Abstract
Traumatic brain injury (TBI) is a common disease that usually causes severe neurological damage, and current treatment is far from satisfactory. The neuroprotective effects of neural stem cell (NSC) transplantation in the injured nervous system have largely been known, but the underlying mechanisms remain unclear, and their limited sources impede their clinical application. Here, we established a rat model of TBI by dropping a weight onto the cortical motor area of the brain and explored the effect of engrafted NSCs (passage 3, derived from the hippocampus of embryonic 12- to 14-d green fluorescent protein transgenic mice) on TBI rats. Moreover, RT-PCR and Western blotting were employed to investigate the possible mechanism associated with NSC grafts. We found rats with TBI exhibited a severe motor and equilibrium dysfunction, while NSC transplantation could partly improve the motor function and significantly reduce cell apoptosis and increase B-cell lymphoma–extra large (Bcl-xL) expression at 7 d postoperation. However, other genes including Bax, B-cell lymphoma 2, Fas ligand, and caspase3 did not exhibit significant differences in expression. Moreover, to test whether Bcl-xL could be used as a therapeutic target, herpes simplex virus (HSV) 1 carrying Bcl-xL recombinant was constructed and injected into the pericontusional cortices. Bcl-xL overexpression not only resulted in a significant improvement in neurological function but also inhibits cell apoptosis, as compared with the TBI rats, and exhibits the same effects as the administration of NSC. The present study therefore indicated that NSC transplantation could promote the recovery of TBI rats in a manner similar to that of Bcl-xL overexpression. Therefore, Bcl-xL overexpression, to some extent, could be considered as a useful strategy to replace NSC grafting in the treatment of TBI in future clinical practices.
Keywords: neural stem cells, traumatic brain injury, neural behavior, cell apoptosis, Bcl-xL overexpression
Introduction
Traumatic brain injury (TBI) is a serious condition that has brought about a heavy burden on patients and society. It is a leading cause of disability worldwide,1,2 with an enormous cost of treatment.3 In the United States alone, about 1,500,000 people sustain TBI annually, resulting from various causes such as traffic accidents and trauma from skating and rugby, and so on. This pushed many researchers to explore therapeutic strategies for this injury. Stem cells, multipotent cells with self-renewal capacity, may provide hope for the treatment of TBI.
Neural stem cells (NSCs), as multipotent cells, could generate cells of neural phenotypes and have the capacity to differentiate into neurons, astrocytes, and oligodendrocytes.4 Several studies demonstrated that NSCs are found in various areas of the rat brain, including the cerebral cortex, subependymal layer, striatum, hippocampus, and midbrain.5–9 Recent findings suggest that NSCs could also be isolated from the cerebral cortex and striatum of humans.10,11 Moreover, engrafted NSCs in the early stage of injury may secrete various neurotrophic factors to maintain the survival of nerve cells in the host12 and differentiate into target cells in the later stage.13 Previously, NSC transplantation has been considered an effective strategy for the treatment of several diseases such as Alzheimer disease (AD), Parkinson disease (PD), and spinal cord injury (SCI),14–19 as well as promoting the molecular mechanisms involved in angiogenesis and the inhibition of astrogliosis and secondary injury processes.18–22 However, the effect of NSC grafts in TBI is continuously waiting to be evaluated, and whether a supplementary method needs to be developed to resolve the limited source for NSC grafts has yet to be determined.
B-cell lymphoma–extra large (Bcl-xL), as a member of the B-cell lymphoma 2 (Bcl-2) antiapoptosis superfamily, shares 44% of its homology with Bcl-2 and participates in many inflammatory diseases.23–29 Recent studies have shown that Bcl-2 protein plays a neuroprotective role and promotes functional recovery in TBI, SCI, and cerebral ischemic injury.23,30–31 Therefore, Bcl-xL may be a potential target for the treatment of several diseases.
In this study, we examined the effect of NSC grafts and investigated the possible molecular mechanism involved in Bcl-xL in TBI rats, then developed a herpes simplex virus-1 (HSV-1)-based Bcl-xL recombinant vector for the treatment of TBI.
Materials and Methods
We used 5 embryonic mice (embryonic day 12 to 14) with transgenic green fluorescent protein (GFP; obtained from the Institute of Neurological Disease, Department of Anesthesiology, Translational Neuroscience Center, West China Hospital, Sichuan University, Chengdu, China) for NSC isolation. Forty adult Sprague Dawley rats (weighing 220 to 250 g) were provided by the Laboratory Animal Center of Sichuan University. Both male and female rat pups were used in this study. All animals were housed in individual cages in a temperature-controlled (21 to 25 °C) and humidity-controlled (45% to 50%) room with a 12-h light/dark cycle and ad libitum access to pellet chow and water. All procedures involving housing and treatment of laboratory animals were in accordance with the regulations of West China Hospital, Sichuan University. All animal care and experimental protocols were approved by the guidelines of the Institutional Medical Experimental Animal Care Committee of Sichuan University, West China Hospital, China.
Groups
This study included 2 main experiments, and the groups were assigned as follows:
First, 30 rats were randomly assigned to 3 groups as described in Table 1: sham operation group (sham), TBI + culture medium injection group (TBI), and TBI + NSC transplantation group (NSCs), with 10 rats in each group.
Table 1.
Animal groups and sample assignment.
| Groups | Treatment | NSS | RT-PCR/WB | TUNEL/IF |
|---|---|---|---|---|
| Sham | Sham operation | 10 | 5 | 5 |
| TBI | TBI + culture medium | 10 | 5 | 5 |
| NSCs | TBI + NSCs | 10 | 5 | 5 |
Abbreviations: TBI, traumatic brain injury; NSCs, neural stem cells; IF, immunofluorescence; WB, Western blotting, RT-PCR, reverse transcription polymerase chain reaction; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; NSS, neurological severity score.
Second, another 15 rats were randomly divided into 3 groups as described in Table 2: TBI group (TBI plus HSV-1-negative vector, i.e., TBI+HSV-1-NC vector), NSC group (TBI plus NSCs, i.e., TBI + NSCs), and Bcl-xL-ORF group (TBI plus Bcl-xL overexpression, i.e., TBI + HSV-1-exBcl-xL), with 5 rats in each group.
Table 2.
Animal groups and sample used.
| Groups | Treatment | NSS | TUNEL/IF |
|---|---|---|---|
| TBI | TBI + culture medium + HSV-1-NC vector | 5 | 5 |
| NSCs | TBI + NSCs | 5 | 5 |
| Bcl-xL-ORF | TBI + HSV-1-exBcl-xL | 5 | 5 |
Abbreviations: TBI, traumatic brain injury; NSCs, neural stem cells; IF, immunofluorescence; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; HSV-1-NC vector, herpes simplex virus-1-negative control vector; Bcl-xL-ORF, B-cell lymphoma-extra large-open reading frame.
Isolation of NSCs
Chloral hydrate (3.6%; TCI Development Co., Ltd, Shanghai, China) was used as anesthesia for GFP transgenic mice (intraperitoneal [ip] injection) in a dosage of 1 mL/100 g. Then, we harvested isolated hippocampus tissue. The culture medium contains Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F12; Hyclone, Logan, UT), added with 1% B27 (Life Technologies, Carlsbad, CA), 20 μg/L basic fibroblast growth factor (Invitrogen, Carlsbad, CA), 1% penicillin, and streptomycin (Hyclone). The cellular density was adjusted to 2.5×106/mL, allocated into a 24-well culture plates precoated with polylysine (40 g/L; Sigma, St Louis, MO). Half of the culture medium was replaced with fresh medium every other day and the formation of neurospheres was observed. All procedures were performed under sterile conditions.
