Abstract
Traumatic brain injury (TBI) is one of the leading causes of death and disability in the population worldwide, with a broad spectrum of symptoms and disabilities. Posttraumatic hyperexcitability is one of the most common neurological disorders that affect people after a head injury. A reliable animal model of posttraumatic hyperexcitability induced by TBI which allows one to test effective treatment strategies is yet to be developed. To address these issues, in the present study, we tested human embryonic stem cell–derived neural stem cell (NSC) transplantation in an animal model of posttraumatic hyperexcitability in which the brain injury was produced in one hemisphere of immunodeficient athymic nude rats by controlled cortical impact, and spontaneous seizures were produced by repeated electrical stimulation (kindling) in the contralateral hemisphere. At 14 wk posttransplantation, we report human NSC (hNSC) survival and differentiation into all 3 neural lineages in both sham and injured animals. We observed twice as many surviving hNSCs in the injured versus sham brain, and worse survival on the kindled side in both groups, indicating that kindling/seizures are detrimental to survival or proliferation of hNSCs. We also replicated our previous finding that hNSCs can ameliorate deficits on the novel place recognition task,33 but such improvements are abolished following kindling. We found no significant differences pre- or post-kindling on the elevated plus maze. No significant correlations were observed between hNSC survival and cognitive performance on either task. Together these findings suggest that Shef6-derived hNSCs may be beneficial as a therapy for TBI, but not in animals or patients with posttraumatic hyperexcitability.
Keywords: traumatic brain injury (TBI), controlled cortical impact (CCI), human neural stem cell, cell transplantation, posttraumatic hyperexcitability, kindling model, spatial–hippocampal memory, emotional memory
Introduction
Traumatic brain injury (TBI) is defined as any injury causing altered brain function or brain pathology as a result of external mechanical force, with grades ranging from no visible damage, to bruising, to edema and hematoma.1 TBI is the leading cause of death and disability in children and young adults worldwide.1 In the United States, more than 1.7 million people experience a TBI that leads to hospitalization.2 While mild TBI (mTBI) accounts for 75% of all TBIs,3 TBI accounts for 30.5% of all injury-related deaths,2 and 43% of patients discharged after acute TBI hospitalization develop long-term disability.4 Thus, an estimated 5.3 million people are living with a long-term TBI-related disability.5 This population costs the United States an estimated cost of 56 to 221 billion each year.6,7
TBI is a disease with a broad spectrum of symptoms and disabilities. Common events that cause TBI include car accidents, falls, violence and abuse, gunshots, explosive blasts and other combat injuries, sport injuries, and any kind of object that penetrates the skull.2,8 As a result of TBI, brain function can be temporarily or permanently impaired. Altered brain function resulting from TBI can have significant chronic effects, including difficulties in concentration and memory, balance and vision changes, sleep disturbance and manifestation of spontaneous seizure.2,9,10
In the present study, we focused on spontaneous seizures, one of the common problems associated with TBI. TBI is often accompanied by the delayed development of posttraumatic epilepsy (PTE), which is manifested as temporal lobe epilepsy (TLE) in as many as 62% of trauma patients11; although others put the risk lower than 60% and suggest that risk of PTE is related to injury severity.12–14 Most seizures happen in the first several days or weeks after a brain injury, although some may occur months or years after the initial injury.15 Post-TBI seizures can be divided into early posttraumatic seizure, when they occur in the first week after injury, and late posttraumatic seizures, when they occur more than 7 days after injury and epilepsy, more than one seizure.15 About 25% of people who have an early posttraumatic seizure will have another seizure months or years later; about 80% of people who had a late posttraumatic seizure will have another seizure.15
Experimental animal models and human TLE are characterized by hippocampal cell loss and mossy fiber sprouting in the dentate gyrus16,17; however, the development of an animal model that more closely mimics human PTE is still in progress. Of these models, fluid percussion injury (FPI) and weight drop have been the most widely investigated models of posttraumatic hyperexcitability,17,18 in contrast to the controlled cortical impact (CCI) injury model. However, regardless of the injury model used, the incidence of spontaneous seizures in post-TBI rodents is approximately 20%19,20 and may be related to injury severity,21 making studies of TBI-induced seizures difficult. At a 20% incidence rate, testing stem cell therapies in a TBI-TLE model would be prohibitively difficult as 80% of animals would be unaffected, yet all animals would have to receive a cell therapy. Since CCI has not been used as a model of injury-induced TLE, in the present study we added a classical kindling component to the initial CCI at 10 wk post-CCI injury, to reliably induce posttraumatic hyperexcitability in all animals in the “kindled” groups. After our study was completed, a group published a protocol for chemical or electrical stimulation of epilepsy post-TBI in a CCI model, reporting that a prior TBI accelerated the time to kindling compared to control animals.22
Kindling is a costly and time-consuming procedure but is one of the most used models of electrical stimulation for the study of chronic seizures.23 It may be induced in rodents by repetitive, systemic administration of convulsants24,25 or by repeated electrical stimulation of sensitive brain regions.24,26 The kindling protocol we used consists of repetitive electrical stimulation in the perforant path (PP), which promotes a gradual and progressive enhancement of electroencephalographic (EEG) activity and behavioral responses, culminating in generalized seizures.27 The progression of the seizure is assessed through a behavioral scale known as “Racine stages,”28 which can be directly correlated with the after discharge (AD) duration obtained from an indwelling electrode in response to an otherwise nonseizure-inducing stimulus.27
Currently, no effective pharmaceutical or rehabilitative treatments exist for either TBI or posttraumatic TLE.29,30 Many groups are evaluating whether stem cells can offer a therapy that can improve the quality and length of life for those suffering from TBIs.31–33 Cell therapies for TBI have recently been reviewed by several independent groups34–36 (see also table 3 in Gold et al.37). Neural stem cell transplantation has been applied in various neurological disease models, including stroke, spinal cord injury, and neurodegenerative disease, based on its ability to replace the lost neural cells and improve functional deficits.38–41 Recently, we reported that the transplantation of human embryonic stem cell (ES)-derived neural stem cells (NSCs) restores cognition in an immunodeficient rat model of TBI.33 In the present study, we first assessed whether kindling alone (after a prior TBI) had an effect on novel place recognition (NPR), elevated plus maze (EPM), AD, and histology; in particular, whether kindling exacerbated the effect of TBI on behavioral end points. Second, we tested the hypothesis that the transplantation of human NSCs (hNSCs) can reduce/prevent the effects of kindling and TBI on NPR, EPM, and AD, and in particular, on the posttraumatic hyperexcitability. We also assessed the effect of kindling on hNSCs transplantation: does kindling increase or decrease the number of surviving hNSCs after TBI and does kindling change the fate of hNSCs? A better understanding of these outcomes and interactions will be important in planning future therapies in patients with TBI-based PTE.
Materials and Methods
Experimental Design
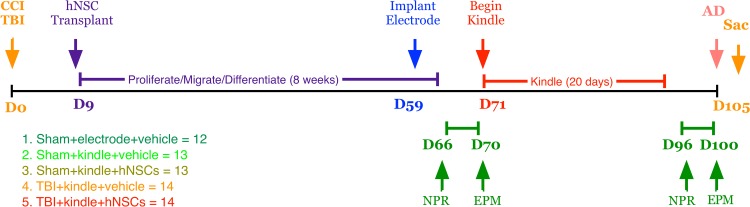
All experiments, including animal housing conditions, surgical procedures, and postoperative care, were in accordance with the Institutional Animal Care and Use Committee guidelines at the University of California–Irvine (UCI). The use of human cells was approved by UCI’s human Stem Cell Review Oversight committee. Sixty-six male athymic nude (ATN) rats (RNU −/−, Cr: NIH-rnu, homozygous, 6 wk old from the National Cancer Institute, Frederick, MD) were randomly divided into 5 experimental groups: sham + electrode + vehicle (n = 12; control group with only craniotomy and implant of electrode for kindling), sham + kindle + vehicle (n = 13), sham + kindle + hNSCs (n = 13), TBI+kindle+vehicle (n = 14), and TBI + kindle + hNSCs (n = 14), prior to the CCI or craniotomy procedure. Figure 1 depicts the experimental time line of hits, cell transplants, behavior, and kindling.
