Abstract
Articular cartilage has a very limited capacity for self-repair, and mesenchymal stem cells (MSCs) have the potential to treat cartilage defects and osteoarthritis. However, in-depth mechanistic studies regarding their applications are required. Here we demonstrated the use of chitosan film culture for promoting chondrogenic differentiation of MSCs. We found that MSCs formed spheres 2 days after seeding on dishes coated with chitosan. When MSCs were induced in a chondrogenic induction medium on chitosan films, the size of the spheres continuously increased for up to 21 days. Alcian blue staining and immunohistochemistry demonstrated the expression of chondrogenic proteins, including aggrecan, type II collagen, and type X collagen at 14 and 21 days of differentiation. Importantly, chitosan, with a medium molecular weight (size: 190–310 kDa), was more suitable than other sizes for inducing chondrogenic differentiation of MSCs in terms of sphere size and expression of chondrogenic proteins and endochondral markers. We identified that the mechanistic target of rapamycin (mTOR) signaling and its downstream S6 kinase (S6K)/S6 were activated in chitosan film culture compared to that of monolayer culture. The activation of mTOR/S6K was continuously upregulated from days 2 to 7 of differentiation. Furthermore, we found that mTOR/S6K signaling was required for chondrogenic differentiation of MSCs in chitosan film culture through rapamycin treatment and mTOR knockdown. In conclusion, we showed the suitability of chitosan film culture for promoting chondrogenic differentiation of MSCs and its potential in the development of new strategies in cartilage tissue engineering.
Keywords: Mesenchymal stem cells (MSCs), Chondrogenesis, Chitosan film culture, Spheroid, Mechanistic target of rapamycin (mTOR)
Introduction
Since adult articular cartilage damaged by trauma or degeneration has a limited capacity for self-repair, treatment of focal defects or diffused degeneration is still problematic for orthopedic surgeons1. Recently, cell-based therapy, that is, implantation of cells or engineered cartilage in damaged areas, has been developed to aid in the repair of articular cartilage defects and degeneration. Brittberg et al. reported that transplantation of ex vivo-expanded autologous chondrocytes successfully treated cartilage defects in the knee2. An alternative approach to using differentiated chondrocytes for cartilage repair is the use of progenitor or stem cells.
Multipotent stromal or mesenchymal stem cells (MSCs), capable of self-renewal and differentiation into various mesenchymal and nonmesenchymal tissues3,4, have emerged as a promising cell source for bone and cartilage tissue engineering5. MSCs in cell pellet or micromass culture were induced in defined medium containing transforming growth factor-β (TGF-β) to undergo chondrogenesis6. Besides, MSCs have also been induced to differentiate into chondrocytes after delivery in extracellular matrices such as alginate or collagen gel7.
However, MSCs often lose their stem cell properties when expanded in conventional two-dimensional (2D) cultures. Recent efforts have been made to develop 3D chitosan film culture for maintaining MSC pluripotent gene expression and differentiation potential8,9. MSCs, when seeded on chitosan films, form spheres 2 days later and maintain the sphere size for up to 7 days. However, it is unknown whether spheres formed by MSCs on chitosan films are suitable for chondrogenic induction. Moreover, how the underlying signaling pathway of MSCs on chitosan films is mediated to affect chondrogenesis remains elusive.
Emerging evidence has shown that mechanistic target of rapamycin (mTOR) modifies cell proliferation and differentiation of many cell types including MSCs10. mTOR signaling specifically regulates peroxisome proliferator-activated receptor γ (PPAR-γ) activity11. Insulin activates the PI3K/Akt pathway that, in turn, activates the mTOR pathway by inactivating the tuberous sclerosis complex 2 (TSC2) and thereby enhances adipogenesis12. Moreover, mTOR inhibition with rapamycin has negative effects on adipogenesis13. However, rapamycin exerts various effects on the osteogenic differentiation of different progenitor or stem cells. It inhibited osteogenic differentiation of primary mouse MSCs or MC3T3-E1 cell lines14, but promoted the process in human embryonic stem cells15 or rat osteoblast-like ROS17/2.8 cells16. However, there have been limited investigations on the effects of mTOR and its downstream signaling on chondrogenic differentiation of MSCs17.
In this study, we explored whether chitosan film culture is suitable for chondrogenesis. We found MSCs, when induced for chondrogenesis, expressed chondrogenic proteins and increased the activation of mTOR and its downstream signal, S6K, in chitosan film culture compared to that of monolayer culture. We further elucidated the effects of mTOR inhibition and knockdown (KD) on chondrogenic differentiation of MSCs in chitosan film culture. Our data will be helpful to develop new strategies in cartilage tissue engineering.