Identification and Differentiation of NSCs In Vitro
After 3 d in vitro, the formation of floating neurospheres was observed. To identify the NSCs, 4% paraformaldehyde (PFA; Suolai Po Biological Technology Co., Ltd, Shanghai, China) was used for cell fixation for 15 to 20 min at 4 °C. H2O2 3% (Suolai Po Biological Technology Co., Ltd) was used to treat the glass slides for inactivating the endogenous peroxidase. After incubating in 0.3% Triton X-100 (Suolai Po Biological Technology Co., Ltd) at 37 °C for 30 min, 5% goat serum (Zhongyu Jinqiao Biotechnology Co., Ltd, Beijing, China) was used to block the nonspecific binding. Subsequently, antirabbit nestin (1:500; Chemicon, Rolling Meadows, IL), neuron-specific nuclear protein (NeuN, 1:2,000; United Chemicon, Inc., Rolling Meadows, IL), and glial fibrillary acidic protein (GFAP, 1:200; United Chemicon, Inc.) monoclonal antibodies were added and incubated at 4 °C for 18 h, respectively. Next, for recognizing neurons, GFAP and nestin antigen were used followed by secondary antibodies. Additionally, routine streptavidin/peroxidase staining and immune-enzyme-linked with 3,3′-diaminobenzidine yellow color staining were also employed. After completing the staining process, distilled water was added to stop the coloration reaction. All slides were observed and images were taken under a microscope (RX50; Ningbo Sunny Instruments Co., Ltd, Yuyao, China). Positive product turned out to be brown-yellow sedimentation. In the negative control group, primary antibody was substituted with 0.01 M phosphate-buffered saline (PBS), while other steps remained the same.
HSV-1-based Virus Preparation
Construction of nonreplicative HSV backbone and intermediate plasmid preparation
HSV-1-based Bcl-xL recombinant vector was constructed by GeneCopoeia Company (GeneCopoeia, Rockville, MD, USA) as previously reported.32,33 First, HSV vector was purchased from SkyBio Company in Beijing, China. In order to acquire the nonreplication HSV, the segments of infectious cell protein 27 (ICP27), ICP4, and ICP34.5 in HSV were deleted using homologous recombination technology. Then, the homologous sequence was amplified and linked into the prokaryotic strain plasmid 72 (pSP72). Afterward, the promoter that contained human cytomegalovirus and other elements including woodchuck hepatitis posttranscriptional regulatory element and polyadenylic acid (Ploy A) were also constructed into the plasmid together, which was finally named as the intermediate plasmid.
Bcl-xL ORF vector generation
For Bcl-xL overexpression, gene sequence of Bcl-xL was acquired from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/ncbisearch/). Then, total RNA extracted from rat brain was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using the primers 5′-CGTGGAAAGCGTAGACAAGG-3′ (sense), 5′-TGAAGAGTGAGCCCAGCAGA-3′ (antisense). Afterward, Bcl-xL complementary DNAs (cDNAs) were acquired and sent to GeneCopoeia Company to construct a recombinant overexpression vector by linking Bcl-xL cDNA to the intermediate plasmid.
Production of recombinant HSV based on the Vero cells transfection
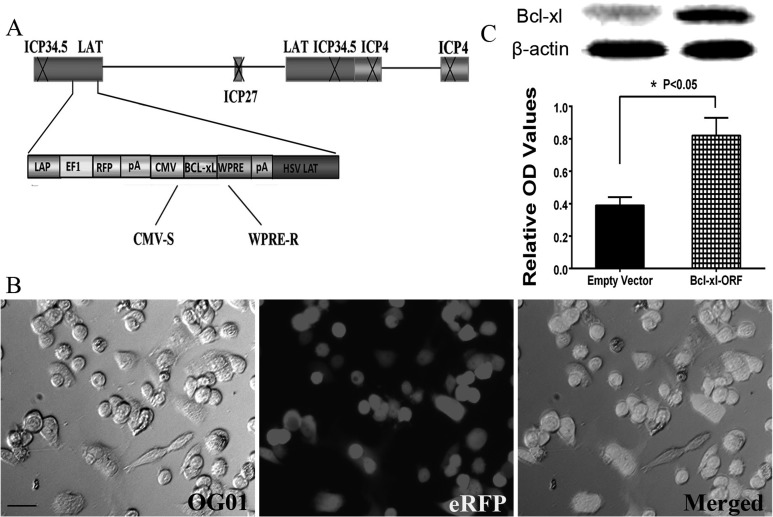
Recombinant HSV was produced as previously described.33 First, as HSV carrying Bcl-xL recombinant has lost its proliferative capacity, in order to help the replication of nonreplicated HSV Bcl-xL recombinant, ICP27 and ICP4 gene were constructed into plasmid. Next, Vero HSV packaging cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Abcam, Cambridge, United Kingdom) and plated in a 6-well plate at the concentration of 1 to 1.5 × 105. After the cells were incubated at 37 °C with 5% CO2 for 1 d and the cell confluency reached 90% to 95%, transfection began according to the manufacturer’s protocol. Briefly, the Bcl-xL recombinant overexpression vector, which expressed the ICP27 and ICP4 proteins required for the packaging of the virus containing the Bcl-xL ORF, was cotransfected into the Vero cell line. After 48 h of transfection, the culture medium was collected and centrifuged at 4 °C, 3,000g, and the supernatant was filtered. Lentiviral stocks were stored at −80°C for further use. Meanwhile, images were obtained using Leica AF6000 (Leica, Wetzlar, Germany) cell station at 48 h after transfection. The successful transfection of the Bcl-xL ORF lentivirus was confirmed by the enhanced red fluorescent protein (eRFP) in the Vero cells.
Generation of TBI model
The modified Feeney method34–36 was used to cause the injury of cortical motor area in rats. Briefly, rats were anesthetized with chloral hydrate (360 mg/kg, ip; TCI Development Co., Ltd) and the head was mounted in a stereotaxic frame. The head was held in a horizontal plane; a midline incision was used for exposure, and a 7- to 8-mm craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at the midpoint between bregma and lambda, ∼3 mm lateral to the midline, overlying the temporoparietal cortex.37 The cerebral cortex was exposed and covered by a sterile clout. An iron cylinder weighing 50 g dropped vertically from a 30 cm height to strike the clout for moderate contusive injury. Sham injury was performed by anesthetizing the animals, making the midline incision, and separating the skin, connective tissue, and aponeurosis from the cranium, before closing the incision. After surgery, the rats were under supportive care.
Cellular transplantation and Bcl-xL-ORF virus injection
Three days before cell transplantation, the rats received cyclosporine A by ip injection (1 mg/200 g; Zhongsheng Ruitai Technology Co., Ltd, Beijing, China) to minimalize immunological rejection reaction. Cellular transplantation was performed at the day of TBI surgery. The density of NSCs was adjusted to 5×105/μL in DMEM/F12. Cells (5 μL/point) were engrafted into a position at 3 mm rostral, caudal, left, and right aside from wound epicenter, respectively. The depth for insertion was 2 mm, and the velocity of injection was 1 μL/min. NSCs suspension 1.5 μL was injected at the deepest point and then the microinjector was elevated twice, 0.5 mm each. After each injection, the injector was held for 1 min. The volume for injection at different depth was as follows: 1.5 μL/2 mm, 2 μL/1.5 mm, and 1.5 μL/1 mm. In the sham operation group, the “skull window” was opened and the scalp was sutured, without the strike injury or cell injections. In the TBI group, rats were subjected to strikes and only culture medium was injected.