Figure 1.
Experimental groups and experimental design. A total of 66 ATN rats were randomly assigned to 1 of 5 groups (Sham + electrode + vehicle, 12; sham + kindle + vehicle, 13; sham + kindle + hNSCs, 13; TBI + kindle + vehicle, 14; and TBI + kindle + hNSCs, 14). The sham animals received a craniotomy, while the TBI animals received a CCI injury at D0. Nine days postsurgery, animals were reanesthetized and received either vehicle or 500,000 hNSCs. Animals were allowed to recover for 50 days before being implanted with an electrode. After 1 wk, animals were tested for the first time on NPR and EPM tasks. Testing was followed by up to 20 days of kindling and then a second NPR and EPM assessment. After discharge (AD) duration was recorded following behavioral tests, and the animals were sacrificed on day 105 (14 wk posttransplantation). All testing were conducted by persons blind to treatment condition. ATN, immunodeficient athymic nude; TBI, traumatic brain injury; CCI, controlled cortical impact; hNSC, human neural stem cell; NPR, novel place recognition; EPM, elevated plus maze; Sac, sacrifice.
CCI
TBIs were produced using a CCI device (TBI 0310; Precision Systems and Instrumentation, Fairfax Station, VA) which uses a pneumatic piston to deliver a unilateral cortical contusion via a 5 mm flat metal impactor tip, with a 2.25 mm impact depth, 4.5 m/s velocity, and 500 ms dwell time. Injuries were produced under 3% isoflurane anesthesia with rats positioned in stereotaxic holder (Leica Microsystem Inc., Buffalo Grove, IL). The stereotaxic coordinates for the craniotomy and/or CCI were anterior/posterior (A/P) −4.5 mm, and medial/lateral (M/L) −3.6 mm in the right hemisphere above the hippocampus. A 6-mm hole was cut with a handheld trephine and the dura exposed. All animals received a craniotomy, but only the TBI groups received a CCI. All animals received lactated Ringer’s (50 mL/kg) subcutaneously as well as buprenorphine (0.5 mg/kg; Hospira Inc., Lake Forest, IL) immediately after surgery and for 2 days thereafter. Due to the immunodeficient nature of ATN rats, an antibiotic (Baytril, 2.5 mg/kg; Western Medical Supply, Arcadia, CA) was administered immediately after surgery and daily for 5 days thereafter.
Preparation and Transplantation of hNSC
Before transplantation, animals were randomly divided into the 5 experimental groups (see Fig. 1). In all, 13 sham and 14 injured animals were transplanted via bilateral injection with xeno-free CD133+/CD34- Shef6 hNSCs at passage 27 (p27), which were prepared via magnetic-activated cell sorting for CD133 and against CD34, as previously reported.42
Both hNSCs and vehicle were prepared fresh the same day as transplantation: 9 days post-TBI. ATN rats were anesthetized with 3% isoflurane and positioned in a stereotaxic holder. A total of 6 injections, 3 per hemisphere, of either hNSCs or vehicle, were made. In the ipsilateral side, the 3 injection sites were made inside the craniotomy hole. On the contralateral side, additional small holes were drilled. A Dremel® rotary tool (Racine, WI), set at 5,000 rpm, was used to create the 3 contralateral injection burr holes. A 5 µL Hamilton syringe (Cat#87930; Hamilton Company, Reno, NV) with a 1” 30G blunt needle was mounted into an UMP-3 (World Precision Instruments, Sarasota, FL) injector connected to a SYS-Micro4 controller (both from World Precision Instruments, Sarasota, FL). The 6 injections were made at the following coordinates: injection #1 M/L 2.50, A/P −2.50, and deep −3.40; injection #2 M/L −4.50, A/P −4.20, and deep −4.00; and injection #3 M/L −5.40, A/P −5.28, and deep −5.40. Cells were first triturated 5 times with a 10-µL pipette, then pulled up into syringe over approximately 10 sec. For each injection, the needle was initially lowered an additional 0.15 mm to create a pocket in the brain. For each of the 6 injections, a dose of 7.5 × 104 cells/µL (1.11 µL per site) was administered over 2 min with a dwell time 4 min after injection. The total dose was 5 × 105 hNSCs. Prior to penetrating the tissues, the syringe was tested each time to ensure it was not clogged by ejecting some of the solution into the air manually through the Micro4 controller. Vehicle injection animals received the same procedure but received 6 cell culture media injections of 1.11 µL. Bone wax was applied to seal each of the burr holes. Animals received lactated Ringer’s (50 mL/kg) subcutaneously immediately after surgery, and buprenorphine (0.5 mg/kg) immediately after surgery and thereafter daily for 5 days.
Kindling
Fifty-nine days post-TBI, and 50 days posttransplantation, all rats were surgically implanted with electrodes under 3% isoflurane (see Fig. 1). The electrode (3.5 mm length and 0.3 mm strip) was stereotaxically positioned contralateral to the injury in the angular bundle of the PP. Implant coordinates were A/P −7.78 mm, L/M 4.59 mm, and dorsal/ventral D/V −2.6 mm from dura and −3.5 mm from the skull surface. Animals are allowed to recover after surgery for 10 days before the kindling regimen. For the kindling protocol, rats were stimulated for 20 days, beginning on day 71 (D71). Rats were stimulated twice a day, morning and afternoon, with a threshold of 400 µA, until they reached stage 5 on the Racine scale – kindling was stopped early if they reach stage 5 three times in a row. As some animals kindled faster than others, 10 days after the last day of the kindling protocol (D91), the animals were stimulated once more (D101) in order to analyze the EEG in response to recent stimulation. All EEG recordings were done using LabChart v8 software (AD Instruments, Colorado Springs, CO) for 5 min. For each stimulation, animals were also evaluated on the Racine scale. The animals were considered kindled if they reached stage 5 three times in a row, which is characterized by rearing and falling.28 For quantitative analyses, we evaluated the duration of the AD, number of spikes in one series, and duration of each spike series (data not shown) on the last day of stimulation. A spike series was defined as a series when there are more than 3 straight uniform spikes.
Behavioral Analysis
In order to see whether kindling and the treatment with hNSCs caused an improvement or impairment of cognitive function, both behavioral tests were performed before (D66–D70) and after kindling (D96–D100), 2 or 3 mo postinjury, respectively, with an interval of 1 mo from each other. All testing were performed by experimenters blind to treatment group.
NPR
An NPR task was used to evaluate spatial–hippocampal-mediated memory. The NPR task consisted of 2 open-field plastic arenas, each measuring 45 × 70 × 70 cm3, which were placed next to each other on the floor in a dedicated behavioral testing room with a video camera set above the arenas. Live tracking of the animals was obtained using EthoVision XT software (version 8; Noldus Information Technology, Leesburg, VA). The discrimination index (DI) was used as the main outcome measurement. DI was calculated as the ratio of total time spent exploring the object in the novel location in relation to the object in the familiar location ([t.novel-t.familiar/t.novel+t.familiar]*100). We first analyzed DI minute by minute for 5 min. However, during NPR analysis, we observed that the animals in the control group (sham + electrode + vehicle) lost interest after the first few minutes, as previously reported.33,43 Thus, we calculated DI only for the first minute of the task.
For the NPR task, we tested 2 animals at the same time, 1 animal for each arena. The same test was done before and after kindling. First, animals were habituated to the testers by 5 min of handling over 5 days, and habituated to the testing environment and open-field arena by exploring the arena freely for 30 min (15 min/day), prior to running the NRP task. The task took place over 2 days. The first day was familiarization testing, in which 2 identical objects were placed in opposing corners of the open field and the animals were allowed 5 min to explore both objects. After 24 h, for the testing phase, the animals were placed back in the same arenas with the 2 identical objects, but 1 was moved to a different location (novel place, to an open corner), while the other object remained at its former spatial location (familiar place). For both the familiarization phase and testing phase, rats were placed in the arenas and allowed to explore freely for 5 min. For both phases, in order to track the exploration of the objects, we used the “head direction to zone” function in EthoVision XT, whereby a rat was considered to be exploring an object when its head was oriented toward it. Arenas and objects were cleaned with 70% ethanol (EtOH) between each trial in order to minimize odor cues.
