Abstract
Bone marrow aspirate concentrates (BMACs) and platelet-rich plasma (PRP) are good sources to control the differentiation of tendon-derived stem cells (TDSCs), but there has been no study about the effect of the BMAC–PRP complex on TDSCs and tendinopathy. The aim of this study was to investigate the effect of BMAC–PRP on the TDSCs and to find the therapeutic effect of BMAC–PRP on the rotator cuff tendon tear. The chondrogenic and osteogenic potential of TDSCs decreased, but the adipogenic potential of TDSCs revealed no significant difference when they were cocultured with BMAC–PRP. Cell proliferation was significantly greater in TDSCs cocultured with BMAC–PRP than in TDSCs. The degree of wound closure (percentage) was different between TDSCs and TDSCs with BMAC–PRP. There was no significant difference in expression of collagen type I and type III in immunocytochemical staining in the presence of BMAC–PRP. Initial visual analog scale (VAS) score was 5.8±1.9, which changed to 5.0±2.3 at 3 weeks and 2.8±2.3 at 3 months after the BMAC–PRP injection (p<0.01). The American Shoulder Elbow Surgeon score changed from 39.4±13.0 at baseline to 52.9±22.9 at 3 weeks and 71.8±19.7 at 3 months after the injection (p<0.01). The initial torn area of the rotator cuff tendon was 30.2±24.5 mm2, and this area was reduced to 22.5±18.9 mm2 at 3 months, but the change was not significant (p > 0.05). The data indicate that BMAC–PRP enhances the proliferation and migration of TDSCs and prevents the aberrant chondrogenic and osteogenic differentiation of TDSCs, which might provide a mechanistic basis for the therapeutic benefits of BMAC–PRP for rotator cuff tendon tear.
Keywords: Bone marrow, Platelet, Rotator Cuff, Tendon, Stem cells
Introduction
The tendon is a complex of highly aligned, closely packed collagen fibrils with few tenocytes and fibroblasts. Collagen fibrils are mainly type I collagen with a small portion of other types of collagen and some proteoglycans, such as decorin, biglycan, and fibromodulin. During tendon development, tenocytes are responsible for the synthesis of the collagen fibrils, and proteoglycans are important components in regulating the assembly of collagen fibrils and acquisition of the biomechanical properties of the tendon1.
Tendinopathy is a common pathological condition of the tendon in clinical settings. In tendinopathy, cells become rounder and more numerous and display an apoptotic feature2. In this condition, diverse changes also occur to the extracellular matrix, resulting in disorganization of collagen fibers and in the tendons losing their unique biomechanical characteristics used to deliver force energy3.
There is much ongoing research into novel therapies to treat tendinopathy. Regenerative medicine research seeks to restore tendon function using growth factors, gene therapy, and tissue engineering techniques4. Recently, the identification of tendon-derived stem cells (TDSCs) has suggested a new paradigm in healing tendons, and providing an adequate environment for the differentiation of these stem cells into normal tenocytes has become an important research issue. Chronic tendinopathy involves calcific tendinitis and fatty degeneration due to the aberrant metaplasia of tenocytes or TDSCs; this increases the importance of the correct control of tendon-related cells5,6.
Bone marrow-derived mesenchymal stem cells (BM-MSCs) and bone marrow aspirate concentrates (BMACs) are good cell sources for use in the repair of damaged structures and have been used to enhance the healing of rotator cuff tears in preclinical and clinical studies7-9. However, in the absence of an adequate scaffold, the survival, reparative capacity, and differentiation capacity of BM-MSCs and BMACs were inadequate to enhance the healing of rotator cuff tears10,11. Therefore, the selection of adequate scaffolds is important in the stem cell-mediated repair of tears including rotator cuff tears.
Platelet-rich plasma (PRP) is plasma enriched with platelets that harbor many growth factors. PRP has been used as a therapeutic material to promote regeneration12,13 and also as a scaffold to enhance the proliferation and multipotency of BMACs14. For degenerative tendinopathy, PRP has regenerative action that involves promoting the correct differentiation and preventing aberrant differentiation of TDSCs15. In addition, studies have been conducted using combinations of PRP with BMACs in settings like diabetic ulcers16, osteochondral issues17-19, and spinal cord injury20. In such cases, the BMAC–PRP complex showed a regenerative potential, demonstrating that PRP has a role in supporting the regenerative action of the BMACs in a scaffold setting.
We hypothesized that BMAC combined with PRP could be used to control the differentiation of TDSCs and increase the regenerative action of TDSCs. However, no study has examined the effects of the BMAC–PRP complex on TDSCs and tendinopathy. Investigating the effects of the BMAC–PRP complex on TDSCs would provide information useful in developing a new treatment for degenerative tendinopathy. Therefore, the aims of this study were to investigate the effect of the BMAC–PRP complex on TDSCs and to assess the therapeutic effects of the BMAC–PRP complex on rotator cuff tendon tear.
Materials and Methods
The appropriate use of biospecimens in research (Approval No. 2014-10-149) and the protocol of clinical procedures (Approval No. 2014-07-173) were approved by our institutional review board (IRB), and written consent was acquired from all of the patients.