Materials and Methods
Cell Culture
The study was approved by the institutional review board (IRB) of Taipei Veterans General Hospital (#2012-05-007A) and followed the guidelines and regulations. The human MSCs were isolated from bone marrow aspirates of three individuals who signed informed consents by a protocol described previously18. The cells were seeded at 100 cells/cm2 and grown in complete culture medium [CCM; α-minimal essential medium (a-MEM; Gibco-BRL, Gaithersburg, MD, USA) supplemented with 16.6% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mm L-glutamine (Gibco-BRL)] with medium change twice per week, and subculture was performed every 10 days. Most of the studies were performed with the use of MSCs derived from three individuals at passage 3. For mTOR inhibition, 0.3% rapamycin (Abcam, Cambridge, MA, USA) was added into the medium.
Chitosan Film Preparation and Culture
Chitosan solution [2.5 ml, 1% (w/v); low molecular weight chitosan (448869; 50–190 kDa), medium molecular weight chitosan (448877; 190–310 kDa), and high molecular weight chitosan (419419; 310–375 kDa); Sigma-Aldrich, St. Louis, MO, USA] dissolved in 0.67% (w/v) acetic acid was added into each well of six-well tissue culture plates (BD Falcon; BD Biosciences, San Jose, CA, USA), and the plates were air dried in a hood for 24 h to form a thin film, after which it was neutralized by 0.5 N NaOH aqueous solution (Sigma-Aldrich) for 2 h. Next, the plates were washed thoroughly with distilled water before being exposed to ultraviolet light overnight. The MSCs were seeded at a density of 2 × 105/well of a six-well plate. The cells were cultured at 37°C in 20% oxygen, and the medium was changed every 2 days. The spheres formed in the culture were photographed, and the long and short diameters of each sphere were measured using ImageJ software [US National Institutes of Health (NIH), Bethesda, MD, USA]. The sphere size was calculated using the formula: size = (long diameter + short diameter)/2.
In Vitro Chondrogenesis
Chondrogenic induction medium (CIM) consisted of α-MEM supplemented with 10 ng/ml recombinant human TGF-β1 protein (R&D Systems, Minneapolis, MN, USA), insulin transferrin selenium solution (ITS-G; Thermo Fisher Scientific, Waltham, MA, USA), 10–7 M dexamethasone (Sigma-Aldrich), 50 μm/ml ascorbic acid-2 phosphate (Sigma-Aldrich), and 40 g/ml L-proline (Sigma-Aldrich). Medium changes were carried out at 2-day intervals, and spheroids were harvested at time points up to 21 days. Chondrogenic spheroids were collected at day 14 or day 21. Samples were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA) for 2 h at room temperature and washed twice with PBS. We used Alcian blue stain [1% Alcian blue (Sigma-Aldrich) in 3% glacial acetic acid solution] and immunohistochemistry to observe chondrogenic differentiation.
Membrane Microarray Assay
Human phospho-MAPK array kit (ARY002B; R&D Systems) consisting of 26 different antibodies spotted in duplicate on a nitrocellulose membrane was used to investigate signaling changes. The detailed protocol and sensitivity thresholds can be found on the manufacturer's website. The intensity of cytokines was presented as relative intensity following the formula: sample - negative control.
Immunohistochemical Staining
Paraffin-embedded sections were deparaffinized in xylene, dehydrated through graded alcohols, and pre-treated with 0.4 mg/ml proteinase K (DAKO, Carpinteria, CA, USA) in Tris-HCl (J.T.Baker, Center Valley, PA, USA) for 15 min at room temperature for optimal antigen retrieval. Residual enzymatic activity was removed by washes in PBS, and nonspecific staining was blocked with PBS containing 10% normal horse serum (Sigma-Aldrich) for 20 min at room temperature. Rabbit polyclonal antibody against human aggrecan (GTX113122; 1:200; GeneTex, Irvine, CA, USA), mouse monoclonal antibody against human type II collagen (CP18; 1:500; Calbiochem, Billerica, MA, USA), and rabbit polyclonal to type X collagen (ab58632; 1:2,000; Abcam) were placed on the sections overnight at 4°C. After extensive washes with PBS, a secondary antibody of biotinylated horse anti-mouse (1:200; Vector Laboratories, Burlingame, CA, USA) was placed on the sections for 30 min at room temperature. Immunostaining was detected by Vectastain ABC reagent (Vector Laboratories), followed by 3,3′-diaminobenzidine (DAB) staining (BioGenex, Fremont, CA, USA). Counterstaining was performed with Mayer's hematoxylin (Sigma-Aldrich).
