Abstract
Studies have shown that the presence of acute inflammation during recovery is indicative of poor outcomes after a traumatic brain injury (TBI); however, the role of chronic inflammation in predicting post-TBI-related symptoms remains poorly understood. The purpose of this study was to compare inflammatory biomarkers (tumor necrosis factor [TNF]-α, interleukin [IL]-6, and IL-10) in active duty personnel who either sustained or did not sustain a TBI. Service members were also assessed for post-traumatic stress disorder (PTSD), depression, and quality of life through self-reported measures. IL-6 and TNF-α concentrations were greater in the TBI group than in the control group. Of those with a TBI, IL-6 and TNF-α concentrations were greater in the high-PTSD group than the low-PTSD group. No significant differences were found in IL-10 or the IL-6/IL-10 ratios between those with low and high PTSD. Exploratory factor analysis was conducted to describe the latent structure of variables relating to emotional and physical health (i.e., Short Form 36 subcomponents, etc.) and their relationships within the TBI group with inflammatory cytokines. Four symptom profiles were found, with the third component most relating to PTSD and depression symptoms and high inflammation. This study indicates that the comorbidity of TBI and PTSD is associated with inflammation in a military sample, emphasizing the necessity for intervention in order to mitigate the risks associated with inflammation.
Keywords: mild traumatic brain injury, inflammation, biomarkers, post-traumatic stress disorder
Introduction
Traumatic brain injuries (TBIs) are the hallmark injuries of Operations Enduring Freedom (OEF) and Iraqi Freedom (OIF)1-3 and are linked to the onset of chronic neurological and psychological symptoms, along with postconcussive disorder, post-traumatic stress disorder (PTSD), depression, and sleep disturbance.3-6 TBIs that occur during deployment include both blunt forces and blast-related injuries, which can result in a broad range of injuries that result in neurological alterations that may result in the loss of consciousness and posttraumatic amnesia.7,8 The neuronal injuries that occur following a TBI often compromise neuronal function9 and likely contribute to susceptibility to behavioral symptoms including PTSD as well as depression; however, the biological links that contribute to these symptoms are not well understood. Previously, we and others have shown that inflammation is associated with PTSD and depression in chronic samples of individuals;10-13 yet, the links between chronic neurological and behavioral symptoms, inflammation, and postacute TBI have not been determined.
Previous studies have linked TBIs in patients with moderate to severe injuries that require emergency care, to acute inflammatory processes that include higher interleukin (IL)-6 in peripheral blood (24 to 48 h following injury),14 which are linked to poor outcomes. Specifically, high concentrations of IL-10, IL-8, and IL-6 found in blood within 24=h of a TBI have been shown to predict mortality.15 Elevated concentrations of IL-10 within 30 h in serum following a TBI have also demonstrated a similar predictive value.16 Elevated levels of IL-6 during acute recovery in cerebrospinal fluid (CSF) have been shown to predict poor recovery at 6 mo.17 Furthermore, in a study of acute TBI, a greater inflammatory load based on a biomarker-based index that included IL-6, IL-10, and tumor necrosis factor (TNF)-α and was linked to poor outcomes at 6 mo following the TBI.18
Therefore, inflammation during acute recovery has been linked to poor outcomes; yet, little is known regarding chronic inflammation in postacute TBI and related neurological and behavioral symptoms. In deployed military personnel, TBIs are linked to the onset of chronic disease and extensive use of medical services.19 The estimated cost of TBI in 2010 was US$76.5 billion.20 Additionally, the average yearly health-care cost for veterans having sustained a TBI is reported to be nearly 4 times greater than the cost for veterans who have not experienced a TBI.21 This suggests that chronic TBIs have a long-lasting effect on health and well-being. Chronic inflammation is common following deployment and, therefore, may be a contributing factor to increases in medical morbidity for the 2.4 million US military personnel who have been deployed.22 This line of research is essential, because many military personnel might not have yet developed the morbidity and mortality risks linked to inflammation,23-27 thus providing a window of opportunity to intervene and reduce the associated risk.
To address this critical issue, we compared inflammatory markers of TNF-α, IL-6, and IL-10 in a sample of military personnel previously deployed to combat stations that reported either a TBI or no TBI. We also examined relationships among comorbid behavioral and neurological symptoms to determine how inflammation may be associated with these often chronic and complex symptoms in military personnel. In using a young military cohort, our study provides the unique opportunity to study these relationships outside the context of confounding inflammatory-related comorbidity associated with aging in older and more complex cohorts.
