Abstract
Telocytes are a novel type of interstitial cell that has been identified in many organs of mammals, but there is little information available on these cells in avian species. This study shows the latest findings associated with telocytes in the muscular layer and lamina propria of the magnum of chicken oviduct analyzed by transmission electron microscopy. Telocytes are characterized by telopodes, which are thin and long prolongations, and a small amount of cytoplasm rich with mitochondria. Spindle- or triangular-shaped telocytes were detected at various locations in the magnum. In the muscular layer, telocytes have direct connection with smooth muscle cells. The cell body of telocytes along with their long telopodes mainly exists in the interstitial space between the smooth muscle bundles, whereas large numbers of short telopodes are scattered in between the smooth muscle cells. In the lamina propria, extremely long telopodes are twisting around each other and are usually collagen embedded. Both in the lamina propria and muscular layer, telocytes have a close relationship with other cell types, such as immune cells and blood vessels. Telopodes appear with dichotomous branching alternating between the podom and podomer, forming a 3D network structure with complex homo- and heterocellular junctions. In addition, a distinctive size of the vesicles is visible around the telopodes and may be released from telopodes because of the close relation between the vesicle and telopode. All characteristics of telocytes in the magnum indicate that telocytes may play a potential, but important, role in the pathogenesis of oviduct diseases.
Keywords: Telocytes (TCs), Oviduct, Chicken, Ultrastructure
Introduction
Telocytes (TCs) are novel interstitial cells of mesenchymal origin initially described in 2005 by Popescu and Faussone-Pellegrini1. First, they were named as interstitial Cajal-like cells (ICLCs); later on, because of their distinction from typical interstitial cells of Cajal (ICCs) based on their morphology, site of existence, ultrastructural features, and immunophenotype1,2, ICLCs were renamed TCs. The main characteristic of TCs is the presence of telopodes (Tps), extending from the cell body. Tps present a moniliform aspect with many dilations, a “bead on a string” look with dilatations called podoms, and thin segments named podomer. Tps can reach exceptional lengths, up to hundreds of micrometers1, and form attachments to various kinds of cells3–10. The cell body of TCs varies in its shape (fusiform–pyriform–triangular) depending on the number of Tps rising directly from the cell body, and it measures between 9 and 15 μm in length11,12. Podoms are possibly involved in calcium uptake/release, accommodating functional units consisting of caveolae, endoplasmic reticulum, and mitochondria8.
Recent studies have also described the gene expression profile, microRNA signature, proteomic analysis, and electrophysiological characteristics of TCs13. It has been proposed that TCs might have a role as mechanical support to make the correct organization of the connective tissue within organs, also in the neurotransmission by spreading the slow waves generated by the pacemaker ICC. In addition, TCs could be involved in intercellular signaling between stromal cells, smooth muscle cells (SMCs), microvessels, immune cells, and nerve bundles by paracrine secretion of signaling molecules and cell-to-cell contacts. At present, transmission electron microscopy (TEM) is the best available method for accurate identification of TCs. By TEM, TCs have been reported in the interstitial space of many mammalian organs, such as hollow organs including the heart, intestine, uterus, pulmonary veins, etc.3–6,8,12,14–16, and parenchymatous organs including the skeletal muscle, pancreas, placenta, mammary gland, etc.7,9,10,17–19.
The avian genital tract, unlike that of mammals, is a unilateral organ. Macroscopically, birds have a single functional oviduct, which is a simple tube coiled up in the left side of the abdominal cavity20,21. The magnum is the anterior and longest part of the oviduct, in which albumen is synthesized and secreted. Almost all the studies of TCs have been carried out in mammalian species; as a result, we were unable to find out any reference in the literature concerning the possible existence of TCs in birds. So the aim of the present study was to identify the TCs for the first time in an oviduct of chicken and investigate their morphological and ultrastructural characteristics and contact with surrounding cells.
