarction scar, and injection site could be identified and evaluated with H&E and Masson's trichrome staining. Figure 3A shows short-axis slices of a representative heart, which correlated with short-axis views obtained by echocardiography. Transplanted NCMs could be identified in recipient hearts at 3 weeks after intramyocardial injection into the border zone of MI as stable grafts with higher density of nuclei and cells compared to surrounding host myocardium (Fig. 3B and C). They were considerably smaller and less organized compared to adult host CMs, but had changed the shape from round (before transplantation) to a longitudinal alignment. Connexin43 (Fig. 3D–F) was detectable within recipient tissue as well as within transplanted NCMs. Additionally, connexin43 was also detectable at the border between the host myocardium and transplanted NCMs, suggesting possible formation of gap junctions and thereby integration of transplanted NCMs into surrounding host myocardium. Staining of α-smooth-muscle-actin (α-SMA) was negative in transplanted NCMs at 3 weeks after intramyocardial injection (Fig. 3G–I), but it was clearly positive in neighboring vascular structures as well as in control slices of native neonatal myocardium (Fig. 3J–L).
Figure 3.
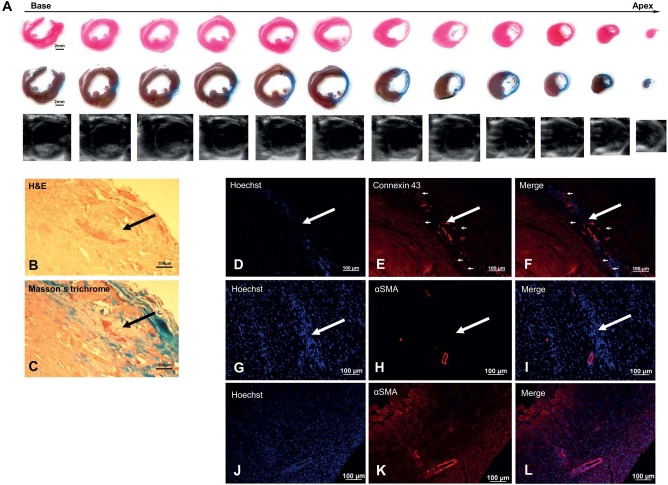
Histological and immunohistochemical analyses of a representative heart at 3 weeks after intramyocardial transplantation of placebo (A, n = 5) or neonatal cardiomyocytes (B–I, n = 10) into border zone of 1-week-old myocardial infarction. Short-axis slices of heart stained with hematoxylin-eosin (H&E; A, first row) or Masson's trichrome (A, second row) correlate well with echocardiographic short-axis views of the same heart (A, third row), all clearly demonstrating a severe transmural scar. In sections stained with H&E (B) and Masson's trichrome (C), transplanted neonatal cardiomyocytes (NCMs, black arrows) could be identified as stable grafts with a higher density of nuclei and cells compared to surrounding host myocardium, and they were smaller and less organized than host CMs. Connexin43 (D–F) was detectable within transplanted NCMs (white arrows) and within host myocardium as well as between both (small white arrows). Staining for α-SMA (G–L) was negative in transplanted NCMs (white arrows), but it was clearly positive in neighboring vascular structures (G–I) as well as in control slices of native neonatal myocardium (J–L).
Electrical Integration and Maturation of Transplanted NCMs
Preliminary experiments showed that freshly isolated NCMs survive in culture for up to 7 days after Hoechst staining, without morphological or functional evidence of cellular damage by the staining. APs of these cells (Fig. 4A and Table 1) showed low maximal diastolic potential of −55.53 ± 1.52 m V, low upstroke velocity (Vmax) of 9.03 ± 1.33 V/s, high AP duration at 20% repolarization (APD20) of 74.13 ± 5.82 ms, and high ratio of APD50 to APD90 (APD50/90) of 64.45 ± 3.80%, as typical for immature CMs (n = 7).
Figure 4.
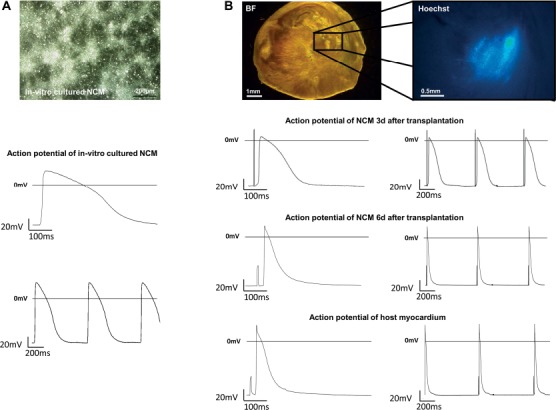
Electrical integration and maturation of transplanted NCMs. (A) Low-magnification phase contrast of representative in vitro cultured NCMs (day 7, n = 7) and their derived action potentials (AP). (B) Representative viable slice of a recipient heart containing NCMs in bright field and Hoechst imaging. AP recordings of NCMs 3 days (top, n = 3) and 6 days after transplantation (middle, n = 18) and of adult recipient CMs (bottom, n = 22) demonstrate a fast electrophysiological maturation.