EPM
The EPM is a task designed to test anxiety responses in animals. Previous literature has suggested that the amygdala and ventral hippocampus both contribute to anxiety response.44 The EPM consisted of a cross-shaped arena, with 2 open arms and 2 closed black plastic arms (Med Associates Inc., Fairfax, VT). To encourage exploratory behavior, the experiment was performed in a dimly lit room with infrared lighting on the EPM using infrared beam detection and an infrared video camera suspended above the plus maze. Each rat was placed in the center of the maze facing one of the open arms and tracked for 5 min using EthoVision XT software. The software recorded total time spent in the open and closed arms as well as in open and closed arm entries (counted when the center point of the rat crossed into either the open and closed arms). The elevated plus maze was cleaned with 70% EtOH between each animal’s assessment to remove odors.
Histology Analyses
At 14 weeks posttransplantation (wpt), animals were euthanized and brain tissue collection and immunohistochemistry were performed as previously described,45 with minor modifications. Rats were anesthetized with a lethal dose of euthasol (100 mg/kg, intraperitoneally [ip]) and transcardially perfused with 50 mL of phosphate-buffered saline (PBS), followed by 300–400 mL of 4% paraformaldehyde (PFA). Brains were dissected and postfixed overnight in 4% PFA and 20% sucrose in PBS at 4°C, frozen at −65°C in isopentane (2-methyl butane), and stored at −80°C.
Cryosection and immunohistochemistry
Brains were embedded in optimal cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA). Brains were cut in coronal sections of, 30 µm thick, using a cryostat and mounted using a CryoJane tape transfer system (Leica Biosystems, Inc., Buffalo Grove, IL). Slides were stored in sealed slide boxes at −80°C or −20°C until use. All the immunostaining procedures were conducted at room temperature.
For 3,3′-diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) immunohistochemistry, sections were removed from the freezer and air-dried at room temperature for 30 min, then sections were washed 3 times (at 5 min each) in Tris 1X (Supelco, Bellefonte, PA) followed by a 20-min incubation in 0.3% hydrogen peroxide/methanol. After one Tris 1X wash for 5 min, sections were lightly permeabilized in 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 15 min, then washed for 30 min with bovine serum albumin (BSA; 20 g per 1 L) and normal serum donkey (150 µL/10 mL; Vector Laboratories) in Tris 1X. Sections were then incubated overnight with the primary antibody. The primary antibody used for DAB staining was an antihuman cytoplasmic monoclonal mouse anti-SC121 (1:8,000; Y40410; Takara, Kusatsu City, Japan). The next day, after two 5-min wash with 0.1% Triton X-100, and 15 min with BSA and normal serum donkey in Tris 1X, the sections were incubated with the secondary antibody biotin-SP-conjugated F(ab)2 purified immunoglobulin G (IgG) secondary antibody (1:500; 715-066-151; Jackson ImmunoResearch, West Grove, PA) preabsorbed against rat mouse donkey goat. After 2 wash (5 min each) in 0.1% Triton X-100 and a wash with BSA in Tris 1X for 15 min, the sections were incubated for 1 h with an avidin–biotinylated peroxidase complex (ABC) using Vectastain Elite ABC kit (PK-6100; Vector Laboratories) prepared according to the manufacturer’s recommendations. ABC incubation was followed by 3 wash in Tris 1X (5 min each), and a 5-min incubation with DAB (SK-4100; Vector Laboratories). Sections were counterstained with cresyl violet (Sigma-Aldrich) and coverslipped using Depex (Sigma-Aldrich) mounting medium and allowed to dry overnight at room temperature. Quantification of the survival of SC121-positive human stem cell was performed using MicroBrightfield Stereo Investigator (version 11.09; MBF Bioscience, Williston, VT).
For fluorescent staining, sections were removed from the freezer and left to dry at room temperature for 30 min, then sections were washed 4 times (5 min each) in PBS, then 1 time (5 min) in 0.1% Triton X-100 in PBS, followed by a 1-h wash with BSA and donkey serum in PBS. Sections were then incubated overnight in primary antibody. The primary antibodies used were monoclonal mouse anti-SC121 (1:500, AB-121-U-050; Takara), polyclonal rabbit anti-nestin (1:1000, ABD69; Millipore, Darmstadt, Germany), polyclonal rabbit anti-Olig2 (1:500, AB9610; Millipore), polyclonal rabbit anti-neuronal nuclei (NeuN) (1:1000, ABN78; Millipore) and polyclonal rabbit anti–glial fibrillary acidic protein (GFAP, 1:1000, Z0334; Agilent Technologies/DAKO, Santa Clara, CA). The next day, sections were washed 3 times (5 min each) in PBS, followed by 1 h in BSA and donkey serum in PBS. Sections were then incubated with fluorescent-purified IgG secondary antibody cyanine (Cy)3-conjugated F(ab’)2 fragment donkey antimouse (1:500, 715-166-151; Jackson ImmunoResearch) or Alexa Fluor 488-conjugated F(ab’)2 fragment donkey anti-rabbit (1:500, 711-546-152; Jackson ImmunoResearch) preabsorbed against the species in which the primary antibody was raised and counterstained with Hoechst (1:500; Life Technologies, Thermo Fisher Scientific, Waltham, MA) to reveal the cell nuclei. After a 5-min wash with PBS, sections were coverslipped using Fluoromount-G® (SouthernBiotech, Birmingham, AL) mounting medium and allowed to dry overnight. Imaging was performed using a Zeiss Observer.Z1 spinning disk system (Axiovision 4.8.2; Carl Zeiss, Thornwood, NY).
Quantification of Histology
Transplanted hNSCs survival analysis
To estimate the total number of human stem cell survival, we used stereology. An optical fractionator probe was used for unbiased stereological analysis. Histological sections, which contained visible cortex and hippocampus, comprised anterior and posterior landmarks of approximately A/P −1.80 mm to A/P −5.28. A section interval of 1/12 was utilized, placing adjacent sections analyzed at 360 µm apart. In order to identify transplanted human cells, sections with DAB anti-SC121 (human cytoplasm) antibody, with cresyl violet counterstain for histological structure identification, were used. Contours were drawn at 2.5× magnification, outlining areas of human cell engraftment, and human cell identification and counting were performed at 63× magnification. The counting frame utilized was 75 ×x 75 µm with a grid size of 125 × 125 µm. The dissector height was set at 12 µm with an upper and lower guard zone of 1 µm. We quantified the estimated population using mean section thickness (only using sites with counts). The Gundersen coefficient of error (CE, m = 1) for all hNSCs transplanted sham animals (n = 13) averaged 0.1 and for all injured animals (n = 14) averaged 0.07.
Transplanted hNSCs fate analysis
To determine the percentage of transplanted human cells differentiating along neural fate pathways, and whether differentiation was effected by kindling, we conducted a proportional analysis of human cell fate. Imaging was performed using a Zeiss Observer.Z1 spinning disk system. An average of 4 images per section across an average of 6 sections were randomly collected and the cell fate was counted using Imaris Scientific 3D/4D image processing and analysis (version 7.6.5; Bitplane AG, Belfast, Ireland). A section interval of 1/12 was utilized, placing adjacent sections analyzed at 360 µm apart from each other. Co-localization was performed using either antibodies specific for neuronal fate (NeuN), astrocyte fate (GFAP), oligodendrocyte fate (Olig2), or undifferentiated cells (nestin) along with an anti-SC121 (human cytoplasm) antibody. Hoechst 33342 was used as a nuclear counterstain. Each fate group consisted of 8 to 10 randomly selected animals; quantification was performed blind to treatment group. An average of 24 images per animal were analyzed (e.g., for SC121/GFAP).