For Bcl-xL-ORF virus injection, in the Bcl-xL-ORF group, the virus injection procedure was the same as NSC transplantation. In the TBI group, rats underwent TBI and were subjected to equal HSV-1-NC vector.
Neurological severity score evaluation
Neurological severity score (NSS) values have been described in detail in previous studies.37,38 During NSS evaluation, subjects were acclimated to the observation fields for 3 d prior to surgery, 5 min per day, so that they often remain motionless when introduced to a new apparatus. In addition, a double-blind method was employed; all scores were determined by 3 assistants who were blind to the subject’s experimental treatment to ensure reliability of score. NSS evaluation was reformed at 7 d postoperation (dpo) and 14 dpo.
Tissue harvest
After 7 and 14 d following NSC transplantation or Bcl-xL-ORF injection, rats in each group were subjected to 4% PFA via left ventricle perfusion. After being fixed by phosphate buffer containing the 4% PFA for 18 to 24 h, samples were immersed into phosphate buffer containing 20% sucrose overnight at 4 °C. Continuous coronal sections were cut in 10 μm thicknesses for the following staining. Fresh cerebral cortex tissue was removed at 7 d after TBI surgery and stored in −80 °C for the following molecular experiments.
Immunofluorescent staining
Frozen sections were observed to evaluate the implanted cells in the host brains under fluorescence microscopy (Leica CM1900; Leica). After detecting the migration of implanted cells, the differentiation was determined by the immunostaining of primary antibodies of neuronal class III β-tubulin (Tuj1, 1:200, mouse; Millipore, Boston, Massachusetts, USA) and GFAP (1:500, mouse, Chemicon), as described previously. The second antibody designed as water-soluble 3H-indocyanine-type bioluminescent-labeled dyes (cy3, 1:200, 115-165-146; Jackson, Bar Harbor, ME) was used. The double-labeled cells including GFP/Tuj1 and GFP/GFAP were seen respectively under the fluorescent microscope.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was used to detect the neuronal apoptosis. Briefly, after the frozen sections were obtained, 100 μL of freshly diluted proteinase K (20 μg/mL; Roche, Basel, Switzerland) was added on each section, followed by a digestion at 37 °C for 15 min. Sections were then immersed in 0.1% diethylpyrocarbonate water for 30 min. After 0.01 M PBS washing, solution 1 and solution 2 from the TUNEL kit (Roche) were merged with ice in a 1:9 volume ratio for making TUNEL reactive mixture. Fresh 3% H2O2 methanol (Suolai Po Biological Technology Co., Ltd) was incubated with the sections under room temperature for 15 min, followed by 3 washes with 0.01 M PBS. A total of 50 μL 5% bovine serum albumin, serving as confining liquid, was added on each section at 37 °C for 30 min. Subsequently, a total of 50 μL transforming agent (Suolai Po Biological Technology Co., Ltd) was added on each section and incubated at 37 °C for 40 min, followed by a wash with 0.01 M PBS 3 times. Tetramethylbenzidine (Suolai Po Biological Technology Co., Ltd) was used for coloration under room temperature. After that, distilled water was added to stop the coloration reaction. The negative control group only received solution 2 without addition of solution 1. Sections were incubated with DNase I (Shanghai Haoran Biological Technology Co., Ltd, Shanghai, China) at room temperature for 10 min as a positive control group. The change of number and the morphology of apoptotic cells from 3 fields of each section were observed under a general light microscope (CX40; Shunyu, Ningbo, China). At least 5 sections from each animal were prepared and 5 animals per group were quantitatively analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD). Three independent experiments were performed and the data were presented as the mean ± standard deviation (SD).
RT-PCR
Total RNA from brain tissue was extracted and reverse transcribed for cDNA using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen), according to the manufacturer’s instructions. The specific primers were designed by Primer 5 software (Premier, Toronto, Ontario, Canada) and synthetized by Takara Bio Inc (Dalian, Liaoning, China; Table 3). PCR reactive system was then established: 2 × PCR Master Mix 12. 5 µL, PCR water nuclear-free 10.5 µL, upriver primer 0.5 µL, downstream primer 0.5 µL, cDNA template 1 µL, 25 µL in total. PCR reactive condition for 35 cycles was as follows: predenaturation, 94 °C for 1 min; reannealing for 1 min; elongation, 72 °C for 1 min. PCR products were electrophoresed with 1% agarose gel electrophoresis, were visualized by Gold view staining (Shang Hai Haoran Biological Technology Co., Ltd), and were analyzed by Alpha Innotech (Bio-Rad, Hercules, CA). The optical density was tested for statistical analysis. β-Actin was used as housekeeping gene for control. Three independent experiments were performed and the data were presented as the mean ± SDs.
Table 3.
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Bcl-xL | 5′CGTGGAAAGCGTAGACAAGG3′ | 5′TGAAGAGTGAGCCCAGCAGA3′ |
| Bax | 5′CATCCAGGATCGAGCAGA3′ | 5′AAGTAGAAGAGGGCAACCAC3′ |
| BCL-2 | 5′GGCATCTTCTCCTTCCAGC3′ | 5′TCCCAGCCTCCGTTATCC3′ |
| FasL | 5′CTGGTGGCTCTGGTTGGA3′ | 5′GCTTAGGGGCTGGCTGTT3′ |
| Caspase3 | 5′AACGAACGGACCTGTGG3′ | 5′GGGTGCGGTAGAGTAAGC3′ |
| β-Actin | 5′TGTGCTGTCCCTGTACGCCTCT3′ | 5′CCTTAATGTCAC-GCACGATTTCC3′ |
Abbreviations: Bcl-xL, B-cell lymphoma-extra large; Bax, associated X protein; Bcl-2, B-cell lymphoma 2; FasL, Fas ligand.
Western blot
Protein was extracted from cerebral cortex tissues by radioimmunoprecipitation assay lysis solution (Beyotime Institute of Biotechnology, Jiangsu, China). Equal quantities of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Beijing Junyi Electrophoresis Co., Ltd, Beijing, China; 3% spacer gel, 12% separation gel) and were electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The PVDF membrane was blocked by Tris-buffered saline Tween-20 (TBST; Shanghai Double-Helix Biotech Co., Ltd, Shanghai, China) containing 5% evaporated milk (Shanghai Double-Helix Biotech Co., Ltd) to prevent unspecific protein-binding sites under room temperature for 2 h. Then the membrane was incubated with Bcl-xL (1:1,000; Abcam) and β-actin antibodies (1:2,000; Abcam) for 18 h at 4 °C, respectively. Horseradish peroxidase–coupled goat antirabbit immunoglobulin G (1:5,000; Millipore), as secondary antibody, was added and incubated for 2 h under room temperature, followed by TBST washout again. The immunoreactive protein was found to be present, as determined by enhanced chemiluminescence reagent and quantified by densitometry.
Statistical Analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL) was used to analyze the data by 1-way analysis of variance (ANOVA) and t test. For multiple group comparison, ANOVA with Tukey post hoc multiple comparisons was applied and the data values were expressed as mean ± SD. P < 0.05 was considered statistically significant.
