Abstract
Somatic cells can be directly converted into induced neural stem cells (iNSCs) by defined transcription factors. However, the therapeutic effect of undifferentiated iNSCs on ischemic stroke has not been demonstrated. In this study, we used a mouse model of transient middle cerebral artery occlusion (tMCAO). iNSCs (5 × 105) were injected directly into the ipsilateral striatum and cortex 24 h after tMCAO. Histological analysis was performed at 7 days, 28 days, and 8 months after tMCAO. We found that iNSC transplantation successfully improved the survival rate of stroke model mice with significant functional recovery from the stroke. The fate of engrafted iNSCs was that the majority of iNSCs had differentiated into astroglial cells but not into neural cells in both the sham-operated brain and the poststroke brain without forming a tumor up to 8 months after tMCAO. Our data suggest that the directly converted iNSCs can be regarded as a candidate of safe cell resource for transplantation therapy in patients suffering from ischemic stroke.
Keywords: Induced neural stem cells (iNSCs), Cerebral ischemia, Cell transplantation, Inflammatory response
Introduction
Stroke is the major cause of death in industrialized nations, and thus a reduction in the quality of life caused by a stroke is a serious challenge. Since only thrombolytic therapy with tissue plasminogen activator has been considered as an effective therapy, stem cell transplantational therapy has recently been highlighted as a second-generation therapy for treating stroke patients.
Somatic cells can be reprogrammed into an embryonic stem cell-like state, the induced pluripotent stem cells (iPSCs), by introducing four transcription factors octamer-binding transcription factor 3/4 (Oct3/4), SRY-related HMG box 2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myc1. iPSCs are considered to be a promising source of cells for regenerative medicine because of their infinite self-renewal ability as well as an unlimited potential to differentiate into any kind of cell. However, the potential risks for tumorigenicity of iPSCs have raised several safety concerns about cell transplantation therapy, especially in the poststroke brain2.
Recently, we and other groups have described the generation of induced neural stem cells (iNSCs) from fibroblasts using technology that allows for direct conversion3–5. Since fibroblasts are directly converted into iNSCs without first passing through an iPSC state, iNSCs have been regarded as a safer cell resource than iPSCs. In a recent study in which the therapeutic effect of iNSC transplantation in the spinal cord injury rat model was evaluated, engrafted iNSCs differentiated into all neuronal lineages, thus enhancing the functional recovery of the spinal cord injury model6. However, the therapeutic effect of iNSCs against stroke has not yet been assessed.
In the current study, therefore, we investigated the therapeutic effect of iNSC transplantation and also monitored engrafted iNSCs after transplantation of undifferentiated murine iNSCs in normal or ischemic striatum/cortex of the mouse brain.
Materials and Methods
Animals and Experimental Groups
Adult (23–27 g, 8-10 weeks old) male C57BL/6N mice were used in this study. All experimental procedures were approved by the Animal Committee of the Okayama University Graduate School of Medicine (OKU-2014298). We studied three experimental groups including the sham + iNSC group (n = 6), the transient middle cerebral artery occlusion (tMCAO) + iNSC group (n = 19), and the tMCAO + vehicle group (n = 19). Each experimental group received intracerebral implantation of saline or murine iNSCs 24 h after sham operation or tMCAO, as described next.
Focal Cerebral Ischemia
In this study, we used a mouse model of tMCAO7 in which the infarcted region is mainly located in the lateral portion of the corpus striatum and cortex. In a pilot study, we compared the survival rate of 45-min tMCAO and 35-min tMCAO in the mouse model and found that over 70% of 45-min tMCAO mice had died by 7 days after tMCAO. For this reason, we selected the 35-min tMCAO mice model in this study. During surgery, mice were anesthetized with a mixture of nitrous oxide, oxygen, and isoflurane (69%/30%/1%), which was administered through an inhalation mask. tMCAO was induced by the intraluminal filament technique7. In brief, the right carotid bifurcation was exposed, and the external carotid artery was coagulated distal to the bifurcation. A silicone-coated 6–0 filament was then inserted through the stump of the external cerebral artery and gently advanced (9.0–10.0 mm) to occlude the middle cerebral artery. After 35 min of occlusion, the filament was gently withdrawn, and the incision was closed. Rectal temperature was maintained at 37.0°C by placing the animals on a heating bed (model BMT-100; Bio Research Center, Tokyo, Japan). A laser Doppler flowmeter probe (MBF3D; Moor Instruments Ltd., Axminster, UK) was attached to the surface of the ipsilateral cortex to monitor regional cerebral blood flow.