Materials and Methods
This was a cross-sectional assessment of 83 active duty personnel belonging to a larger military cohort referred to the Madigan Sleep Disorders Clinic for sleep disturbance. This study was approved by institutional review board (#145947) at Madigan Army Medical Center in Tacoma, Washington. Self-reported measures were used to assess for postconcussive symptoms, PTSD, depression, and quality of life (QOL). Informed consent was obtained from each participant prior to any baseline measurements. All participants were deployed within 16 mo prior to sample collection. Exclusion criteria included the following: (1) history of drug or alcohol abuse in the previous year, (2) current severe medical conditions requiring chronic treatment (e.g., cancer, diabetes, human immunodeficiency virus [HIV], and autoimmune disorders) or a severe psychiatric condition (i.e., schizophrenia, bipolar disorder), and (3) severe neurological disorders (e.g., multiple sclerosis, seizure disorders, and history of stroke).
Procedures for Determining TBI Groups
Sixty-three individuals with a history of TBI (TBI+) were identified by self-reporting TBI on the Warrior Administered Retrospective Casualty Assessment Tool (WARCAT). Twenty participants without a self-reported TBI on the WARCAT were classified as controls (TBI−). The WARCAT assesses war-related and postdeployment injuries by investigating the mechanism of injury as well as evaluating immediate symptoms or loss of consciousness after injury, all of which may indicate that a subject has sustained a TBI.28 Controls were matched to TBI cases as much as possible on critical variables that would have influenced biomarkers including age, body mass index (BMI), gender, race, time since deployment, and military rank.
Collection Methods
Plasma samples were obtained from nonfasting subjects into ethylenediaminetetraacetic acid tubes, transported on ice, centrifuged, aliquoted, and frozen at −80 °C. Subject availability varied such that collection times ranged from 9:00 AM to 4:00 PM (mean = 11:36 AM; SD = 1h54min). Samples were thawed and analyzed in a single batch.
Symptom and QOL Measures
PTSD symptoms were measured using the PTSD Checklist Military Version, which has a score ranging from 0 to 80. Higher scores indicate higher severity, with a score over 50 meeting criteria for a positive PTSD screen.29 Depression symptoms were measured using the Quick Inventory of Depressive Symptomatology, which has a score ranging from 0 to 7. Higher scores indicate higher severity, and a score over 13 indicates a clinical diagnosis of depression.30
The Short Form 36 (SF-36) was used to assess QOL on 8 self-reported outcomes including physical functioning; role limitations caused by physical health; and role limitations caused by emotional problems, social functioning, emotional well-being, energy/fatigue, pain, and general health perceptions. Combined scores from each outcome yield a total possible score ranging from 0 to 100, with higher scores reflecting a more favorable health state. The SF-36 was derived from the RAND 36-Item Health Survey, the most widely utilized health-related QOL measure used with traumatized individuals that is regarded as both valid and reliable (test–retest correlation coefficient = .86).31
The WARCAT was used to assess war-related and postdeployment injuries.28 The WARCAT is a 20-item, self-administered tool used to ascertain details regarding postconcussive symptoms and mechanisms of injury commonly associated with TBI. Indication of any alteration in consciousness related to mechanisms of injury including blast exposure and blunt trauma is associated with a positive TBI screen.
Laboratory Analysis
Concentrations of TNF-α, IL-6, and IL-10 in plasma samples were analyzed using Simoa™ (Quanterix, Lexington, MA), a high-definition-1 analyzer, which is a paramagnetic bead-based enzyme-linked immunosorbent assay. This method has been previously described.32 The TNF-α, IL-6, and IL-10 assays have a low limit of detection of 0.126 pg/mL, 0.330 pg/mL, and 0.034 pg/mL, respectively. The reported coefficient of variation (intra- and interplate) values were below 10% for all analytes.
Statistical Methods
All statistical analyses were completed using IBM SPSS Statistics Version 23 (IBM, Armonk, NY). Demographic, military, and clinical characteristics group comparisons were made using independent samples t tests and χ2 tests for continuous and nominal data, respectively. Cytokine concentration levels were compared between groups using independent samples Mann–Whitney U tests. For biomarker data only, outliers were removed from group analyses using the Grubbs test. An exploratory factor analysis (EFA) was done to describe the latent structure of variables and the correlations between resultant components within the TBI group. We used the principle component analysis extraction method and promax rotation. Cases were excluded listwise. Multicollinearity was assessed by the determinant of the correlation matrix. If the determinant was greater than 0.00001, then multicollinearity was not considered a significant issue.33 We determined whether our data were appropriate for EFA by using the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity. Data were considered appropriate if the KMO was >0.7 and Bartlett’s test was significant at P < 0.05.34 Components were retained if the eigenvalues were greater than 1. Component scores for each individual were obtained using the regression method.
