Abstract
Safe and stable cryopreservation is critical for research involving human embryonic stem cells (hESCs). Dimethyl sulfoxide (DMSO) is a popular cryoprotective agent; however, its cytotoxicity cannot be ignored. Thus, there is a need for an alternate cryoprotectant. We reported previously that a novel cryopreservation reagent, StemCell Keep™ (SCK), was effective for cryopreserving human induced pluripotent stem cells (hiPSCs) by vitrification. Because hESCs and hiPSCs are not identical, the current study examined the use of SCK on hESCs. hESCs cryopreserved with SCK were thawed and cultured on SNL 76/7 cells, which were derived from a mouse fibroblast STO cell line transformed with neomycin resistance and murine LIF genes. After cryopreservation, cultured hESCs were assessed for their attachment ability and characterized by alkaline phosphatase (AP) and immunocytochemical (ICC) staining, fluorescence-activated cell sorting (FACS), reverse transcription polymerase chain reaction (RT-PCR), and karyotyping. The proliferation of SCK-cryopreserved hESCs cultured on SNL cells, or in feeder-free conditions, was higher than that of cells preserved in a solution of 2 M DMSO, 1 M acetamide, and 3 M propylene glycol (DAP). The cell number with SCK-cryopreserved hESCs was about twice that of hESCs cryopreserved in DAP. The pluripotency of SCK-cryopreserved hESCs was similar to that of DAP-cryopreserved hESCs based on AP staining. Data from ICC, FACS, and RT-PCR analyses showed that stem cell markers were continually expressed on SCK-cryopreserved hESCs. The teratoma assay showed that SCK-cryopreserved hESCs differentiated into three germ layers. Furthermore, SCK-cryopreserved hESCs had normal karyotypes. These data indicate that SCK was effective for cryopreservation of hESCs by vitrification.
Keywords: Cryopreservation, Human embryonic stem cells (hESCs), Vitrification, Dimethyl sulfoxide free, Polyampholytes
Introduction
Cryopreservation is used for the long-term storage of biological materials containing cells. The two primary cryopreservation techniques are rapid and slow freezing. Rapid cooling leads to the formation of amor-phous ice; the transition of water to the glassy state can be done directly without crystallization, which is called vitrification (39). Human embryonic stem cells (hESCs) can be cryopreserved successfully by vitrification (26). Researchers have focused on developing containers with a reduced amount of solution to improve vitrification (27,34). However, the protocol is impractical for large-scale use because it requires open pulled straws and manual selection of stem cell colonies.
Fujioka et al. developed a simple, large-scale vitrification method for primate ESCs in a solution of 2 M dimethyl sulfoxide (DMSO), 1 M acetamide, and 3 M propylene glycol (DAP) (5). However, hESCs are not maintained effectively in an undifferentiated state when using DMSO (11). There are numerous studies examining vitrification without DMSO, including one by Kuleshova et al., who reported the vitrification of human oocytes without DMSO (12). A chemically defined solution without DMSO has been developed to vitrify hESCs in a cryotube with greater efficiency (23). However, this solution is insufficiently effective, indicating that further work is needed to improve the composition of vitrification solutions for use with hESCs. Some polymeric additives have been reported to stabilize the glassy state including our previous study (22).
We previously investigated the effects of adding a devitrification inhibitor compound, carboxylated e-poly-L-lysine (CPLL), to ethylene glycol-based vitrification solutions (17). CPLL was developed as a low-toxicity, highly efficient, novel cryoprotective alternative to DMSO (16,18). Our proprietary chemically defined cryopreservative agent, StemCell Keep™ (SCK), contains 10% CPLL, 6.5 M ethylene glycol, and 0.7 M sucrose without serum, and is effective for the cryopreservation of human induced pluripotent stem cells (hiPSCs) (17). However, hiPSCs and hESCs are not identical. Thus, the current study examined the efficacy of SCK in cryopreserving hESCs by assessing the growth, morphology, and some molecular characteristics of vitrified hESCs. Recently, DMSO was found to alter the epigenetic DNA methylation profile of mouse embryoid bodies and to be a strong inducer of DNA hydroxymethylation in preosteoblastic MC3T3-E1 cells (1,4,9,35). Thus, we also evaluated the expression of relevant mRNAs in hESCs vitrified with SCK.
Materials and Methods
hESC Culture
The hESC lines, KhES1 and KhES3, established by Suemori et al. (32), were purchased from the RIKEN BioResource Center (Tsukuba, Japan). These cell lines were used in conformance with The Guidelines for Derivation and Utilization of Human Embryonic Stem Cells (2009) of the Ministry of Education, Culture, Sports, Science, and Technology, Japan, after approval from the institutional review board (IRB) of the Institute of Frontier Medical Sciences, Kyoto University. hESCs were maintained on a feeder layer of SNL 76/7 cells (DS Pharma Biomedical, Osaka, Japan). SNL 76/7 cells, established by McMahon and Bradley (20), are clonally derived from a mouse fibroblast STO cell line transformed with neomycin resistance and murine leukemia inhibitory factor (LIF) genes. SNL cells serve as feeder cells for ESC growth and have also been used in mouse or hiPSC cultures (24,33). Furthermore, SNL cells maintain ESCs or iPSCs in the undifferentiated state.
SNL cells were mitotically inactivated by treatment with mitomycin C (10 μg/ml; 2-4 h; Kyowa Hakko Kirin Co. Ltd., Tokyo, Japan) prior to the addition of hESCs. SNL cells were maintained on a 0.1% gelatin (Nacalai Tesque, Kyoto, Japan)-coated dish in high-glucose Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque) containing 7% fetal bovine serum (FBS; MP Biomedicals, LLC Solon, OH, USA) and 1% penicillin-streptomycin (Nacalai Tesque). hESCs were cultured in human pluripotent stem cell (hPSC) medium (20% knockout serum replacement; Life Technologies, Carlsbad, CA, USA) containing 2 mM L-glutamine (Nacalai Tesque), 0.1 mM minimum essential medium (MEM) with nonessential amino acids (Nacalai Tesque), and 0.1 mM 2-mercaptoethanol (Life Technologies) with 5 ng/ml basic fibroblast growth factor (bFGF; Wako Pure Chemical, Osaka, Japan). hESCs in feeder cell-free cultures were maintained on Matrigel-coated plates (BD Biosciences, Franklin Lakes, NJ, USA) in SNL-conditioned medium (the supernatant of SNL cells cultured in hPSC medium) containing 5 ng/ ml bFGF. hESC cultures were maintained at 37°C in a 5% CO2 incubator. The cells were subcultured every 3 to 5 days using 0.25% trypsin and 1 mg/ml collagenase (Life Technologies) with 20% knockout serum replacement and 1 mM CaCl2 (Nacalai Tesque) in phosphate-buffered saline (PBS; CTK buffer; Nacalai Tesque). When hESCs were cultured in feeder cell-free conditions, aggregated cells were further treated with TrypLE Select (Life Technologies) to produce a single-cell suspension, scattered on Matrigel-coated plates (BD Biosciences), and maintained in SNL-conditioned medium with 5 ng/ml bFGF.