Generation of iNSCs
Murine iNSC lines were established from 5 × 104 fibroblasts transduced with replication-defective retroviral particles coding for Sox2, Klf4, c-Myc, and BRAIN-4 (Brn4). Briefly, the transduced fibroblasts were cultured in standard NSC medium, which is Dulbecco's modified Eagle's medium (DMEM)/F-12 supplemented with N2 or B27 supplements (Invitrogen, Carlsbad, CA, USA), 10 ng/ml epidermal growth factor (EGF; PeproTech, Rocky Hill, NJ, USA), 10 ng/ml basic fibroblast growth factor (bFGF; PeproTech), and 1× L-glutamine with penicillin/streptomycin (Invitrogen). Four to five weeks after transduction, iNSC clusters were observed and expanded as previously reported4. Finally, iNSC lines were established and labeled with retroviral vector-encoding enhanced green fluorescent protein (EGFP).
In Vitro Differentiation of iNSCs
For neuronal differentiation, 1 × 105 iNSCs were plated on gelatin-coated four-well dishes that were incubated in NSC medium without EGF. Four days later, the medium was replaced by NSC medium without bFGF and EGF. For glial differentiation, 1 × 105 iNSCs were plated on gelatin-coated four-well dishes and incubated in NSC medium without bFGF and EGF. Four days later, the medium was replaced by NSC medium containing 1% (v/v) fetal bovine serum (FBS) in the absence of cytokines.
Transplantation of iNSCs
The EGFP-labeled iNSCs were collected, washed three times with phosphate-buffered saline (PBS), and pelleted by centrifugation at 200 × g for 5 min. The pellets were resuspended in 15 μl of Hank's balanced salt solution (HBSS) and placed on ice. Two microliters of the cell suspension (5 × 105 cells) or 2 μl of saline was stereotaxically injected into the ipsilateral striatum and cortex (anterior, lateral, and depth: −0.5, 2.5, and 1.5–2.5 mm, respectively)8. This position approximates the ischemic boundary zone. An immunosuppressive compound, cyclosporine A (Novartis International, Basel, Switzerland), was applied intraperitoneally to all animals immediately after implantation of iNSCs or saline and every other day before animals were sacrificed with a 10 mg/kg/day dose.
Histochemistry
The surviving mice [the sham + iNSC group (n = 0, 3, 3), the tMCAO + iNSC group (n = 4, 6, 4), and the tMCAO + vehicle group (n = 4, 5, 0)] were sacrificed at 7 days, 28 days, and 8 months after the induction of cerebral ischemia, respectively. Each animal was anesthetized by intraperitoneal (IP) injection of pentobarbital (20 mg/kg) and then perfused with chilled PBS, followed by 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer. After postfixation overnight, 50-μm-thick sections were cut with a vibrating blade microtome (VT1000S; Leica, Wetzlar, Germany). For immunohistochemistry, the following primary antibodies were used: rabbit anti-nestin antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-Sox2 antibody (1:100; Santa Cruz Biotechnology); rabbit anti-microtubule-associated protein 2 (MAP2) antibody (1:200; Millipore, Billerica, MA, USA); rabbit anti-β III tubulin (Tuj1) antibody (1:50; Covance Research, Princeton, NJ, USA); mouse anti-galactocerebroside (GalC) antibody (1:400; Millipore); rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:500; Dako, Glostrup, Denmark); rabbit anti-Iba1 antibody (1:1,000; Wako, Osaka, Japan); mouse anti-NeuN antibody (1:100; Millipore); and mouse anti-O4 antibody (1:100; R&D Systems, Minneapolis, MN, USA). The antibodies against nestin, Sox2, MAP2, Tuj1, GalC, GFAP, NeuN, and O4 were detected by secondary antibodies conjugated with Alexa Fluor 488 or 555™ (Thermo Fisher Scientific, Waltham, MA, USA). To estimate the expression of GFAP and Iba1, sections were incubated with the respective first antibody and then with an appropriate biotin-labeled secondary antibody (1:500). After incubation with the ABC Elite complex (Vector Laboratories, Burlingame, CA, USA), the signal was visualized with diaminobenzidine tetrahydrochloride.