Table 1.
Results of Electrophysiological Measurements After Cultivation or Transplantation of NCMs
| n | Amplitude (mV) | Maximum Diastolic Potential (mV) | Frequency (Hz) | APD20 (ms) | APD50 (ms) | APD90 (ms) | APD50/90 (%) | Vmax (V/s) | Delay (ms) | Maximum Frequency (Hz) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| In vitro | 7 | 71.31 ± 2.77*** | -55.53 ± 1.52*** | 2.15 ± 0.05*** | 74.13 ± 5.82*** | 140.76 ± 8.84*** | 218.09 ± 1.27*** | 64.45 ± 3.80*** | 9.03 ± 1.33*** | n/a | n/a |
| NCM 3d | 3 | 79.38 ± 13.88 | -56.59 ± 14.28* | 1.70 ± 0.26** | 72.40 ± 8.90*** | 137.66 ± 20.22*** | 199.44 ± 16.59*** | 68.28 ± 4.90*** | 16.23 ± 7.02*** | 36.27 ± 11.59** | 1.70 ± 0.26*** |
| NCM 6d | 18 | 86.94 ± 2.04 | -71.93 ± 2.00 | 1.9 ± 0.02 | 12.82 ± 1.86* | 26.63 ± 2.73 | 67.59 ± 4.14 | 38.55 + 1.47 | 131.34 ± 7.60 | 12.15 ± 0.92 | 12.13 ± 0.88 |
| Host | 22 | 91.25 ± 2.43 | -73.71 ± 1.70 | 2.00 ± 0.01 | 8.66 ± 0.96 | 22.85 ± 1.85 | 61.40 ± 4.05 | 37.09 ± 1.32 | 159.33 ± 13.02 | 11.85 + 0.61 | 12.61 ± 0.61 |
p<0.05;
p<0.01;
p<0.001 versus host.
Transplanted NCMs could be identified within viable slices of recipient hearts by fluorescence of Hoechst dye applied prior to transplantation. Three days after intramyocardial injection, transplanted NCMs were electrophysiologically integrated into host myocardium following a maximal stimulation frequency of 1.70 ± 0.26 Hz without conduction blocks. Host CMs followed maximal stimulation frequency of 12.61 ± 0.61 Hz without conduction blocks (p < 0.001, n = 3).
APs of transplanted NCMs at 3 days (NCM 3d) after intramyocardial injection were similar to those of freshly isolated NCMs (Fig. 4B and Table 1).
In contrast, transplanted NCMs at 6 days after intramyocardial injection (NCM 6d, n = 18) were much more similar to host CMs (n = 22) in both maximal stimulation frequency followed without conduction blocks (indicating quality of electrical integration) and action potential morphology (indicating maturity) (Fig. 4B and Table 1). The only AP parameter that was different between NCM 6d and host CMs was APD20 (12.82 ± 1.86 ms in transplanted NCM 6d vs. 8.66 ± 0.96 ms in host CMs; p = 0.04).
Delay of electrical activation, which quantifies deceleration of excitation spread in transplanted cells and therefore is another parameter to evaluate electrical coupling with surrounding tissue, declined from 36.27 ± 11.59 ms in transplanted NCM 3d (p < 0.001 vs. host) to 12.15 ± 0.92 ms in transplanted NCM 6d, approximating value of host CMs (11.85 ± 0.61ms, ns vs. host d6).
Cardiac Function
Measurements of cardiac function 1 week after coronary artery ligation were invasively performed with P-V catheter in 33 rats and noninvasively with echocardiography in 37 rats. Criteria for exclusion from further analyses were insufficient ischemic injury [ejection fraction (EF) > 50% or no visible scar, seven animals excluded from all analyses] or technical problems leading to implausible values [left ventricular end diastolic pressure (LVEDP) > 25 mmHg, three animals excluded from P-V analyses only]. Exclusion criteria were chosen according to echocardiography of five additional healthy animals resulting in EF = 59 ± 3% without any significant change throughout the observation period of 3 weeks (-0.2 ± 2.4%).