Differential Scanning Calorimetry (DSC)
To compare the stabilities of the glassy states between DAP and SCK, thermal analysis was performed using a differential scanning calorimeter (DSC8500; Perkin Elmer, Waltham, MA, USA). Ten microliters of each solution was placed on the aluminum pan. The pan was set on the calorimeter sample chamber and cooled to −150°at 10°C/min and 30°C/min.
Vitrification and Warming of hESCs
The SCK and DAP vitrification solutions were purchased from Bioverde (Kyoto, Japan) and Wako Pure Chemicals, respectively. hESCs were maintained on 6-cm dishes until 80% confluent (when the cell number was approximately 1-2 × 10° cells/dish). hESCs were then rinsed with PBS and treated with CTK buffer. Detached hESC colonies were dissociated into clumps by pipetting. The hESC clumps were collected, centrifuged at 190 × g, suspended in 200 μl of vitrification solution in a cryotube, and immersed in liquid nitrogen. Vitrified hESCs were thawed by adding 1 ml of hPSC medium to the cryotube. hESCs were then transferred into a conical tube with 10 ml of hPSC medium for centrifugation. hESCs were cultured on SNL cells in hPSC medium with 5 ng/ ml bFGF and 10 μM Y-27632 (Wako Pure Chemicals) for 24 h. The medium was then changed to hPSC medium with bFGF but without Y-27632.
Attachment Assay of hESCs
One tube of cryopreserved hESCs was warmed and cultured in a 6-cm dish on the SNL feeder cell layer. Their attachment ability on the bottom of the dish after 1, 2, and 5 days of culturing was observed. Photos were taken under an inverted microscope (Nikon, Tokyo, Japan) at 40x magnification. The number of hESC colonies in four fields was counted visually after marking them with dots. For feeder cell-free culture conditions, cryopreserved hESCs were thawed and cultured initially on SNL cells until 80% confluent. The hESCs were then disaggregated and cultured on Matrigel-coated plates for 1 day. The colonies formed were observed under the inverted microscope, and photos were taken to count the number of hESC colonies.
Alkaline Phosphatase (AP) Staining
The Alkaline Phosphatase Leukocyte kit (Sigma-Aldrich, St. Louis, MO, USA) was used to assess AP activity. Cultured hESCs were washed with PBS, fixed with 4% paraformaldehyde (PFA; Nacalai Tesque) for 10 min, and washed with distilled water. A mixture of sodium nitrite (1 ml) and FRV-alkaline solution (1 ml) in the Alkaline Phosphatase Leukocyte kit (Sigma-Aldrich) was incubated for 2 min. Distilled water (45 ml) and naphthol AS-BI alkaline solution (1 ml) from the kit were then added to the mixture and reacted with hESCs as per the manufacturer's instructions. After staining, samples were washedter and air dried. Images of AP+ colonies were obtained with Pick-in Master PM2 colony counter (Microtec Co. Ltd., Chiba, Japan) and counted.
Immunocytochemical Analysis
hESCs were rinsed with PBS, fixed with 4% PFA, and washed. For the intranuclear antigens octamer-binding transcription factor 3/4 (Oct3/4; Santa Cruz Biotechnology, Dallas, TX, USA) and Nanog (ReproCELL, Yokohama, Japan), the cells were permeabilized with Perm/Wash buffer I (BD Phosflow; BD Biosciences) for 15 min. After three washes with 2% FBS in PBS, the cells were incubated with diluted primary antibodies at 4°C overnight. The primary antibodies used were Oct3/4 (Santa Cruz Biotechnology), SSEA4 (Millipore, Billerica, MA, USA), Nanog (ReproCELL), and Tra-1-60 (Millipore). Primary antibodies were diluted to 1:200. Secondary antibodies (diluted to 1:300-1:500) were added to the hESCs for 1 h in the dark after removing the primary antibodies and washing the cells. The samples were then washed three times with 2% FBS in PBS, and one drop of mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) was added. Cells were observed with a fluorescence microscope (Keyence, Osaka, Japan). Negative controls not treated with primary antibodies were prepared using the same procedure.
Fluorescence-Activated Cell Sorting (FACS) Analysis
hESCs were trypsinized into a single-cell suspension, fixed with 4% PFA, and washed. hESCs were then permeabilized and incubated with diluted primary anti-bodies for Tra-1-60 and Nanog for 1 h on ice. The primary antibodies were diluted to 1:100. After washing, the diluted secondary antibodies (1:100) were added at 4°C for 30 min in the dark. hESCs were then washed and suspended in cold 2% FBS in PBS for analyses using FACS Calibur (BD Biosciences).
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analyses
Total RNA from hESCs was purified using RNeasy (Qiagen, Venlo, The Netherlands) according to the manufacturer's instructions. After measuring the concentration of total RNA, cDNA was synthesized using the Transcriptor Universal cDNA Master mix (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions. The expression of each mRNA was determined with their primers and template cDNAs using StepOne Plus (Life Technologies). Eight Homo sapiens genes were assessed: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), SRY (sex-determining region Y)-box 2 (SOX2), POU domain class 5F1 (OCT3/4), Kruppel-like factor 4 (KLF4), v-myc myelocytomatosis viral oncogene homolog (c-MYC), RNA exonuclease 2 homolog (REX2), epithelial cadherin 1 (ECAD), and epithelial cell adhesion molecule (EPCAM).
The primer sequences were the following: GAPDH, 5′-TGTTGCCATCAATGACCCCTT and 3′-CTCCACG ACGTACTCAGCG; SOX2, 5′-CACCATGTACAACAT GATGGAGACGGA and 3′-CGGTATTTATAATCCGG GTGC; OCT3/4, 5′-CACCATGGCGGGACACCTGGC TTCAG and 3′-CCTCTTCTGCTTCAGGAGCTT; KLF4, 5′-CACCATGGCTGTCAGTGACGCGCTGCT and 3′-G GCGCGCTGGCAGGGCCGCTG; c-MYC, 5′-CACCAT GCCCCTCAACGTTAGCTTCAC and 3′-GCTCCACA TACAGTCCTGGAT; REX2, 5′-CAGATCCTAAACAG CTCGCAGAAT and 3′-GCGTACGCAAATTAAAGTC CAGA; ECAD, 5′-TCATCGATGGAGATGGAACA and 3′-GGAGGCTCATCAGCATCTTC; EPCAM, 5′-GCTG GTGTGTGAACACTGCT and 3′-ACGCGTTGTGATCT CCTTCT.