Behavioral Analysis
At 24 h after transplantation of iNSCs, the surviving mice were tested every 7 days for behavioral changes and scored, as described by Bederson, but with minor modifications9 as follows: 0, no observable neurologic deficits; 1, failure to extend the right forepaw; 2, circling to the contralateral side; 3, falling to the right; 4, unable to walk spontaneously. A corner test was also carried out to detect the impairment of sensorimotor function, which is correlated with ischemic lesion volume and the neurologic score10. Briefly, one mouse was placed between two boards, which were attached to an edge at a 30° angle to each other. The mouse turned either left or right, but an ischemic mouse preferentially turns toward the non-impaired side. After this test was repeated 10 times in each mouse, the number of right turns was recorded. In addition, the mice were evaluated by a rotarod test on the same day. Mice were habituated by exposing them to a rotating rod 3 days before tMCAO. Initially, mice were placed on a rod that was not rotated. The speed was slowly accelerated to 45 rpm over a period of 5 min. The mice were allowed a maximum of three trials to remain on the rotarod for 5 min for each trial. The evaluation was terminated when mice fell from the rotarod or reached the criterion level. The maximum time that the animal remained on the rod was recorded.
Quantitative Analysis
For the semiquantitative evaluation of histochemical staining, such as for GFAP and Iba1, stained sections were selected from three levels of the caudate putamen (1.0, 0.5, and 0 mm rostral to the bregma)8 of each animal. Three areas in the peri-infarct cortex were randomly chosen in each section and captured at 200× magnification with a microscope (BX51; Olympus, Tokyo, Japan). Staining intensity was measured using Scion Image software (Scion Corp.).
For the cell type-specific marker/EGFP double-labeling immunohistochemistry (see Fig. 4), four areas where EGFP-labeled iNSCs were transplanted were randomly chosen and captured at 100× magnification with a confocal laser microscope (LSM780; Zeiss, Oberkocken, Germany).
Figure 4.
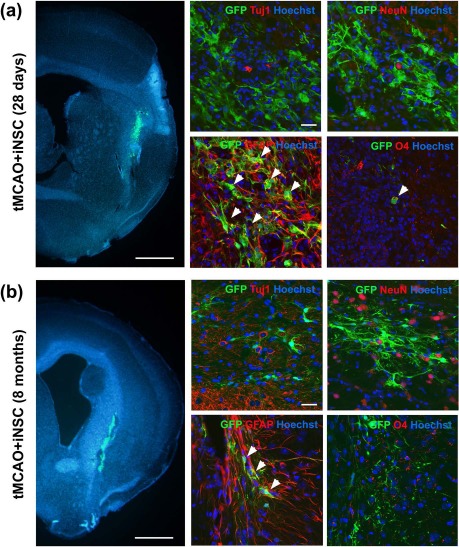
iNSCs mainly differentiated into glial cells in the postischemic brain. (a, b) Left: Representative images of abundant encoding enhanced green fluorescent protein (EGFP+) iNSCs (green) that survived in the postischemic striatal lesions at 28 days (a) and 8 months (b) after iNSC transplantation. Scale bars: 1 mm. Right: iNSCs could mainly differentiate into glial cells in the brain, as shown by immunostaining using antibodies against EGFP+ rabbit anti-β III tubulin (Tuj1), NeuN, GFAP, and O4. Scale bars: 50 μm. GFP, green fluorescent protein.
Statistical Analysis
Statistical analyses were performed with the Ystat 2002 software (Igaku Tosho Shuppan Co., Ltd., Tokyo, Japan) together with the Microsoft Excel software (Microsoft Corp., Redmond, WA, USA). Values were expressed as means ± standard deviation (SD). Differences in behavioral analysis and intensity of GFAP and Iba1 staining were evaluated for statistical significance by non-repeated-measures analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) test. In all statistical analyses, significance was assumed at p < 0.05.