Results
TBI versus Control
Demographic, military, and clinical characteristics of the 83 participants used in this analysis are described in Table 1. The TBI group (N = 63) as a whole was matched to a control group (N = 20) on demographic (age, BMI, gender, race, and education) and military (service branch, military rank, and time elapsed since last deployment) characteristics.
Table 1.
Demographic, Military, and Clinical Characteristics.
| Cases (n = 63) | Controls (n = 20) | χ2/t | P Value | |
|---|---|---|---|---|
| Demographic and military characteristics | ||||
| Age | ||||
| Age: mean (SD) | 33.2 (9.0) | 31.6 (8.0) | 0.694 | 0.489 |
| Body mass index (BMI) | ||||
| BMI: mean (SD) | 29.7 (4.1) | 27.9 (4.3) | 1.611 | 0.111 |
| Gender | ||||
| Male: n (%) | 63 (100) | 19 (95) | 0.241 | |
| Race | ||||
| White: n (%) | 41 (66) | 12 (67) | 1.00 | |
| Nonwhite: n (%) | 21 (34) | 6 (33) | ||
| Education | ||||
| Some college: n (%) | 37 (62) | 7 (41) | 0.17 | |
| No college: n (%) | 23 (38) | 10 (59) | ||
| Service branch | ||||
| Air Force: n (%) | 2 (3) | 0 | 1.412 | 0.49 |
| Army: n (%) | 60 (95) | 18 (95) | ||
| Navy: n (%) | 1 (2) | 1 (5) | ||
| Military rank | ||||
| Junior: n (%) | 21 (37) | 8 (44) | 0.875 | 0.65 |
| NCO: n (%) | 34 (60) | 10 (56) | ||
| Officer: n (%) | 2 (3) | 0 | ||
| Time elapsed since deployment | ||||
| <6 Months: n (%) | 13 (21) | 6 (32) | 0.36 | |
| ≥6 Months: n (%) | 50 (79) | 13 (68) | ||
| Clinical characteristics | ||||
| Depression (QIDS): n (%) | 12.4 (4.9) | 7.7 (4.3) | 3.917 | 0.000 |
| PTSD Total (PCL-M): n (%) | 48.7 (15.1) | 32.5 (11.5) | 4.400 | 0.000 |
| Quality of life (Short Form 36) | ||||
| Bodily pain: n (%) | 50.5 (25.6) | 63.4 (22.1) | −1.539 | 0.057 |
| Emotional well- being: n (%) | 52.6 (19.5) | 71.7 (20.1) | −3.539 | 0.001 |
| Energy/fatigue: n (%) | 25.6 (16.9) | 38.3 (18.6) | −2.759 | 0.007 |
| Health perceptions: n (%) | 49.1 (18.1) | 67.2 (22.0) | −3.565 | 0.001 |
| Physical functioning: n (%) | 67.85 (23.7) | 77.3 (26.2) | −1.450 | 0.151 |
| Role limits— emotional: n (%) | 45.6 (43.6) | 70.3 (41.1) | −2.146 | 0.035 |
| Role limits— physical: n (%) | 31.7 (38.4) | 56.9 (41.8) | −2.413 | 0.018 |
| Social functioning: n (%) | 51.5 (24.8) | 71.6 (21.7) | −3.114 | 0.003 |
Abbreviations: NCO, noncommissioned officer; PTSD, post-traumatic stress disorder; PCL-M, PTSD Checklist Military Version; QIDS, Quick Inventory of Depressive Symptomatology; SD, standard deviation. Bold values signify p < 0.05 statistical significance.