Data were analyzed by the comparative Ct method (15,28). The equation of this method is as follows: fold change (relative expression) = 2-AACt
= [(Ct gene of interest - Ct internal control) sample A - (Ct gene of interest - Ct internal control) sample B]
Sample A: mRNA from SCK-cryopreserved hESCs Sample B: mRNA from DAP-cryopreserved hESCs Internal control gene: SOX2
Genes of interest: GAPDH, OCT3/4, KLF4, c-MYC, REX2, ECAD, EPCAM
Teratoma Formation in Severe Combined Immunodeficiency (SCID) Mice
hESCs cultured on SNL feeder cells were enzymatically dissociated, and 2 × 106 cells were injected subcutaneously into 5-week-old male SCID mice (CB17/Icr-Prkdc scid/CrlCrlj; CLEA, Osaka, Japan). After 8 weeks, the teratomas were removed, fixed in 4% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). All procedures received prior approval from the animal research committee of the Institute for Frontier Medical Sciences, Kyoto University.
Karyotype Analysis
Karyotype analysis was performed by SRL (Kyoto, Japan). In brief, hESCs were incubated at 37°C for 30 min in hPSC medium containing 0.1 mg/ml colcemid. The cells were then trypsinized, suspended, and incubated in 0.075 M KCl at 37°C for 25 min prior to fixation. Metaphase spreads were prepared on glass slides, G-banded by brief exposure to trypsin, and stained with Giemsa and Leishman solutions.
Statistical Analysis
All results are expressed as means ± standard deviation (SD). To compare data among more than three groups, we used one-way analysis of variance (ANOVA) followed by the Tukey-Kramer post hoc test. To compare data between two groups, Student's t-tests were used. Statistical analyses were performed by commercialized software (Excel Statistics; SSRI Co. Ltd., Tokyo, Japan). Values of p < 0.05 were considered statistically significant.
Results
Confirmation of Vitrification by DSC
DSC measurements were done to confirm vitrification. When the cryotube containing 200 μl of vitrification solution was immersed directly into liquid nitrogen, the cooling rate was about −270°C/min when measured with a thermocouple. The warming rate when prewarmed medium was added into the cryotube was approximately 2,500°C/min. These cooling and warming rates were calculated by the slope between 0 and −150°C/min in the temperature-time plots. Both vitrification solutions showed clear glass transition around −120°C when the cooling rate was about −30°C/min (Fig. 1). This was much slower than the actual cooling rate. In contrast, crystallization was observed in only DAP when the cooling rate decreased to −10°C/min, suggesting better inhibition of crystallization in SCK.
Figure 1.
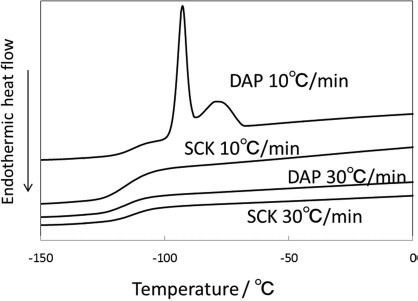
Differential scanning calorimetry (DSC) thermograms of propylene glycol (DAP) and StemCell Keep™ (SCK) for cooling at 10°C/min and 30°C/min.
The Attachment Ability of hESCs Cryopreserved with SCK
In contrast to mouse stem cells or other common human cells, hPSCs are seriously damaged by cryopreservation. Thus, it is critical to develop methods to achieve good cell growth and viability after thawing. The numbers of colonies after 1 and 2 days of culture when using hESCs cryopreserved with SCK were 4.8 ± 1.3 and 15.0 ± 2.9, respectively. hESCs that were cryopreserved with DAP developed 0.5 ± 0.6 and 7.3 ± 4.6 colonies after 1 and 2 days, respectively. This was significantly less than the SCK-cryopreserved hESCs (p < 0.05 for day 2) (Fig. 2a-q).
Figure 2.
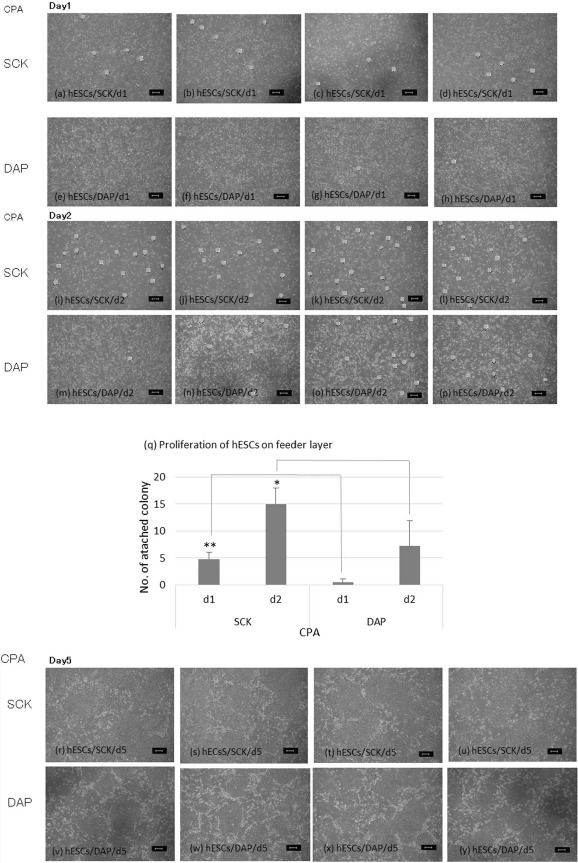
The attachment ability of human embryonic stem cells (hESCs) cryopreserved with SCK or DAP and cultured on a feeder cell layer. Freeze-thawed hESCs were cultured on a layer of SNL feeder cells, and their attachment to the bottom of a culture dish was observed under an inverted microscope. hESC colonies were photographed at 40x magnification. The number of colonies was counted by marking them with dots. hESC colonies after cryopreservation with SCK (a-d) or DAP (e-h) and culturing for 1 day (Day1). hESC colonies after cryopreservation with SCK (i-l) or DAP (m-p) and culturing for 2 days (Day2). (q) Comparison of the number of hESC colonies after cryopreservation with SCK or DAP and culturing for 1 or 2 days. Data are presented as means ± SD. Statistical evaluations were done by the Tukey-Kramer test. *p < 0.05, statistically significant difference. hESC colonies after cryopreservation with SCK (r-u) or DAP (v-y) and culturing for 5 days (Day5) (r-y). Both 5-day cultures had many colonies, and the numbers were not determined. Scale bars: 100 um. CPA, cryoprotective agent.