Results
Murine iNSCs were established from mouse fibroblasts using a combination of transcriptional factors (Sox2, Klf4, c-Myc, and Brn4) for transplantation therapy as in our previous studies3,4,6,11. The majority of iNSCs expressed NSC markers such as nestin and Sox2 (Fig. 1a). An in vitro differentiation assay showed that the directly converted iNSCs differentiated into both neuronal and glial lineages (Fig. 1b and c), indicating that iNSCs generated in the current study were fully reprogrammed into an NSC-like state, as in our previous studies3,4,6,11.
Figure 1.
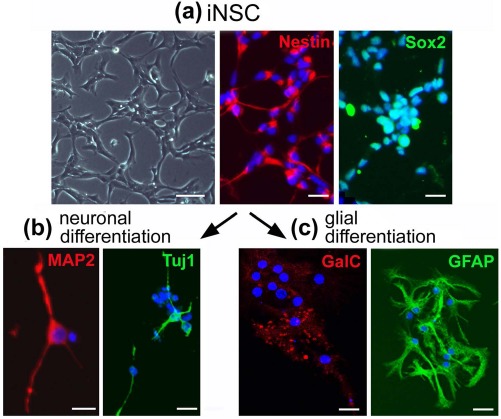
Induced neural stem cells (iNSCs) can differentiate into both neuronal and glial lineages in vitro. (a) Left: Morphology of undifferentiated iNSCs as assessed by bright-field microscopy. Scale bars: 50 μm. Right: Immunofluorescence microscopy images of undifferentiated iNSCs, using antibodies against nestin and (sex-determining region Y)-box 2 (Sox2). Scale bars: 50 μm. (b) Immunofluorescence microscopy images of neuronal differentiated iNSCs using antibodies against microtubule-associated protein 2 (MAP2) and rabbit anti-β III tubulin (Tuj1). Scale bars: 20 μm. (c) Immunofluorescence microscopy images of glial differentiated iNSCs using antibodies against the oligodendrocyte marker, anti-galactocerebroside (GalC), and an astrocyte marker, glial fibrillary acidic protein (GFAP). Scale bars: 20 μm.
To examine the therapeutic effect of iNSCs, undifferentiated iNSCs (5 × 105 cells, 70.2% nestin+, 61.5% Sox2+, 0% Tuj1+, and 72.9% GFAP+) were transplanted into the ipsilateral striatum/cortex 24 h after the induction of cerebral ischemia. Behavioral and histological analyses were then performed. The tMCAO + iNSC group showed a significantly higher survival rate than the tMCAO + vehicle group (p < 0.05) (Fig. 2a). In addition, the tMCAO + iNSC group showed enhanced functional recovery relative to the tMCAO + vehicle group as evidenced by a reduced Bederson's score as well as a higher corner test score (p < 0.05) (Fig. 2b and c). The rotarod test results also suggest that the tMCAO + iNSC group may have improved functional recovery, although it was not statistically significant (Fig. 2d). In terms of infarct volume, there was no statistically significant difference between the tMCAO + vehicle group and the tMCAO + iNSC group at 7 days after MCAO (Fig. 2e). Taken together, these results indicate that iNSCs may have a protective effect in the acute phase of stroke upon transplantation into the tMCAO disease model.
Figure 2.