All remaining 30 animals in echocardiographic analyses and 23 animals in P-V analyses showed a homogenously reduced ejection fraction of 39.9 ± 1.4% in echocardiography and 39.2 ± 3.6% in P-V catheter at 1 week after MI. Repeated analyses after 3 weeks (4 weeks after MI, 3 weeks after intramyocardial injection) allowed detection of individual changes, and P-V catheter revealed a further decrease in EF of −18.5 ± 5.7% in Ctrl (n = 10; p = 0.01 over time) but no significant changes in EF of treated groups with +11.7 ± 10.3% in low NCM (n = 7) and +18.1 ± 11.9% in high NCM (n = 6) resulting in a highly significant dose dependence for NCM treatment (Fig. 5A). Stroke volumes tended to further decrease in Ctrl with −14.8 ± 6.8 μl (p = 0.06 over time), remained constant in low NCM with +30.9 ± 28.9 μl, tended to increase in high NCM with +35.8 ± 17.4 μl (p = 0.09 over time), and showed a trend for dose dependence (p = 0.06) (Fig. 5B). Cardiac output also tended to further decrease in Ctrl (-4 132 ± 1 904 μl/min, p = 0.06 over time), whereas it remained constant in low NCM (+9 213 ± 8 660 μl/min) and slightly tended to increase in high NCM (+9 993 ± 5 938 μl/min, p = 0.15), resulting in a trend for dose dependence (p = 0.08) (Fig. 5C).
Figure 5.
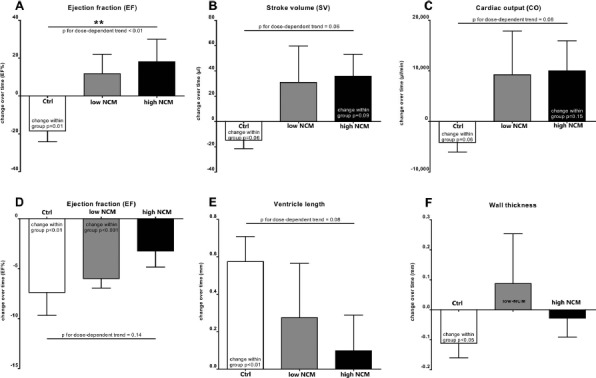
Cardiac function of infarcted rat hearts. Change over time of ejection fraction (EF, A), stroke volume (SV, B), and cardiac output (CO, C) determined by repeated invasive pressure–volume catheter (Millar; Ctrl: n = 10, low NCM: n = 7, high NCM: n = 6). Change over time of EF (D), ventricle length (E), and wall thickness (F) determined by repeated noninvasive echocardiography (Ctrl: n = 13, low NCM: n = 8, high NCM: n = 9). All performed at 1 week after myocardial infarction (MI) (i.e., prior to intramyocardial injection) and repeated at 4 weeks after MI (i.e., 3 weeks after intramyocardial injection). Cardiac cell replacement therapy dose-dependently stabilized function of infarcted hearts (**p < 0.01).
Repeated echocardiography showed similar results. Ejection fraction decreased highly significantly in Ctrl with −7.4 ± 2.3% (n = 13; p < 0.01 over time) and in low NCM with −6.0 ± 1.0% (n = 8; p < 0.001 over time), but it did not show any significant change in high NCM with −3.2 ± 1.6% (n = 9), which translated into a slight trend for dose dependence (p = 0.14) (Fig. 5D). Additional measurements in echocardiography showed that ventricle length increased highly significantly in Ctrl with +0.57 ± 0.13 mm (p < 0.01 over time) in contrast to constant values in treated groups (low NCM: +0.28 ± 0.29 mm, high NCM: +0.10 ± 0.19 mm), resulting in a trend for dose dependence (p = 0.08) (Fig. 5E). Furthermore, wall thickness decreased significantly in Ctrl with −0.11 ± 0.05 mm (p < 0.05 over time), but it remained constant in cell-treated groups (low NCM: +0.09 ± 0.17 mm, high NCM: −0.03 ± 0.06 mm) (Fig. 5F).
During dobutamine-induced stress, heart rate was significantly increased in all animals, and invasive as well as noninvasive measurements showed a similar contractile reserve in all experimental groups.
Heart/Lung Weight and Cardiac Biomarkers
There was no significant difference between groups in heart weight, heart-to-body weight ratio, lung weight, and lung-to-body weight ratio. However, a slight trend was seen for lower lung weights in pooled data of all cell-treated animals versus Ctrl (0.81 ± 0.01 g vs. 1.37 ± 0.43 g, p = 0.14). Lung weight as well as lung-to-body weight ratio of exemplary healthy rats were almost identical to those of cell-treated groups.