Materials and Methods
Twelve egg-laying Chinese Three-Yellow hens weighing 2.0–2.5 kg and purchased from Nanjing Farm (Nanjing, P.R. China) were used in the present study. All protocols were approved by the Chinese Committee for Animal Use for Research and Education [SYXK (SU) 2011-0036]. The birds were sacrificed by cervical dislocation. Then we collected the oviduct samples very carefully, immediately after sacrifice. In this study, tissue blocks (magnum) were quickly fixed using the immersion fixation technique in 2.5% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M phosphate-buffered saline (PBS, pH 7.3) overnight. The tissues were then cut to 1-mm3 small pieces and fixed overnight in the same fixative at 4°C, rinsed three times for 8 min in 0.1 M PBS, and postfixed in 1% osmium tetra oxide (OsO4) (Sigma-Aldrich) in 0.1 M PBS for 2 h. The specimens were washed in distilled water and stained en bloc with 2% aqueous uranyl acetate (Sigma-Aldrich), then washed in distilled water 3 × 5 min, dehydrated in ethanol, and embedded in Epon 812 R (Merck, Whitehouse Station, NJ, USA). The ultrathin sections were mounted on copper grids and contrasted with uranyl acetate and lead citrate (Sigma-Aldrich). The sections were examined and photographed with a high-resolution digital camera (16 megapixels; H-7650; Hitachi, Tokyo, Japan) connected to the TEM.
Results
Ultrastructure of the entire thickness of the wall of the magnum of chicken oviduct was performed. TEM showed the existence of TCs in the muscular layer (Figs. 1 and 2), as well as within the lamina propria of the mucosa (Figs. 3 and 4). In all samples, TCs were identified according to the existence of their Tps. The long, slender Tps, occasionally convoluted with dilations, were seen with distinctive podoms and podomers (Figs. 1–6), which is the most important identifiable feature of TCs. The nucleus of TCs appeared as irregular in shape and contained clusters of heterochromatin attached to the nuclear envelope periphery (Figs. 1–4). The shape of the TCs changed from spindle to triangular, depending on the number of Tps arising directly from the body (Figs. 1–4). In the dilated processes (podom), the vesicle (V), rough endoplasmic reticulum (rER), and mitochondria were clearly observed (Fig. 5A).
Figure 1.

Transmission electron microscopic image of the muscular layer of chicken oviduct magnum. (A) The cell body of telocytes (TCs) mainly exists in the interstitial space between the smooth muscle bundles, and their telopode (Tp; arrowhead) is very long. (B) The vesicles (Vs), rough endoplasmic reticulum (rER), and mitochondria (m) are clearly visible in the podom (arrowhead). (C) Tps bordering the smooth muscle bundles along with their alternation of podom (arrowhead) and podomer located within the muscular layer (SMC, smooth muscle cell; BV, blood vessel).
Figure 2.
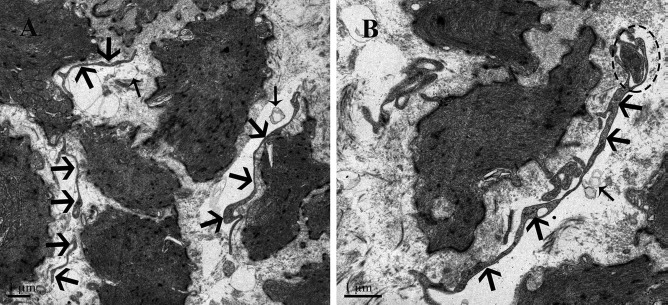
Transmission electron microscopic image of the muscular layer of chicken oviduct magnum. (A) Large number of short Tps (thick arrowhead) are scattered in between the SMCs except the cell body of TCs. (B) A Tp (thick arrowhead) located between the SMCs and divided in two branches in a dichotomous pattern, which forms the homocellular junction (dashed oval) with the podom of the other Tp. The vesicles (thin arrowhead) are present near the Tp.
Figure 3.