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA according to the manufacturer's specifications. RNA was reversely transcribed in 20 μl using 0.5 μg of oligo dT (Invitrogen) and 200 U of SuperScript® III RT (Invitrogen) for 30 min at 50°C, followed by 2 min at 94°C to inactivate the reverse transcriptase. The quantitative real-time PCR (qRT-PCR) was performed using cDNA as the template in a 20–μl reaction mixture containing FastStart SYBR Green Master (Roche Applied Science, Penzberg, Germany) and a specific primer pair of each cDNA according to the published sequences. PCRs were prepared in duplicate and heated to 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 1 min, and extension at 72°C for 20 s with a specific primer pair of each cDNA following by sequences. The primer sets were as follows: aggrecan, TCGAGGACAGCGAGGCC (forward) and TCGAGGGTGTAGCGTGTAGAGA (reverse); type II collagen, GGCAATAGCAGGTTCACGTACA (forward) and CGATAACAGTCTTGCCCCACTT (reverse); type X collagen, CAAGGCACCATCTCCAGGAA (forward) and AAAGGGTATTTGTGGCAGCATATT (reverse). The results were analyzed by the software supplied with the ABI Step One Real-Time PCR system machine (Thermo Fisher Scientific), and the gene expressions were calculated relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (DCT) and then relative to controls (DDCT) by the fluorescence threshold of the amplification reaction and the comparative CT method.
Western Blotting
Cells were lysed and protein extracted using M-PER™ (Pierce, Rockford, IL, USA) plus Halt Protease Inhibitor Cocktail (Pierce), and protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce). Aliquots of protein lysates were separated on sodium dodecyl sulfate (SDS; BioShop, Burlington, ON, CA)-10% polyacrylamide gels and transferred onto polyvinylidene difluoride (PVDF; PerkinElmer, Waltham, MA, USA) membrane, which was blocked with 5% blotting grade milk (Bio-Rad, Hercules, CA, USA) in TBST [20 mm Tris-HCl (pH 7.6; J.T.Baker), 137 mm NaCl (Sigma-Aldrich), and 1% Tween 20 (Sigma-Aldrich)]. The membrane was then hybridized with the indicated primary antibodies followed by corresponding secondary antibodies and then detected using a chemiluminescence assay (Millipore, Billerica, MA, USA). Membranes were exposed to X-ray film to visualize the bands (Amersham Pharmacia Biotech, Piscataway, NJ, USA). In this study we used primary antibodies to detect mTOR (2983S; 1:1,000; Cell Signaling, Danvers, MA, USA), phospho-mTOR (S2448; 1:1,000; Bioworld Technology, St. Louis Park, MN, USA), S6K (2708S; 1:1,000; Cell Signaling), phospho-S6K (9234S; 1:1,000; Cell Signaling), S6 (2217S; 1:1,000; Cell Signaling), and phospho-S6 (9323S; 1:1,000; Cell Signaling), and Image result for GAPDH (GTX100118; 1:1,000; GeneTex) was used as a loading control. Membranes were washed three times with TBST for 10 min and then probed with their respective secondary antibodies. The signals were imaged with enhanced chemiluminescence (ECL; Bionovas, Toronto, ON, Canada).
Lentiviral-Mediated RNAi
All the RNAi reagents were obtained from the National RNAi Core Facility supported by the National Science Council in Taiwan. Expression plasmids and the bacteria clone for mTOR shRNA (Accession No. NM 004958; TRCN0000038677 and TRCN0000199323) were used to generate recombinant lentiviral particles. Subconfluent cells (3 × 105 per well of a six-well plate) were infected with lentivirus in the presence of 8 μg/ml polybrene (Sigma-Aldrich) at a multiplicity of infection (MOI) of 3. At 24 h after infection, we removed the medium and replaced it with fresh growth medium containing 3 μg/ml puromycin (Sigma-Aldrich) to select infected cells for 48 h.
Statistical Analysis
All values were expressed as mean ± standard error of the mean (SEM). Comparisons between two groups were analyzed by Student's t-test, while more than three groups were analyzed by one-way analysis of variance (ANOVA) with Bonferroni post hoc test using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). A value of p < 0.05 was considered statistically significant.
Results
MSCs Formed Spheres in Chitosan Film Culture
After 2 days of culturing on chitosan films, MSCs formed spheres. To explore whether chitosan film culture is suitable for chondrogenesis, we employed these spheres for chondrogenesis in CIM for up to 21 days. Moreover, efforts were made to optimize chitosan molecular weight for inducing chondrogenic differentiation of MSCs. Before induction in CIM, the spheres were small in size (Fig. 1A). However, the average size of spheres increased with the increase in differentiation period (Fig. 1A). The size of spheres formed on high or medium molecular weight chitosan films was greater than that on low molecular weight chitosan films (Fig. 1B). These data suggest that MSCs form spheres on chitosan films, and the size of spheres increased with the induction period.