Statistical Analysis
All behavioral, histological, and stereological analyses were performed blindly by experimenters. Statistical analyses were performed using Prism (version 7.0b; GraphPad Software, La Jolla, CA). Behavioral data (NPR and EPM) were analyzed either by 1-way or 2-way analysis of variance (ANOVA). One-way ANOVA was used when comparing the injury groups. Two-way ANOVA was used when comparing the same group, pre- and post-kindling (of the latter, data not shown). Stereological quantifications were analyzed via unpaired 2-tailed t tests (surviving hNSCs). Errors are standard error of the mean of averaged results and significance was defined as p < 0.05 for all statistical analyses. T-test results were reported as the t ratio (t), degrees of freedom (df), and the p value. Correlation between hNSCs survival and behavior (NPR and EPM) scores was analyzed by Pearson correlation analysis.
Results
Sham and Injured ATN Rats Exhibit Long-Term Survival of hNSCs Shef6 p27, with Higher Survival in the Hemisphere Ipsilateral to the Injury
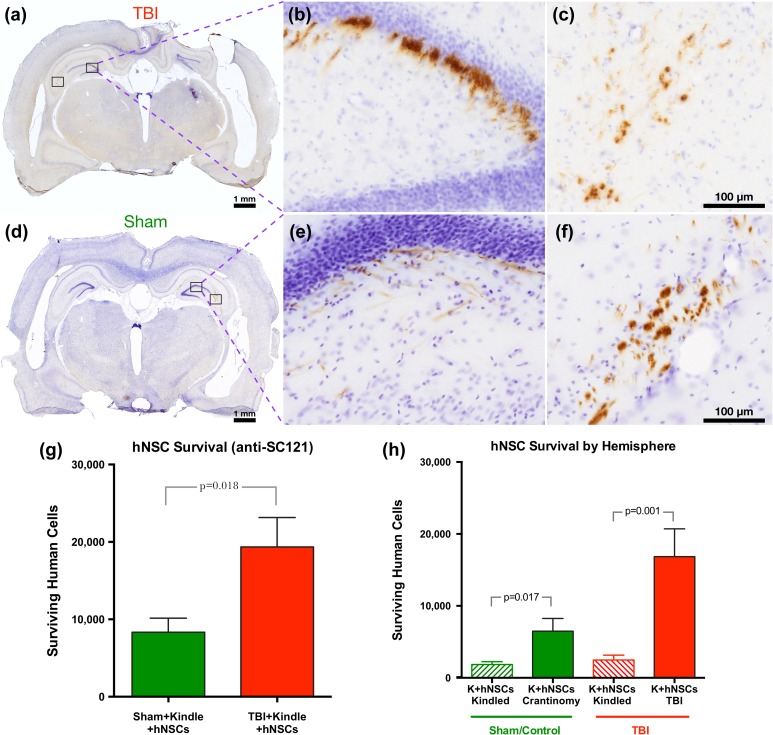
Nine days post-TBI, sham and injured ATN rats were transplanted with hNSCs Shef6 (p27) in the injury hemisphere (TBI for injury animals and craniotomy for sham animals) and contralateral hemisphere (side that was kindled). In total, 250,000 cells were transplanted in each hemisphere for a total dose of 500,000 cells. At 14 wpt (and 4 days after the final AD recording), the hNSCs were detected in the hippocampus on both injured and contralateral hemispheres (Fig. 2) of both TBI (Fig. 2a–c) and sham (Fig. 2d–f) animals. On the injured side of TBI animals, the hNSCs were found concentrated along the injury site. Of the initial 500,000 hNSCs transplanted, just 8,337 ± 1,812 for sham and 19,344 ± 3,816 for TBI animals survived long-term (Fig. 2g). Since the kindling was done on the contralateral hemisphere compared to the injury, we quantified the survival of the transplanted hNSCs in each hemisphere and found that, for both groups, the “injury” hemisphere contained a higher number of hNSCs that survived long-term compared to the contralateral “kindled” hemisphere (Fig. 2h).
Figure 2.
Long-term survival (14 wpt) of hNSCs is significantly greater in TBI animals than in shams; survival was also better in the nonkindled hemisphere. At 14 wpt and 1 wk after stimulating to measure AD, transplanted hNSCs show long-term survival in the brains of both sham and TBI ATN rats. (a–c) Immunohistochemistry of transplanted hNSCs with the human cytoplasmic protein marker, anti-SC121, showing survival and engraftment in both injured (a) and sham (d) brains in photomontage collected at 2.5×. Boxed areas in part figures a and d shown at higher magnification in b, c e, and f, respectively, taken at 20×. (g) Stereological quantification of transplanted hNSCs in sham (n = 13, 8,337 ± 1,812) and in TBI animals (n = 14, 19,344 ± 3,816) shows a significant difference in cell survival between experimental groups (2-tailed t test t25 = 2.54, p = 0.0177). (h) Stereological quantification of transplanted hNSCs shows a significant difference between the kindled side and the contralateral side in both groups (Sham—2-tailed t test t24 = 2.56, p = 0.017; TBI—2-tailed t test t26 = 3.65 p = 0.001). Data are presented as mean ± standard error of the mean (SEM), with TBI animals demonstrating significantly higher survival. Animals were kindled on the contralateral side of the injury (CCI injured for TBI animals and craniotomy for sham). wpt,: weeks posttransplantation; hNSC, human neural stem cell; TBI, traumatic brain injury; AD, after discharge; ATN, immunodeficient athymic nude; anti-SC121, human cytoplasmic marker; CCI, controlled cortical impact; K, kindled.
Shef6 p27 hNSCs Differentiated into All 3 Neural Lineages
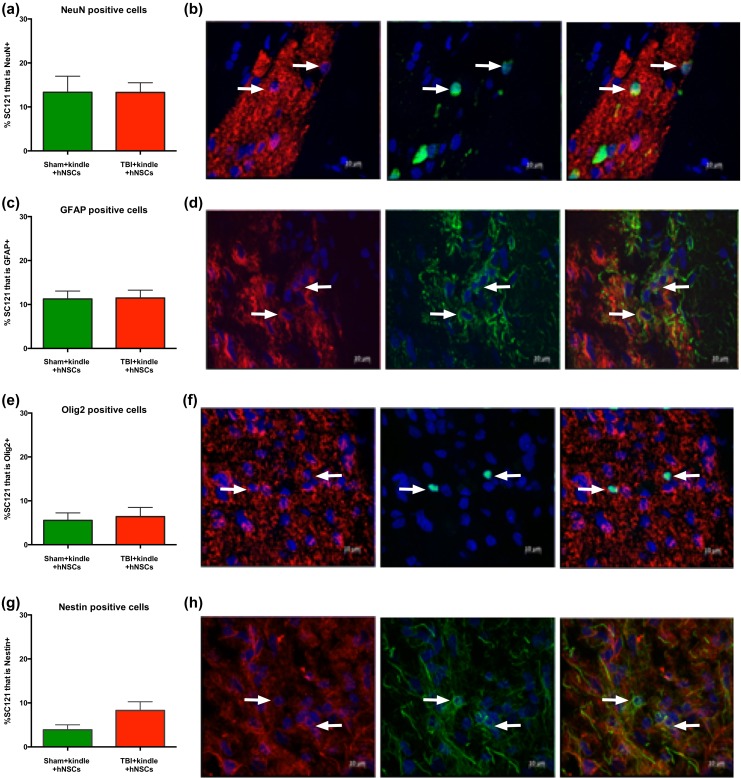
Proportional cell fate analyses showed that, in both sham and injured animals, the transplanted hNSCs differentiate into cells of all 3 neuronal lineages and that cell fate was not significantly affected by the injury (Fig. 3). The predominant terminal fate was neuronal (NeuN+; sham 13.3% ± 3.6%; injured 13.3% ± 2.2%; Fig. 3a–b), followed by astroglial (GFAP+) (sham 11.2% ± 1.8%; injured 11.5% ± 1.7%; Fig. 3c–d). A smaller percentage of oligodendroglial cells/progenitors (Olig2+; sham 5.5% ± 1.7%; injured 6.4% ± 2.1%; Fig. 3e–f) was observed and a small percentage remained in an undifferentiated state (e.g., nestin+; sham 3.9% ± 1.1%; injured 8.3% ± 1.9%; Fig. 3g–h). The proportional cell fates observed here, at 14 wtp and following kindling, were similar to proportional cell fates observed in our previous study using these same cells (Table 1).33
Figure 3.