In Vitro Study
Acquisition of TDSCs from Patients with Degenerative Tendinopathy
Human tendon tissues were acquired from surgical procedures in one patient with lateral epicondylopathy. The donor was a woman 58 years of age (Pt-1) who had experienced severe pain and discomfort intractable to conservative management. She had not received a steroid injection during the 3 months before the surgery. Tendon tissue was extracted from the common extensor carpi radialis tendon. This tissue was cut into 1-mm × 1-mm pieces, and the sheath was removed. After the tendon tissue was weighed, it was immersed in 1 ml of phosphate-buffered saline (PBS) containing 3 mg of collagenase type I (Catalog No. C0130; Sigma-Aldrich, St. Louis, MO, USA) and 4 mg of dispase (Catalog No. D4693; Sigma-Aldrich) per 100 mg of tissue within 20 min after extraction. The tissue was digested at 37°C for 2 h. The digested solution was then centrifuged at 800 × g for 15 min (Centrifuge 5810 R; Eppendorf, Hamburg, Germany), and the supernatant was discarded. The pellet was suspended in 5 ml of Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotics (Gibco, Waltham, MA, USA) in a T25 flask. The suspended solution was incubated at 37°C in a 5% CO2 incubator, and the medium was changed every other day. After culture expansion to 80% confluence, the cells were washed three times with PBS and treated with trypsin-ethylenediaminetetraacetic acid (EDTA) buffer (Catalog No. 25200-056; Gibco) for 3 min at 37°C in a 5% CO2 incubator. The cells were suspended in DMEM with 20% FBS and 1% penicillin/streptomycin and were diluted to 1 cell/pl by counting using a hemocytometer. One hundred microliters of the solution was cultured in a 10-mm × 10-mm dish at 37°C and 5% CO2. After the boundaries were marked in the single-cell adhesion cultures, the cells continued to be cultured in DMEM containing 20% FBS and 1% penicillin/streptomycin to form individual cell colonies. After about 10 days of culture, several colonies that had formed were extracted with local application of trypsin-EDTA solution to each colony marked area using a micropipette. Cells extracted from each area by local trypsinization were collected into a 24-well culture dish and cultured with DMEM containing 20% FBS and 1% penicillin/streptomycin. All TDSCs in this study were used between passages 4 and 6 with polyclonal origin.
Extraction of BMACs and PRP and Coculture with TDSCs
A 52-year-old woman (Pt-2) diagnosed with a rotator cuff tendon tear was recruited for the extraction of BMACs and PRP. For the extraction of BMACs, she lay on the table in a prone position. After the iliac crest was localized by palpation, the area was sterilized by povidone iodine and the skin and periosteum were anesthetized using 1% lidocaine. A bone marrow aspiration needle penetrated into the iliac bone and progressed to the bone marrow site. Bone marrow aspirates were acquired from the bone marrow and centrifuged using a BIOMET MarrowStim™ Mini kit (Biomet Biologics and Biomaterials, Inc., Warsaw, IN, USA) to isolate the concentrated BMACs. Peripheral blood (30 ml) was acquired from the antecubital vein and was centrifuged using a BIOMET GPS™ III kit (Biomet Biologics and Biomaterials, Inc.) to extract the PRP.
To determine whether BMAC and PRP could promote tenogenic differentiation in vitro, TDSCs were cocultured directly with BMACs and PRP. TDSCs at P3 were seeded at 1 × 106 cells in a T75 flask and stored at 37°C in a 5% CO2 incubator. After 24 h, 450 μl of BMACs and PRP was injected without posttreatment. Cultures were incubated with DMEM containing 20% FBS. In the negative controls, the TDSCs were seeded alone, at the same density described above, in a T75 flask in DMEM containing 20% FBS. The culture flasks were incubated aerobically at 37°C in a humidified atmosphere containing 5% CO2 for 7 days. In both conditions, the appropriate medium was changed every 2 to 3 days. The whole experiment was conducted on three different samples and in triplicate.
Identification and Characteristics of Human TDSCs
Immunocytochemical Staining
TDSCs were plated at a density of 2 × 103 cells/cm2 on a 22-mm × 22-mm cover glass and cultured in complete culture medium until the cells reached 70% confluence at 37°C and 5% CO2. The cells were fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature, quenched with 0.25% Triton X-100 (Sigma-Aldrich) in PBS for 10 min at room temperature, blocked with 1% bovine serum albumin (Sigma-Aldrich) in PBS for 30 min at room temperature, and incubated with primary antibody overnight at 4°C. The primary antibodies and the titers used were as follows: rabbit polyclonal anti-tenomodulin (1:100; Catalog No. sc-98875; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-nucleostemin (1:500; Catalog No. ab70346; Abcam, Cambridge, UK), rabbit polyclonal anti-octamer-binding transcription factor 4 (OCT4; 1:100; Catalog No. ab18976; Abcam), and mouse monoclonal anti-stage-specific embryonic antigen-4 (SSEA4; 1:500; Catalog No. ab16287; Abcam). The blocking solution was substituted for primary antibody in the negative controls. After being washed with PBS, the cells were incubated with donkey polyclonal anti-rabbit IgG antibody (Alexa Fluor® 488; 1:1,000; Catalog No. ab150073; Abcam) or donkey polyclonal anti-mouse IgG antibody (Alexa Fluor® 594; 1:1,000; Catalog No. ab150108; Abcam) for 2 h at room temperature in the dark. The cells were then rinsed in PBS and mounted with Fluoroshield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Catalog No. ab104139; Abcam). For good reproducibility and comparability, all incubation times and conditions were strictly controlled. The cells were examined under confocal microscopy using a model LSM 700 microscope (Carl Zeiss Meditec, Jena, Germany).
For the identification of TDSCs, immunofluorescence staining for tenomodulin, the specific cell marker of tendon-derived cells, was performed using tenomodulin as the primary antibody and Alexa Fluor 488 as the secondary antibody. To determine the properties of the stem cells in the TDSCs, the stem cell markers OCT4, SSEA4, and nucleostemin were examined by immunofluorescence expression. For the negative control, the primary antibody was omitted under the same conditions.