When culturing hESCs for 5 days, the colonies became large and overlaid and could not be counted (Fig. 2r-y). It was difficult to make single-cell suspensions from feeder cell-free cultures because the hESCs were very weak immediately after thawing. Thus, cryopreserved hESCs were cultured at a single time on an SNL feeder cell layer and then disaggregated into the feeder cell-free condition. The number of colonies from hESCs cryopreserved with SCK and treated in this way was 24 ± 5.3, which was significantly higher than that of hESCs cryopreserved with DAP (9.0 ± 2.6, p < 0.05) (Fig. 3). These data indicate that hESCs cryopreserved with SCK had a better attachment ability than hESCs cryopreserved with DAP.
Figure 3.
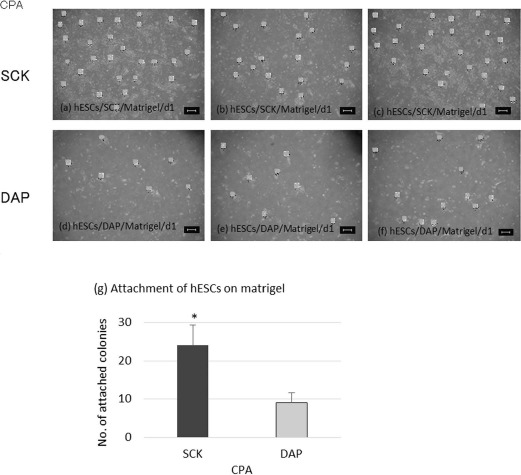
The ability of hESCs cryopreserved with SCK or a solution of 2 M dimethyl sulfoxide (DMSO), 1 M acetamide, and 3 M DAP to attach to the bottom of Matrigel-coated culture dishes. Freeze-thawed hESCs were cultured on a layer of feeder cells for several days, and then disaggregated and cultured on Matrigel-coated dishes for 1 day. Cells were then observed for their adherence under an inverted microscope. hESC colonies were photographed at 40x magnification. The number of colonies was counted by marking them with dots. hESC colonies had a star-shaped morphology. hESCs after 1 day (d1) of cryopreservation with (SCK) (a-c) or DAP (d-f). (g) Comparison of the number of hESC colonies following cryopreservation with SCK or DAP. Data are presented as means ± SD. Statistical evaluations were done by Student's t-test. *p < 0.05, statistically significant difference. Scale bars: 100 um. CPA, cryoprotective agent.
Characterization of hESCs with AP Staining
AP staining is a simple assay to evaluate pluripotency and was used to identify the formation of undifferentiated colonies. The AP+ colony counts of hESCs cryopreserved with SCK and cultured for 3, 4, 5, and 6 days were 161 ± 93, 209 ± 54, 203 ± 80, and 245 ± 28, respectively. Colony numbers for hESCs cryopreserved with DAP were 135 ± 53, 126 ± 54, 219 ± 22, and 257 ± 45, for 3, 4, 5, and 6 days, respectively (Fig. 4a-d and e-h). Notably, when we seeded hESCs at the same numbers without vitrification, colony numbers increased to more than 2,000 after 6 days. As shown in Figure 4i, the proliferation profiles of the AP+ colonies in both groups were similar, although the number of colonies from cells cultured after SCK cryo-preservation for 4 days was significantly higher (p < 0.05). Magnification (50x) of hESCs stained for AP showed that the cells cryopreserved with SCK had larger AP+ colonies than hESCs cryopreserved with DAP (Fig. 4j-k). The number and viability of hESCs cryopreserved with SCK and cultured for 4 days were 6.3 × 105 ± 8.5 × 104 and 96 ± 1%, respectively. The number and viability of hESCs cryopreserved with DAP were 2.9 × 105 ± 2.8 × 104 and 92 ± 3%, respectively. The number of cells in the SCK group was significantly higher than that of the DAP group (p < 0.05) (Fig. 4l and m). Overall, these data indicated that hESCs cryopreserved with SCK retained multipotency and cell growth equal to hESCs cryopreserved with DAP.
Figure 4.
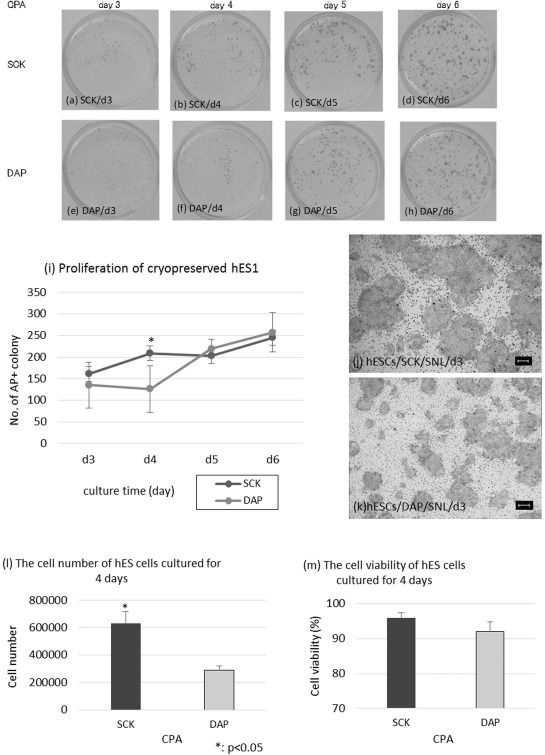
Proliferation profiles of human embryonic stem cells (hESCs) cryopreserved with SCK or a solution of 2 M dimethyl sulfoxide, 1 M acetamide, and 3 M DAP using AP staining. AP-stained hESCs cryopreserved with SCK (a-d) or DAP (e-h) after 3, 4, 5, and 6 days of culturing (day 3, day 4, day 5, and day 6). (i) Comparison of the number of AP+ hESC colonies after preservation with SCK or DAP and culturing for 3, 4, 5, and 6 days. AP+ colonies were counted in four fields at low magnification (10x). hESC colonies 3 days after cryopreservation in SCK (j) or DAP (k). AP staining was observed under an inverted microscopy at 40x magnification. The number (l) and viability (m) of hESCs cultured for 4 days after cryopreservation in SCK or DAP. All data are presented as means ± SD. Statistical evaluations were done by Student's t-test, *p < 0.05, statistically significant difference. Scale bars: 100 um. CPA, cryoprotective agent.