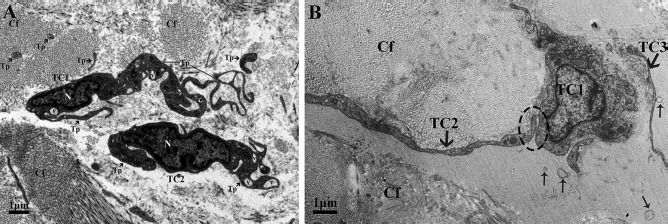
Transmission electron microscopic image of the lamina propria of chicken oviduct magnum. (A) Two TCs with their long Tps are located in the lamina propria embedded in collagen fibers (Cfs). The nucleus (N) appeared irregular in shape and contained clusters of heterochromatin attached to the periphery of the nuclear envelope. The vesicles (Vs) are also clearly visible. (B) The long Tp of TC2 is in close contact with the cell body of TC1 within the lamina propria and embedded in the Cfs. Shedding Vs (thin arrowhead) are often captured.
Figure 4.
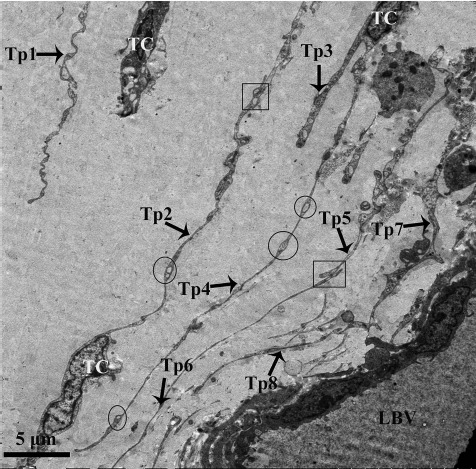
Transmission electron microscopic image of the lamina propria of chicken oviduct magnum. At least eight extremely long Tps from different TCs are visible in the lamina propria embedded in Cfs, near the lumen of blood vessel (LBV). Tps are very long with alternation of podom (circular areas) and podomer. Square areas show the close contact between the Tps with each other.
Figure 6.
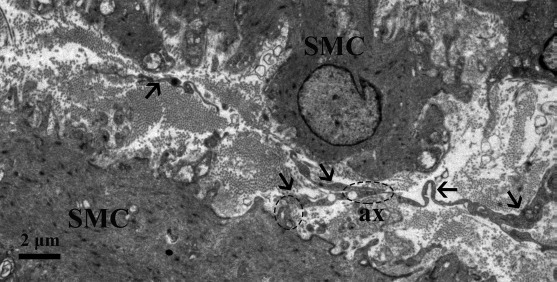
Transmission electron microscopic image of the muscular layer of chicken oviduct magnum. Several Tps (arrowhead) often lie in close membrane-to-membrane contact with SMCs and nerve ending. Further, it also forms the homocellular junction with other Tps (dashed oval region) and heterocellular junctions with SMC (dashed circular areas) (ax, axon).
Figure 5.
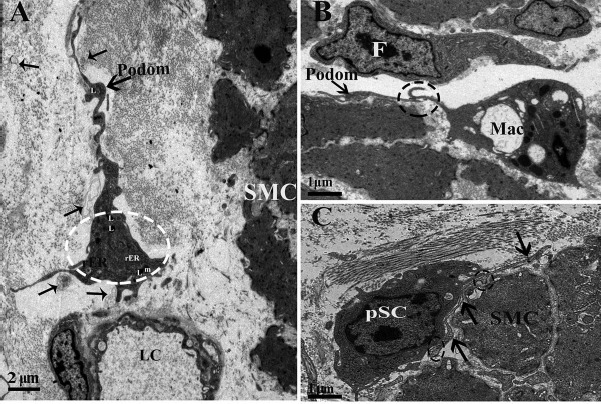
Transmission electron microscopic image of the muscular layer of chicken oviduct magnum. (A) The TC exists around the lumen of capillary (LC) and SMCs. The lysosomes (Ls) are also seen in the cell body of the TC (dashed circular areas). Shedding vesicles (thin arrowhead) are visible. (B) The dashed circular area shows the close contact between the Tp and macrophage (Mac) cell located between the SMCs. The internal structure of the fibroblast (F) can be seen, including the extended rough rER. (C) The TP (arrowhead) is present between the stem cell and SMCs and forms the heterocellular junction (close membrane-to-membrane contact) with the potential stem cell (pSC; dashed circular area) (m, mitochondria).