Human neural stem cell transplantation into either sham or TBI rat brain shows neuronal, astrocytes, and oligodendrocytes differentiation or remain undifferentiated. hNSCs Shef6 P27 via bilateral injection (9-day postinjury for TBI animals) at 13 wk posttransplantation and 4 wk post-kindling differentiate into either (a–b) mature neurons (NeuN+; sham, n = 10, 13.3% ± 3.6%; injured, n = 10, 13.3% ± 2.2%; 2-tailed t test t18 = 0.01, p = 0.99), (c–d) astrocytes (GFAP+; sham, n = 10 11.2% ± 1.8%; injured, n = 10, 11.5% ± 1.7%; 2-tailed t test t18 = 0.087, p = 0.93), and (e–f) oligodendrocytes (Olig2+) (sham, n = 8, 5.5% ± 1.7%; injured rats, n = 8, 6.4% ± 2.1%; 2-tailed t test t14 = 0.317, p = 0.75) or remain in an undifferentiated state (g–h) (nestin+; sham, n = 8, 3.9% ± 1.1%; injured, n = 8, 8.3% ± 1.9%, 2-tailed t test t14 = 1.90, p = 0.07). In red, human stem cells and in green, the various lineage cell types. White arrows show co-localized cells. No significant differences between sham and injured groups after kindling in terms of differentiation into any of the 3 lineage cell types, or undifferentiated cells, were observed. Data are presented as mean ± standard error of the mean (SEM). TBI, traumatic brain injury; hNSC, human neural stem cell; GFAP, glial acidic fibrillary protein.
Table 1.
Comparisons of Terminal Cell Fate in the Present Study (Beretta) vs Haus et al 2016 Using the Same Shef6 hNSCs at p27.
| Antibodies for Fate | Beretta | Haus |
|---|---|---|
| NeuN+ | 13.3% | 18.6% |
| GFAP+ | 11.5% | 13.2% |
| Olig2+ | 6.4% | 11.3% |
| Nestin+ | 8.3% | 9.7% |
Note: GFAP, glial fibrillary acidic protein; hNSCs, human neural stem cells.
hNSCs Transplantation Improved Long-Term Cognitive Function on the NPR Task: This Effect Was Abolished After Kindling
In this study, one goal was to examine the behavioral effect of kindling on sham animals and injured animals in the context of hNSC transplantation. Our aim was to test whether hNSCs can reduce the deleterious effects of TBI and kindling on cognitive and emotional tasks. Cognitive and emotional effects of TBI were evaluated using NPR and EPM tasks, respectively. Animals were behaviorally assessed before and after kindling (2 mo and 3 mo postinjury, respectively). We predicted that nontransplanted injured and kindled animals would exhibit worse cognitive and emotional deficits compared to hNSC transplanted injured and kindled animals. Comparison of the 3 sham groups on both the NPR and EPM tasks, both pre-kindling and post-kindling via 1-way ANOVAs, revealed no significant differences between the control groups, while one outlier was identified via a nonlinear regression outlier removal test in Prism (the ROUT test), and excluded. The 3 sham/control groups were then pooled as controls for further comparison to the respective treatment groups (see Table 2).
Table 2.
Comparison of the 3 Sham Groups via 1-way ANOVA and Multiple Comparisons Corrected via Holm-Sidak.
| Experiment | F | df | p Value |
|---|---|---|---|
| NPR—pre-kindling | 2.91 | F(2, 32) | 0.069 |
| NPR—post-kindling | 4.41 | F(2, 30) | 0.021 |
| EPM—pre-kindling | 0.73 | F(2, 35) | 0.488 |
| EPM—post-kindling | 2.73 | F(2, 35) | 0.079 |
Note: The controls are not significantly different from each other and were thus pooled. ANOVA, analysis of variance; NPR, novel place recognition; EPM, elevated plus maze.
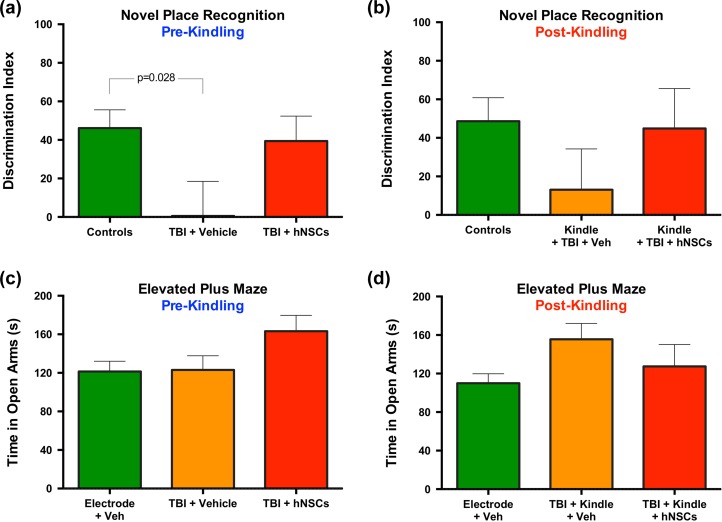
Comparison of the pooled control/sham groups to TBI + vehicle and TBI + hNSCs groups’ performance on the NPR task via 1-way ANOVA revealed a significant difference between the controls and the TBI + vehicle group on the DI, F(2, 59) = 3.28, p = 0.047, pre-kindling (Fig. 4). A Holm-Sidak correction for multiple comparisons showed a significant difference between controls and TBI + vehicle treated animals (p = 0.028), but no difference between TBI + vehicle and TBI + hNSCs treated animals (p = 0.144) nor controls and TBI + hNSCs treated animals (p = 0.710). Thus, TBI animals performed worse on the NPR task in comparison to controls (as reported previously in Haus et al.) 33; however, hNSC treatment returned animals with TBI animals to cognitive performance statistically indistinguishable from control levels (Fig. 4a). This negative effect on the NPR task following TBI, and restoration of function following hNSC transplantation, was lost following kindling (Fig. 4b), where a 1-way ANOVA indicated no significant differences, F(2, 55) = 1.11, p = 0.336, between treatment groups and controls.
Figure 4.
TBI results in a significant deficit on the novel place recognition (NPR) task in comparison to controls; this deficit is reversed following hNSC transplantation. At 2 to 3 mo postinjury, before and after kindling, respectively, rats were assessed via NPR and EPM tasks by experimenters blind to treatment. (a–b) Comparison of the pooled sham/control groups to TBI + vehicle and TBI + hNSCS group performance on the NPR task via 1-way ANOVA revealed a significant difference between the controls and the TBI + vehicle group on the DI, F(2, 59) = 3.28, p = 0.047) pre-kindling. A Holm-Sidak correction for multiple comparisons showed a significant difference between controls and TBI + vehicle treated animals (p = 0.028), but no difference between TBI + vehicle and TBI + hNCS treated animals (p = 0.144) nor controls and TBI + hNSCs treated animals (p = 0.710). (a) TBI animals performed worse on the NPR task in comparison to controls pre-kindling; however, hNSC treatment returned animals with TBI to cognitive performance statistically indistinguishable from control levels (as reported previously in Haus33). (b) This negative effect on the NPR task following TBI, and restoration of function following hNSC transplantation, was lost following kindling, where a 1-way ANOVA indicated no significant differences, F(2, 55) = 1.11, p = 0.336, between either treatment group and controls. (c–d) On the EPM, no significant differences between any groups were detected via 1-way ANOVA either pre-kindling, F(2, 62) = 2.40, p = 0.099, or post-kindling, F(2, 62) = 2.35, p = 0.103. TBI, traumatic brain injury; hNSC, human neural stem cell; EPM, elevated plus maze; ANOVA, analysis of variance; DI, discrimination index; Veh, vehicle.