Characterization of hESCs by Immunocytochemical Staining and FACS Analyses
Oct3/4, SSEA4, and Tra-1-60 are common stem cell markers. The pluripotency of hESCs cryopreserved with SCK was investigated by measuring these markers using immunocytochemical staining. Fresh hESCs were positive for all markers (Fig. 5a-c, g-i, and m-o). hESCs cryopreserved with DAP were also positive for these markers (Fig. 5d-f, j-l, and p-r). Furthermore, disaggregated hESCs cryopreserved with SCK were positive for Nanog and Tra-1-60, by FACS analyses, at 98% and 76%, respectively. hESCs cryopreserved with DAP were 96% and 75% positive for Nanog and Tra-1-60, respectively (Fig. 6). These data indicated that hESCs cryopreserved with SCK or DAP expressed common stem cell markers after vitrification.
Figure 5.
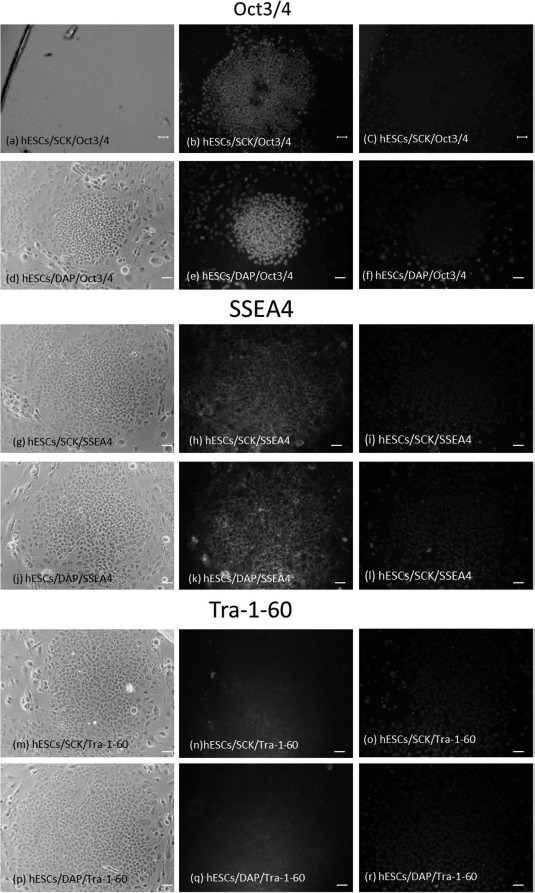
Immunocytochemical analyses of hESCs cryopreserved in SCK or DAP for the stem cell markers octamer-binding transcription factor 3/4 (Oct3/4), stage-specific embryonic antigen-4 (SSEA4), and Tra-1-60. Images of hESCs in bright-field, immunofluorescence, and 4′,6-diamidino-2-phenylindole (DAPI) staining were obtained on a microscope at a magnification of 40x. Scale bars: 100 um. hESCs were fixed with 4% PFA, permeabilized, and reacted with primary antibodies against Oct3/4, SSEA4, or Tra-1-60 at 4° C overnight, and further reacted with fluorescently tagged secondary antibodies in the dark. Mounting medium containing DAPI was added, and cells were observed on a fluorescence microscope. (a-c) hESC/SCK/Oct3/4. (d-f) hESC/DAP/Oct3/4. (g-i) hESC/SCK/ SSEA4. (j-l) hESC/DAP/SSEA4. (m-o) hESC/SCK/Tra-1-60. (p-r) hESC/DAP/Tra-1-60.
Figure 6.
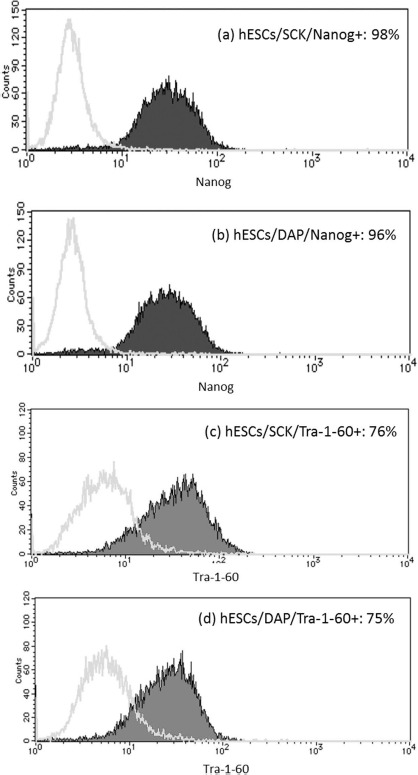
FACS analyses of the expression of Nanog and Tra-1-60 in human embryonic stem cells (hESCs) cryopreserved with SCK or a solution of 2 M DMSO, 1 M acetamide, and 3 M DAP. Freeze-thawed hESCs were cultured on a layer of feeder cells for 5 days, disaggregated by TrypLE Select, fixed with 4% PFA, and permeabilized. Cells were then reacted with primary antibodies against Nanog and Tra-1-60 for 1 h and further reacted with fluorescently tagged secondary antibodies in the dark. The control was hESCs without primary antibody treatment. hESCs were analyzed using the ABI FACSCalibur. Data were analyzed by Quest software and presented as the control (open histogram), and samples were treated with primary antibodies (closed histogram). (a) hESC/SCK/ Nanog. (b) hESC/DAP/Nanog. (c) hESC/SCK/Tra-1-60. (d) hESC/DAP/Tra-1-60.
RT-PCR Analyses of hESCs Cryopreserved with SCK
Expression of the stem cell markers OCT3/4, SOX2, KLF4, c-MYC, REX2, and ECAD, and EPCAM was investigated by RT-PCR. A control gene for the comparative Ct method was selected in order to evaluate RT-PCR data (Table 1). The 2-Ct SCK/2-Ct DAP ratio for GAPDH was 2.6 and was not stable and thus not an appropriate control for this experiment. However, this ratio with SOX2 was 1.1 and was the most stable of the eight genes tested. SOX2 was, therefore, used as the control gene.
Table 1.