In the muscular layer, we observed that the cell body of the TCs mainly exists in the interstitial space between the smooth muscle bundles and their very long Tps (Fig. 1), whereas a large number of short Tps were scattered in between the SMCs, except the cell body of TCs (Fig. 2). These short Tps were also detected as discontinuous segments with alternation of podom and podomer (Figs. 2 and 5B). Through repeated observation, we speculated that the large number of short Tps was derived from the TCs within the smooth muscle bundles. A distinctive size of vesicles was found near the Tps, including exosomes (diameter: 60- to 100-nm vesicles), ectosomes (diameter: 100- to 250-nm vesicles), and microvesicles (diameter: 250–350 nm up to 1 μm). In addition to this, we also saw some Tps located between the SMCs divided into branches in a dichotomous pattern, which formed the homocellular junction with the podom of the other Tps (Fig. 2B). The TCs were embedded in the collagen fibers (Cfs), existed around the blood vessels (BVs) and SMCs, and some lysosomes (Ls) were found in their podom (Fig. 5A). Our ultrastructural findings showed the close contact between the Tps and processes of macrophage (Mac) cells that are located between the SMCs (Fig. 5B). The Tps often lie in close membrane-to-membrane nerve ending contact with SMCs; further, they also formed the homocellular junction with other Tps and heterocellular junctions with SMCs (Fig. 6). Tps are often located between the potential stem cell (pSC) and smooth muscles and result from the heterocellular junction between them (Fig. 5C). The fibroblast from Figure 5B is obviously different from TCs because of the large, dilated rER cisternae, which practically fill its cytoplasm.
In the lamina propria, extremely long Tps were twisting around each other and were usually collagen embedded (Figs. 3, 4, 7, and 8). The vesicles were also visible around the Tps (Figs. 3B and 7) and may be released from Tps because of the close relation between the vesicle and Tps (Fig. 3B). The Tps with labyrinthine-like structures overlapping with each other secreted large numbers of vesicles compared to straight Tps (Fig. 7). The Tps existed near the lymphocyte, plasma cell, and blood vessels and formed close contact with the Mac cell (Fig. 8A). Electron microscopy also clearly shows several Tps surrounding the nerve ending and blood vessels (Fig. 8B).
Figure 7.
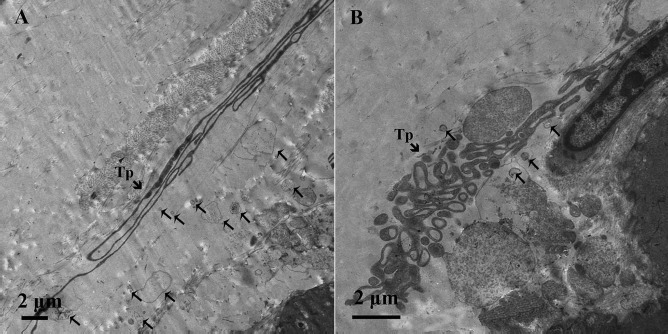
Transmission electron microscopic image of the lamina propria of chicken oviduct magnum. (A) Extremely long Tps were twisting around each other and were collagen embedded. (B) The Tps show the labyrinthine structure by overlapping each other. These Tps seem to secrete large number of vesicles (thin arrow) compared to straight Tps.
Figure 8.
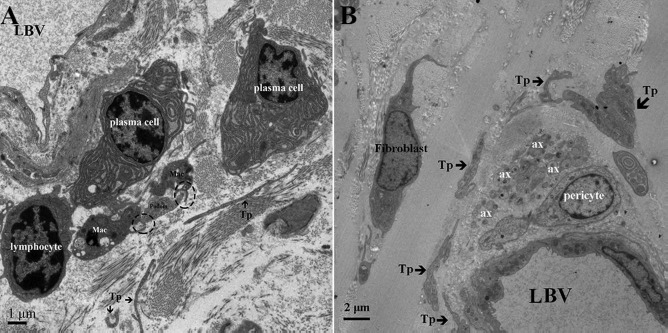
Transmission electron microscopic image of the lamina propria of chicken oviduct magnum. (A) Some short Tps are scattered around the lymphocyte, plasma cell, and LBV. The Tp forms close membrane-to-membrane contact with the Mac (dashed circular area) and is located within the lamina propria. (B) Several Tps are present in close vicinity of the nerve ending and lumen LBV in the lamina propria (ax. axon; Mac, macrophage).