Fluorescence-Activated Cell Sorting (FACS) Analysis
TDSCs were harvested at the third passage by trypsinization. After two washes with PBS, 1 × 105 TDSCs and BM-MSCs obtained after three passages were incubated with phycoerythrin (PE)-conjugated mouse-specific antibody to human CD34 (Catalog No. ab46970; Abcam) and CD90 (Catalog No. ab95700; Abcam) and with fluorescein isothiocyanate (FITC)-conjugated mouse-specific antibodies to human CD45 (Catalog No. ab27287; Abcam) and CD105 (Catalog No. ab18278; Abcam) for 1 h at room temperature. Nonspecific FITC or PE-conjugated IgG were used as an isotype control. After being washed with PBS and being centrifuged at 400 × g for 10 min, the stained cells were resuspended in 500 ml of ice-cold PBS and analyzed using a FACSCalibur machine (BD Biosciences, San Jose, CA, USA); 1 × 104 events were counted for each sample. The percentage of cells showing a positive signal and the mean geometric fluorescence value of the positive population were analyzed using the Cell Quest software (BD Biosciences).
Multidifferentiation Potential
To confirm the multilineage properties, TDSCs were grown in adipogenic, chondrogenic, and osteogenic media, and their differentiation was evaluated by examining various cell markers. Osteogenic Differentiation Assay. Tendon-derived cells were plated at a density of 5 × 103 cells/cm2 in a 24-well plate and cultured in standard growth medium (DMEM+ 10% FBS) until the cells reached confluence. They were then incubated for 21 days in standard growth medium or osteogenic medium (Catalog No. A10072-01; Gibco). The medium was changed every 2 to 3 days. Osteogenesis was evaluated by an Alizarin red S assay. Briefly, the medium was removed from the cell culture plates, and the cells were washed in PBS. The cells were then fixed in 4% PFA for 30 min at room temperature. Subsequently, the cells were washed three times with PBS and incubated with 2% Alizarin red S stain solution (Catalog No. 0223; ScienCell Research Laboratories, Carlsbad, CA, USA) for 30 min, followed by three washes with distilled water. Stained samples were examined under a light microscope. Stained calcium deposits of the cells appeared red.
Adipogenic Differentiation Assay
TDSCs were plated at the same density as indicated for the osteogenic assay. The medium was replaced with standard growth medium (DMEM + 10% FBS) or adipogenic medium (Catalog No. A10070-01; Gibco). The cells were cultured for an additional 21 days, and the medium was changed every 2 to 3 days. Adipogenesis was evaluated by an Oil red O assay. Briefly, the medium was removed from the cell culture plate, and the cells were washed in PBS. The cells were fixed in 4% PFA for 30 min at room temperature, washed three times with PBS, incubated with 0.3% Oil red O solution (Catalog No. O1391; Sigma-Aldrich) for 50 min, and washed three times with distilled water. Stained samples were examined under a light microscope. Stained lipid droplets of the adipocytes appeared red.
Chondrogenic Differentiation Assay
For chondrogenic differentiation, a pellet culture system was used21,22. About 8 × 105 cells were pelleted into a micromass by centrifugation at 800 × g for 10 min in a 15-ml conical polypropylene tube and cultured in standard growth medium or chondrogenic medium (Catalog No. A10071-01; Gibco) at 37°C and 5% CO2 for 28 days, with the medium changed every 2 to 3 days. The cell pellet acquired at that time was fixed in 4% PFA, dehydrated, and embedded in paraffin. Sections were cut at a thickness of 5 μm and stained with hematoxylin and Safranin O (Koma Biotech, Daejeon, South Korea) after deparaffination as described below. Safranin O staining was assayed using a NovaUltra™ Safranin O stain kit (Catalog No. IW-3011; IHC World, Ellicott City, MD, USA). The section was stained in hematoxylin solution for 10 min and then washed in running tap water for 10 min. Subsequently, the section was stained in Safranin O solution for 5 min, rinsed with distilled water, stained with Fast Green solution (Koma Biotech) for 10 min, and rinsed quickly with acetic acid solution for 10-15 s. Stained samples were examined under a digital slide scanner (Aperio AT; Leica Microsystems, Wetzlar, Germany).
Immunohistochemical Staining
Paraffin-embedded sections were deparaffinized in xylene (Koma Biotech) and dehydrated through a graded series of alcohol. Antigen retrieval was done with target retrieval solution pH 6.0 citrate (Catalog No. S2031; Dako, Glostrup, Denmark) at 121°C and high pressure for 7 min for type II collagen detection. To block the endogenous peroxidase activity, a peroxidase blocking solution (Catalog No. S2023; Dako) was used for 15 min at room temperature. After being blocked with protein block serum-free solution (Catalog No. X0909; Dako) for 20 min at room temperature, the sections were incubated with rabbit polyclonal antibody against type II collagen (1:200; Catalog No. ab34712; Abcam) for an hour at room temperature. Type II collagen was localized by incubation with EnVision®+ System-HRP Labelled Polymer Anti-Rabbit (Catalog No. K4003; Dako) for 1 h at room temperature, followed by liquid DAB + substrate chromogen system (Catalog No. K3468; Dako). Afterward, the sections were rinsed, counterstained with hematoxylin (Catalog No. S3309; Dako), dehydrated with a graded series of ethanol and xylene, and mounted. For the negative control, blocking solution was used instead of primary antibody. For the positive control, knee joint cartilage from mice (Orient Bio Inc., Gapyeong, South Korea) was used. The histology was evaluated using a digital slide scanner.