Biomarkers of Inflammation
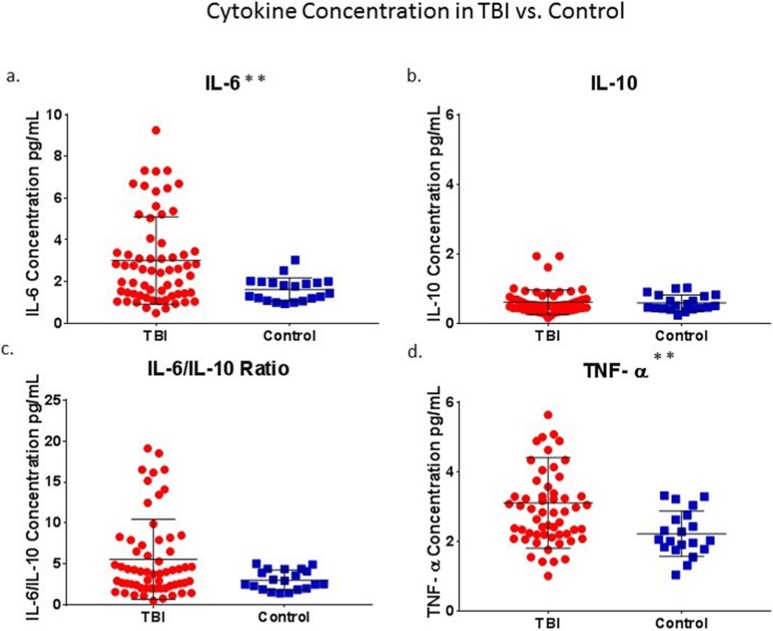
Cytokine concentrations (pg/mL) for both control and TBI groups followed nonnormal distributions as assessed by Kolmogorov–Smirnov and Shapiro–Wilk tests of normality. The Grubbs test was used to remove outliers. No outliers were found within the control group. Within the TBI group, no outliers were found for IL-6, 3 outliers were removed for IL-10 (3.31, 3.31, and 5.00), and 2 outliers were removed for TNF-α (10.16, 12.61). Significant group differences were found for IL-6 and TNF-α, but not for IL-10 or for IL-6/IL-10 ratios (Fig. 1). Mann–Whitney U tests showed that IL-6 concentrations were greater in the TBI group, N = 63 (3.01 ± 2.10), compared to the control group, N = 20 (1.62 ± 0.56, P = 0.007), and TNF-α concentrations were greater in the TBI group, n = 59 (3.10 ± 1.30), compared to the control group, n = 20 (2.22 ± 0.65, P = 0.003). Since all outliers belonged to the TBI group, and all outlying values were 3 standard deviations above the mean, inclusion of these outliers resulted in even greater group differences.
Figure 1.
TBI versus control: Significant group differences were found for IL-6 and TNF-α (a and d) but not for IL-10 or for IL-6/IL-10 ratios (b and c). Asterisk denote statistical significance as follows: *P < 0.05, ** P < 0.01. TBI, traumatic brain injury; TNF, tumor necrosis factor; IL, interleukin.
TBI: Low PTSD versus High PTSD
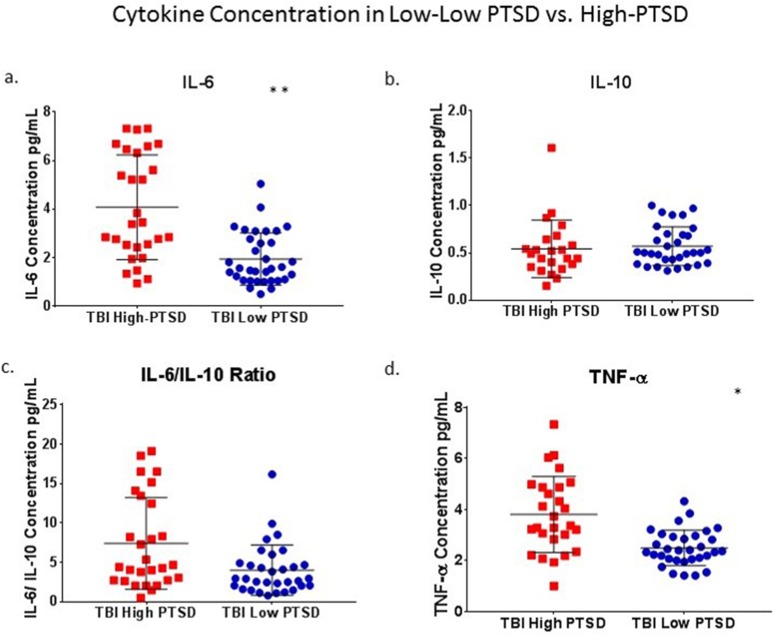
Within the TBI group, those with low PTSD (n = 35) were compared to those with high PTSD (n = 28). Significant differences were found for IL-6 and TNF-α, but not for IL-10 or IL-6/IL-10 ratios (Fig. 2). Mann–Whitney U tests showed that IL-6 concentrations were greater in the high-PTSD group, n = 28 (4.08 ± 2.16), compared to low-PTSD group, n = 34 (1.94 ± 1.07, P = 0.001), and TNF-α concentrations were greater in the high-PTSD group, n = 27 (3.81 ± 1.49), compared to the low-PTSD group, n = 32 (2.49 ± 0.70, P = 0.013).
Figure 2.