Discussion
This is the first study to identify TCs in chicken oviduct. Here the general features of TCs were identified in the magnum of chicken oviduct by TEM, as this technique assures the precise identification of TCs and is in line with the diagnostic criteria of TCs already reported in mammalian species1,22–24. On the basis of our study, about two to three Tps were observed in a single section, but it mostly depended on the section angle and site. It was very difficult to observe three-dimensional convolutions of Tps full length in a section. As measured on TEM images, the lengths of Tps were tens to hundreds of micrometers long13. TCs have slender-shaped cell bodies with a thin rim of cytoplasm surrounding the nucleus and long thin cytoplasmic processes that swiftly come out from the cellular body as displayed in our TEM images. Our findings are in line with previous findings for mammalian species.
In our study, the TCs were found between the SMCs and within the bundles of smooth muscles, so we hypothesized that they were involved in the contraction of oviduct to facilitate the transport of sperm and egg. These were similar to the enteric TCs described by Pieri et al. in the muscularis externa of the human gut (cells described at that time as ICLCs)25. They showed that ICLCs were intermingled with ICCs, to be CD34+ and c-kit−. Pieri et al. and Faussone-Pellegrini hypothesized that these cells might directly or indirectly be involved in gut motility25,26. Popescu et al. also revealed that the TCs exhibit electrical activity like ICCs27 in the human fallopian tube; however, they are different from pacemaker ICCs28,29 and can be distinguished from ICCs by their morphology, site of existence, ultrastructural features, and the presence of Tps1.
Carmona et al.14 suggested that TCs were involved in intercellular signaling, for their strategic position with other cells, nerve endings and blood capillaries, and their three-dimensional network of TPs. Our results clearly demonstrate the presence of Tps in close vicinity to the BVs and nerve endings. In addition, TCs were also found in contact with different cells through their long Tps to maintain intercellular signaling3–11,30–37.
For example, TCs were found in close vicinity of plasma cells in this study. Plasma cells synthesized and secreted large amounts of antibodies into circulation. It was observed that the initial burst of antibody production gradually decreased as the stimulus was removed38, so it can be proposed that TCs might be involved in the stimulation of plasma cells to synthesize antibodies and are involved in humoral immunity39,41.
In previous studies, TCs were found to be located near stem cell niches in the lung and had a close relationship with these stem cell niches, suggesting that TCs might play a role in the nurturing, communicating, or stimulating of stem cells11,13,42–46. Popescu and colleagues9,13,47 stated that TCs might be involved in tissue reparation and regeneration. Our results are in accordance with these studies and demonstrated close and direct membrane-to-membrane contact of TCs with stem cells. We can speculate that when the oviduct tissues are injured by physical, biological, or chemical factors, TCs might be activated to stimulate and promote the proliferation and differentiation of stem cells to be associated with oviduct tissue injury and initiate repair3,48,49. Our finding of TCs embedded in collagen fibers similar to those of Rusu et al.50 and Ceafalan et al.11, suggesting that TCs might be involved in homeostasis, remodeling, regeneration, and repair of tissues. It is becoming increasingly evident that stem cells and TCs may work in tandem19,42,50–54.
In addition, the results of the present study clearly found that the TCs released vesicles and exosomes. Some studies5,51,55–58 stated that TCs generally form and release vesicles (or exosomes), which might indicate the potential participation of TCs in intercellular communication. As in the heart, TCs interact with cardiomyocytes by heterocellular communication, direct contact (intercellular junctions), and indirect contact by shed extracellular vesicles. These vesicles transport RNA or DNA to neighboring cells or target cells, inducing epigenetic changes51,56. So it is suggested that released vesicles and exosomes may be involved in intercellular communication in chicken oviduct. In this phenomenon, the role of TCs cannot be overlooked in the pathology.