Migration Assay
The migration capacity of TDSCs was assessed using a scratch assay. TDSCs were seeded at a density of 2 × 104 cells per well in a 12-well flat-bottom plate. Confluent growth was attained during incubation in 10% FBS-supplemented DMEM for 4 days. A P1000 pipette tip was used to scratch off an area of TDSCs from the confluent growth. The scratch was kept to a limited width by maintaining the pipette tip at an angle of about 30°. The same procedure was repeated for all plates. The cells were rinsed in the medium, and then the medium was replaced with 10% FBS-supplemented DMEM.
For quantification of the scratch assay, image capture and data analysis were performed. Images at time zero (t=0 h) were captured to record the initial areas of the wounds, and the recovery of the wounded monolayers due to cell migration toward the denuded area was evaluated at 24 h (t=Δ h). The images were captured using an inverted phase-contract microscope equipped with a 4x objective. The area of each wound was quantified by ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA] using the polygon selection mode. The migration of cells toward the wounds was expressed as percentage of wound closure:
% of wound closure=[(At=0h - At=h-At=Δh)/At=0h] × 100%, where At=0h is the area of wound measured immediately after scratching, and At=Δh is the area of wound measured 24 h after scratching23.
Cell Proliferation Assay.
Doubling Time
TDSC proliferation was assessed as population doubling time (PDT), defined as the total culture time (8 days) divided by the PD24. PD was determined at six passages as:
PD=log2 (Nn/N0), where N0 is the number of cells seeded initially, and Nn is the number of cells harvested at the end of a period of growth (8 days).
PDT is the period of time required for the cells to double. To calculate the PDT, the time period in which the cells grew was divided by the PD value that corresponded to the same time period:
PDT (hours) = (time period)/PD
PDT was measured three times and averaged for increased reliability.
CCK-8 Assay
Cell proliferation was measured at 1, 3, 5, 7, and 9 days after cell seeding using Cell Counting Kit-8® solution (CCK-8; Dojindo, Gaithersburg, MD, USA) according to the manufacturer's protocol. Cells were seeded at a concentration of 1 × 103 cells/100 μl per well in 96-well culture plates and treated with 10 μl/well of Cell Counting Kit-8® solution during the last 3 h of the culture. The optical densities of the wells were measured at 450 nm using a microplate reader. The proliferation analyses of each group and the whole experimental process were performed in triplicate.
Expression of Type I and Type III Collagen
To evaluate the regenerative capacity of TDSCs, the expression of type I and type III collagen was investigated by immunocytochemical staining. Immunocytochemical staining of both collagen types was conducted using rabbit polyclonal anti-collagen I (1:500; Catalog No. ab292; Abcam) and mouse monoclonal anti-collagen III (1:100; Catalog No. NBP1-05119; Novus Biologicals, Littleton, CO, USA).
Clinical Study
The clinical study was conducted as a prospective, single-blind study. Patients who visited our outpatient department from August 2014 to July 2016 and were diagnosed with partial rotator cuff tear were included in our study. Twelve patients including Pt-2 participated in the clinical study as the study group and underwent extraction of their BMACs and PRP. Inclusion criteria were (1) no history of shoulder surgery during the past 3 months, (2) no abnormal findings in simple radiography, (3) rotator cuff partial tear diagnosed with ultrasound, (4) no abnormalities in blood coagulation and routine laboratory examination, and (5) no history of steroid injection during the past 3 months. The patients had experienced shoulder pain for more than 3 months and had not demonstrated any improvement by oral medication and physical modalities. Ultrasound imaging was performed in the modified Crass position, and the width and depth (both in mm) of the tear size were measured (Fig. 1A). Their product represented the torn area (mm2).
Figure 1.
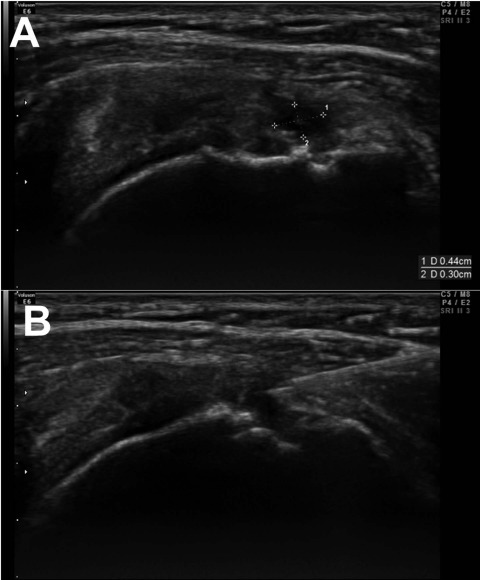
Tear size was measured by width (mm) and depth (mm) (A), and the bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex injection was done under ultrasound guidance (B).
Injection of BMAC–PRP Into Rotator Cuff Tendon Tears
After acquiring BMACs and PRP with the aforementioned protocol, 2 ml of BMACs was mixed with 1 ml of PRP in a 5-ml syringe. The patient lay on an examination table in a supine position to receive the BMAC–PRP injection at the tear site under ultrasound guidance (Fig. 1B).
Evaluation of Shoulder Function
For evaluation of shoulder function, the visual analog scale (VAS) and manual muscle test (MMT) scores of the shoulder abductor were measured by one experienced physician (P.J.W.), and the American Shoulder and Elbow Surgeons (ASES) scores were recorded before, 3 weeks after, and 3 months after the injection. At 3 months after the injection, ultrasound imaging was repeated for the measurement of tear size (mm). The original version of the ASES was used25.
Statistical Analyses
For the in vitro study, each experiment was performed at least three times, and each treatment was conducted with three replicates. The data are expressed as mean±standard error of the mean (SEM). The independent t-test was used for statistical analysis. Statistical significance was established at p<0.05. In the clinical study, repeated-measures analysis of variance (ANOVA) and paired t-tests were conducted for all of the parameters, and p<0.05 was considered statistically significant. For statistical analyses, SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was used.