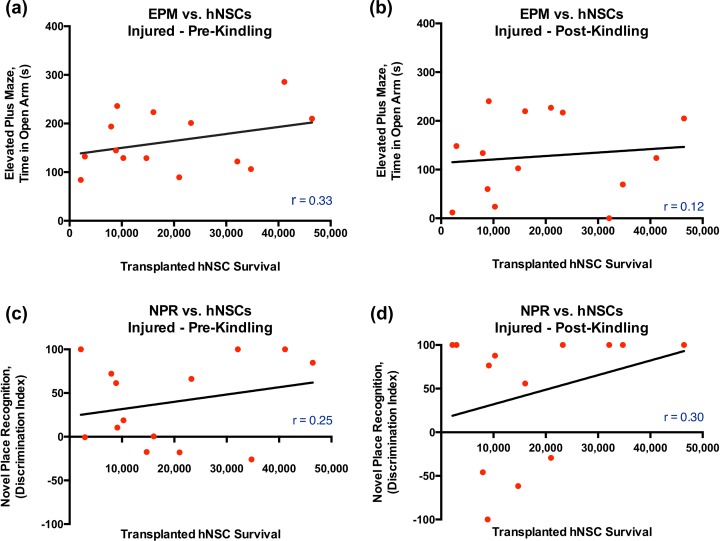
In contrast to the significant difference in performance on the more cognitive NPR task, comparison of the TBI groups (with vehicle or hNSCs) on a more emotion-focused task, the EPM, revealed no significant differences between any groups via 1-way ANOVA either pre-kindling, F(2, 62) = 2.40, p = 0.099, or post-kindling, F(2, 62) = 2.35, p = 0.103 (Fig. 4c–d). The lack of significant group effects on the EPM may have been the result of differing levels of cell engraftment within animals in the same group. However, correlations of transplanted hNSC survival number with performance on either cognitive (NPR) or emotional tasks (EPM) were not statistically significant (Fig. 5a–d).
Figure 5.
There were no significant correlations between the number of surviving transplanted hNSC and performance on either the EPM or NPR in TBI animals. (a–d) A Pearson correlation analysis showed no significant correlations pre-kindling (a, c) or post-kindling (b, d) between surviving transplanted hNSC quantity and either the time in open arms on the EPM (a–b) or the discrimination index score on the NPR (c–d) in animals with TBI (EPM pre-kindling, n = 14, Pearson r = 0.33, p = 0.12; EPM post-kindling n = 14, Pearson r = 0.12, p = 0.34; NPR pre-kindling, n = 14, Pearson r = 0.25, p = 0.20; NPR post-kindling, n = 13, Pearson r = 0.3, p = 0.16, 1-tailed t test). Similar correlation analysis showed no significant correlations in sham/control, with the exception on post-kindling NPR performance (see Fig. 7d). TBI, traumatic brain injury; hNSC, human neural stem cell; EPM, elevated plus maze; NPR, novel place recognition.
hNSC Transplantation in ATN Rats Doesn’t Have an Effect on Kindling
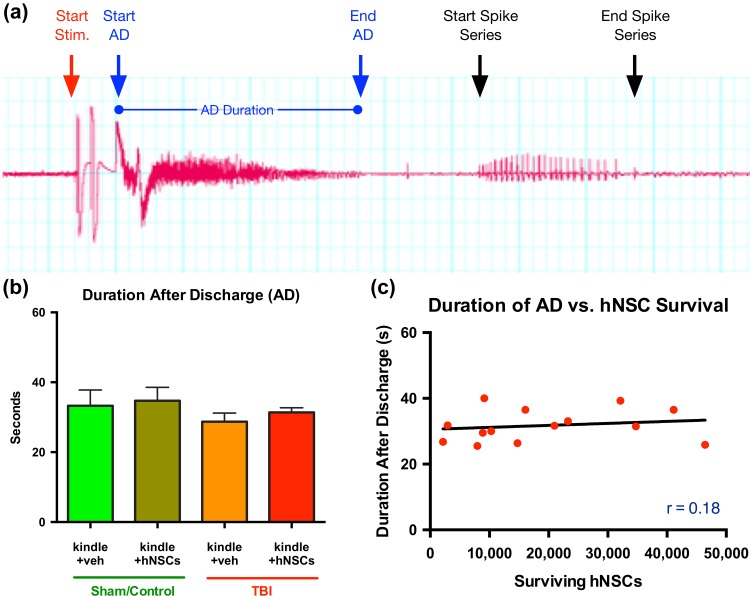
In order to evaluate whether kindling can enhance the development of spontaneous seizures in a CCI TBI animal model, or whether kindling effects the survival of transplanted hNSCs, and whether hNSCc can prevent or reduce the development of kindling-induced seizures at 10 wk post-TBI (9 wpt), animals were stimulated for 20 days, twice daily, morning and afternoon, and again 10 days after the last day of kindling (D91). Animals were stimulated once more, at D101, to record their final AD duration. For analyses, we assessed the duration of the AD for all the kindled groups (Fig. 6), days of stimulation required to induce kindling, and number of spikes in a spike series poststimulation. Figure 6a shows the representative EEG recording at the start of stimulation, start of AD, end of AD, and start and end of spike series. We predicted that hNSCs in transplanted animals, both sham or injured, would reduce the duration of AD compared with the vehicle group, but our results show that AD duration is not significantly different among the different groups. In particular, the presence of hNSCs does not affect the AD duration between the different groups (Fig. 6b). Correlations of transplanted hNSCs survival with the duration of an animal’s respective AD (at D101) were not statistically significant (Fig. 6c). No differences were observed in the other parameters either.
Figure 6.
hNSCs transplantation doesn’t affect the after discharge (AD) duration in kindled animals. (a) Representative EEG recording showing start of stimulation, start of AD, end of AD, and start and end of spike series. AD duration was calculated using LabChart software blind to treatment group. (b) A 2-way repeated-measure ANOVA shows no significant difference in duration of AD between the different groups. (C) Pearson correlation analysis showed no significant correlations between duration of the AD and number of surviving hNSCs (injured, Pearson r = 0.18, p = 0.54; 1-tailed t test). Similarly linear regression analysis presented no significant correlations in sham (data not shown). hNSC, human neural stem cell; EEG, electroencephalogram; ANOVA, analysis of variance; Stim., stimulation; veh, vehicle.
Surviving hNSC Number Does Not Correlate with Performance on Either the EPM or NPR Task
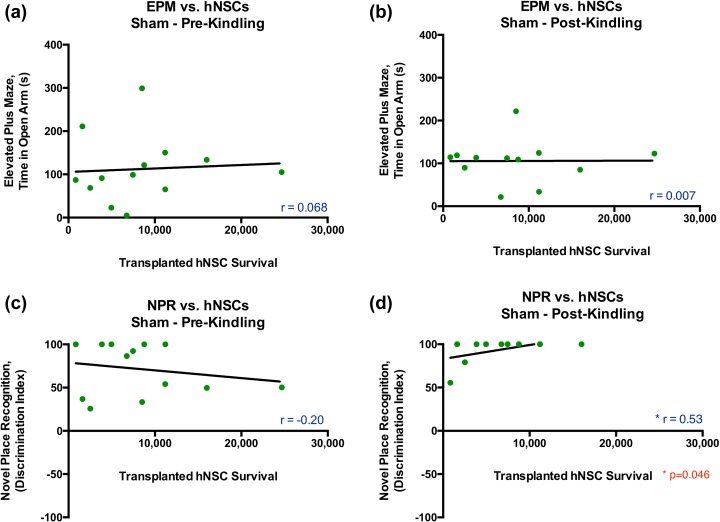
Pearson correlation analyses showed no significant correlations pre-kindling or post-kindling between surviving transplanted hNSC number and either the time in open arms on the EPM or the DI score on the NPR in uninjured animals (Fig. 7). While the correlation between cell number and NPR post-kindling is “significant,” due to a threshold effect on the DI and lack of spread in cell survival (see Fig. 7d), little can be interpreted from these results.
Figure 7.