(TBI) Low PTSD versus high PTSD: Within the TBI group, those with low PTSD (n = 35) were compared to those with high PTSD (n = 28). Significant differences were found for IL-6 and TNF-α (a and d) but not for IL-10 or IL-6/IL-10 ratios (b and c). Asterisk denote statistical significance as follows: *P < 0.05, **P < 0.01. TBI, traumatic brain injury; PTSD, posttraumatic stress disorder; IL, interleukin; TNF, tumor necrosis factor.
EFA: TBI
An EFA was conducted to describe the latent structure of variables relating to physical and emotional health and the correlations between resultant components within the TBI group. Variables in the analysis included SF-36 subcomponents as well as symptoms of PTSD and depression and inflammatory biomarkers of IL-6, IL-10, IL-6/IL10 ratios, and TNF-α concentrations.
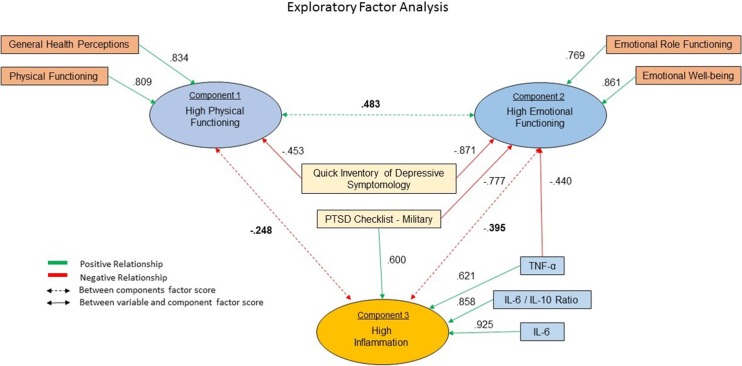
Fifty participants had no missing data and were therefore included in the analysis (low PTSD, n = 28; high PTSD, n = 22). The determinant was 0.00004, KMO = 0.746, and Bartlett’s test was significant, P < 0.0001, and therefore, we found our data appropriate for EFA. Communalities were all greater than 0.584. Four components had eigenvalues greater than 1 and cumulatively accounted for 75.1% of the variance. Component 1 accounted for 42.7%, component 2 accounted for 13.2%, component 3 accounted for 10.8%, and component 4 accounted for 8.4% of the variance (see Fig. 3).
Figure 3.
(TBI) Exploratory factor analysis: Exploratory factor analysis was conducted to describe the latent structure of variables relating to emotional and physical health (i.e., Short Form 36 subcomponents, etc.) and their relationships within the TBI group with inflammatory cytokines. Four components had eigenvalues greater than 1 and cumulatively accounted for 75.1% of the variance. (Component 1 accounted for 42.7%, component 2 accounted for 13.2%, component 3 accounted for 10.8%, and component 4 accounted for 8.4% of the variance). Components 1, 2, and 3 are described in this schematic. As shown, component 3 greatly differs from components 1 and 2, with high: PTSD, depression, TNF-α, IL-6, IL-6/IL-10 ratios and is primarily described by variables relating to high inflammation and poor mental health: IL-6 (0.925), IL-6/IL-10 ratios (0.858), TNF-α (0.621), PTSD (0.600), and depression (0.428). PTSD, posttraumatic stress disorder; IL, interleukin; TNF, tumor necrosis factor.
Component 1 is primarily described by variables in the SF-36 including high (physical role functioning, general health perceptions, physical functioning, social functioning, and vitality) and low (bodily pain and depression). In contrast, component 2 is primarily described by low (PTSD, depression, TNF-α, and bodily pain) and high (emotional functioning, emotional well-being, emotional role functioning, social functioning, vitality, and physical role functioning). Component 3 greatly differs from components 1 and 2, with high (PTSD, depression, TNF-α, IL-6, IL-6/IL-10 ratios) and is primarily described by variables relating to high inflammation and poor mental health: IL-6 (0.925), IL-6/IL-10 ratios (0.858), TNF-α (0.621), PTSD (0.600), and depression (0.428).
Discussion
Here we report that TBIs sustained during deployment relate to high inflammation; however, our major finding is that this relationship is driven by the very high levels of inflammation in those military personnel with comorbid PTSD and to a lesser extent, comorbid depression. This finding is unique, as previous studies have not examined the relationships among comorbid symptoms and their link to higher inflammation in military cohorts with TBIs. These findings highlight the need to intervene in military personnel and veterans with comorbid PTSD and TBIs in order to mitigate the long-term morbidity and mortality risks related to chronic inflammation.