Figure 1.
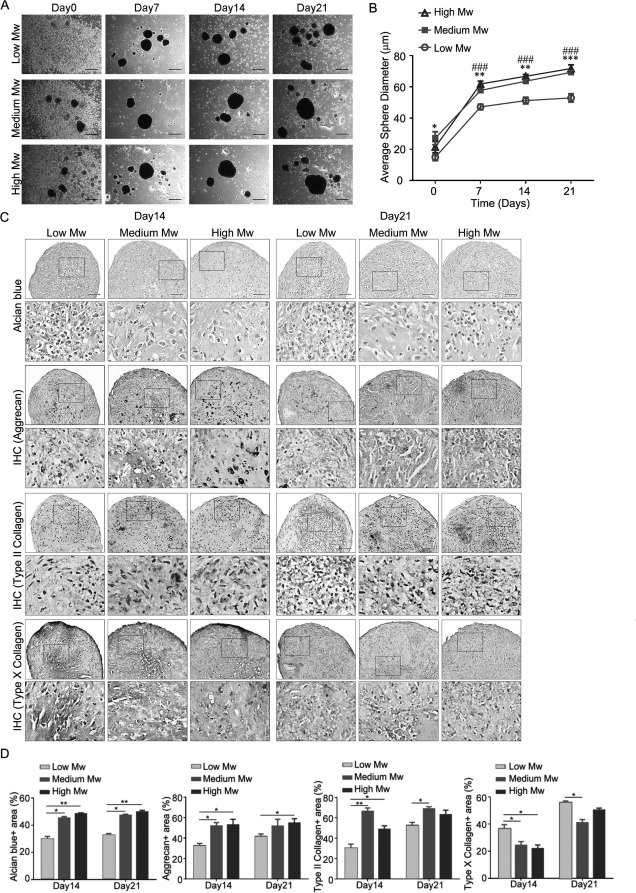
Effects of different molecular weight chitosan films on morphology and chondrogenic protein expression in MSC spheroid. Aliquots of 2× 105 mesenchymal stem cells (MSCs) in complete culture medium were seeded to each well of a six-well plate, coated with different molecular weight (Mw) chitosan. Medium was replaced with chondrogenic induction medium 2 days later (Day0). (A) Morphology, (B) average spheroid diameter, and (C) Alcian blue or immunohistochemistry of indicated proteins in MSC spheroids on different Mw chitosan films for indicated time of differentiation. (D) Quantification of the data in (C). Quantification data are presented as mean+standard error of the mean (SEM) (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ###p < 0.001 (medium Mw vs. low Mw) as determined by one-way analysis of variance (ANOVA). Scale bar: 500 μm.
MSC Spheres Induced for Chondrogenesis on Chitosan Films Expressed Chondrogenic Proteins
We then examined whether spheres were formed by MSCs on chitosan films and induced chondrogenesis for 14 and 21 days, after which the MSCs underwent chondrogenesis and expressed chondrogenic proteins. Alcian blue staining revealed that MSC spheres on medium and high molecular weight chitosan films had greater Alcian blue-stained percentages than on low molecular weight chitosan films both at 14 and 21 days (Fig. 1C and D). Immunohistochemistry demonstrated that MSC spheres on medium and high molecular weight chitosan films expressed more aggrecan and type II collagen, but less type X collagen than on low molecular weight chitosan films at 14 days (Fig. 1C and D). Moreover, MSC spheres on medium molecular weight chitosan films showed higher type II and lower type X collagen expression than that on low molecular weight chitosan films at 21 days. Therefore, we chose MSC spheres on medium molecular weight chitosan films to explore the underlying signaling pathway involved in the chondrogenic differentiation of MSCs on chitosan film culture.
Signaling Pathway Differences Between Monolayer and Chitosan Film Culture
Comparison of transcriptomes between MSC monolayer and spheroid culture revealed considerable differences in genes associated with cell communication and signaling19. We used a human phospho-MAPK array and Western blot analysis to identify and confirm the signaling differences between MSC monolayer and chitosan film culture 2 days after seeding (Fig. 2A). The result showed that in comparison to 26 pathways, mTOR signaling was the most upregulated in spheroid rather than in monolayer culture. Consequently, we examined the role of mTOR signaling in the chondrogenic differentiation of MSCs in chitosan film culture. Western blot analysis revealed that the activation of mTOR and its downstream signaling S6K/S6 increased with an increase in the induction period (Fig. 2B). Inhibition of mTOR with rapamycin blocked S6K/S6 activation (Fig. 3A), reduced sphere size (Fig. 3B), decreased aggrecan and Col2a1 mRNA and protein levels, but increased Col10a1 mRNA and protein levels (Fig. 3C and D), compared to that of vehicle controls.