Results
In Vitro Study: Effects of BMAC–PRP on TDSCs
Identification of TDSCs From Human Tendon Tissue
Extracted tendon tissue was 102 mg in weight, and after incubation for 11 days, about 1.33 × 106 cells were acquired. Immunocytochemical staining of tenomodulin allowed the identification of tenogenic lineage cells, and the immunocytochemical staining of nucleostemin, OCT4, and SSEA4 assayed the stemness of TDSCs acquired from human tendon tissue (Fig. 2). Human TDSCs were characterized with respect to the expression of various surface antigens. Over 96% of the TDSCs expressed MSC-specific markers, including CD90 and CD105. They were negative for the hematopoietic stem cell marker CD34 and the leukocyte marker CD45 (Fig. 2).
Figure 2.
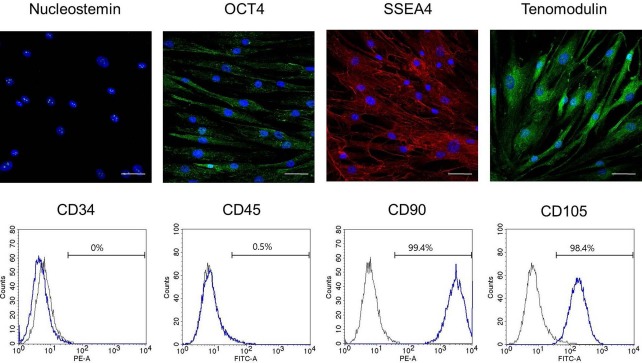
Immunocytochemical staining for tendon-derived stem cells (TDSCs) and expression of surface antigens for identification of TDSCs. OCT4, octamer-binding transcription factor 4; SSEA4, stage-specific embryonic antigen-4; FITC, fluorescein isothiocyanate; PE, phycoerythrin. Scale bars: 50 μm.
Multidifferentiation Potential
We conducted an experiment on osteogenic, chondrogenic, and adipogenic differentiation to evaluate the effects of BMAC–PRP on the multidifferentiation of TDSCs. The multidifferentiation potential of TDSCs was confirmed by the osteogenic, chondrogenic, and adipogenic induction. To compare the degrees of adipogenic differentiation, Oil red O staining was performed 21 days after the adipogenic induction. No significant difference was found between the BMAC–PRP and control groups in the Oil red O staining of adipocyte lipid droplets. Alizarin red S staining at 21 days after osteogenic induction showed a significant decrease in staining in the BMAC–PRP group compared to the control group. Chondrogenic differentiation was measured by Safranin O staining and the immunofluorescence staining of type II collagen after the induction with chondrogenic media through the pellet culture. Differentiation of TDSCs to chondrocytes and the expression of type II collagen were lower in the BMAC–PRP group compared to the control group. Figures 3 and 4 show the multidifferentiation potentials between the BMAC–PRP and control groups.
Figure 3.
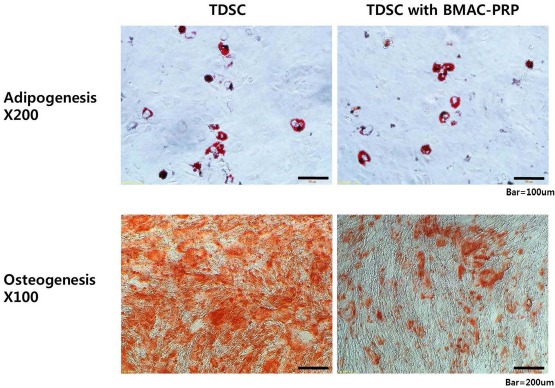
Multidifferentiation potential of tendon-derived stem cells (TDSCs) was confirmed by the osteogenic and adipogenic induction. Osteogenic potential of TDSCs decreased, but the adipogenic potential of TDSCs revealed no significant difference when they were cocultured with bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex.
Figure 4.
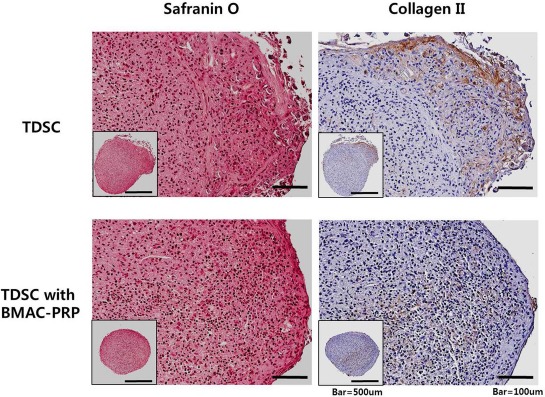
Chondrogenic differentiation potential of tendon-derived stem cells (TDSCs) was confirmed by the chondrogenic induction. Chondrogenic potential of TDSCs decreased when they were cocultured with bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex.
Cell Proliferation
The PDT of the TDSCs and TDSCs cocultured with BMAC–PRP were 48.4±1.4 and 46.6±1.9 h, respectively, which suggests that BMAC–PRP decreased the doubling time of the TDSCs.
Proliferation using the CCK-8 assay increased with incubation time in both TDSCs and TDSCs cocultured with BMAC–PRP (Fig. 5). After 1 day of incubation, the cell proliferation was identical in the TDSCs and TDSCs cocultured with BMAC–PRP. The cell proliferation was significantly greater in TDSCs cocultured with BMAC–PRP than in TDSCs alone at 3, 5, 7, and 9 days.
Figure 5.