Blood from exemplary animals was analyzed for heart failure biomarkers using Multiplex assay and ELISA. None of the six tested cytokines showed significantly different levels in Ctrl (n = 5), low NCM (n = 6), and high NCM (n = 6) at 4 weeks after MI (3 weeks after intramyocardial injection).
Discussion
Cardiac cell replacement therapy is a promising approach to restore cardiac function in heart failure by replacing lost myocardium with new contractile cells, which ideally should be or become CMs. To assess the true potential of this therapy, it is very important to thoroughly investigate the following most crucial issues:
-
1.
Persistence and survival of transplanted CMs within infarcted hearts, since only sufficient numbers of new cells can lead to potential therapeutic effects.
-
2.
Integration of transplanted CMs into host myocardium and adoption or preservation of a mature cardiac phenotype, since only a functioning syncytium of new cells and host myocardium allows synchronous contraction.
-
3.
Changes in cardiac function over time produced by transplantation of different doses of CMs in comparison to control transplantations, if possible determined in repeated functional analyses, since only relevant intraindividual and dose-dependent changes over time can be ascribed to cardiac cell replacement therapy.
Therefore, we investigated all these crucial issues for the first time in one study using the very reliable model of MI through LAD ligation in rats followed by intramyocardial injection of syngeneic NCMs, which were shown to have a significant persistence over time9. Besides the very robust method of quantitative real-time PCR to determine persistence of transplanted cells within recipient hearts, we performed intense electrophysiological analyses of transplanted cells within host myocardium with sharp-electrode measurements in viable slices of recipient hearts as well as histological and immunohistochemical analyses to determine integration and phenotype of transplanted cells. Finally, differentially treated animals underwent detailed functional analyses with repeated invasive and noninvasive measurements of cardiac function.
Transplanted NCMs Persist and Survive in Relevant Numbers Within Infarcted Recipient Heart
Number of cells successfully implanted into injured heart and their persistence within the heart is the first essential factor for effective cardiac cell replacement therapy. In the present study, more than 8% of transplanted NCMs persisted and survived 3 weeks after intramyocardial injection into infarcted syngeneic rat hearts. This finding is consistent with our and other earlier studies9,22,23. Transplanted NCMs show much higher persistence and survival compared to bone marrow stem cells (BM-MSCs)1,3 and also compared to transplanted adult CMs7 or ESCs or iPSCs24,26. By increasing applied cell number from 5 to 25 million NCMs, the absolute cell number of detectable cells was increased fivefold, while the percentage of surviving cells did not change. This suggests that in methods used here, persistence and survival of transplanted cells was not limited due to space and nutrient deficiency, as described by Zhang et al.23. Importantly for subsequent functional analyses of dose dependence, we could demonstrate that after receiving a fivefold higher number of NCMs at the time of transplantation, hearts in high NCM still contain a significantly and approximately fivefold higher number of persisting and surviving NCMs at the time of second and final functional measurements compared to hearts in low NCM.
Exemplary histological analysis of transplanted NCMs showed some morphological signs of maturation as larger cell volume and incipient longitudinal orientation, in contrast to the phenotype of freshly isolated NCMs. Negative staining for α-SMA, which is detectable in early/immature CMs, confirmed increasing structural maturation of NCMs after transplantation. Moreover, connexin43, as an important component of gap junctions and structural basis of electro-physiological coupling, could be shown not only within the graft of transplanted NCMs and within host myocardium but also between graft and host tissue. Notwithstanding these signs of integration of transplanted NCMs into host myocardium, transplanted NCMs still were significantly smaller than host CMs, indicating that their structural maturation seemed to be incomplete. Consistently, Sato et al.26 and Müller-Ehmsen et al.3 observed a considerable increase in length of transplanted neonatal rat CMs as well as clear sarcomeres at 2–4 weeks after transplantation, but without reaching the size of host CMs within the observation period of up to 6 weeks. In contrast to the present study and the results of Yao et al.27, Etzion et al.8 detected α-SMA positivity in transplanted rat FCMs at 2 months after transplantation. One reason for this different finding may be that in this study, rat FCMs had been transplanted into the infarct scar and therefore lacked direct contact with the host myocardium. Halbach et al. found that transplanted mouse FCMs showed much faster structural and electrophysiological maturation when surrounded by host myocardium than after transplantation into the scar of cryoinjury21,28.
Electrical Integration and Electrophysiological Maturity of Transplanted NCMs
Electrophysiological integration into host myocardium and adoption or preservation of a mature cardiac phenotype are important to enable a functional syncytium of transplanted and host CMs and therefore for a safe and effective cardiac cell replacement therapy. Rubart et al.29 showed that transplanted FCMs can integrate electrically into healthy hearts by imaging cellular calcium transients using two-photon molecular excitation laser scanning microscopy. Roell et al.30 and Sato et al.26 observed a significant maturation of FCMs and NCMs after transplantation into healthy and infarcted hearts. However, hearts were dissociated in these studies to enable electrophysiological measurements of transplanted cells, which therefore were isolated from surrounding host tissue. Thus, no information about electrical integration of studied CMs was achieved.