Figure 2.
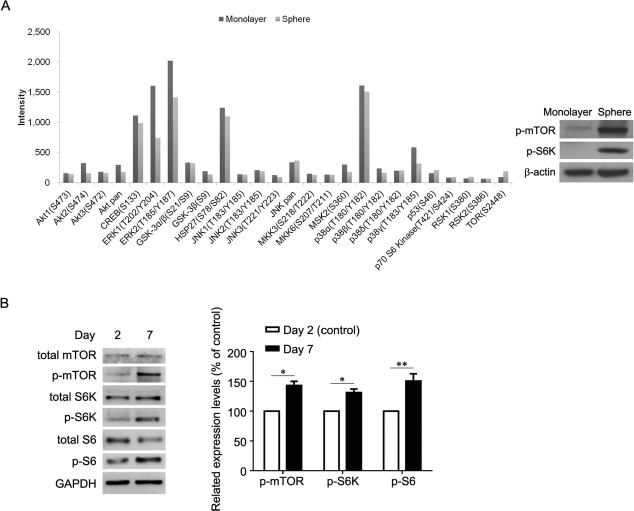
Signaling differences between monolayer and spheroid culture. (A, left) Human phospho-MAPK array and (A, right) Western blot analysis for the signaling differences between monolayer and spheroid culture. (B, left) Western blot analysis and (B, right) its quantification for indicated periods of chondrogenesis. ∗p < 0.05, ∗∗p < 0.01 as determined by paired Student's t-test. Monolayer, monolayer culture; Sphere, spheroid culture; Day 2, chondrogenic induction medium cultured for 2 days; Day 7, chondrogenic induction medium cultured for 7 days.
Figure 3.
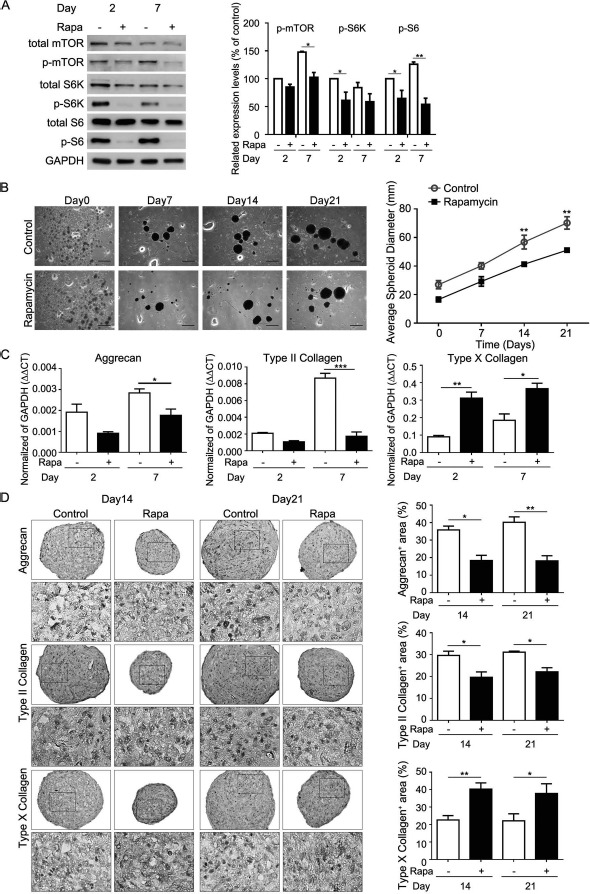
Effects of rapamycin on size and chondrogenic protein expression of MSC spheroid on chitosan films. Aliquots of 2× 105 MSCs in complete culture medium were seeded to each well of a six-well plate, coated with chitosan. Medium was replaced with chondrogenic induction medium in the absence or presence of rapamycin (Rapa) 2 days later (Day0). (A, left) Western blot analysis of mTOR-related signaling proteins and (A, right) its quantification at indicated time periods. ∗p < 0.05, ∗∗p < 0.01 as determined by paired Student's t-test. (B, left) Morphology, (B, right) average spheroid diameter, and (C) quantitative RT-PCR for mRNA levels. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by paired Student's t-test. (D, left) Immunohistochemistry for indicated proteins of MSC spheroids at indicated time periods. (D, right) Quantification data of immunohistochemistry. Data are presented as mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by unpaired Student's t-test. Scale bar: 500 μm. Day2, chondrogenic induction medium cultured for 2 days; Day7, chondrogenic induction medium cultured for 7 days; Day 14, chondrogenic induction medium cultured for 14 days; Day21, chondrogenic induction medium cultured for 21 days; Rapa, chondrogenic induction medium in the presence of rapamycin; Control, chondrogenic induction medium in the absence of rapamycin.