Results
NSC Morphology and Identification
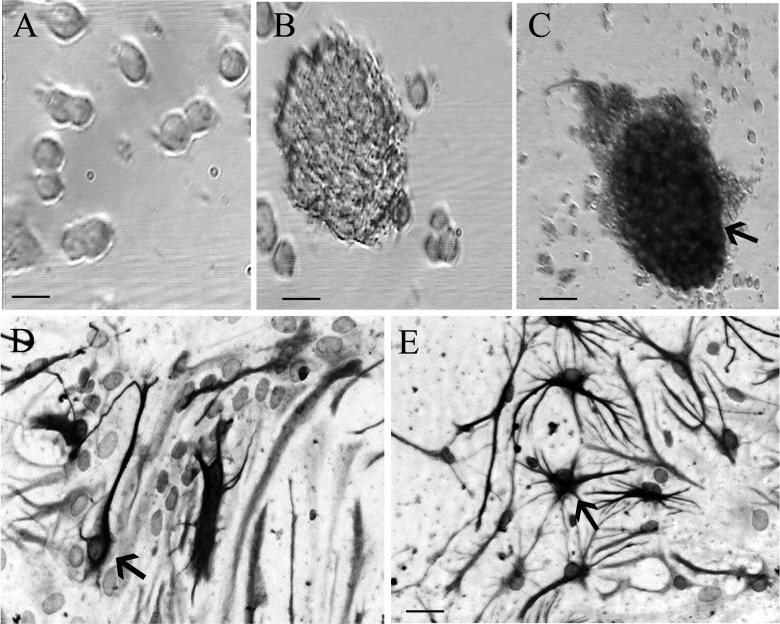
During the formation of the neurospheres, their sizes increased gradually. At 12 h of primary culture, the single-cell suspension mainly exhibited a single cell, with a small size and transparent form that was round or oval in shape (Fig. 1a). After being cultured for 3 d, tens to several tens of cells aggregated to form neurospheres that gradually enlarged over time (Fig. 1b). After passage 1, there were the same growth spheres as at 3 d of primary culture. Moreover, immunoenzyme-linked staining of undifferentiated NSCs by nestin antigen in the neurospheres confirms the character of the NSCs (Fig. 1c). In addition, we detected the capacity of NSCs to differentiate into neurons and astrocytes in vitro by NeuN and GFAP detection (Fig. 1d, e).
Figure 1.
Morphology and identification of neural stem cells (NSCs). (a) The morphology of cells at 12 h of primary culture. (b) The neurospheres composed of hundreds of cells at 3 d after culture under microscope in bright field. (c, d, and e) Three days after culture, respectively. Nestin (marker of NSCs), NeuN (marker of neurons), and glial fibrillary acidic protein (GFAP; marker of astrocytes) immunopositive cells were observed under the light microscope, respectively. Scale bar = 25 μm in a, b, d, e. Scale bar = 50 μm in c.
Survival and Differentiation of the Implanted Cells
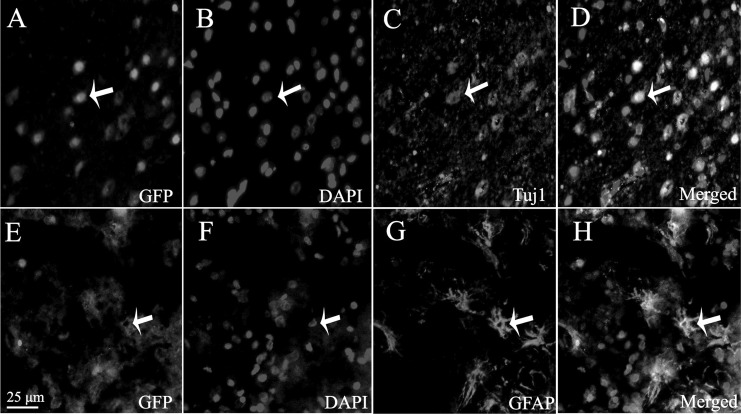
As the implanted NSCs were derived from GFP mice, we could trace the implanted cells under a fluorescent microscope (Leica Microsystems GmbH). GFP-positive cells were observed in host brains around the injured region, which indicated that implanted NSCs could survive and migrate in recipient rats (Fig. 2a, e). Immunofluorescent staining analysis demonstrated that 1 wk postinjection, a few engrafted NSCs had differentiated into immature neurons, confirmed by coexpression of the neuronal marker, Tuj1 (Fig. 2b to d), whereas a few implanted cells with GFP staining could express GFAP-positive marker, indicating part of the engrafted NSCs had differentiated toward astrocytes (Fig. 2f to h).
Figure 2.
Survival and differentiation of transplanted neural stem cells (NSCs) in host brain. (a) Green fluorescent protein (GFP)–positive cells were observed in host brain under fluorescence microscope. (b) Cell nucleus stained by 4,6-diamidino-2-phenyl indole (DAPI). (c) The number of Tuj1-positive cells. (d) The merged picture. (e) Green fluorescent protein (GFP)–positive (green) cells observed in host brain under fluorescence microscope. (f) The cell nucleus stained by DAPI. (g) The differentiated GFAP-like cells. (h) The merged picture. Scale bar = 25 μm in a to h.
Neurological Function Evaluation by NSS
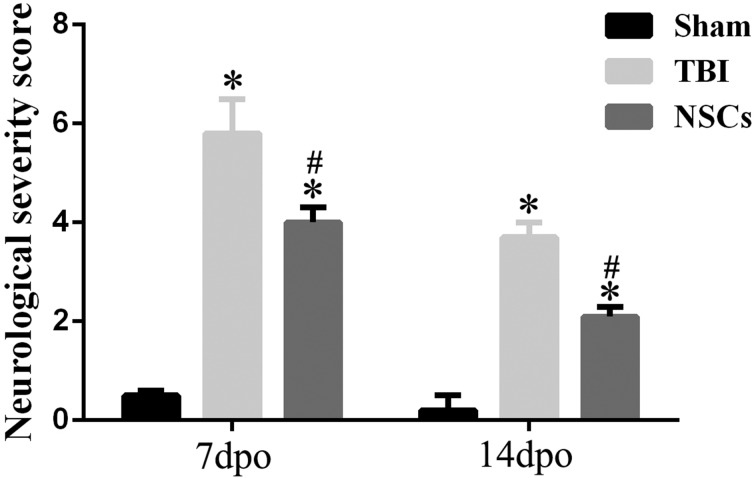
Rats immediately presented convulsions and paraplegia after TBI surgery. We also found that in TBI rats, forelimbs and bodies lost equilibrium function, thereby they were vulnerable to fall down. Three dpo, there was no significant difference between the TBI group and the NSC engrafted group (data not shown). However, by 7 dpo and 14 dpo, the NSS was significantly decreased by NSC grafting (P < 0.05), when compared with only the TBI group (Fig. 3).
Figure 3.
Effect of neural stem cell (NSC) transplantation on neurological functional repair. The figure shows neurological severity score (NSS) test 7 and 14 d after traumatic brain injury (TBI) surgery. The findings showed that TBI induced a significant increase in NSS, while the value of NSS was significantly decreased by NSC implantation. Data are presented as means ± standard deviation (SD). #P < 0.05 versus TBI; *P < 0.05 versus sham.
Effect of NSC Grafts on Neural Apoptosis
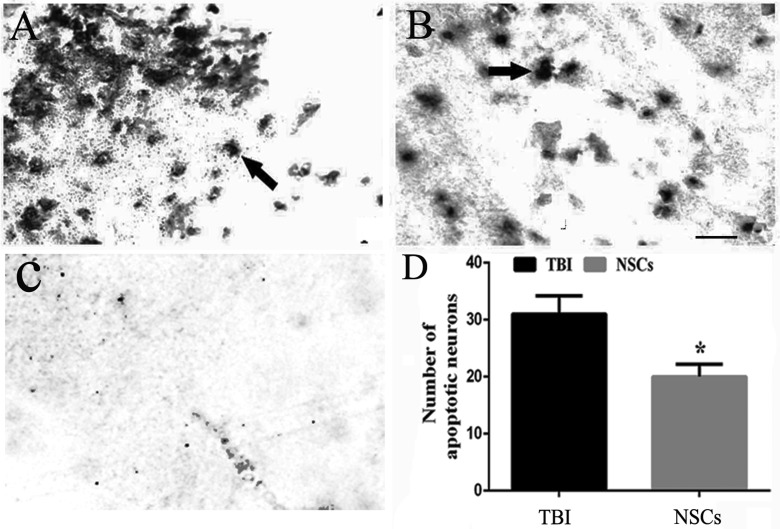
TUNEL staining showed that few apoptotic cells were observed in the sham operated group. In contrast, cellular apoptosis was obviously observed in TBI rats, whereas the number of apoptotic cells was significantly reduced in rats subjected to the administration of NSCs. This confirmed that NSC transplantation could promote neural survival and prevent neuronal apoptosis in host (P < 0.05; Fig. 4a to d).