Here integration and maturation of transplanted NCMs was examined using sharp-electrode measurements within viable slices of recipient hearts, which allows to sustain nearly in vivo conditions and to elucidate electrophysiological properties of transplanted CMs within the surrounding host tissue14,21,28. However, since slicing technique revealed to be impossible for rat hearts after ligation infarction due to severe fibrosis of the tissue, we had to use healthy hearts for these experiments.
For the first time, we were able to show not only a structural7 but also an electrical integration of transplanted NCMs as early as 3 days after transplantation as indicated by following a stimulation applied to distant host myocardium. This was further improved at 6 days after transplantation, when maximal stimulation frequency without conduction blocks was similar to host CMs, which indicates an optimal electrical integration into the host myocardium. In contrast to other groups, we observed a marked and rapid electrophysiological maturation of transplanted cells over time. While NCMs possessed quite immature AP properties 3 days after transplantation, already at 6 days NCMs showed almost adult electrophysiological properties. Sato et al.26 found signs of immaturity regarding contraction amplitude and APD50 in rat NCMs at 2 weeks after transplantation, but no more differences between transplanted NCMs and adult host CMs at 4 weeks after transplantation. They suspected that immature cells and their relatively slow maturation may cause arrhythmias and therefore might be a problem and limitation of cell therapy.
Halbach et al.21,28 were able to show a time course of electrophysiological integration and maturation of mouse FCMs after intramyocardial transplantation into the border zone of cryoinjury with almost adult AP properties after 12 days. In contrast, induced pluripotent stem cell-derived CMs (iPSC-CMs) still showed very immature action potentials at 6–12 days after transplantation similar to in vitro cultured iPSC-CMs. Even after 6–8 months considerable differences in amplitude, upstroke velocity, and APD50/90 ratio between iPSC-CMs and host CMs remained14. Our data indicate that NCMs reach an optimal electrophysiological integration and maturation indistinguishable from host CMs much faster than transplanted FCMs, ESC-CMs, or iPSC-CMs (if given at all in the latter). A reason for this may be the higher maturity at the time of isolation and transplantation. One might speculate that using even more mature CMs in cardiac cell replacement therapy could allow to achieve this optimal state even earlier and may result in improved therapeutic effects. However, persistence and survival of more mature cells, like adult CMs, is dramatically lower and therefore limits this approach. Certainly, future research is needed to overcome these obstacles. One potentially useful strategy may be an “electrical preconditioning” by electrical stimulation of immature cells prior to transplantation31.
Transplanted NCMs Dose-Dependently Stabilize Systolic Function of Infarcted Recipient Hearts
Robust and reliable functional analysis is needed to assess the true therapeutic potential of cardiac cell replacement therapy and is facilitated by repeated measurements, which is therefore mandatory and standard in clinical trials. In our present study with repeated invasive and noninvasive functional analysis, we were able to show a dose-dependent stabilization of systolic left ventricular function and some signs of improvement within 4 weeks after MI and 3 weeks after cell transplantation of NCMs, as well as less left ventricular dilatation and wall thinning in cell-treated versus control hearts. In contrast, control group showed the natural progress of heart failure with progressive left ventricular remodeling and deterioration. In previous studies with transplanted CMs, Yao et al.27 described a higher ejection fraction, a trend to larger stroke volumes, and a trend to smaller end-systolic and end-diastolic volumes in angiography at 10 months after MI and transplantation of rat FCMs versus placebo. Similar results were observed for transplantation of NCMs after 6 months9, for ES derived (after 1–2 months)11,32 and for iPSC-CMs33.
Nevertheless, long-term persistence and survival of transplanted stem cell-derived CMs is extremely low and most likely limits their therapeutic effects. As Etzion et al.8 already discussed, the number of remaining CMs in the heart has apparently a decisive influence on real cardiac outcome, which we could show as dose dependence in the present project. Despite improved cardiac function, Kawamura et al.33 detected only a small number of human iPSC-CMs 8 weeks after transplantation into infarcted pig hearts by fluorescence in situ hybridization (FISH). To improve persistence of ES-CMs, Kolossov et al.32 transplanted these cells with syngeneic fibroblasts, and Laflamme et al.34 mixed the cells with pro-survival factors.