We had hoped to use immunohistochemistry and light microscopic techniques to localize and especially quantitate the numbers of TCs in chicken oviduct for a better understanding of the role of TCs in avian species. As we know, CD34 labeling remains the best available choice for the single immunohistochemical identification of TCs; however, sometimes it may be confused with endothelial cells in general, so CD34/PDGFRa or CD34/CD31 labeling by double immunohistochemistry can be an accurately oriented diagnosis1,13,59. However, our attempt regrettably failed due to the lack of available easy choice-specific avian antibodies in the market.
In conclusion, we proposed that the TCs could play an important role in the stimulation and functioning of the chicken oviduct. We positively fulfilled our aim and clearly identified TCs' morphological and ultrastructural characteristics within the interstitial spaces of the magnum. TCs connected with each other and with blood vessels, collagen fibers, stem cells, smooth muscles, lymphocytes, and plasma cells and had lysosomes. This added new data for the existence of TCs in chicken oviduct. There is still a need to further explore potential biofunctions of TCs in certain oviduct pathological conditions and mechanisms of interactions between TCs and other cells.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Nos. 31402155 and 31672505), Natural Science Foundation of Jiangsu Province of China (Youth Project, No. BK20130681), the Fundamental Research Funds for the Central Universities of China (No. KJ2013025), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, P.R. China. The authors declare no conflicts of interest.
References
- 1.Popescu L.M., Faussone-Pellegrini M.S.. TELOCYTES—A case of serendipity: The winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010; 14(4): 729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faussone-Pellegrini M.S., Popescu L.M.. Telocytes. Biomol Concepts 2011; 2(6): 481–5. [DOI] [PubMed] [Google Scholar]
- 3.Cretoiu S.M., Cretoiu D., Marin A., Radu B.M., Popescu L.M.. Telocytes: Ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction 2013; 145(4): 357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gherghiceanu M., Manole C.G., Popescu L.M.. Telocytes in endocardium: Electron microscope evidence. J Cell Mol Med. 2010; 14(9): 2330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gherghiceanu M., Popescu L.M.. Heterocellular communication in the heart: Electron tomography of telocyte-myocyte junctions. J Cell Mol Med. 2011; 15(4): 1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manole C.G., Cismasiu V., Gherghiceanu M., Popescu L.M.. Experimental acute myocardial infarction: Telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011; 15(11): 2284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolescu M.I., Bucur A., Dinca O., Rusu M.C., Popescu L.M.. Telocytes in parotid glands. Anat Rec. 2012; 295(3): 378–85. [DOI] [PubMed] [Google Scholar]
- 8.Popescu L.M., Manole C.G., Gherghiceanu M., Ardelean A., Nicolescu M.I., Hinescu M.E., Kostin S.. Telocytes in human epicardium. J Cell Mol Med. 2010; 14(8): 2085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popescu L.M., Manole E., Serboiu C.S., Manole C.G., Suciu L.C., Gherghiceanu M., Popescu B.O.. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. J Cell Mol Med. 2011; 15(6): 1379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suciu L., Popescu L.M., Gherghiceanu M., Regalia T., Nicolescu M.I., Hinescu M.E., Faussone-Pellegrini M.S.. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010; 192(5): 325–39. [DOI] [PubMed] [Google Scholar]
- 11.Ceafalan L., Gherghiceanu M., Popescu L.M., Simionescu O.. Telocytes in human skin—Are they involved in skin regeneration? J Cell Mol Med. 2012; 16(7): 1405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cretoiu D., Cretoiu S.M., Simionescu A.A., Popescu L.M.. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012; 27(7): 1067–78. [DOI] [PubMed] [Google Scholar]
- 13.Cretoiu S.M., Popescu L.M.. Telocytes revisited. Biomol Concepts 2014; 5(5): 353–69. [DOI] [PubMed] [Google Scholar]