Figure 4.
Cell apoptosis in host brain. (a and b) Terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate nick end labeling (TUNEL) staining of the cerebral cortex in the traumatic brain injury (TBI) group and the NSC group, respectively. (c) The staining in negative control. (d) The representative bar graph of the TUNEL-positive cells. Data are presented as means ± standard deviation (SD). *P < 0.05 versus TBI. Arrow showed the apoptotic cells. Scale bars = 25 μm.
Alternations of Bcl-xL in the Host Cerebral Cortex
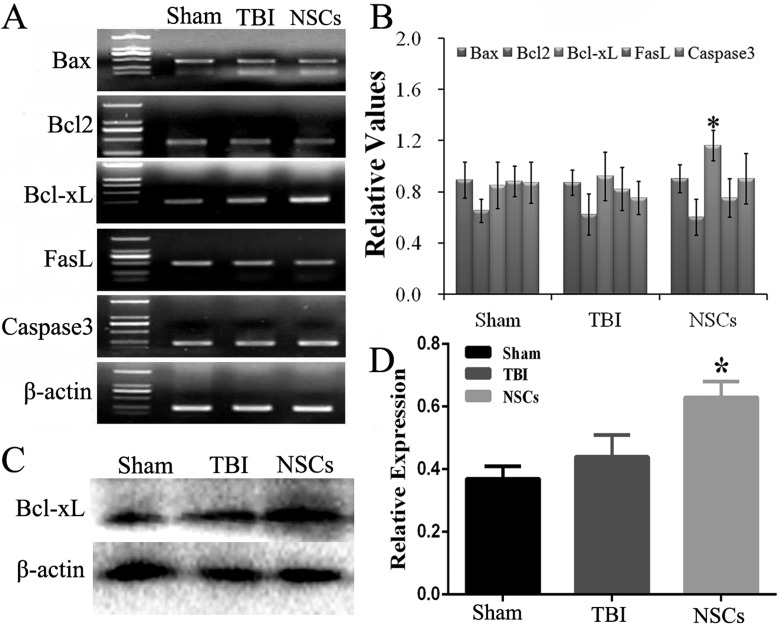
To determine the key genes associated with NSC transplantation after TBI, we examined the expression of several genes 7 d after NSC transplantation. RT-PCR results demonstrated that the expression of Bcl-xL messenger RNA (mRNA) was significantly increased following NSC transplantation compared with that of the TBI group (P < 0.05), but the expression of other genes (associated X protein [Bax], Bcl2, Fas ligand [FasL], and caspase3) was not significantly different (Fig. 5a to b).
Figure 5.
Change of apoptosis genes in host brain. (a) The changes on mRNA of B-cell lymphoma-extra large (Bcl-xL), BCL2-associated X protein (Bax), B-cell lymphoma 2 (Bcl2), Fas ligand (FasL), and caspase3 in host cerebral cortex, which were examined by reverse transcription-polymerase chain reaction (RT-PCR), and quantitative analysis was shown in (b). (c and d) The evaluation of Bcl-xL protein by Western blot and quantitative analysis, respectively. Data are presented as means ± standard deviation (SD). #P < 0.05 versus TBI; *P < 0.05 versus sham.
Meanwhile, Western blot was performed to identify the level of Bcl-xL. The results showed the content of Bcl-xL protein was also significantly increased in cortex following NSC transplantation (P < 0.05; Fig. 5c, d).
Comparison of the Effect of Bcl-xL-ORF Injection and NSC Transplantation on Functional Recovery after TBI
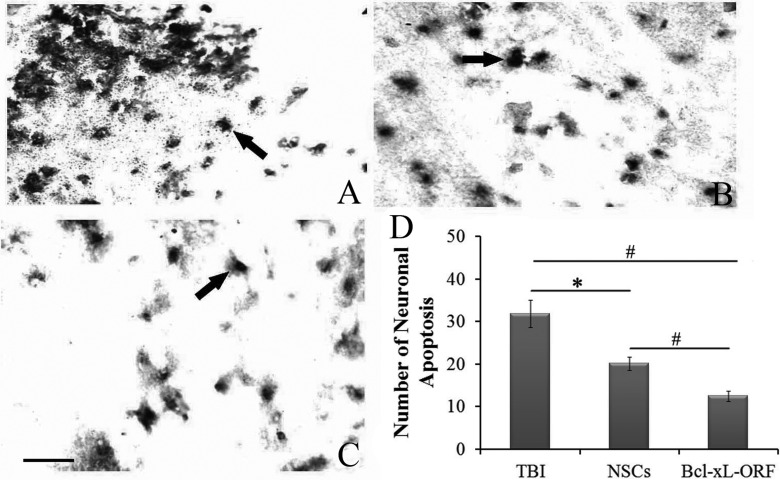
To investigate the effect of Bcl-xL on the repair of neurological function and detect whether Bcl-xL overexpression could be used a viable treatment for TBI, we constructed an HSV-1-based vector of Bcl-xL to upregulate Bcl-xL expression (Fig. 6a). In vitro, we confirmed that products of Bcl-xL ORF virus could efficiently transfect the OG01 cells and express the eRFP (Fig. 6b). After injection of HSV-1-Bcl-xL-ORF, the protein expression of Bcl-xL in the cerebral cortex was higher in the Bcl-xL-ORF group as compared with the HSV empty vector group (Fig. 6c). Moreover, we compared the effects of NSC transplantation and Bcl-xL-ORF therapy on the neurological function improvement indicated by NSS evaluation, and the consequence is similar (Fig. 7). Moreover, TUNEL staining showed that neuronal apoptosis induced by TBI was significantly reduced in NSC graft and Bcl-xL overexpression rats (Fig. 8a to d). Comparing the NSC group with the Bcl-xL-ORF group, the NSS score in the Bcl-xL-ORF group was further decreased, and there were fewer apoptotic cells in the Bcl-xL-ORF group than in the NSCs group at 7 dpo (P < 0.05; Figs. 7, 8a to d, 9).
Figure 6.
Construction and identification of the herpes simplex virus (HSV) B-cell lymphoma-extra large (Bcl-xL) overexpression vector. (a) Clone representation of the HSV-1-based Bcl-xL recombinant vector. The schematic of the defective HSV-based vector was produced by deleting the infectious cell protein 4 (ICP4), ICP27, and ICP34.5 genes in the wild-type HSV-1. The vector also contained the elements of human cytomegalovirus (CMV) promoter, woodchuck hepatitis posttranscriptional regulatory element (WPRE), and a polyA (pA) region. (b) Vero cells were transfected by HSV-Bcl-xL-open reading frame (ORF). Scale bar = 25 μm. (c) The protein expression of Bcl-xL after HSV-empty vector and HSV-Bcl-xL-ORF was transfected into the cerebral cortex, indicated by Western blot, and the protein expression of Bcl-xL in the Bcl-xL-ORF group is significantly higher than that in the empty vector group. *P < 0.05.