Comparison of the Threshold Cycle (Ct) Between GAPDH and SOX2 for RT-PCR Analyses of hESCs Cryopreserved With SCK or DAP
| (1) The 2-Ct value ratio of GAPDH between SCK and DAP | |||
|---|---|---|---|
| Ct (SCK) | 2-Ct | Ct (DAP) | 2-Ct |
| 15.33 | 2.43E-05 | 16.75 | 9.04E-06 |
| 15.23 | 2.60E-05 | 16.55 | 1.05E-05 |
| 15.22 | 2.63E-05 | 16.56 | 1.03E-05 |
| Mean ± SD | (2.6E-05) ± (1.1E-06) | (9.9E-06) ± (7.8E-07) | |
| 2-Ct SCK/2-Ct DAP = 2.6 | |||
The comparative Ct method requires a stable reference gene for evaluating mRNA expression. The 2-Ct SCK/2-Ct DAP ratios of the GAPDH and SOX2 genes were obtained from the RT-PCR data. The ratio with SOX2 was more stable than that with GAPDH and was used as the control gene.
Five genes (OCT3/4, c-MYC, REX2, ECAD, and EPCAM) of hESCs cryopreserved with SCK were expressed at significantly higher levels than those of hESCs cryopreserved with DAP. Relative to SOX2, the expression levels of OCT3/4, c-MYC, REX2, ECAD, and EPCAM were 1.41 ± 0.12, 2.31 ± 0.1, 2.04 ± 0.15, 2.17 ± 0.11, and 1.17 ± 0.09, respectively. The relative expression of KLF4 (0.58 ± 0.05) was much lower (Fig. 7). The relative expression of GAPDH in the SCK group was as high as 2.3 ± 0.05. These data indicate that four stem cell marker genes of hESCs cryopreserved with SCK showed a high expression.
Figure 7.
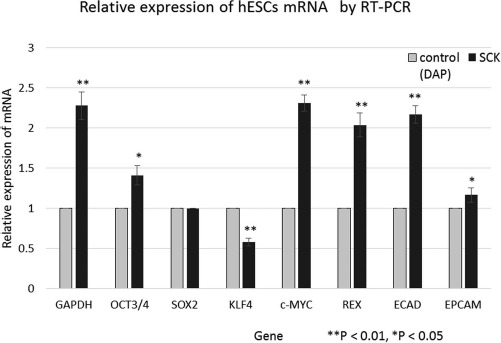
Relative expression of eight genes in cryopreserved human embryonic stem cells (hESCs). RNA was extracted from hESCs cryopreserved with SCK or a solution of 2 M DMSO, 1 M acetamide, and 3 M DAP. cDNA was then synthesized, and the expression of eight Homo sapiens genes was assessed: GAPDH, SOX2, OCT3/4, KLF4, c-MYC, REX2, ECAD, and EPCAM, determined by RT-PCR. The 2-Ct SCK/2-Ct DAP ratio was calculated for each gene. SOX2 was used as the control gene. Data for the comparative Ct method are shown as mean fold changes ± SD of the expression of each gene. **p < 0.01, *p < 0.05, statistically significant difference versus DAP.
Teratoma Formation and In Vitro Differentiation
hESCs cryopreserved with SCK were injected into SCID mice. The resulting teratomas included neural crest (Fig. 8a and b, endoderm), cartilage (Fig. 8c and d, mesoderm), and muscle and intestinal tissues (Fig. 8e and f, ectoderm). These data showed that hESCs cryopreserved with SCK had multipotency and could differentiate into three germ layers.
Figure 8.
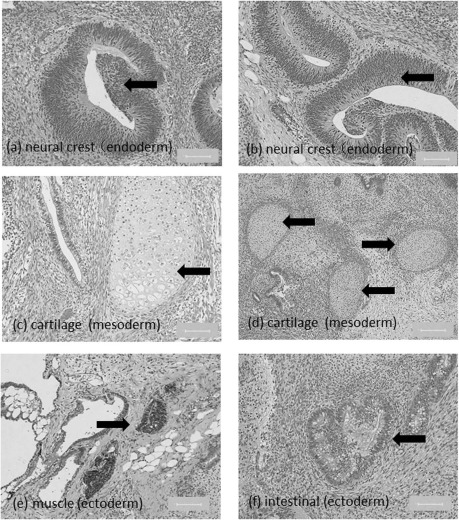
Pluripotency of hESCs cryopreserved with SCK. hESCs developed into a teratoma when transplanted subcutaneously into SCID mice. (a, b) Hematoxylin and eosin (H&E)-stained sections of neural crests, (c, d) cartilage, (e) muscle-like tissue, and (f) intestinal-like tissue. Scale bars: 100 μm.
Karyotype Analyses
hESC1 and hESC3 cells cryopreserved with SCK showed normal karyotypes (Fig. 9), which were consistent with the original data (http://www.shigen.nig.ac.jp/escell/human/about_khes.jsp).
Figure 9.
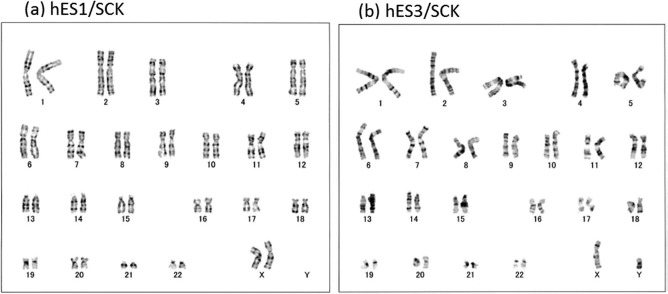
Karyograms of hESCs cryopreserved with SCK. Karyotypes of (a) hESC1 and (b) hESC3 after vitrification with SCK. The karyograms were created by taking a photo of a G-banded metaphase obtained from hESCs. Both karyograms show normal profiles of KhES1 and KhES3.
These results indicate that hESCs cryopreserved with SCK retained their normal genetic background.
Discussion
hESCs are derived from the preimplantation embryo and exhibit both self-renewal and pluripotency. hiPSCs are established from differentiated cells that are induced to return to a pluripotent state by manipulating them to express pluripotent genes. As a result, hESCs and hiPSCs are not identical, and hiPSCs are often called ES-like cells. Because hESCs and hiPSCs are fragile and easy to differentiate, it is important that both cell types are maintained in a stable state during culture and storage. For these reasons, we tested the efficacy of SCK on hESC cryopreservation by vitrification even though we had already reported that SCK was applicable to hiPSCs. The results show that this solution was able to effectively preserve hESCs. hESCs cryopreserved with SCK retained both excellent multipotency and self-renewal ability after vitrification.