Although there is an established link between TBIs and PTSD in military personnel deployed to OIF and OEF, little is known regarding the health impact of these comorbid conditions. While previous studies link chronic PTSD in military personnel to inflammation,35-37 the long-term outcomes following TBIs and relationships to PTSD are not determined. Chronic inflammation is one of the most well-established morbidity and mortality risk factors,38,39 playing a key role in the pathophysiology and progression of metabolic syndrome, a cluster of premorbid conditions associated with metabolic dysfunction,40 resulting in risk factors for stroke,41 type 2 diabetes,42 and cardiovascular disease.40,43 Chronic activation of the innate and adaptive immune system in the postacute phases of TBI provides a possible link to inflammatory-related comorbidity.14,44 A chronic inflammatory state is the hallmark of a range of metabolic diseases and has been implicated in the pathogenesis of diabetes and cardiovascular disease. The notion that TBI may result in chronic inflammation highlights a possible direct link to comorbid metabolic disorders.40 Having PTSD more than doubles all-cause medical mortality in Vietnam veterans,45 suggesting that these morbidity risks in younger cohorts may magnify as they age.
There are also consequences of chronic inflammation on neuronal function which can even result in neuronal loss46 and may contribute to the maintenance of these more long-term behavioral symptoms following TBI.47 Chronic inflammation is known to increase the permeability of the blood–brain barrier (BBB), increasing the risk for mood and cognitive declines as well as neurodegenerative processes during aging.48 There is also evidence that inflammatory cytokine overactivity including IL-6 and TNF-α within the central nervous system results in microglia overactivation, destruction of noninjured neurons, increased permeability of the BBB, and overall neuronal loss.49 In preclinical studies, blocking both IL-6 and TNF-α in the hippocampus and hypothalamus following acute stress was shown to ameliorate the risk for PTSD like symptom onset,50 indicating that excessive central inflammatory activity contributes to neuronal changes related to PTSD onset. In clinical studies of PTSD, inflammatory overactivity is related to alterations in the function of the neuroendocrine system,51,52 suggesting that interactive processes that lead to these alterations in function compromise neuronal health. PTSD is associated with overall volume reductions in neurons, with the hippocampus most often reported as reduced in volume and function;53,54 chronic inflammation may be implicated in these neurological function changes. In military personnel with PTSD, peripheral concentrations of the soluble receptor II for TNF were significantly higher and related to a lesser hippocampal volume,37 providing evidence of the link between inflammation and neuronal loss. Together these studies link inflammation to neuronal impairment and loss, suggesting that the inflammation observed in this study of younger military personnel with TBIs and comorbid PTSD may have implications to neuronal viability.
Although our findings highlight the impact of comorbid PTSD and TBIs on inflammatory biomarkers, the findings are limited by a relatively small sample size that included mostly young males. However, our finding that the relationship between comorbid PTSD and TBIs is present in a young, relatively healthy cohort highlights the need to intervene to prevent the often irreversible risks related to chronic inflammation. These findings are limited by the exclusion of 13 subjects from the EFA. In having excluded these subjects, we were unable to determine how their inclusion might have impacted the results of analysis and therefore what degree of bias, if any, their exclusion may have introduced. These findings are also limited by a cross-sectional design that precludes a better understanding of the temporal relationship between TBIs and behavioral neurological symptoms and inflammation. Therefore, additional larger more prospective studies are warranted to determine how inflammation relates to these often comorbid symptoms following TBIs. This line of research is important, as here we show that TBIs and comorbid TBIs are associated with inflammation, which is linked to both physical and behavioral health declines. Since there are more than 2 million Americans deployed to OEF or OIF, and a quarter of these military personnel or veterans have PTSD or TBIs, protecting these individuals from the chronic impact of inflammation becomes essential as these individuals age and become at greater risk for morbidity and health mortality risks.
Footnotes
Ethical Approval: This study was approved by institutional review board (#145947) at Madigan Army Medical Center in Tacoma, Washington.
Statement of Informed Consent: Informed consent was obtained from each participant prior to any baseline measurements.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was performed at the Madigan Army Medical Center, Tacoma, WA. Support for this work included funding from the Intramural department of the National Institutes of Health, National Institutes of Nursing Research, and the Department of Defense in the Center for Neuroscience and Regenerative Medicine.
References
- 1. Fisher H. US military casualty statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service; 2010 http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA535410. Accessed June 20th, 2016.