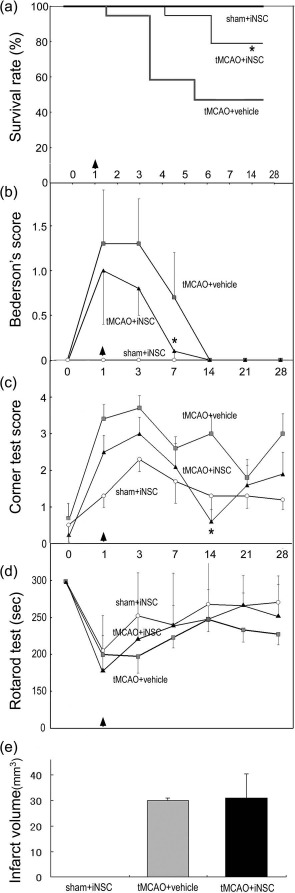
Mice survival and behavioral analysis after iNSC transplantation. (a) Survival rate of the three experimental groups after cerebral ischemia. iNSC transplantation significantly improved the survival rate (∗p < 0.05). Experimental groups include sham + iNSC (n = 6), transient middle cerebral artery occlusion (tMCAO) + iNSC (n = 19), and tMCAO + vehicle (n = 19). (b, c) The tMCAO + iNSC group showed improved functional recovery after stroke compared with the tMCAO + vehicle group in terms of both Bederson's score (∗p < 0.05) and the corner test (∗p < 0.05). (d) The rotarod test was also performed in the three experimental groups. (e) There was no statistically significant difference in the infarct volume at 7 days after MCAO between the tMCAO + vehicle group and the tMCAO + iNSC group.
GFAP expression was significantly suppressed in both the peri-infarct cortex and striatum of the iNSC-transplanted mice 7 days after the stroke. However, there was no significant difference between both groups 28 days after the stroke (p < 0.05) (Fig. 3a–m). Moreover, the expression of Iba1, a microglial marker, also seems to have been suppressed in iNSC-transplanted mice 7 days after the stroke, although not significantly (Fig. 3n).
Figure 3.
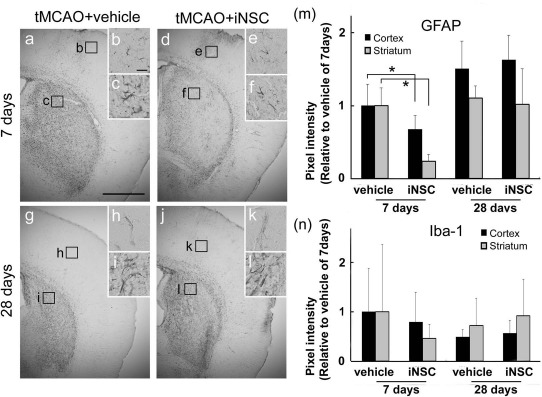
iNSC transplantation suppressed the inflammatory response in mice brain. (a–l) Immunohistochemical staining for glial fibrillary acidic protein (GFAP) in the ipsilateral side of a poststroke brain. Scale bar: 1 mm. (b, c, e, f, h, i, k, l) High-magnification views of the field indicated by the boxed areas in (a), (d), (g), and (j), respectively. Scale bar: 50 μm. (m, n) Relative intensity of GFAP and Iba1 in the peri-infarcted lesion of the cortex was measured on days 7 and 28 of transplantation (∗p < 0.05).
Engrafted iNSCs were prelabeled with EGFP in both sham-operated and poststroke brains. On day 28 of transplantation, the majority of EGFP+ cells expressed the astrocytic marker GFAP in both the sham + iNSC group (218 cells/251 EGFP+ cells) and the tMCAO + iNSC group (224 cells/249 EGFP+ cells) (Fig. 4a). A relatively small number of EGFP+ cells also expressed O4, an oligodendrocyte marker, in both experimental groups (9 cells/266 EGFP+ cells, 7 cells/156 EGFP+ cells). However, EGFP+ iNSCs that coexpressed neuronal markers such as Tuj1 (0/380 in sham; 0/433 in tMCAO), MAP2 (0/316 in sham; 0/181 in tMCAO), and doublecortin (Dcx; 0/282 in sham; 0/320 in tMCAO) were not detected in both experimental groups. A similar pattern of differentiation was also observed in the iNSC derivatives expressing EGFP even at 8 months after transplantation (Fig. 4b). No signs of tumor formation were observed at the site of transplantation in both the penumbra and surrounding regions (data not shown) from a total of seven mice [sham + iNSC group (n = 3); tMCAO + iNSC group (n = 4)] up to 8 months after transplantation in the Nissl-stained sections.