There are two cryopreservation methods used for hESCs and hiPSCs: vitrification and slow freezing. Vitrification is based on the notion that the homogeneous nucleation temperature is a kinetic restriction and occurs if the temperature interval within which nucleation occurs can be bypassed rapidly. Thus, ice crystallization in and outside cells can be prevented by vitrification through superfast cooling or usage of highly concentrated solutions (2,3,7,14,25,38,39). In contrast, the slow freezing method freezes cells suspended in the cryopreservation agent by gradually decreasing the temperature to −80°C. Although this method is easier than vitrification and popular for the storage of mammalian cells, it sometimes damages cells because of intracellular moisture crystallization resulting in low cell viability and growth after thawing. This deficiency of slow freezing is a serious concern for hiPSCs and hESCs, and thus the vitrification method has been considered more suitable for mammalian pluripotent stem cells. Recently, the slow freezing method has been improved, and excellent cryopreservation reagents have been developed using various antifreeze agents or cryoprotectants (8,13,21,29). Unfortunately, these new cryopreservation reagents are not yet ideal for maintaining pluripotency and proliferation of hiPSCs and hESCs.
Although DMSO is a popular cryoprotectant and can easily infiltrate into cells at freezing and inhibit intra-cellular moisture crystallization, DMSO has cytotoxicity and can induce differentiation. Thus, DMSO-free or DMSO-diminished cryoprotective agents are desirable for hPSCs. The solution we developed, SCK, includes CPLL (molecular weight around 6,000) as the cryoprotectant instead of DMSO. CPLL is used widely as a food additive and is less cytotoxic than DMSO. e-Poly-L-lysine was first reported by Hiraki (6) and Shima et al. to have antimicrobial activity (30,31). We further developed the carboxylated form of e-poly-L-lysine as the novel cryopreservative reagent, SCK, and reported its cryopreservative effect on hiPSCs (17). In the current work, we showed that SCK was also applicable to the cryopreservation of hESCs by vitrification. As shown in Figure 1, it is evident that ice crystal formation was inhibited more in SCK than DAP. This was because of the ability of CPLL to inhibit ice crystallization as has been reported by our group (19,37).
hESCs cryopreserved with SCK showed a strong attachment on SNL cells and steady proliferation. These cells also retained an excellent attachment ability and growth after they were transferred to Matrigel-coated plates (Figs. 2 and 3). This indicated that SCK could effectively cryopreserve hESCs by vitrification. SCK has some viscidity caused by CPLL. However, this problem can be overcome by mixing hESC clumps well with SCK before freezing.
AP staining data showed that hESCs cryopreserved with SCK retained their pluripotency and a similar cell proliferation profile to that of cells cryopreserved with DAP after culturing for 3 to 6 days (Fig. 4i). However, the actual number of hESCs that were viable after cryopreservation with SCK was about twice as high as those preserved with DAP. Furthermore, there were larger colonies following SCK than DAP cryopreservation of hESCs (Fig. 4j and k). This may be caused by the cryoprotective ability of SCK. The pluripotency of hESCs cryopreserved with SCK was shown by the expression of the stem cell markers Oct3/4, SSEA4, Tra-1-60, and Nanog, and was similar to hESCs cryopreserved with DAP. Importantly, hESCs cryopreserved with SCK showed the ability to differentiate into three different germ layers in the teratoma formation assay.
The expression of relevant mRNAs was analyzed by RT-PCR to assess the genetic stability of stem cell markers in hESCs. The comparative Ct method was used for analyses with SOX2 as the internal control because of its stability. GAPDH, a housekeeping gene, is usually used as the reference gene for RT-PCR. However, as shown in Table 1, the ratio of GAPDH between the SCK- and DAP-cryopreserved hESCs was regarded as unstable, and thus GAPDH was not applicable in this situation. Other researchers have also reported that GAPDH was unsuitable as a control because of its instability (10,36).
The expression of OCT3/4, c-MYC, REX2, and ECAD mRNAs by hESCs cryopreserved with SCK was significantly higher than that of hESCs cryopreserved with DAP (Fig. 7). These RT-PCR data, and the normal karyotype profile of the SCK group, indicate the genetic stability of the pluripotency of the cells preserved with SCK. Even though vitrification reduced the number of attached cells compared with fresh hESCs, SCK provided better preservation than DAP.
In conclusion, we evaluated vitrification using SCK and demonstrated its high efficacy for the cryopreservation and banking of hESCs. This vitrification technique does not require DMSO or animal-derived materials, indicating its potential to find widespread use for the preservation of hPSCs for clinical and industrial applications.
Acknowledgments
The authors thank Hirofumi Suemori and Naoki Nakajima for their excellent advice, and Satomi Koshiba, Hiromi Mori, and Hiroyuki Okihana for their excellent technical assistance. This study was supported, in part, by Grant-in-Aid KAKENHI (25242050) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. K.M. and S.-H.H. are cofounders of Bioverde Inc. A.O. is an employee of Bioverde Inc.
References
- 1.Adler S.; Pellizzer C.; Paparella M.; Hartung T.; Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol. In Vitro 20: 265–271; 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baharvand H.; Salekdeh G. H.; Taei A.; Mollamohammadi S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protoc. 5: 588–594; 2010. [DOI] [PubMed] [Google Scholar]
- 3.Beier A. F.; Schulz J. C.; Zimmermann H. Cryopreservation with a twist—Towards a sterile, serum-free surface-based vitrification of hESCs. Cryobiology 66: 8–16; 2013. [DOI] [PubMed] [Google Scholar]
- 4.Chen H. H.; Chuang C. Y.; Lee W. C.; Huang H. P.; Wu H. C.; Ho H. N.; Chen Y. J.; Kuoet H. C. Surface marker epithelial cell adhesion molecule and e-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev. Rep. 7: 722–735; 2011. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka T.; Yasuchika K.; Nakamura Y.; Nakatsuji N.; Suemori F. A simple and efficient cryopreservation method for primate embryonic stem cells. Int. J. Dev. Biol. 48: 1149–1154; 2004. [DOI] [PubMed] [Google Scholar]
- 6.Hiraki J. [The base and application of e-poly-L-lysine]. J. Antibact. Antifung. Agents 23: 349–354; 1995. [Google Scholar]