Repeated functional measurements using echocardiography and P-V catheter after MI (before transplantation) and after cardiac cell replacement therapy enabled us to follow individual changes of heart function. Ejection fraction remained stable or improved in most rats of cell-treated groups (69%), whereas it declined in most animals of the control group (80%, p < 0.05 with chi-square test). Nevertheless, our study failed to show a significant improvement of any tested parameter of systolic left ventricular function by cardiac cell replacement therapy in infarcted rat hearts in the course of time, which could have been hoped for regarding efficient engraftment, integration, and maturation of transplanted new CMs. However, current evidence-based pharmacological treatment like angiotensin-converting enzyme (ACE)-inhibitor captopril also did not improve cardiac function in small animal infarction models, but it attenuated dramatic deterioration seen in untreated animals35. Therefore, we believe that dose-dependent stabilization in systolic left ventricular function by cardiac cell replacement therapy seen in our study might still translate into a significant clinical benefit in patients, especially when further optimized and of course combined with all optimal therapies currently recommended.
Lung Weight and Levels of Cardiac Biomarkers as Systemic Indicators of Heart Failure
High lung weight and high lung-to-body weight ratio are strong indicators of congestive heart failure after MI in small animal experiments36. In the present study, mean lung weight as well as mean lung-to-body weight ratio of placebo-control animals was approximately 70% higher than in cell-treated animals, but these differences did not reach statistical significance presumably due to low sample numbers in this analysis. However, both values were similarly low in cell-treated animals at 4 weeks after MI compared to exemplary healthy controls, which might therefore be taken as a sign of a well-compensated heart failure in cell-treated animals versus placebo controls. In contrast, six cardiac biomarkers tested had similar levels in all groups and therefore seem to be less sensitive systemic indicators of heart failure in our experimental setting.
Conclusions
We performed a detailed placebo-controlled functional study using repeated invasive and noninvasive analyses of cardiac function to investigate the therapeutic effects of cardiac cell replacement therapy with transplantation of different doses of neonatal rat CMs into infarcted syngeneic rat hearts. Furthermore, we quantified persistence and evaluated integration and maturation of transplanted cells. Within 4 weeks after MI and 3 weeks after transplantation, we were able to show that transplanted NCMs:
-
a)
persist and survive within the infarcted heart in dose-dependent numbers,
-
b)
electrically integrate into host myocardium to a similar state like host CMs,
-
c)
electrophysiologically mature to a state almost indistinguishable from host CMs, and
-
d)
dose-dependently stabilize left ventricular function of infarcted hearts in contrast to significant further deterioration in the placebo controls.
Although this exact cell type is not relevant for clinical use, our findings are reassuring that after all cardiac cell replacement therapy is still a promising therapy for ischemic heart disease.
However, future investigations need to optimize the use of clinically, more relevant types of CMs in cardiac cell replacement therapy, like derivatives of iPSCs, in order to thoroughly elucidate their true therapeutic potential.
Acknowledgments
We thank Dr. Alexander Ghanem for his support with echocardiography, Dr. Jan Mauer and Dr. Gunther Rappel for their support with Multiplex analysis, as well as support provided by workshops and the animal facility of Institute of Neurophysiology and Institute of Experimental Medicine of University Hospital of Cologne. This work was supported by the German Heart Foundation/German Foundation of Heart Research (F/02/12); by the Koeln Fortune Program/Faculty of Medicine, University of Cologne; by the Federal Ministry of Education and Research (BMBF; 01GN0947-TP3); and by the Marga und Walter Boll-Stiftung (210-03-12). The authors declare no conflicts of interest.