Figure 7.
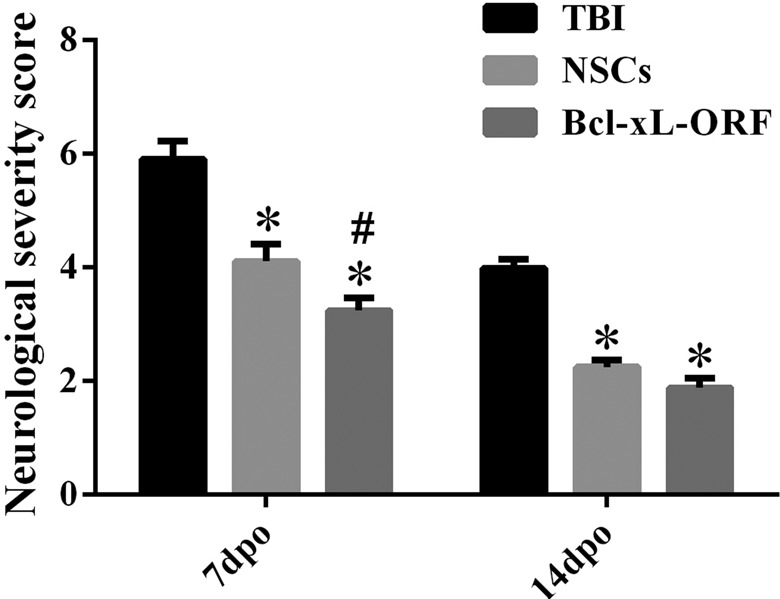
Effect of B-cell lymphoma-extra large (Bcl-xL)-open reading frame (ORF) on neurological functional repair as compared with neural stem cell (NSC) transplantation. The representative bar graph shows neurological severity score (NSS) test after 7 and 14 d of traumatic brain injury (TBI) surgery. The results exhibited that TBI induced a significant increase in NSS, while the value of NSS was significantly decreased by NSC implantation or Bcl-xL-ORF injection. Moreover, the NSS scores in the Bcl-xL-ORF group were lower than that in the NSC group at 7 d postoperation (dpo). Data are presented as means ± standard deviation (SD). #P < 0.05 versus TBI; *P < 0.05 versus sham.
Figure 8.
Effect of B-cell lymphoma-extra large (Bcl-xL)-open reading frame (ORF) on cell apoptosis. The changes of cells apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining in host brain are shown in TBI group (a) and neural stem cell (NSC) group (b) and Bcl-xL-ORF group (c). The arrows in (a-c) show the apoptotic cells. (d) The quantitative analysis of the cell apoptosis. Data are presented as means ± standard deviation (SD). #P < 0.01; *P < 0.05. Scale bars = 25 μm (a-c).
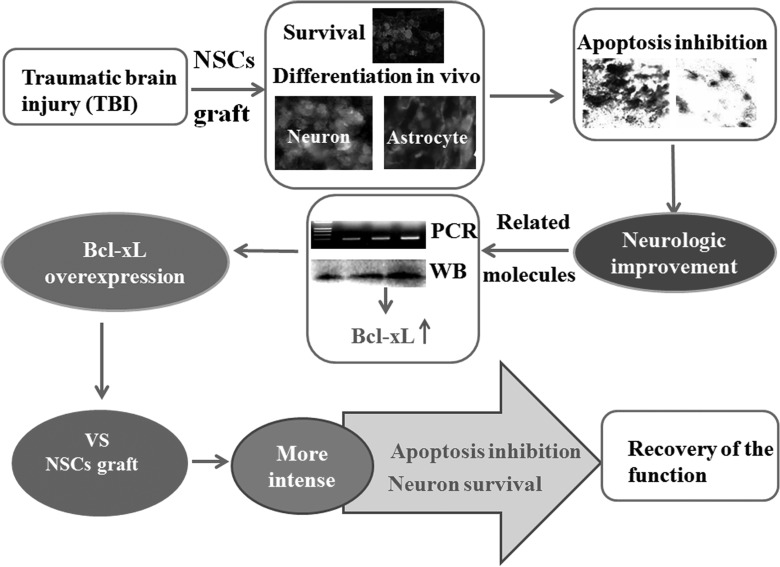
Figure 9.
Model of the underlying mechanism of how neural stem cell (NSC) transplantation induced functional recovery in traumatic brain injury (TBI) rats as well as comparison with B-cell lymphoma-extra large (Bcl-xL) overexpression. At the first day of TBI, NSCs were transplanted into the host brain tissue and demonstrated to survive and differentiate into neurons and astrocytes in vivo. In addition, after NSC transplantation, the cell apoptosis was decreased and the neurologic function was improved. Real-time quantitative polymerase chain reaction (RT-PCR) and Western blot (WB) exhibited that Bcl-xL was closely related to the neuroprotective role of NSC transplantation after TBI. Thereafter, the comparison of Bcl-xL overexpressing and NSCs transplantation showed that Bcl-xL overexpression could inhibit cell apoptosis and promote neuronal survival more intensely. These indicate overexpressing Bcl-xL, as an alternative for stem cell therapy, may be a novel treatment of TBI patients.
Discussion
In this study, we demonstrated that NSCs sourced from GFP transgenic mice transplanted into TBI rats could survive, migrate, and differentiate into neurons and astrocytes near the epicenter of the injury cortex in TBI rats under immunosuppression conditions. After NSC transplantation, TBI rats exhibited a significant decrease in neurological function defects and the number of cellular apoptosis was attenuated, compared with the TBI group. We hypothesized that the NSC transplantation effects might be associated with regulation of apoptosis. To demonstrate this, several apoptosis genes including Bax, Bcl2, FasL, caspase3, Bcl-xL were detected, and a significant upregulation of Bcl-xL was noted in NSC-implanted TBI model. Subsequently, to further demonstrate the therapeutic potential of Bcl-xL in TBI, we overexpressed Bcl-xL in the cerebral cortex after TBI and compared the effects with those of NSC transplantation. The results showed that Bcl-xL overexpression could reduce the cell apoptosis and the effect is much better than that in NSC transplantation. Therefore, it could be used as an optimal strategy for the treatment of TBI (Fig. 9).
The Reason for the Choice of NSCs Derived from GFP Transgenic Mice
In this study, we isolated and cultured NSCs derived from the hippocampus of GFP-labeled transgenic mice in order to trace the transplanted cells conveniently. Previous studies have demonstrated that engrafted NSCs are resident in the host’s cerebral cortex, subependymal layer, striatum, hippocampus, and midbrain in the rat brain.5–9 In order to protect transplanted cells from immune attack, cyclosporine A, an immunosuppressive agent, was effectively administrated in this experiment. We found that transplanted GFP-NSCs from mice could survive in the brain tissues of rats, which indicates our method is viable.
TBI Model Establishment
TBI induced blood–brain barrier breakage, cerebral hemorrhage, and ischemia, which led to increasing intracranial pressure and the release of excitatory amino acids.2 As a consequence, cytoplasm membrane and myelin sheath degeneration happened, which resulted in neurological function deficits.39 In our study, a weight-drop injury mimicking clinical cerebral contusive injury dramatically increased NSS scores and neuronal loss, which suggested that the TBI model was successfully generated in rats. The generation of a TBI model provides useful experimental animals for later cell transplantation.