- 14.Cantarero Carmona I., Luesma Bartolome M.J., Junquera Escribano C.. Identification of telocytes in the lamina propria of rat duodenum: Transmission electron microscopy. J Cell Mol Med. 2011; 15(1): 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherghiceanu M., Hinescu M.E., Andrei F., Mandache E., Macarie C.E., Faussone-Pellegrini M.S., Popescu L.M.. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008; 12(5): 1777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusu M.C., Pop F., Hostiuc S., Curca G.C., Jianu A.M., Paduraru D.. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012; 27(6): 807–16. [DOI] [PubMed] [Google Scholar]
- 17.Bosco C., Diaz E., Gutierrez R., Gonzalez J., Perez J.. Ganglionar nervous cells and telocytes in the pancreas of octodon degus extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton Neurosci. 2013; 177(2): 224–30. [DOI] [PubMed] [Google Scholar]
- 18.Gherghiceanu M., Popescu L.M.. Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma. Transmission electron microscope (TEM) identification. J Cell Mol Med. 2005; 9(4): 893–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popescu L.M., Hinescu M., Ionescu N., Ciontea S.M., Cretoiu D., Ardeleanu C.. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005; 9(1): 169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Wang W., Feng Y., Li M., Bao H., Chen Q.. The sympathetic postganglionic and sensory innervation of oviducal magnum in hen: A choleratoxin subunit B-conjugated horseradish peroxidase study. J Anat. 2007; 210(4): 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez M.S., Moya A., Lopez L., Arias J.L.. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 2001; 19(8): 793–803. [DOI] [PubMed] [Google Scholar]
- 22.Cretoiu S.M., Cretoiu D., Suciu L., Popescu L.M.. Interstitial Cajal-like cells of human fallopian tube express estrogen and progesterone receptors. J Mol Histol. 2009; 40(5–6): 387–94. [DOI] [PubMed] [Google Scholar]
- 23.Roatesi I., Radu B.M., Cretoiu D., Cretoiu S.M.. Uterine telocytes: A review of current knowledge. Biol Reprod. 2015; 93(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 24.Yang J., Chi C., Liu Z., Yang G., Shen Z.J., Yang X.J.. Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis. J Cell Mol Med. 2015; 19(7): 1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pieri L., Vannucchi M.G., Faussone-Pellegrini M.S.. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008; 12(5): 1944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faussone-Pellegrini M.S.. Interplay among enteric neurons, interstitial cells of Cajal, resident and not resident connective tissue cells. J Cell Mol Med. 2009; 13(7): 1191–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu L.M., Ciontea S.M., Cretoiu D., Hinescu M.E., Radu E., Ionescu N., Ceausu M., Gherghiceanu M., Braga R.I., Vasilescu F.. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005; 9(2): 479–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allix S., Reyes-Gomez E., Aubin-Houzelstein G., Noël D., Tiret L., Panthier J.J., Bernex F.. Uterine contractions depend on KIT-positive interstitial cells in the mouse: Genetic and pharmacological evidence. Biol Reprod. 2008; 79(3): 510–7. [DOI] [PubMed] [Google Scholar]
- 29.Sanders K.M., Koh S.D., Ward S.M.. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68: 307–43. [DOI] [PubMed] [Google Scholar]
- 30.Burridge K., Guilluy C.. Focal adhesions, stress fibers and mechanical tension. Exp Cell Res. 2015; 343(1): 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X., Zheng Y., Manole C.G., Wang X., Wang Q.. Telocytes in human oesophagus. J Cell Mol Med. 2013; 17(11): 1506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolescu M.I., Popescu L.M.. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012; 41(6): 949–56. [DOI] [PubMed] [Google Scholar]
- 33.Popescu L.M., Ciontea S.M., Cretoiu D.. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann NY Acad Sci. 2007; 1101(1): 139–65. [DOI] [PubMed] [Google Scholar]
- 34.Rusu M.C., Jianu A.M., Mirancea N., Didilescu A.C., Manoiu V.S., Paduraru D.. Tracheal telocytes. J Cell Mol Med. 2012; 16(2): 401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullah S., Yang P., Zhang L., Zhang Q., Liu Y., Chen W., Waqas Y., Le Y., Chen B., Chen Q.. Identification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J Cell Mol Med. 2014; 18(12): 2385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vannucchi M.G., Traini C., Guasti D., Giulio D.P., Faussone-Pellegrini M.S.. Telocytes subtypes in human urinary bladder. J Cell Mol Med. 2014; 18(10): 2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y., Li H., Manole C.G., Sun A., Ge J., Wang X.. Telocytes in trachea and lungs. J Cell Mol Med. 2011; 15(10): 2262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manz R.A., Thiel A., Radbruch A.. Lifetime of plasma cells in the bone marrow. Nature 1997; 388(6638): 133–4. [DOI] [PubMed] [Google Scholar]