References
- 1.Tossios P., Krausgrill B., Schmidt M., Fischer T., Halbach M., Fries J.W., Fahnenstich S., Frommolt P., Heppelmann I., Schmidt A., Schomäcker K., Fischer J.H., Bloch W., Mehlhorn U., Schwinger R.H., Müller-Ehmsen J.. Role of balloon occlusion for mononuclear bone marrow cell deposition after intracoronary injection in pigs with reperfused myocardial infarction. Eur Heart J. 2008; 29: 1911–21. [DOI] [PubMed] [Google Scholar]
- 2.Wen Y., Meng L., Ding Y., Ouyang J.. Autologous transplantation of blood-derived stem/progenitor cells for ischaemic heart disease. Int J Clin Pract. 2011; 65(8): 858–65. [DOI] [PubMed] [Google Scholar]
- 3.Müller-Ehmsen J., Krausgrill B., Burst V., Schenk K., Neisen U.C., Fries J.W., Fleischmann B.K., Hescheler J., Schwinger R.H.. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006; 41: 876–84. [DOI] [PubMed] [Google Scholar]
- 4.Krausgrill B., Vantler M., Burst V., Raths M., Halbach M., Frank K., Schynkowski S., Schenk K., Hescheler J., Rosenkranz S., Müller-Ehmsen J.. Influence of cell treatment with PDGF-BB and reperfusion on cardiac persistence of mononuclear and mesenchymal bone marrow cells after transplantation into acute myocardial infarction in rats. Cell Transplant. 2009; 18: 847–53. [DOI] [PubMed] [Google Scholar]
- 5.Wu K.H., Mo X.M., Han Z.C., Zhou B.. Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg. 2011; 92(5): 1917–25. [DOI] [PubMed] [Google Scholar]
- 6.Nygren J.M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B.K., and Jacobsen S.E.. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004; 10: 494–501. [DOI] [PubMed] [Google Scholar]
- 7.Reinecke H., Zhang M., Bartosek T., Murry C.E.. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation 1999; 100(2): 193–202. [DOI] [PubMed] [Google Scholar]
- 8.Etzion S., Battler A., Barbash I.M., Cagnano E., Zarin P., Granot Y., Kedes L.H., Kloner R.A., Leor J.. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol. 2001; 33(7): 1321–30. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Ehmsen J., Peterson K.L., Kedes L., Whittaker P., Dow J.S., Long T.I., Laird P.W., Kloner R.A.. Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation 2002; 105(14): 1720–6. [DOI] [PubMed] [Google Scholar]
- 10.Planat-Bénard V., Menard C., André M., Puceat M., Perez A., Garcia-Verdugo J.M., Pénicaud L., Casteilla L.. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004; 94(2): 223–9. [DOI] [PubMed] [Google Scholar]
- 11.van Laake L.W., Passier R., Doevendans P.A., Mummery C.L.. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008; 102: 1008–10. [DOI] [PubMed] [Google Scholar]
- 12.Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., Gantz J.A., Fugate J.A., Muskheli V., Gough G.M., Vogel K.W., Astley C.A., Hotchkiss C.E., Baldessari A., Pabon L., Reinecke H., Gill E.A., Nelson V., Kiem H.P., Laflamme M.A., Murry C.E.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014; 510(7504): 273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murry C.E., Chong J.J., Laflamme M.A.. Letter by Murry et al regarding article, “Embryonic stem cell-derived cardiac myocytes are not ready for human trials”. Circ Res. 2014; 115(10): e28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbach M., Peinkofer G., Baumgartner S., Maass M., Wiedey M., Neef K., Krausgrill B., Ladage D., Fatima A., Saric T., Hescheler J., Müller-Ehmsen J.. Electrophysiological integration and action potential properties of transplanted cardiomyocytes derived from induced pluripotent stem cells. Cardiovasc Res. 2013; 100(3): 432–40. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker P., Zhang H.P., and Kloner R.A.. Biphasic survival response to amlodipine after myocardial infarction in rats: Association with cardiac vascular remodelling. Cardiovasc Pathol. 2000; 9: 85–93. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem A., Troatz C., Elhafi N., Dewald O., Heeschen C., Nickenig G., Stypmann J., Tiemann K.. Quantitation of myocardial borderzone using reconstructive 3-D echocardiography after chronic infarction in rats – Incremental value of low-dose dobutamin. Ultrasound Med Biol. 2008; 34(4): 559–66. [DOI] [PubMed] [Google Scholar]
- 17.Kanno S., Lerner D.L., Schuessler R.B., Betsuyaku T., Yamada K.A., Saffitz J.E., Kovacs A.. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr. 2002; 15: 601–9. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P., Nagayama T., Mukhopadhyay P., Bátkai S., Kass D.A.. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008; 3(9): 1422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillekamp F., Reppel M., Dinkelacker V., Duan Y., Jazmati N., Bloch W., Brockmeier K., Hescheler J., Fleischmann B.K., Koehling R.. Establishment and characterization of a mouse embryonic heart slice preparation. Cell Physiol Biochem. 2005; 16(1–3): 127–32. [DOI] [PubMed] [Google Scholar]