The Effects of NSC Transplantation on Neurological Function Recovery
Implanted NSCs needed an optimal microenvironment for survival and differentiation. We detected GFP-positive cells near the injured brain after transplantation; the observation showed that implanted NSCs could survive in the host brain. Previously, studies showed that NSCs grafted in an injured brain could promote neuronal functional recovery. Implanted NSCs have the capacity to stimulate host neurogenesis in the adult mammalian brain40,41 and promote damaged tissue regeneration in the central nervous system (CNS).42 Although potential side effects make existing cell transplantation therapies an ethical issue,43 several pieces of evidence have shown that cell transplantation could play a crucial role in promoting repair processes. It is well known that NSCs, as undifferentiated cells, can self-renew and differentiate into many types of cells in the CNS, including neurons, astrocytes, and oligodendrocytes. Therefore, the therapeutic effects of NSC transplantation are related to its capacity of differentiation. The cells (neurons, astrocytes, and oligodendrocytes) derived from NSCs are involved in various processes, including remyelination, trophic action, repair, and regeneration.44 Increasing evidence has demonstrated that NSC transplantation is a promising treatment for several neurodegenerative diseases including AD, PD, and SCI.15–19 For the application of NSC transplantation in AD, NSCs were injected into bilateral hippocampal regions. As a result, spatial learning and memory function in all implanted mice were improved, and proliferation, migration, and differentiation of NSCs were found. Furthermore, long-term potentiation was enhanced and expression of proteins related to cognitive function in neurons was increased, but there were no changes in Aβ pathology.17,45 Human NSC transplantation also improves cognitive function, which is associated with enhancing endogenous synaptogenesis.46 In the PD mouse model, it has been reported that after transplantation into the right ascending nigrostriatal dopaminergic pathway or striatum, NSC grafts transplanted alone or with Glialcelltine Derived Neurotrophic Factor (GDNF) integrated in the striatum and mitigated the contralateral rotations, therefore improving the symptoms of PD via regulation of the host microenvironment or through improving ceruloplasmin expression.18,47,48 In addition, NSCs transduced with tyrosine hydroxylase cotransfected with Rattus norvegicus POU class 3 homeobox 4 (Pou3f4/Brn4) could generate more mature dopaminergic neurons.49 In addition, studies have shown that hind limb motor function was improved significantly after NSC transplantation. This recovery might be due partly to the reduction in tissue loss during secondary injury processes and diminished glial scarring.20,21 Additionally, NSC transplantation can not only fill lesion cavities and extend axons robustly but also form ectopic colonies in different areas of the nervous system.50,51 It has also been revealed that NSC transplantation may promote angiogenesis by inducing vascular endothelial growth factor expression and reducing astrogliosis, thus improving functional recovery of limb movements after SCI.19,22 Moreover, in SCI mice, NSC transplantation markedly reduced the expression of several pro-inflammatory cytokines, such as tumor necrosis factor α, interleukin 1β (IL-1β), IL-6.52 In our study, we confirmed that NSCs had differentiated into neurons and astrocytes, which therefore provided new evidence to at least partially explain the effect of NSCs on functional improvement. We found that the underlying molecular mechanisms after NSC transplantation were involved in cell apoptosis and the regulation of apoptotic genes.
Bcl-xL Upregulation Served as an Important Mechanism for the Protective Role of NSC Transplantation Associated with Inhibition of Cell Apoptosis
To explore the possible genes involved in NSC grafting in the TBI model, brain tissues were prepared for a PCR experiment at day 7 after NSC transplantation. Through the comparative analysis of gene expressions between the TBI group and the TBI + NSC group, we found the mRNA level of Bcl-xL was markedly increased following NSC transplantation, while no differences were detected in Bax, Bcl2, FasL, and caspase3 mRNA levels. Moreover, the level of Bcl-xL protein was in accordance with its gene expression, indicated by a Western blot. As previously reported, on one hand, Bcl-xL could prevent apoptosis by binding to and sequestering proapoptotic factors and signals so they could not reach their target. On the other hand, Bcl-xL could induce proapoptotic transcription factor p53 expression.53 In addition, increasing Bcl-xL/Bax complex production can reduce cytochrome c release and inhibit caspase3 activation.54 Therefore, the neuroprotective action of NSC transplantation could partially depend on antiapoptotic protein upregulation. Our data demonstrated that NSC grafting effectively increased the expression of Bcl-xL, and Bcl-xL expression may be associated with inhibition of neuronal apoptosis in TBI rats.
Bcl-xL Overexpression, as a Replacement for NSC Transplantation, Could Be a Potential Therapeutic Strategy for TBI
In this study, we found that Bcl-xL expression was upregulated, suggesting it played a vital role in the inhibition of apoptosis after NSC transplantation. Although NSC grafting has been shown to play a neuroprotective role in the CNS,15,16 the source and immunological rejection of NSCs are still obstacles in the clinic. Researchers therefore need to find other effective strategies for disease intervention. In this study, we discovered that the upregulation of Bcl-xL is associated with NSC transplantation, which suggested the overexpression of Bcl-xL may have therapeutic roles after TBI. Recently, with stable chemical and physical properties, low immunogenicity of capsid proteins, high safety profile in the host, and the feasibility of clinical scale production,33,55 virus-transduced gene therapy, such as with HSV and lentiviruses, has been demonstrated to be a potential viable option for the treatment of various diseases. Accordingly, an HSV-1-based vector for Bcl-xL was employed to upregulate Bcl-xL expression in our study. Our data demonstrated that compared with the NSC-grafted group, the injection of Bcl-xL-ORF virus could effectively reduce cellular apoptosis and ultimately improve neurological function. Meanwhile, Bcl-xL overexpression exhibited an effective therapeutic role in the TBI rat model. Therefore, Bcl-xL overexpression may be considered as a potential treatment for TBI in future clinical practices.
Conclusion
Our study revealed that NSC grafts played an antiapoptotic role in recovery of neurological function in a murine TBI model, and the mechanisms underlying recovery are associated with Bcl-xL upregulation. Furthermore, Bcl-xL overexpression by the HSV-1-based method showed a positive therapeutic role in TBI rats. In investigating the mechanisms of action, we concluded that Bcl-xL overexpression could effectively decrease apoptosis and promote neurological recovery. Therefore, to some extent, administration of Bcl-xL, similar to NSC transplantation, may be used as a novel therapeutic strategy for TBI patients in future clinical trials.
Footnotes
Author Contributions: Ai-Lan Pang, Liu-Lin Xiong contributed equally to this work.
Ethical Approval: The protocols in this study were approved by the relevant ethics committee (see Materials and Methods).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (CN; No. 2014BAI01B10), by the Program for IRTSTYN, together with the program Innovative Research Team in Science and Technology in Yunnan province and the grant from the National Natural Science Foundation of China (Grant No. 81360267).