Discussion
In the current study, we showed that engrafted iNSCs significantly improved the survival rate of stroke model mice with enhanced functional recovery (Fig. 2). Recent evidence has suggested that the therapeutic effect of transplanted neural stem cells may be primarily derived from neurotrophic effects that downregulate the inflammatory response and also suppress glial scar formation12. Our series of immunohistochemical analyses with an astroglial marker (for GFAP) or a microglial marker (for Iba1 antibody) suggest that the suppressed inflammatory response is mediated by engrafted iNSCs. A previous article already reported that iNSCs expressed some neurotrophic factors, including glial cell line-derived neurotrophic factor (GDNF) and hepatocyte growth factor (HGF)6; thus, neurotropic factors that are secreted from iNSCs might be related to the therapeutic effect of iNSCs in the stroke model (Fig. 3).
In the current study, we confirmed that the established iNSCs differentiated into both neural and glial lineages in vitro. In contrast to the in vitro data, we detected mainly glial differentiation (89.9% GFAP+, 4.5% O4+ at 28 days) in vivo. There was no significant difference between the sham + iNSC group and the tMCAO + iNSC group in terms of differentiation pattern, indicating that the differentiation patterns observed in this study were independent of stroke-related conditions. Related to this biased differentiation in vivo, there are two possibilities to explain the distinct cell fate commitment between in vitro and in vivo. One possible hypothesis is the different conditions in vitro and in vivo. Kumamaru et al. revealed that the in vivo environment can dramatically modify the RNA expression pattern of grafted neural stem/progenitor cells13. Another possible explanation is the characteristics of the cell line. In the present study, we employed one iNSC line without inducing differentiation before cell transplantation. Thus, in order to clarify this issue, another experimental setting using more iNSC lines with multiple modes of differentiation needs to be established upon transplantation into stroke models.
We previously reported that a pathologic in vivo environment may raise tumorigenicity or increase the tumor size of transplanted iPSCs14. In the present study, we attempted to check the tumorigenicity of transplanted iNSCs. However, we were unable to observe any tumor formation even after 8 months of cell transplantation. These data suggest that iNSCs may be safer than iPSCs and can thus be regarded as a candidate of promising and safe cell resource for cell replacement therapy for stroke patients.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research (B) 25293202, (C) 15K09316, and Challenging Research 15K15527 and Young Research 15K21181, and by Grants-in-Aid from the Research Committees (H. Mizusawa, K. Nakashima, M. Nishizawa, H. Sasaki, and M. Aoki) from the Ministry of Health, Labour and Welfare of Japan. The authors declare no conflicts of interest.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–76. [DOI] [PubMed] [Google Scholar]
- 2.Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010; 30(8): 1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Scholer HR,. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012; 10(4): 465–72. [DOI] [PubMed] [Google Scholar]
- 4.Kim SM, Flasskamp H, Hermann A, Arauzo-Bravo MJ, Lee SC, Lee SH, Seo EH, Storch A, Lee HT, Scholer HR, Tapia N, Han DW,. Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc. 2014; 9(4): 871–81. [DOI] [PubMed] [Google Scholar]
- 5.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, Brustle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012; 10(4): 473–9. [DOI] [PubMed] [Google Scholar]
- 6.Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, Ko K, Shin CY, Lee HT, Scholer HR, Hyun JK, Han DW. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014; 289(47): 32512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K,. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006; 26(24): 6627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchiteftonic atlas of the C57BL/6 and 129/Sv mouse brains. Amsterdam (The Netherlands): Elsevier; 2000. [Google Scholar]
- 9.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986; 17(3): 472–6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 2002; 117(2): 207–14. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Sun W, Han DW, Lim DJ, Lee J. Induced neural stem cells have protective effects on cortical neuronal cells in vitro. Neurol Sci. 2015; 36(4): 527–34. [DOI] [PubMed] [Google Scholar]
- 12.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM,. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009; 132(Pt 8): 2239–51. [DOI] [PubMed] [Google Scholar]
- 13.Kumamaru H, Ohkawa Y, Saiwai H, Yamada H, Kubota K, Kobayakawa K, Akashi K, Okano H, Iwamoto Y, Okada S. Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat Commun. 2012; 3: 1140. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2010; 20(6): 883–91. [DOI] [PubMed] [Google Scholar]