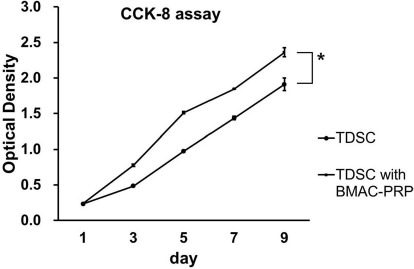
Cell proliferation measured using Cell Counting Kit-8® (CCK-8) assay. After 1 day of incubation, cell proliferation was nearly identical in tendon-derived stem cells (TDSCs) and TDSCs cocultured with bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex. Cell proliferation was significantly greater in TDSCs cocultured with BMAC–PRP than in TDSCs at 3, 5, 7, and 9 days. *p < 0.05.
Migration Assay
TDSCs were separated by scratching, and the migration of the two fronts toward each other was observed at 24 h (Fig. 6). The degree of wound closure (percentage) was quantified by the ImageJ program. The degree of wound closure in the TDSCs cocultured with BMAC–PRP was 53.08±6.58%, while that in the TDSCs alone was 50.21±6.20%.
Figure 6.
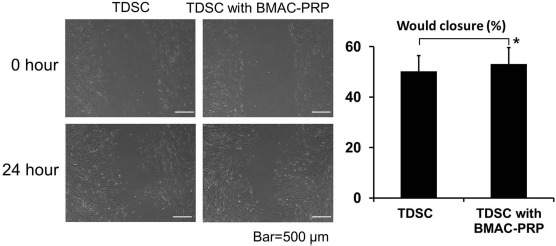
As a surrogate read for wound healing, tendon-derived stem cells (TDSCs) were separated by scratching, and the extents of migration of the two fronts toward each other were observed at 24 and 48 h. Degree of wound closure (percentage) was quantified by the ImageJ program. Wound healing was significantly different between TDSCs and TDSCs with bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex. *p < 0.05.
Expression of Type I and Type III Collagens in TDSCs
Immunocytochemical staining revealed no significant differences in the expression of type I and type III collagen in the presence versus the absence of BMAC–PRP (Fig. 7).
Figure 7.
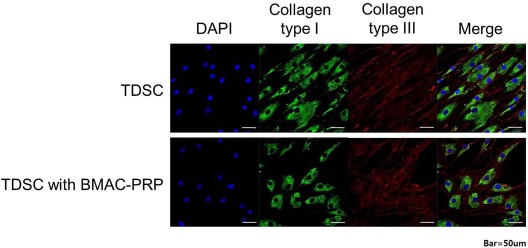
Immunocytochemical staining of collagen type I and type III. There was no significant difference in expression of collagen type I and type III in immunocytochemical staining in the presence of bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex. DAPI, 4′,6-diamidino-2-pheylindole.
Clinical Study: Effect of BMAC–PRP on Rotator Cuff Tendon
Clinical Symptoms
The initial VAS score was 5.8±1.9 in the BMAC–PRP group, which changed to 5.0±2.3 at 3 weeks and 2.8±2.3 at 3 months after the BMAC–PRP injection (F = 38.08, p < 0.01). The ASES score in the BMAC–PRP group changed from 39.4±13.0 at baseline to 52.9±22.9 at 3 weeks and 71.8±19.7 at 3 months after the injection (F = 14.51, p < 0.01). The changes in VAS and ASES scores according to the elapsed time are presented in Figure 8. The MMT of the shoulder abductor was grade 3 in three patients, grade 4 in four patients, and grade 5 in five patients at baseline, which changed to grade 3 in four patients, grade 4 in three patients, and grade 5 in five patients at 3 weeks, and to grade 4 in five patients and grade 5 in seven patients at 3 months (data not shown).
Figure 8.
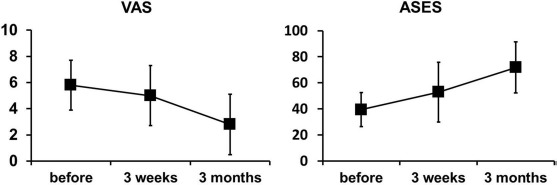
Visual analog scale (VAS) and American Shoulder Elbow Surgeon (ASES) score 3 weeks and 3 months after bone marrow aspirate concentrate–platelet-rich plasma (BMAC–PRP) complex injection.
Ultrasound Determination of Tear Size
The initial torn area of the rotator cuff tendon was 30.2±24.5 mm2, and this area was reduced to 22.5±18.9 mm2 at 3 months, but the change was not statistically significant (p > 0.05). There were no complications or side effects during or after the procedure, and no patient complained of persistent pain except the acute pain immediately after bone marrow aspiration and BMAC–PRP injection. This pain subsided within 4 h.
Discussion
BMAC–PRP enhanced the proliferation of TDSCs and migration of TDSCs to the damaged site. In addition, BMAC–PRP inhibited aberrant differentiation into osteocytes and chondrocytes. These observations might suggest the effects of BMAC–PRP in patients with rotator cuff tendon tears on the improvement of shoulder function and pain reduction.
We acquired tendon tissues from a patient with lateral epicondylopathy who did not have rotator cuff tendinopathy. Although TDSCs from one cell source can have different characteristics from TDSCs from other cell sources26, we could not perform a tendon biopsy from a patient with rotator cuff tendinopathy due to ethical reasons.
In this study, there was no clear separation of the individual roles of PRP and BMAC in the healing of tendon tears because we did not have groups who used only PRP or only BMAC. We propose that both BMAC and PRP might play a role in the healing of the tendon tears; several studies have shown the healing potential of the BMAC–PRP combination, and PRP might play a role in supporting the regenerative potentials of BMAC, as PRP is known to be a good scaffold containing many supportive growth factors.