References
- 1. Chang TP, Nager AL. Pediatric traumatic brain injury: the utility of beta-natriuretic peptide. J Trauma. 2010;68(6):1401–1405. [DOI] [PubMed] [Google Scholar]
- 2. Namjoshi DR, Good C, Cheng WH, Panenka W, Richards D, Cripton PA, Wellington CL. Towards clinical management of traumatic brain injury: a review of models and mechanisms from a biomechanical perspective. Dis Model Mech. 2013;6(6):1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dewall J. The ABCs of TBI. Evidence-based guidelines for adult traumatic brain injury care. JEMS. 2010;35(4):54–61. [DOI] [PubMed] [Google Scholar]
- 4. Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106(32):13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372(6503):263–266. [DOI] [PubMed] [Google Scholar]
- 6. Zhou FC, Duguid JR, Edenberg HJ, McClintick J, Young P, Nelson P. DNA microarray analysis of differential gene expression of 6-year-old rat neural striatal progenitor cells during early differentiation. Restor Neurol Neurosci. 2001;18(2-3):95–104. [PubMed] [Google Scholar]
- 7. Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22(5):1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14(5):595–614. [DOI] [PubMed] [Google Scholar]
- 9. Tomishima M. Midbrain dopamine neurons from hESCs. StemBook (Internet). Cambridge (MA): Harvard Stem Cell Institute; 2008. –2012. https://www.ncbi.nlm.nih.gov/pubmed/23658980. [PubMed] [Google Scholar]
- 10. Lee da Y, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansouri S, Ortsäter H, PintorGallego O, Darsalia V, Sjöholm A, Patrone C. Pituitary adenylate cyclase-activating polypeptide counteracts the impaired adult neural stem cell viability induced by palmitate. J Neurosci Res. 2012;90(4):759–768. [DOI] [PubMed] [Google Scholar]
- 12. Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. [DOI] [PubMed] [Google Scholar]
- 13. Bergstrom T, Forsbery-Nilsson K. Neural stem cells: brain building blocks and beyond. Ups J Med Sci. 2012;117(2):132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: new perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2014;141(1):21–31. [DOI] [PubMed] [Google Scholar]
- 15. Piccini P, Pavese N, Hagell P, Reimer J, Björklund A, Oertel WH, Quinn NP, Brooks DJ, Lindvall O. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain. 2005;28(Pt 12):2977–2986. [DOI] [PubMed] [Google Scholar]
- 16. Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, Tarensenko YI, Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol. 2006;201(2):281–292. [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Wang PJ, Sha HY, Ni J, Li MH, Gu GJ. Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer’s disease. Mol Neurobiol. 2014;50(2):423–437. [DOI] [PubMed] [Google Scholar]
- 18. Zuo FX, Bao XJ, Sun XC, Wu J, Bai QR, Chen G, Li XY, Zhou QY, Yang YF, Shen Q, et al. Transplantation of human neural stem cells in a Parkinsonian model exerts neuroprotection via regulation of the host microenvironment. Int J Mol Sci. 2015;16(11):26473–26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Guo GH, Wang GS, Guan CX, Yue L. Influence of neural stem cell transplantation on angiogenesis in rats with spinal cord injury. Genet Mol Res. 2014;13(3):6083–6092. [DOI] [PubMed] [Google Scholar]
- 20. Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289(47):32512–32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015;24(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilcox JT, Satkunendrarajah K, Zuccato JA, Nassiri F, Fehlings MG. Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury. Stem Cells Transl Med. 2014;3(10):1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao L, Liu X, Liang J, Han S, Wang Y, Yin Y, Luo Y, Li J. Phosphorylation of p38 MAPK mediates hypoxic preconditioning-induced neuroprotection against cerebral ischemic injury via mitochondria translocation of Bcl-xL in mice. Brain Res. 2013;1503:78–88. [DOI] [PubMed] [Google Scholar]
- 24. Guo Y, Xu X, Li Q, Li Z, Du F. Anti-inflammation effects of picroside 2 in cerebral ischemic injury rats. Behav Brain Funct. 2010;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu G, Song J, Guo Y, Wang T, Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct. 2013;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. [DOI] [PubMed] [Google Scholar]
- 27. Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29(23):6149–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parrondo R, de Las Pozas A, Reiner T, Perez-Stable C. ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. Peer J. 2013;1:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res. 2013;73(4):1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SI, Kim BG, Hwang DH, Kim HM, Kim SU. Overexpression of Bcl-XL in human neural stem cells promotes graft survival and functional recovery following transplantation in spinal cord injury. J Neurosci Res. 2009;87(14):3186–3197. [DOI] [PubMed] [Google Scholar]
- 31. Umschwief G, Shein NA, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30(3):616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xing RX, Zhu LL, Yang M, Liu F. BDNF administration in skeletal muscle is effective to promote locomotor function improvement in SCT rats. Ibrain. 2015;1(5):36–40. [Google Scholar]
- 33. Chen ZW, Wang HP, Yuan FM, Zhang X, Dong XJ, Xie RS, Tian C, Li BS, Sun ZW, Zhou LH, et al. Releasing of herpes simplex virus carrying NGF in subarachnoid space promotes the functional repair in spinal cord injured rats. Curr Gene Ther. 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34. Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5(1):1–15. [DOI] [PubMed] [Google Scholar]
- 35. Olanow CW. Deprenyl in the treatment of Parkinson’s disease: clinical effects and speculations on mechanism of action. J Neural Transm Suppl. 1996;48:75–84. [DOI] [PubMed] [Google Scholar]
- 36. Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine (Phila Pa 1976). 2000;25(14):1859–1866. [DOI] [PubMed] [Google Scholar]
- 37. Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS., Jr Subacute neural stem cell therapy for traumatic brain injury. J Surg Res. 2009;153(2):188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28(4):365–378. [DOI] [PubMed] [Google Scholar]
- 39. Krieger DW. Therapeutic drug approach to stimulate clinical recovery after brain injury. Front Neurol Neurosci. 2013;32:76–87. [DOI] [PubMed] [Google Scholar]
- 40. Jaber M, Benoit-Marand M, Prestoz L, Gaillard A. Cell transplantation in the damaged adult brain. Rev Neurol (Paris). 2013;169(11):838–843. [DOI] [PubMed] [Google Scholar]
- 41. Yi BR, Kim SU, Choi KC. Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab Anim Res. 2013;29(3):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okano H, Sawamoto K. Neural stem cells: involvement in adult neurogenesis and CNS repair. Philos Trans R Soc Lond B Biol Sci. 2008;363(1500):2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winkler C, Kirik D, Björklund A. Cell transplantation in Parkinson’s disease: how can we make it work? Trends Neurosci. 2005;28(2):86–92. [DOI] [PubMed] [Google Scholar]
- 44. Okano H. Neural stem cells and strategies for the regeneration of the central nervous system. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(4):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang W, Gu G, Shen X. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer’s disease-like pathology. Neurobiol Aging. 2015;36(3):1282–1292. [DOI] [PubMed] [Google Scholar]
- 46. Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, LaFerla FM, Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 2015;25(7):813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duan K, Wang X, Yang Z, Wang B, Wang M, Zhang H, Deng X. Therapeutic effect of GDNF gene-modified mesencephalic neural stem cell transplantation in a rat model of Parkinson disease [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(1):32–38. [PubMed] [Google Scholar]
- 48. Xiao JJ, Yin M, Wang ZJ, Wang XP. Transplanted neural stem cells: playing a neuroprotective role by ceruloplasmin in the substantia nigra of PD model rats? Oxid Med Cell Longev. 2015;2015:618631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan X, Zhang L, Zhu H, Qin J, Tian M, Dong C, Li H, Jin G. Brn4 and TH synergistically promote the differentiation of neural stem cells into dopaminergic neurons. Neurosci Lett. 2014;571:23–28. [DOI] [PubMed] [Google Scholar]
- 50. Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014;257:186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Steward O, Sharp KG, Yee KM. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J Neurosci. 2014;34(42):14013–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng Z, Zhu W, Cao K. Anti-Inflammatory mechanism of neural stem cell transplantation in spinal injury. Int J Mol Sci. 2016;17(9). pii: E1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Michels J, Kepp O, Senovilla L, Lissa D, Castedo M, Kroemer G, Galluzzi L. Functions of BCL-X L at the interface between cell death and metabolism. Int J Cell Biol. 2013;2013:705294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012;9(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qi YH, Yao WL, Zhang CH. Effect of lentivirus-mediated RNA interference of APC-Cdh1 expression on spinal cord injury in rats. Genet Mol Res. 2014;13(1):1366–1372. [DOI] [PubMed] [Google Scholar]