- 7.Hunt C. J. Cryopreservation of human stem cells for clinical application: A review. Transfus. Med. Hemother. 38: 107–123; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imaizumi K.; Nishishita N.; Muramatsu M.; Yamamoto T.; Takenaka C.; Kawamata S.; Kobayashi K.; Nishikawa S.; Akuta T. A simple and highly effective method for slow-freezing human pluripotent stem cells using dimethyl sulfoxide, hydroxyethyl starch and ethylene glycol. PLoS One 9: 1–11; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwatani M.; Ikegami K.; Hattori N.; Tanaka S.; Yagi S.; Shiota K. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 24: 2549–2556; 2006. [DOI] [PubMed] [Google Scholar]
- 10.Jacob F.; Guertler R.; Naim S.; Nixdorf S.; Fedier A.; Hacker N. F.; Heinzelmann-Schwarz V. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One 8: 1–10; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katkov I. I.; Kim M. S.; Bajpai R.; Altman Y. S.; Mercola M.; Loring J. F.; Terskikh A. V.; Snyder E. Y.; Levine F. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology 53: 194–205; 2006. [DOI] [PubMed] [Google Scholar]
- 12.Kuleshova L.; Gianaroli L.; Magli C.; Ferraretti A.; Trounson A. Birth following vitrification of a small number of human oocytes: Case report. Hum. Reprod. 14: 3077–3079; 1999. [DOI] [PubMed] [Google Scholar]
- 13.Lee J. Y.; Lee J. E.; Kim D. K.; Yoon T. K.; Chung H. M.; Lee D. R. High concentration of synthetic serum, step-wise equilibration and slow cooling as an efficient technique for large-scale cryopreservation of human embryonic stem cells. Fertil. Steril. 93: 976–985; 2010. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.; Tan J. C.; Li L. S. Comparison of three methods for cryopreservation of human embryonic stem cells. Fertil. Steril. 93: 999–1005; 2010. [DOI] [PubMed] [Google Scholar]
- 15.Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta C (T) method. Methods 25: 402–408; 2001. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura K.; Hyon S. H. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials 30: 4842–4849; 2009. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura K.; Bae J. Y.; Kim H. H.; Hyon S. H. Effective vitrification of human induced pluripotent stem cells using carboxylated e-poly-L-lysine. Cryobiology 63: 76–83; 2011. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K.; Bae J. Y.; Hyon S. H. Polyampholytes as cryoprotective agents for mammalian cell cryopreservation. Cell Transplant. 19: 691–699; 2010. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura K.; Kawamoto K.; Takeuchi M.; Yoshimura S.; Tanaka D.; Hyon S. H. Cryopreservation of a two-dimensional monolayer using a slow vitrification method with polyampholyte to inhibit ice crystal formation. ACS Biomater. Sci. Eng. 2(6): 1023–1029; 2016. [DOI] [PubMed] [Google Scholar]
- 20.McMahon A. P.; Bradley A. The Wnt-1 (int-l) protooncogene is required for development of a large region of the mouse brain. Cell 62: 1073–1085; 1990. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki T.; Nakatsuji N.; Suemori H. Optimization of slow cooling cryopreservation for human pluripotent stem cells. Genesis 52: 49–55; 2014. [DOI] [PubMed] [Google Scholar]
- 22.Mukaida T.; Wada S.; Takahashi K.; Pedro P. B.; An T. Z.; Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum. Reprod. 13: 2874–2879; 1998. [DOI] [PubMed] [Google Scholar]
- 23.Nishigaki T.; Teramura Y.; Suemori H.; Iwata H. Cryopreservation of primate embryonic stem cells with chemically-defined solution without Me2SO. Cryobiology 60: 159–164; 2010. [DOI] [PubMed] [Google Scholar]
- 24.Okita K.; Ichisaka T.; Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317; 2007. [DOI] [PubMed] [Google Scholar]
- 25.Pruksananonda K.; Rungsiwiwut R.; Numchaisrika P.; Ahnonkitpanit V.; Isarasena N.; Virutamasen P. Eighteen-year cryopreservation does not negatively affect the pluripotency of human embryos: Evidence from embryonic stem cell derivation. BioResearch 1: 166–173; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reubinoff B. E.; Pera M. F.; Vajta G.; Trouson A. T. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum. Reprod. 16: 2187–2194; 2001. [DOI] [PubMed] [Google Scholar]
- 27.Richards M.; Fong C. Y.; Tan S.; Chan W. K.; Bongso A. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells 22: 779–789; 2004. [DOI] [PubMed] [Google Scholar]
- 28.Schmittgen T. D.; Livak K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3: 1101–1108; 2008. [DOI] [PubMed] [Google Scholar]
- 29.Sharp D.; Picken A.; Morris T. J.; Hewitt C. J.; Coopman K.; Slater N. K. Amphipathic polymer-mediated uptake of trehalose for dimethyl sulfoxide-free human cell cryo-preservation. Cryobiology 67: 305–311; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shima S.; Fukuhara Y.; Sakai H. μ Inactivation of bacteriophages by e-poly-L-lysine produced by Streptomyces. Agric. Biol. Chem. 46: 1917–1919; 1982. [Google Scholar]
- 31.Shima S.; Matsuoka H.; Iwamoto T. Antimicrobial action of e-poly-L-lysine. J. Antibiotics 37: 1449–1455; 1984. [DOI] [PubMed] [Google Scholar]
- 32.Suemori H.; Yasuchika Y.; Hasegawa K.; Fujioka T.; Tsuneyoshi N.; Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem. Biophys. Res. Commun. 345: 926–932; 2006. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K.; Okita K.; Nakagawa M.; Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2: 3081–3089; 2007. [DOI] [PubMed] [Google Scholar]
- 34.Tan F. C.; Lee K. H.; Gouk S. S.; Magalhaes R.; Poonepalli A.; Hande M. P.; Dawe G. S.; Kuleshova L. L. Optimization of cryopreservation of stem cells cultured as neurospheres: Comparison between vitrification, slow-cooling and rapid cooling “freezing” protocols. Cryo Letters 28: 445–460; 2007. [PubMed] [Google Scholar]
- 35.Thaler R.; Spitzer S.; Karlic H.; Klaushofer K.; Varga F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics 7: 1–17; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veazey K. J.; Golding M. C. Selection of stable reference genes for quantitative RT-PCR comparisons of mouse embryonic and extra-embryonic stem cells. PLoS One 6: 1–10; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorontsov D. A.; Sazaki G.; Hyon S. H.; Matsumura K.; Furukawa Y. Antifreeze effect of carboxylated e-poly-L-lysine on the growth kinetics of ice crystals. J. Phys. Chem. B 118: 10240–10249; 2014. [DOI] [PubMed] [Google Scholar]
- 38.Wagh V.; Meganathan K.; Jagtap S.; Gaspar J. A.; Winkler J.; Spitkovsky D.; Hescheler J.; Sachinidis A. Effects of crypreservation on the transcriptome of human embryonic stem cells after thawing and culturing. Stem Cell Rev. Rep. 7: 506–517; 2011. [DOI] [PubMed] [Google Scholar]
- 39.Zhmakin A. I. Fundamentals of cryobiology. Physical phenomena and mathematical models. Berlin (Germany): Springer-Verlag; 2009. [Google Scholar]