Xu et al.13 demonstrated that PRP promoted the proliferation of TDSCs and facilitated cell migration in both an in vitro study and an animal study, which supports our findings. In addition, several studies support our findings in the proliferation increase seen for TDSCs in the presence of the BMAC–PRP combination. Crowe et al.27 showed that PRP with adipose-derived stem cells enhanced tendon healing by the migration of cells to the injured area and cellular proliferation, similar to our study. Chiou et al.28 also studied the therapeutic effects of PRP and adipose-derived stem cells on tendon healing in a tendinopathy model.
Presently, the BMAC–PRP complex decreased the chondrogenic and osteogenic potentials of TDSCs. Our results were consistent with the description of decreased osteogenic and chondrogenic potentials in Zhang and Wang's study29. Mishra et al.30 demonstrated that buffered PRP enhanced MSC proliferation, which was consistent with our study, but also that it increased chondrogenic potentials, which was contradictory to our study. This contradiction might be due to the concentration ratio differences between BMAC–PRP and PRP along with TDSCs in the two studies. Considering that a separate study displayed different TDSC characteristics according to the concentration of PRP31, this explanation might be plausible.
The therapeutic benefits of BMACs on tendon healing have been reported in animal models, but the studies did not reveal the mechanism of the therapeutic effect9,32-35. We also did not investigate the mechanism of BMAC action on TDSCs, but we think that BMAC might affect tendon healing by enhancing the TDSC proliferation and inhibiting the aberrant differentiation of TDSCs.
The expression levels of type I and type III collagen did not show definite changes in immunocytochemical staining after BMAC–PRP injection, although a more quantitative analysis, such as Western blotting, was not performed in our study. Considering that previous studies have demonstrated that tenocytes cultured with collagen and PRP show higher expression of type I and III collagen13,36, further studies for the quantitative analysis of collagen expression must be conducted to elucidate the effect of the BMAC–PRP combination on the expression of collagen.
We did not conduct animal studies to explore the effect of the BMAC–PRP combination on rotator cuff tendon healing because our PRP and BMAC extraction kits were for human use, not for animal use. Preparations from different PRP and BMAC extraction kits might lead to different results, which can confound the conclusions drawn. Previous animal studies have demonstrated the effects of PRP or MSCs on rotator cuff tears12,37-39, so we think that BMAC–PRP will show a similar positive response in an animal model.
In this study, we did not use transcription factors or growth factors to enhance the function of BMAC or PRP. Considering that PRP combined with a transforming growth factor-β (TGF-β) inhibitor and a transducer of ERBB2 combined with BMAC enhance tendon healing40,41, further studies using these factors will be helpful.
We injected BMAC–PRP into the tear site under ultrasound guidance and found that the clinical symptoms were improved, and the tear size was reduced. Park et al.42 demonstrated that local injection of umbilical cord blood-derived MSCs was associated with a significant reduction in tear size, which is consistent with our results. The authors did not investigate the therapeutic mechanism of the MSCs but suggested the differentiation of cells capable of synthesizing tendon matrix or the secretion of factors as a therapeutic mechanism. Although we did not trace the injected BMACs and PRP, we think that enhancing the proliferation and migration and inhibiting the aberrant differentiation of the TDSCs might be a mechanism for the therapeutic benefits of BMAC–PRP on rotator cuff tendon tears.
Our study had some limitations. There was no control group in the rotator cuff tear experiments with which to compare the improvement of symptoms and tear size changes. However, considering that rotator cuff tears do not improve spontaneously43, our findings imply the healing effects of BMAC–PRP on rotator cuff tendon tears. The other limitation was that TDSCs used were from a single donor. However, we had difficulty in acquiring the human tendon tissues and isolating human TDSCs from them because the isolating procedure must be done within 30 min of the operation for isolating the TDSCs from the resected tendon tissues.
We found that BMAC–PRP enhanced the proliferation and migration of TDSCs and prevented the aberrant chondrogenic and osteogenic differentiation of TDSCs, which might provide a mechanistic basis for the therapeutic benefits of BMAC–PRP for rotator cuff tendon tear. A large-scale, multicenter, randomized controlled, prospective phase III study is required to confirm the findings of our study and to support the use of the BMAC–PRP combination protocols for rotator cuff tendon tear cases and as a new therapeutic approach.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Nos. HI15C-3060-010115 and HI14C3484). English correction was done by a native English-speaking medical editor at eWORLDEDITING, a specialized English correction company (http://www.eworldediting.com/). There is no commercial party related to this experiment. The authors declare no conflicts of interest.