There were no significant correlations between the number of surviving transplanted hNSCs and performance on either the EPM or NPR in sham/control animals. (a–d) A Pearson correlation analysis showed no significant correlations pre-kindling (a, c) or post-kindling (b, d) between surviving transplanted hNSC quantity and either the time in open arms on the EPM (A-B) or the discrimination index score on the NPR (C) in uninjured animals. (EPM pre-kindling, n = 134, Pearson r = 0.068, p = 0.413; EPM post-kindling n = 12, Pearson r = 0.007, p = 0.492; NPR pre-kindling, n = 12, Pearson r = 0.20, p = 0.261; NPR post-kindling, n = 11, Pearson r = 0.53, p = 0.046, 1-tailed t test). (d) While this last correlation is significant, due to a threshold effect on the discrimination index and lack of spread in cell survival, little can be interpreted from these results. hNSC, human neural stem cell; EPM, elevated plus maze; NPR, novel place recognition.
Discussion
TBIs are known to cause disabilities in cognitive processes, sensation, movement, memory, and emotion in both rats and humans alike,37,46 with a high incidence in the population. Further, TBIs account for 20% of symptomatic epilepsy in the general civilian population,20 and 10% of individuals with severe TBI exhibit PTE.47 Several laboratories have reported acute seizures and hippocampal hyperexcitability to stimulation or proconvulsant drug exposure in TBI models21, but just a few previous studies have investigated an animal model of spontaneous chronic seizures17,19–22 after experimental head injury. FPI has been the most widely investigated as a model of posttraumatic hyperexcitability.17,19,21 In contrast, until 2016, the CCI model of TBI had not been used in a model of induced PTE.17,22 In the present study, we tested whether CCI and hippocampal kindling (a model of experimental epilepsy) can induce hippocampal hyperexcitability. In this animal model, we then assessed the effect of human ES-derived NSC transplantation on cognitive and emotional tasks, and a battery of kindling parameters.
At 14 wpt, we found that sham and injured ATN rats maintained long-term survival of hNSCs. Paradoxically, we observed 2-fold greater survival in injured animals than sham animals. While it is often presumed that the injured microenvironment is hostile to the survival of transplanted cells,48 our experience is that the time posttransplant in spinal cord injury models does not affect cell survival.45,49 We have historical data suggesting that fetal-derived hNSC survival is slightly better in uninjured than in injured central nervous system tissue at 14 wpt, and that this difference is decreasing over time post-transplant, as cells proliferate (i.e., the number of 5-bromo-20-deoxyuridine-positive hNSCs is higher in uninjured tissue at 7 dpi and 14 dpi, but not at 28 dpi).50 We also have an “historical” comparison using Shef6-derived hNSCs that can be made from the Haus et al. study.33 In Haus et al.,33 ATN rats of the same age and from the same breeder were given the same injury or sham procedure, but none were subjected to kindling. Animals in Haus 2016 received Shef6 hNSCs at p27, as in the present study, however Haus animals received a dose of 250,000 cells, and animals in our study received 500,000 cells. At 20 wpt in the contralateral hemisphere, Haus et al.33 reported that 21,846 ±10,408 hNSCs survived (or 8.7%) long-term. We observed a survival of just 2,487 cells in the contralateral/kindled hemisphere of the initial 250,000 dose received on that side. The overall average of 19,344 cells per animal following a dose of 500,000 cells is less than 3%t survival. However, on the nonkindled side, 7.7% of the 250,000 cells survived, comparable to that observed in Haus et al.33 If we were to extrapolate equivocal cell survival in both studies (e.g., simply “double” the number of surviving cells from Haus et al.33), we can see that kindling most likely had a significant detrimental effect on the survival of hNSCs. This presumption is also supported by the hemispheric differences in survival of cells on the kindled and nonkindled side (Fig. 2h).
Why did kindling decrease the number of surviving hNSCs in this TBI model? Little is known about the effect of kindling or seizures on exogenous stem cells. However, others have reported that seizures can increase endogenous neurogenesis, leading to aberrant network reorganization, in adult rats.51 Functional electrical stimulation, at a subseizure threshold, also facilitates an increase in nestin-positive precursors in animals with a prior stroke.52 One might predict that the kindling received in the present study would have facilitated survival and proliferation of exogenously administrated stem cells, however the opposite effect was observed. Because we observed less than half the number of surviving cells in the kindled hemisphere compared to the nonkindled hemisphere, it is likely that the kindling was directly toxic to the hNSCs or reduced their proliferation. We might postulate that kindling accelerated their differentiation53 and thus less cells remained in a proliferative state once kindling began at D71, thus reducing the overall numbers of engrafted hNSCs when scarified at later time points.
We also report that hNSCs differentiate into all 3 neural lineages (Fig. 3) and that this differentiation is unaffected by whether the animal received a TBI or sham surgery (although both groups were kindled; Fig. 3.). Again, without an hNSC transplanted, uninjured, and nonkindled control, it is impossible to demonstrate whether kindling altered the fate in the present experiment. But comparing the fate of the present kindling experiment to that in the Haus et al.33 study, which used identical late passage P27 Shef6 hNSCs and assessed fate at 20 wpt, we observed 13% of hNSCs differentiating into NeuN+ neurons in the present study and 19% in Haus et al.; 12% GFAP+ in the present study and 13% in Haus et al., 6% Olig2+ and 11% in Haus et al., and 8% nestin+ and 10% in Haus et al. (Fig. 7; see Table 1). This suggests that exposure to kindling 2 mo posttransplant did not significantly alter the fate of hNSC in the injured microenvironment. Alternatively, perhaps 2 mo posttransplant is too late to alter the dynamics of Shef6 hNSC fate choice in this injury paradigm.
We have previously reported that ATN TBI rats exhibit long-term cognitive impairments in a hippocampal-dependent spatial memory task (NPR) compared to sham animals at 2 mo postinjury. Additionally, after transplantation of hNSCs, ATN TBI animals show improvement in the NPR task at 8 wk compared to ATN TBI animals that received the vehicle.33 In the present study, we replicated the beneficial effect of Shef6 hNSCs on NPR performance. However, this improvement in comparison to TBI animals that received a vehicle injection was abolished after exposure of animals to kindling (Fig. 4). We did not observe improvement in emotional function following hNSC transplantation as assessed via EPM tasks. Nor did we observe significant correlations between transplanted hNSC survival number and NPR nor EPM scores in injured animals. It is likely that these tasks were underpowered and would benefit from additional animals, should future studies be conducted using this model.
Given the moderate improvement in the NPR task we observed both here and previously with Shef6 hNSCs and the relatively low level of long-term engraftment, one can speculate the mechanism of action of these cells. It is possible that the hNSC used here integrate with the host tissue and either remyelinate dis-myelinated axons and/or form synaptic connections with the host. Alternatively, the human cells could be providing trophic support of the injured host or modulating the host inflammatory response. These mechanisms are not mutually exclusive. However, given the low level of final engraftment observed here (3%), and our prior data demonstrating that ablation of human fetal NSCs via diphtheria toxin in a contusion model of SCI abolished the behavioral efficacy of the cells in a model with ∼200% engraftment,39, it seems more likely that the efficacy observed in TBI was not via integration with the host. However, this possibility should be tested more directly via a diphtheria experiment in TBI animals receiving hNSCs. Diphtheria toxin is 100,000 more toxic to human cells than rodent cells; thus diphtheria can be given to animals after human stem cell engraftment and the human cells can be selectively ablated while the rodent remains unharmed. If the improvements in the NPR task in the hNSC-treated group were retained post-diphtheria treatment, this would suggest that the hNSCs exerted their effect by restoring host circuitry and not by functionally integrating with host cells.
Finally, our results show that the AD duration is not significantly different among the different kindled groups, whether they received hNSCs or vehicle. The lack of an effect on AD could indicate that transplanted hNSCs do not integrate with host circuits in a manner that effects AD duration, or conversely, that there aren’t sufficient surviving cells to alter neural circuits. Taken together, these data suggest that, while hNSCs have no effect on posttraumatic injury hyperexcitability, they can improve performance on a hippocampal-based memory task, NPR; this is a replication of the Haus et al. study.33
Additional work in a TBI model is necessary to improve posttransplant cell survival (perhaps via coadministration of an immunosuppressant such as Asilo-GM1) to optimize cell administration and timing of transplantation. For example, would a great delay in transplantation lead to great cell survival and still improve cognitive function? Further, it would be beneficial to compare the efficacy of these Shef6 hNSCs to other cell populations (iPS, fetal, or ES derived) before an expensive clinical trial is undertaken with less than optimal cells, timing, or delivery methods.