Similarly, mTOR KD suppressed mTOR protein level and blocked S6K/S6 activation (Fig. 4A), reduced sphere size (Fig. 4B), decreased aggrecan and Col2a1 mRNA and protein levels, but increased Col10a1 mRNA and protein levels (Fig. 4C and D), compared to that of control shRNAs. Together, these results suggest that mTOR is upregulated and required during the chondrogenic differentiation of MSCs in chitosan film culture.
Figure 4.
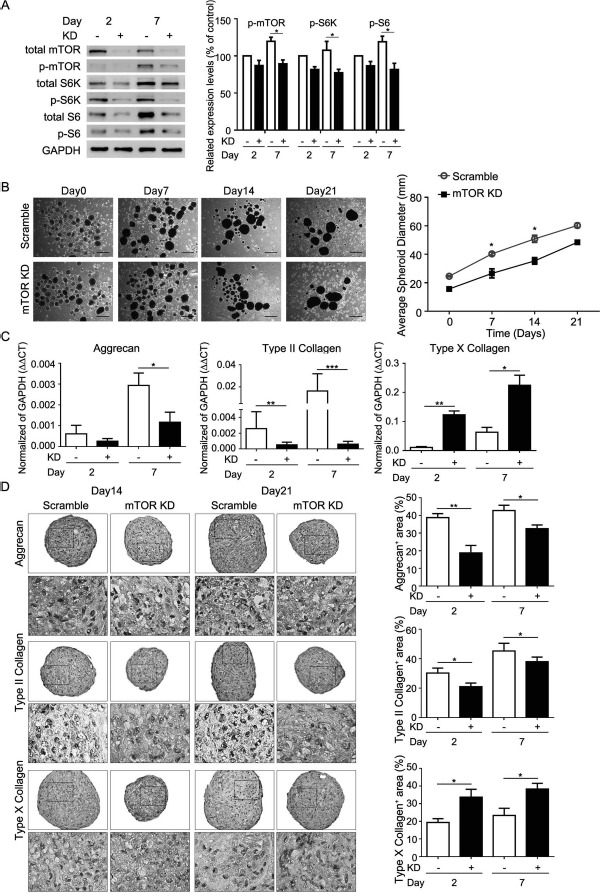
Effects of mTOR knockdown (KD) on size and chondrogenic protein expression of MSC spheroid on chitosan films. Aliquots of 2 × 105 MSCs with or without mTOR KD in complete culture medium were seeded to each well of a six-well plate, coated with chitosan. Medium was replaced with chondrogenic induction medium 2 days later (Day0). (A, left) Western blot analysis of mTOR-related signaling proteins and (A, right) its quantification at indicated time periods. ∗p < 0.05 as determined by paired Student's t-test. (B, left) Morphology, (B, right) average spheroid diameter, (C) quantitative RT-PCR for mRNA levels, and (D, left) immunohistochemistry for indicated proteins of MSC spheroids at indicated time periods. (D, right) Quantification data of immunohistochemistry. Data are presented as mean + SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by unpaired Student's t-test. Scale bar: 500 μm. Day2, chondrogenic induction medium cultured for 2 days; Day7, chondrogenic induction medium cultured for 7 days; Day14, chondrogenic induction medium cultured for 14 days; Day21, chondrogenic induction medium cultured for 21 days; KD, MSCs with mTOR KD; Scramble, MSCs without mTOR KD.
Discussion
Articular cartilage self-repairing capacity is limited due to joint load bearing, synovial inflammation, and the paucity of endogenous stem or progenitor cells for repair20. Although stem cells, such as MSCs, have potential in the treatment of numerous diseases including tissue injury and immune disorders21, in-depth studies regarding their application and mechanisms are required. In the current study, we demonstrated the suitability of chitosan film culture for promoting the chondrogenic differentiation of MSCs. Importantly, chitosan with medium molecular weight has been found to be more effective than others in promoting the chondrogenic differentiation of MSC in terms of sphere size, expression of chondrogenic proteins, and endochondral markers. Moreover, we identified that the mTOR signaling and its downstream S6K/S6 is required for successful chondrogenic differentiation of MSCs in chitosan film culture.