References
- 1.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: Regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005; 5: 5–21. [PubMed] [Google Scholar]
- 2.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008; 466: 1528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JH. Mechanobiology of tendon. J Biomech. 2006; 39: 1563–82. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Maffulli N. Tendinopathy and tendon injury: The future. Disabil Rehabil. 2008; 30: 1733–15. [DOI] [PubMed] [Google Scholar]
- 5.Kang JR, Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg. 2012; 21: 175–80. [DOI] [PubMed] [Google Scholar]
- 6.Oliva F, Via AG, Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011; 39: 1282–9. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int Orthop. 2014; 38: 1811–8. [DOI] [PubMed] [Google Scholar]
- 9.Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012; 40: 1259–68. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Lee HJ, Ok JH, Park JS, Kim DW. Survivorship of implanted bone marrow-derived mesenchymal stem cells in acute rotator cuff tear. J Shoulder Elbow Surg. 2013; 22: 1037–15. [DOI] [PubMed] [Google Scholar]
- 11.Mora MV, Iban MA, Heredia JD, Laakso RB, Cuellar R, Arranz MG. Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells 2015; 7: 691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SJ, Lee SM, Kim JE, Kim SH, Jung Y. Effect of platelet-rich plasma with self-assembled peptide on the rotator cuff tear model in rat. J Tissue Eng Regen Med. 2017; 11(1): 77–85. [DOI] [PubMed] [Google Scholar]
- 13.Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y, Xu Z, Pan X, Yang L. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2017; 11(4): 1173–84. [DOI] [PubMed] [Google Scholar]
- 14.Andia I, Rubio-Azpeitia E. Angiogenic and innate immune responses triggered by PRP in tendon cells are not modified by hyperuricemia. Muscles Ligaments Tendons J. 2014; 4: 292–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Wang JH. PRP treatment effects on degenerative tendinopathy—An in vitro model study. Muscles Ligaments Tendons J. 2014; 4: 10–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Lian Z, Yin X, Li H, Jia L, He X, Yan Y, Liu N, Wan K, Li X, Lin S. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol. 2014; 26: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, Hakimi M, Wild M. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One 2013; 8: e71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JR, Houdek MT, Sierra RJ. Use of concentrated bone marrow aspirate and platelet rich plasma during minimally invasive decompression of the femoral head in the treatment of osteonecrosis. Croat Med J. 2013; 54: 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun JH, Han SH, Choi SH, Lee MH, Lee SJ, Song SU, Oh N. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. J Biomed Mater Res B Appl Biomater. 2014; 102: 1021–30. [DOI] [PubMed] [Google Scholar]
- 20.Zhao T, Yan W, Xu K, Qi Y, Dai X, Shi Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy 2013; 15: 792–804. [DOI] [PubMed] [Google Scholar]
- 21.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A 2010; 16: 1549–58. [DOI] [PubMed] [Google Scholar]
- 22.Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A 2012; 18: 840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue PY, Leung EP, Mak NK, Wong RN. A simplified method for quantifying cell migration/wound healing in 96-well plates. J Biomed Screen. 2010; 15: 427–33. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskeletal Disord. 2010; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, Iannotti JP, Mow VC, Sidles JA, Zuckerman JD. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994; 3: 347–52. [DOI] [PubMed] [Google Scholar]
- 26.Lui PP. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ—Update and future development. Stem Cell Res Ther. 2015; 6: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowe CS, Chiou G, McGoldrick R, Hui K, Pham H, Chang J. Tendon regeneration with a novel tendon hydrogel: In vitro effects of platelet-rich plasma on rat adipose-derived stem cells. Plast Reconstr Surg. 2015; 135: 981e–9e. [DOI] [PubMed] [Google Scholar]
- 28.Chiou GJ, Crowe C, McGoldrick R, Hui K, Pham H, Chang J. Optimization of an injectable tendon hydrogel: The effects of platelet-rich plasma and adipose-derived stem cells on tendon healing in vivo. Tissue Eng Part A 2015; 21: 1579–86. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010; 38: 2477–86. [DOI] [PubMed] [Google Scholar]
- 30.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, Jacobs CR. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 2009; 15: 431–5.19216642 [Google Scholar]
- 31.Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells—Implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015; 6: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 2013; 8: e75697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanazawa T, Soejima T, Noguchi K, Tabuchi K, Noyama M, Nakamura K, Shiba N. Tendon-to-bone healing using autologous bone marrow-derived mesenchymal stem cells in ACL reconstruction without a tibial bone tunnel-A histological study. Muscles Ligaments Tendons J. 2014; 4: 201–6. [PMC free article] [PubMed] [Google Scholar]
- 34.He M, Gan AW, Lim AY, Goh JC, Hui JH, Chong AK. Bone marrow derived mesenchymal stem cell augmentation of rabbit flexor tendon healing. Hand Surg. 2015; 20: 421–9. [DOI] [PubMed] [Google Scholar]
- 35.Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012; 13: 13605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012; 40: 1035–45. [DOI] [PubMed] [Google Scholar]
- 37.Valencia Mora M, Antuna Antuna S, Garcia Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury 2014; 45(suppl 4): S22–7. [DOI] [PubMed] [Google Scholar]
- 38.Lamplot JD, Angeline M, Angeles J, Beederman M, Wagner E, Rastegar F, Scott B, Skjong C, Mass D, Kang R, Ho S, Shi LL. Distinct effects of platelet-rich plasma and BMP13 on rotator cuff tendon injury healing in a rat model. Am J Sports Med. 2014; 42: 2877–87. [DOI] [PubMed] [Google Scholar]
- 39.Chen HS, Su YT, Chan TM, Su YJ, Syu WS, Harn HJ, Lin SZ, Chiu SC. Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant. 2015; 24: 509–20. [DOI] [PubMed] [Google Scholar]
- 40.Kelc R, Trapecar M, Gradisnik L, Rupnik MS, Vogrin M. Platelet-rich plasma, especially when combined with a TGF-beta inhibitor promotes proliferation, viability and myogenic differentiation of myoblasts in vitro. PLoS One 2015; 10: e0117302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Zhang Y, Lu Y, Wang Y, Kou X, Lou Y, Kang Y. TOB1 deficiency enhances the effect of bone marrow-derived mesenchymal stem cells on tendon-bone healing in a rat rotator cuff repair model. Cell Physiol Biochem. 2016; 38: 319–29. [DOI] [PubMed] [Google Scholar]
- 42.Park GY, Kwon DR, Lee SC. Regeneration of full-thickness rotator cuff tendon tear after ultrasound-guided injection with umbilical cord blood-derived mesenchymal stem cells in a rabbit model. Stem Cells Transl Med. 2015; 4: 1344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: A three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013; 95: 1249–55. [DOI] [PubMed] [Google Scholar]