- 39.Calame K.L., Lin K.I., Tunyaplin C.. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003; 21(1): 205–30. [DOI] [PubMed] [Google Scholar]
- 40.Manz R.A., Arce S., Cassese G., Hauser A.E., Hiepe F., Radbruch A.. Humoral immunity and long-lived plasma cells. Curr Opin Immunol. 2002; 14(4): 517–21. [DOI] [PubMed] [Google Scholar]
- 41.Slifka M.K., Antia R., Whitmire J.K., Ahmed R.. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8(3): 363–72. [DOI] [PubMed] [Google Scholar]
- 42.Bani D., Formigli L., Gherghiceanu M., Faussone-Pellegrini M.S.. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J Cell Mol Med. 2010; 14(10): 2531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cretoiu D., Hummel E., Zimmermann H., Gherghiceanu M., Popescu L.M.. Human cardiac telocytes: 3D imaging by FIB-SEM tomography. J Cell Mol Med. 2014; 18(11): 2157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gherghiceanu M., Popescu L.M.. Cardiac telocytes—Their junctions and functional implications. Cell Tissue Res. 2012; 348(2): 265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popescu L.M., Curici A., Wang E., Zhang H., Hu S., Gherghiceanu M.. Telocytes and putative stem cells in ageing human heart. J Cell Mol Med. 2015; 19(1): 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y., Bai C., Wang X.. Potential significance of telocytes in the pathogenesis of lung diseases. Expert Rev Respir Med. 2012; 6(1): 45–9. [DOI] [PubMed] [Google Scholar]
- 47.Popescu L.M., Gherghiceanu M., Manole C.G., Faussone-Pellegrini M.S.. Cardiac renewing: Interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009; 13(5): 866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bei Y., Wang F., Yang C., Xiao J.. Telocytes in regenerative medicine. J Cell Mol Med. 2015; 19(7): 1441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luesma M.J., Gherghiceanu M., Popescu L.M.. Telocytes and stem cells in limbus and uvea of mouse eye. J Cell Mol Med. 2013; 17(8): 1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rusu M.C., Mirancea N., Manoiu V.S., Valcu M., Nicolescu M.I., Paduraru D.. Skin telocytes. Ann Anat. 2012; 194(4): 359–67. [DOI] [PubMed] [Google Scholar]
- 51.Cismasiu V.B., Popescu L.M.. Telocytes transfer extracellular vesicles loaded with microRNAs to stem cells. J Cell Mol Med. 2015; 19(2): 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinescu M.E., Gherghiceanu M., Suciu L., Popescu L.M.. Telocytes in pleura: Two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011; 343(2): 389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manetti M., Guiducci S., Ruffo M., Rosa I., Faussone-Pellegrini M.S., Matucci-Cerinic M., Ibba-Manneschi L.. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013; 17(4): 482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolescu M.I., Manole C.G.. Telocytes and stem cells in regenerative medicine. FASEB J. 2013; 27: 752–4. [Google Scholar]
- 55.Cantarero I., Luesma M.J., Junquera C.. The primary cilium of telocytes in the vasculature: Electron microscope imaging. J Cell Mol Med. 2011; 15(12): 2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cismasiu V.B., Radu E., Popescu L.M.. miR-193 expression differentiates telocytes from other stromal cells. J Cell Mol Med. 2011; 15(5): 1071–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fertig E.T., Gherghiceanu M., Popescu L.M.. Extracellular vesicles release by cardiac telocytes: Electron microscopy and electron tomography. J Cell Mol Med. 2014; 18(10): 1938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smythies J., Edelstein L.. Telocytes, exosomes, gap junctions and the cytoskeleton: The makings of a primitive nervous system? Front Cell Neurosci. 2014; 7(1): 278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vannucchi M.G., Traini C., Manetti M., Ibba-Manneschi L., Faussone-Pellegrini M.S.. Telocytes express PDGFRα in the human gastrointestinal tract. J Cell Mol Med. 2013; 17(9): 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]