- 2. Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma Inj Infect Crit Care. 2008;64(2):295–299. [DOI] [PubMed] [Google Scholar]
- 3. Armed Forces Health Surveillance Center. Insomnia, active component, US Armed Forces, January 2000-December 2009. 2010;12–15. http://www.health.mil/Reference-Center/Reports/2010/01/01/Medical-Surveillance-Monthly-Report-Volume-17-Number-5. Accessed June 20th, 2016.
- 4. Seelig AD, Jacobson IG, Smith B, Hooper TI, Boyko EJ, Gackstetter GD, Gehrman P, Macera CA, Smith TC. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33(12):1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai JC. Neurological and neurobehavioral sequelae of obstructive sleep apnea. NeuroRehabilitation. 2010;26(1):85–94. [DOI] [PubMed] [Google Scholar]
- 6. Capaldi VF, 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil Med. 2011;176(8):879–888. [DOI] [PubMed] [Google Scholar]
- 7. DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. N Engl J Med. 2005;352(13):1335–1342. [DOI] [PubMed] [Google Scholar]
- 8. Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, Jaffee MS, Moore DF. Case report of a soldier with primary blast brain injury. Neuroimage. 2009;47(Suppl 2):T152–T153. [DOI] [PubMed] [Google Scholar]
- 9. Kou Z, Vandevord PJ. Traumatic white matter injury and glial activation: from basic science to clinics. Glia. 2014;62(11):1831–1855. [DOI] [PubMed] [Google Scholar]
- 10. Maes M, Mihaylova I, Kubera M, Ringel K. Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):169–175. [DOI] [PubMed] [Google Scholar]
- 11. Gill J, Luckenbaugh D, Charney D, Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry. 2010;68(11):999–1006. [DOI] [PubMed] [Google Scholar]
- 12. Gill J, Lee H, Barr T, Baxter T, Heinzelmann M, Rak H, Mysliwiec V. Lower health related quality of life in U.S. military personnel is associated with service-related disorders and inflammation. Psychiatry Res. 2014;216(1):116–122. [DOI] [PubMed] [Google Scholar]
- 13. Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M, Yan L, Baccarelli A. PTSD and DNA methylation in select immune function gene promoter regions: a repeated measures case-control study of U.S. military service members. Front Psychiatry. 2013;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plesnila N. The immune system in traumatic brain injury. Curr Opin Pharmacol. 2016;26:110–117. [DOI] [PubMed] [Google Scholar]
- 15. Ferreira LC, Regner A, Miotto KD, Moura SS, Ikuta N, Vargas AE, Chies JA, Simon D. Increased levels of interleukin-6 -8, and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 2014;28(10):1311–1316. [DOI] [PubMed] [Google Scholar]
- 16. Schneider Soares FM, Menezes de Souza N, Libório Schwarzbold M, Paim Diaz A, Costa Nunes J, Hohl A, Nunes Abreu da Silva P, Vieira J, Lisboa de Souza R, Moré Bertotti M, et al. Interleukin-10 is an independent biomarker of severe traumatic brain injury prognosis. Neuroimmunomodulation. 2012;19(6):377–385. [DOI] [PubMed] [Google Scholar]
- 17. Kumar RG, Rubin JE, Berger RP, Kochanek PM, Wagner AK. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain Behav Immun. 2016;53:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santarsieri M, Kumar RG, Kochanek PM, Berga S, Wagner AK. Variable neuroendocrine–immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain Behav Immun. 2015;45:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pugh MJ, Finley EP, Copeland LA, Wang CP, Noel PH, Amuan ME, Parsons HM, Wells M, Elizondo B, Pugh JA. Complex comorbidity clusters in OEF/OIF veterans: the polytrauma clinical triad and beyond. Med Care. 2014;52(2):172–181. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. 2014. Injury prevention & control: traumatic brain injury & concussion. http://www.cdc.gov/TraumaticBrainInjury/severe.html. Accessed June 20th, 2016.