- 20.Halbach M., Pillekamp F., Brockmeier K., Hescheler J., Muller-Ehmsen J., Reppel M.. Ventricular slices of adult mouse hearts—A new multicellular in vitro model for electrophysiological studies. Cell Physiol Biochem. 2006; 18: 1–8. [DOI] [PubMed] [Google Scholar]
- 21.Halbach M., Pfannkuche K., Pillekamp F., Ziomka A., Hannes T., Reppel M., Hescheler J., Müller-Ehmsen J.. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007; 101(5): 484–92. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Ehmsen J., Whittaker P., Kloner R.A., Dow J.S., Sakoda T., Long T.I., Laird P.W., Kedes L.. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002; 34: 107–16. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., Murry C.E.. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001; 33(5): 907–21. [DOI] [PubMed] [Google Scholar]
- 24.Krausgrill B., Soemantri S.P., Knirsch F., Maass M., Sahito R.G., Ladage D., Halbach M., Hescheler J., Saric T., Muller-Ehmsen J.. Influence of co-injected biomaterials or fibroblasts on engraftment and persistence of cardiomyocytes derived from murine embryonic stem cells after intramyocardial injection. Eur Heart J. 2012; 33(Supplement 1): 433(Abstract at the annual congress of European Society of Cardiology).21868475 [Google Scholar]
- 25.Maass M., Krausgrill B., Steigerwald C., Fatima A., Drey F., Neef K., Choi Y-H, Hescheler J., Saric T., Muller-Ehmsen J.. Cotransplantation of non-cardiomyocytes increases persistence of highly purified murine induced pluripotent stem cell derived cardiomyocytes after intramyocardial injection into syngeneic mouse hearts. Eur Heart J. 2012; 33(Supplement 1): (Abstract at the annual congress of European Society of Cardiology). [Google Scholar]
- 26.Sato M., Carr C.A., Stuckey D.J., Ishii H., Kanda G.K., Terracciano C.M., Siedlecka U., Tatton L., Watt S.M., Martin-Rendon E., Clarke K., Harding S.E.. Functional and morphological maturation of implanted neonatal cardiomyocytes as a comparator for cell therapy. Stem Cells Dev. 2010; 19(7): 1025–34. [DOI] [PubMed] [Google Scholar]
- 27.Yao M., Dieterle T., Hale S.L., Dow J.S., Kedes L.H., Peterson K.L., Kloner R.A.. Long-term outcome of fetal cell transplantation on postinfarction ventricular remodeling and function. J Mol Cell Cardiol. 2003; 35(6): 661–70. [DOI] [PubMed] [Google Scholar]
- 28.Halbach M., Krausgrill B., Hannes T., Wiedey M., Peinkofer G., Baumgartner S., Sahito R.G., Pfannkuche K., Pillekamp F., Reppel M., Müller-Ehmsen J., Hescheler J.. Time-course of the electrophysiological maturation and integration of transplanted cardiomyocytes. J Mol Cell Cardiol. 2012; 53(3): 401–8. [DOI] [PubMed] [Google Scholar]
- 29.Rubart M., Pasumarthi K.B., Nakajima H., Soonpaa M.H., Nakajima H.O., Field L.J.. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003; 92(11): 1217–24. [DOI] [PubMed] [Google Scholar]
- 30.Roell W., Lu Z.J., Bloch W., Siedner S., Tiemann K., Xia Y., Stoecker E., Fleischmann M., Bohlen H., Stehle R., Kolossov E., Brem G., Addicks K., Pfitzer G., Welz A., Hescheler J., Fleischmann B.K.. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation 2002; 105(20): 2435–41. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner S., Halbach M., Krausgrill B., Maass M., Srinivasan S.P., Sahito R.G., Peinkofer G., Nguemo F., Müller-Ehmsen J., Hescheler J.. Electrophysiological and morphological maturation of murine fetal cardiomyocytes during electrical stimulation in vitro. J Cardiovasc Pharmacol Ther. 2015; 20(1): 104–12. [DOI] [PubMed] [Google Scholar]
- 32.Kolossov E., Bostani T., Roell W., Breitbach M., Pillekamp F., Nygren J.M., Sasse P., Rubenchik O., Fries J.W., Wenzel D., Geisen C., Xia Y., Lu Z., Duan Y., Kettenhofen R., Jovinge S., Bloch W., Bohlen H., Welz A., Hescheler J., Jacobsen S.E., Fleischmann B.K.. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006; 203(10): 2315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T., Kawamura T., Kuratani T., Daimon T., Shimizu T., Okano T., Sawa Y.. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012; 126(11 Suppl 1): S29–37. [DOI] [PubMed] [Google Scholar]
- 34.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S., O'Sullivan C., Collins L., Chen Y., Minami E., Gill E.A., Ueno S., Yuan C., Gold J., Murry C.E.. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007; 25(9): 1015–24. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer J.M., Pfeffer M.A., Braunwald E.. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985; 57(1): 84–95. [DOI] [PubMed] [Google Scholar]
- 36.Jasmin J.F., Calderone A., Leung T.K., Villeneuve L., Dupuis J.. Lung structural remodeling and pulmonary hypertension after myocardial infarction: Complete reversal with irbesartan. Cardiovasc Res. 2003; 58(3): 621–31. [DOI] [PubMed] [Google Scholar]