Spheroid culture, which mimics in vivo 3D microenvironments, offers many advantages over monolayer culture22. Hanging drop protocol has been applied for MSC spheroid culture and shows better capacity for enhancing their anti-inflammatory properties19 and promoting proliferation, migration, and extracellular matrix invasion of endothelial cells23. Besides hanging drop protocol, spheroids or aggregates formed by centrifugation or a conical tube-based format have been applied in vitro to demonstrate the chondrogenic potential of MSCs24. However, these protocols are not suitable for MSC-based cartilage tissue engineering, since MSCs in these protocols are forced to form spheroids, and the densities are dependent on the initial cell number used to form spheroids. In the current study, MSCs on chitosan films automatically formed spheroids with appropriate cell densities. Moreover, MSCs in chitosan film culture express pluripotent genes and maintain stem cell properties8,9. More importantly, these MSCs differentiate into chondrocytes when induced for chondrogenesis as shown in the current study.
The effects of chitosan molecular weight on physical and biological properties of collagen/chitosan scaffolds have been studied1. High molecular weight chitosan significantly prolonged the biodegradation of collagen/chitosan scaffolds, while low molecular weight chitosan was more effective in promoting mouse fibroblast proliferation. In contrast, cell growth of the Saos-2 osteoblast cell line over 7 days on chitosan scaffolds was proportional to chitosan molecular weight3. The current study showed that the spheroid size of MSCs cultured on medium and high molecular weight chitosan plates was greater than that on low molecular weight chitosan plates. Moreover, the expression of aggrecan and type II collagen was increased, while type X collagen was decreased in MSC spheres on medium and high molecular weight chitosan plates in comparison with low molecular weight chitosan plates. These data together suggest that the effects of chitosan molecular weight on its physicochemical characteristics and biological properties should be well studied to optimize its application on tissue engineering.
mTOR is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription25. The mTOR/S6K1 signaling pathway is required for adipogenesis13 and osteoblast proliferation and differentiation14,26. Although mTOR modulation contributes to chondrogenesis, possibly through its ability to regulate Indian hedgehog27, its activation during the chondrogenic differentiation of MSCs has not been thoroughly investigated. Recently, the effects of the mTOR/S6K pathway on chondrocyte proliferation and differentiation have been studied via the TSCI gene deletion in the growth plate of a developing long bone, which induces mTORC1 hyperactivation and causes uncoupling of the normal proliferation and differentiation program, resulting in chondrodysplasia in mice28. The mechanistic approaches show that mTORC1 downstream kinase S6K1 is crucial to regulate Gli2/parathyroid hormone-related peptide (PTHrP) during endochondral ossification. In the current study, we induced the chondrogenic differentiation of MSCs cultured on chitosan films to explore the roles of mTOR and its downstream signals in chondrogenesis. Our results show that mTOR/S6K is activated upon seeding on chitosan films compared to that in monolayer culture. Moreover, we showed that the activation was continuously upregulated from days 2 to 7. More interestingly, we found that mTOR/S6K signaling was required for the expression of chondrogenic proteins, such as aggrecan and type II collagen in MSCs through rapamycin treatment and mTOR KD. Similarly, we also found that rapamycin treatment and mTOR KD also enhanced the expression of type X collagen, a marker of endochondral ossification. Our data also suggest that MSCs in chitosan film culture may serve as an in vitro model to study endochondral ossification.
The effects of rapamycin and mTOR on inhibiting MSC chondrogenesis in the current study seem similar, which needs further discussion. The mTOR kinase is present in two functionally and structurally distinct complexes termed TORC1 and TORC2, the former is rapamycin sensitive, while the latter is not directly inhibited by rapamycin29. Thus, rapamycin mainly inhibited mTORC1 in the current study, while mTOR KD equally suppressed both mTORC1 and mTORC2. Because mTORC2 phosphorylates Akt to activate mTORC1 indirectly, the end effect of mTOR KD is also mTROC1 activation. Thus, it would be expected to observe that rapamycin and mTOR KD affected chondrogenesis of MSCs in chitosan culture similarly.