- 21. Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, Sayer NA. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq war veteran VA users. Med Care. 2012;50(4):342–346. [DOI] [PubMed] [Google Scholar]
- 22. Edwards RD. A review of war costs in Iraq and Afghanistan. The National Bureau of Economic Research Working Paper Series Cambridge (MA): National Bureau of Economic Research; 2010. p. 38 doi:10.3386/w16163. [Google Scholar]
- 23. Paulus EJ, Argo TR, Egge JA. The impact of posttraumatic stress disorder on blood pressure and heart rate in a veteran population. J Trauma Stress. 2013;26(1):169–172. [DOI] [PubMed] [Google Scholar]
- 24. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol. 2012;31(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherrer JF, Chrusciel T, Zeringue A, Garfield LD, Hauptman PJ, Lustman PJ, Freedland KE, Carney RM, Bucholz KK, Owen R, et al. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. Am Heart J. 2010;159(5):772–779. [DOI] [PubMed] [Google Scholar]
- 26. Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70(6):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phillips AC, Batty GD, Gale CR, Deary IJ, Osborn D, MacIntyre K, Carroll D. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the Vietnam experience study. Psychosom Med. 2009;71(4):395–403. [DOI] [PubMed] [Google Scholar]
- 28. Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24(1):14–23. [DOI] [PubMed] [Google Scholar]
- 29. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. [DOI] [PubMed] [Google Scholar]
- 31. MacKenzie EJ, Sacco WJ, Luchter S, Ditunno JF, Staz CF, Gruen GS, Marion DW, Schwab WC. ; Pennsylvania Study Group on Functional Outcomes Following Trauma. Validating the Functional Capacity Index as a measure of outcome following blunt multiple trauma. Qual Life Res. 2002;11(8):797–808. [DOI] [PubMed] [Google Scholar]
- 32. Mondello S, Buki A, Barzo P, Randall J, Provuncher G, Hanlon D, Wilson D, Kobeissy F, Jeromin A. CSF and plasma amyloid-beta temporal profiles and relationships with neurological status and mortality after severe traumatic brain injury. Sci Rep. 2014;4:6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Field A. Discovering statistics using SPSS. London (UK): Sage; 2009. [Google Scholar]
- 34. Field A. Discovering statistics using IBM SPSS statistics London (UK): Sage; 2013. [Google Scholar]
- 35. Groer MW, Kane B, Williams SN, Duffy A. Relationship of PTSD symptoms with combat exposure, stress, and inflammation in American soldiers. Biol Res Nurs. 2015;17(3):303–310. [DOI] [PubMed] [Google Scholar]
- 36. Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. [DOI] [PubMed] [Google Scholar]
- 37. O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, Weiner MW, Neylan TC. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 2015;51:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lustenberger T, Kern M, Relja B, Wutzler S, Störmann P, Marzi I. The effect of brain injury on the inflammatory response following severe trauma. Immunobiology. 2016;221(3):427–431. [DOI] [PubMed] [Google Scholar]
- 39. Sun J, Axelsson J, Machowska A, Heimbürger O, Bárány P, Lindholm B, Lindström K, Stenvinkel P, Qureshi AR. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol. 2016;11(7):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci. 2016;13(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grenon SM, Owens CD, Alley H, Perez S, Whooley MA, Neylan TC, Aschbacher K, Gasper WJ, Hilton JF, Cohen BE. Posttraumatic stress disorder is associated with worse endothelial function among veterans. J Am Heart Assoc. 2016;5(3):e003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dallmeier D, Larson MG, Wang N, Fontes JD, Benjamin EJ, Fox CS. Addition of inflammatory biomarkers did not improve diabetes prediction in the community: the Framingham heart study. J Am Heart Assoc. 2012;1(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brewer-Smyth K. Obesity, traumatic brain injury, childhood abuse, and suicide attempts in females at risk. Rehabil Nurs. 2014;39(4):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rathbone AT, Tharmaradinam S, Jiang S, Rathbone MP, Kumbhare DA. A review of the neuro- and systemic inflammatory responses in post concussion symptoms: introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav Immun. 2015;46:1–16. [DOI] [PubMed] [Google Scholar]
- 45. Schlenger WE, Corry NH, Williams CS, Kulka RA, Mulvaney-Day N, DeBakey S, Murphy CM, Marmar CR. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. Am J Epidemiol. 2015;182(12):980–990. [DOI] [PubMed] [Google Scholar]
- 46. Oosthuizen F, Wegener G, Harvey BH. Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr Dis Treat. 2005;1(2):109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22(9):411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 49. Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628(Pt B):288–297. [DOI] [PubMed] [Google Scholar]
- 50. Levkovitz Y, Fenchel D, Kaplan Z, Zohar J, Cohen H. Early post–stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. European Neuropsychopharmacology. 2015;25(1):124–132. [DOI] [PubMed] [Google Scholar]
- 51. Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Zuiden M, Heijnen CJ, Maas M, Amarouchi K, Vermetten E, Geuze E, Kavelaars A. Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: a prospective study. Psychoneuroendocrinology. 2012;37(11):1822–1836. [DOI] [PubMed] [Google Scholar]
- 53. Sussman D, Pang EW, Jetly R, Dunkley BT, Taylor MJ. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neuroscience. 2016;17(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SA. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. 2015;233(3):436–442. [DOI] [PubMed] [Google Scholar]