Footnotes
Ethical Approval: The protocols in this study were approved by the relevant ethics committee (see Materials and Methods).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded by an Early Translation Award grant from CIRM (TR2-01767) to BJC, a CIRM Bridges award to L Klig at CUSLB (TB1-01182) to support KMC, and a fellowship from the University of Milano-Bicocca to SB.
References
- 1. Basso A, Previgliano I, Servadei F. Traumatic brain injuries In: Aarli JA, Avanzini G, Bertolote JM, de Boer H, Breivik H, Dua T, Graham N, Janca A, Kesselring J, Mathers C, Muscetta A, Prilipko L, Saraceno B, Saxena S, Steiner TJ, editors. Neurological disorders: public health challenges. Geneva (Switzerland: ): WHO Press, World Health Organization; 2006. p. 164–175. [Google Scholar]
- 2. Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths 2002-2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. p. 11–21. [Google Scholar]
- 3. Gerberding J, Binder S. Report to congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention; 2003. p. 1–37. [Google Scholar]
- 4. Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. [DOI] [PubMed] [Google Scholar]
- 5. Langlois JA, Sattin RW. Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC). J Head Trauma Rehabil. 2005;20(3):187–188. [DOI] [PubMed] [Google Scholar]
- 6. Finkelstein E, Corso P, Miller T. The incidence and economic burden of injuries in the United States. New York (NY): Oxford University Press; 2006. [Google Scholar]
- 7. Coronado V, McGuire L, Sarmiento K, Bell J, Lionbarger M, Jones C, Geller A, Khoury N, Xu L. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res. 2012;43(4):299–307. [DOI] [PubMed] [Google Scholar]
- 8. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. [DOI] [PubMed] [Google Scholar]
- 9. Cifu D, Hurley R, Peterson M, Cornis-Pop M, Rikli P, Ruff R, Scott S, Sigford B, Silva K, Tortorice K, et al. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46(6):CP1–68. [PubMed] [Google Scholar]
- 10. Menon DK, Schwab K, Wright DW, Maas AI, Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640.21044706 [Google Scholar]
- 11. Hudak AM, Trivedi K, Harper CR, Booker K, Caesar RR, Agostini M, Van Ness PC, Diaz-Arrastia R. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J Head Trauma Rehabil. 2004;19(4):290–295. [DOI] [PubMed] [Google Scholar]
- 12. D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004;17(6):731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kharatishvili I, Pitkanen A. Posttraumatic epilepsy. Curr Opin Neurol. 2010;23(2):183–188. [DOI] [PubMed] [Google Scholar]
- 14. Kharatishvili I, Pitkanen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90(1-2):47–59. [DOI] [PubMed] [Google Scholar]
- 15. Englander J, Cifu DX, Diaz-Arrastia R. Information/education page. Seizures and traumatic brain injury. Arch Phys Med Rehabil. 2014;95(6):1223–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majores M, Schoch S, Lie A, Becker AJ. Molecular neuropathology of temporal lobe epilepsy: complementary approaches in animal models and human disease tissue. Epilepsia 2007;48(Suppl 2):4–12. [DOI] [PubMed] [Google Scholar]
- 17. Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol. 2009;215(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21(21):8523–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 2006;140(2):685–697. [DOI] [PubMed] [Google Scholar]
- 20. Echegoyen J, Armstrong C, Morgan RJ, Soltesz I. Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res. 2009;85(1):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 2004;127(Pt 2):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eslami M, Ghanbari E, Sayyah M, Etemadi F, Choopani S, Soleimani M, Amiri Z, Hadjighassem M. Traumatic brain injury accelerates kindling epileptogenesis in rats. Neurol Res. 2016;38(3):269–274. [DOI] [PubMed] [Google Scholar]
- 23. Glushakov AV, Glushakova OY, Dore S, Carney PR, Hayes RL. Animal models of posttraumatic seizures and epilepsy. Methods Mol Biol. September 2016;1462:481–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32(6):778–782. [DOI] [PubMed] [Google Scholar]
- 25. Ergül Erkec Ö, Arihan O. Pentylenetetrazole kindling epilespsy model. Epilespi. 2015;21(1):6–12. [Google Scholar]
- 26. Ghafouri S, Fathollahi Y, Javan M, Shojaei A, Asgari A, Mirnajafi-Zadeh J. Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Res. October 2016;126:37–44. [DOI] [PubMed] [Google Scholar]
- 27. Morales JC, Alvarez-Ferradas C, Roncagliolo M, Fuenzalida M, Wellmann M, Nualart FJ, Bonansco C. A new rapid kindling variant for induction of cortical epileptogenesis in freely moving rats. Front Cell Neurosci. July 2014;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. [DOI] [PubMed] [Google Scholar]
- 29. Watson NF, Barber JK, Doherty MJ, Miller JW, Temkin NR. Does glucocorticoid administration prevent late seizures after head injury? Epilepsia. 2004;45(6):690–694. [DOI] [PubMed] [Google Scholar]
- 30. Kirmani BF, Robinson DM, Fonkem E, Graf K, Huang JH. Role of anticonvulsants in the management of posttraumatic epilepsy. Front Neurol. March 2016;7(32):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456(3):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walker PA, Shah SK, Harting MT, Cox CS., Jr Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis Model Mech. 2009;2(1-2):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haus DL, Lopez-Velazquez L, Gold EM, Cunningham KM, Perez H, Anderson AJ, Cummings BJ. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol. July 2016;281:1–16. [DOI] [PubMed] [Google Scholar]
- 34. Chang J, Phelan M, Cummings BJ. A meta-analysis of efficacy in pre-clinical human stem cell therapies for traumatic brain injury. Exp Neurol. September 2015;273:225–233. [DOI] [PubMed] [Google Scholar]
- 35. Peng W, Sun J, Sheng C, Wang Z, Wang Y, Zhang C, Fan R. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res Ther. 2015;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmed AI, Gajavelli S, Spurlock MS, Chieng LO, Bullock MR. Stem cells for therapy in TBI. J R Army Med Corps. 2016;162(2):98–102. [DOI] [PubMed] [Google Scholar]
- 37. Gold EM, Su D, Lopez-Velazquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 2013;8(4):483–516. [DOI] [PubMed] [Google Scholar]
- 38. Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, Jeong SW, Kim SU, Lee SK, Shin HS, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023(2):213–221. [DOI] [PubMed] [Google Scholar]
- 39. Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102(39):14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hefferan MP, Galik J, Kakinohana O, Sekerkova G, Santucci C, Marsala S, Navarro R, Hruska-Plochan M, Johe K, Feldman E, et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS One. 2012;7(8): e42614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, LaFerla FM, Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 2015;25(7):813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haus DL, Nguyen HX, Gold EM, Kamei N, Perez H, Moore HD, Anderson AJ, Cummings BJ. CD133-enriched xeno-free human embryonic-derived neural stem cells expand rapidly in culture and do not form teratomas in immunodeficient mice. Stem Cell Res. 2014;13(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9(2):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118(1):63–78. [DOI] [PubMed] [Google Scholar]
- 45. Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS One. 2009;4(6): e5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20–24. [DOI] [PubMed] [Google Scholar]
- 48. Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol. 2003;14(3):191–198. [DOI] [PubMed] [Google Scholar]
- 49. Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5(8): e12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sontag CJ, Uchida N, Cummings BJ, Anderson AJ. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Reports. 2014;2(5):620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiang Y, Liu H, Yan T, Zhuang Z, Jin D, Peng Y. Functional electrical stimulation-facilitated proliferation and regeneration of neural precursor cells in the brains of rats with cerebral infarction. Neural Regen Res. 2014;9(3):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pires F, Ferreira Q, Rodrigues CA, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta. 2015;1850(6):1158–1168. [DOI] [PubMed] [Google Scholar]