Besides S6K, 4EBP1 is also a downstream signal of mTOR30; therefore, we considered whether 4EBP1 is involved in the chondrogenic differentiation of MSCs. In addition, mTOR regulates many cellular mechanical processes, including senescence and autophagy, which are involved in reprogramming somatic cells to pluripotency, implicating its role in energy metabolism, stem cell renewal, and aging31. We also considered whether mTOR-regulated senescence and autophagy were involved in the chondrogenic differentiation of MSCs cultured on chitosan films. Moreover, it is necessary to demonstrate whether the positive effects of chitosan film culture on the chondrogenic differentiation of MSCs observed in this study could be replicated in in vivo MSC cartilage tissue engineering in future research. In conclusion, we showed the suit ability of chitosan film culture for inducing the chondrogenic differentiation of MSCs and its potential use in the development of new strategies for cartilage tissue engineering.
Acknowledgments
The study was supported by grants from the Ministry of Science and Technology (MOST 103-2321-B-010-024 and MOST 103-2321-B-010-018) and the Academia Sinica Translational Medicine Program (5202401023–2325). Space and administrative supports were received from the Institute of Biomedical Sciences, Academia Sinica. The authors declare no conflicts of interest.
References
- 1.Wakitani S, Goto T, Young RG, Mansour JM, Goldberg VM, Caplan AI. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 1998; 4: 429–44. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994; 331: 889–95. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science 1997; 276: 71–4. [DOI] [PubMed] [Google Scholar]
- 4.Hung SC, Cheng H, Pan CY, Tsai MJ, Kao LS, Ma HL. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells 2002; 20: 522–9. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005; 11: 1198–211. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7. [DOI] [PubMed] [Google Scholar]
- 7.Ma HL, Hung SC, Lin SY, Chen YL, Lo WH. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res A 2003; 64: 273–81. [DOI] [PubMed] [Google Scholar]
- 8.Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 2012; 33: 1748–58. [DOI] [PubMed] [Google Scholar]
- 9.Huang GS, Dai LG, Yen BL, Hsu SH. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011; 32: 6929–45. [DOI] [PubMed] [Google Scholar]
- 10.Xiang X, Zhao J, Xu G, Li Y, Zhang W. mTOR and the differentiation of mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 2011; 43: 501–10. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004; 53: 2748–56. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 2009; 4: e6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HJ, Park J, Lee HW, Lee YS, Kim JB. Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem Biophys Res Commun. 2004; 321: 942–8. [DOI] [PubMed] [Google Scholar]
- 14.Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008; 103: 434–46. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 2010; 31: 7012–20. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T, Tokuda M, Tomizawa K, Matsui H, Itano T, Konishi R, Nagahata S, Hatase O. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun. 1998; 249: 226–30. [DOI] [PubMed] [Google Scholar]
- 17.Phornphutkul C, Wu KY, Auyeung V, Chen Q, Gruppuso PA. mTOR signaling contributes to chondrocyte differentiation. Dev Dyn. 2008; 237: 702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SP, Chiu FY, Wang Y, Yen ML, Kao SY, Hung SC. RB maintains quiescence and prevents premature senescence through upregulation of DNMT1 in mesenchymal stromal cells. Stem Cell Reports 2014; 3: 975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 2010; 107: 13724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science 2012; 338: 917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol. 2009; 217: 318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidrol X, Fouque B, Ghenim L, Haguet V, Picollet-D'hahan N, Schaack B,. 2D and 3D cell microarrays in pharmacology. Curr Opin Pharmacol. 2009; 9: 664–8. [DOI] [PubMed] [Google Scholar]
- 23.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007; 25: 1761–8. [DOI] [PubMed] [Google Scholar]
- 24.Welter JF, Solchaga LA, Penick KJ. Simplification of aggregate culture of human mesenchymal stem cells as a chondrogenic screening assay. Biotechniques 2007; 42: 732, 734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung C-M, Garcia-Haro L, Sparks CA, Guertin DA,. mTOR-dependent cell survival mechanisms. Cold Spring Harb Perspect Biol. 2012; 4: a008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, Cho YS. Rapamycin promotes the osteoblastic differentiation of humonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010; 19: 557–68. [DOI] [PubMed] [Google Scholar]
- 27.Phornphutkul C, Wu KY, Auyeung V, Chen Q, Gruppuso PA. mTOR signaling contributes to chondrocyte differentiation. Dev Dyn. 2008; 237: 702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan B, Zhang Z, Jin D, Cai C, Jia C, Liu W, Wang T, Li S, Zhang H, Huang B, Lai P, Wang H, Liu A, Cai D, Juang Y, Bai X. mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation. Nat Commun. 2016; 7: 11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM,. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002; 110: 163–75. [DOI] [PubMed] [Google Scholar]
- 30.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002; 16: 1472–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez JA, Vellon L, Oliveras-Ferraros C, Cufi S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: A roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle 2011; 10: 3658–77. [DOI] [PubMed] [Google Scholar]


