Abstract
Artificial bones made of β-tricalcium phosphate (β-TCP) combined with bone marrow-derived mesenchymal stromal cells (BM-MSCs) are used for effective reconstruction of bone defects caused by genetic defects, traumatic injury, or surgical resection of bone tumors. However, the selection of constructs with high osteogenic potential before implantation is challenging. The purpose of this study was to determine whether the calcium concentration in BM-MSC culture medium can be used as a nondestructive and simple osteogenic marker for selecting tissue-engineered grafts constructed using β-TCP and BM-MSCs. We prepared three cell passages of BM-MSCs derived from three 7-week-old, male Fischer 344 rats; the cells were cultured in osteoinductive medium in the presence of β-TCP for 15 days. The medium was replaced with fresh medium on day 1 in culture and subsequently changed every 48 h; it was collected for measurement of osteocalcin secretion and calcium concentration by enzyme-linked immunosorbent assay and X-ray fluorescence spectrometry, respectively. After cultivation, the constructs were implanted subcutaneously into the backs of recipient rats. Four weeks after implantation, the alkaline phosphatase (ALP) activity and osteocalcin content of the constructs were measured. A strong inverse correlation was observed between the calcium concentration in the medium and the ALP activity and osteocalcin content of the constructs, with Pearson's correlation coefficients of 0.92 and 0.90, respectively. These results indicate that tissue-engineered bone with high osteogenic ability can be selected before implantation based on low calcium content of the culture medium, resulting in successful bone formation after implantation. This nondestructive, simple method shows great promise for assessing the osteogenic ability of tissue-engineered bone.
Keywords: Nondestructive, Osteogenic marker, Calcium concentration, Mesenchymal stromal cells, Tissue engineering
Introduction
Treatment of bone defects caused by genetic defects, traumatic injury, or surgical resection of bone tumors is generally complicated and challenging because of the need for transfer of tissues such as bone autografts or free flaps1. Autologous bone grafts are the gold standard for the treatment of bone defects because they do not elicit immunological rejection and possess all the essential components required for new bone formation2. However, harvesting autologous bone damages the otherwise healthy collection site, typically the pelvis, and the amount of bone harvested is generally limited3.
Artificial bone graft materials such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) have been used for the reconstruction of bone defects in clinical cases4,5. Although these artificial bones generally are osteoconductive, they rarely show osteoinduction6. Therefore, large bone defects are difficult to reconstruct using artificial bone alone1. In a quest to overcome these problems, useful techniques that combine artificial bones with bone marrow-derived mesenchymal stromal cells (BM-MSCs), which show remarkable osteogenesis after implantation, have been developed7–11. We previously reported an efficient technique for preparing artificial bones with high osteogenic capacity, in which the artificial bones were combined with an osteogenic matrix cell sheet5,12. However, it has been reported that the in vivo osteogenic potential of human BM-MSC-loaded artificial bone based on HA or β-TCP depends on the osteogenic potential of the BM-MSCs13. This finding indicated that the selection of tissue-engineered constructs with high osteogenic potential is important for achieving stable bone regeneration in clinical cases.
Ideally, an in vitro method for construct selection should be rapid, simple, and nondestructive. Nondestructive methods for evaluating osteoblast differentiation in vitro by measuring calcein added to the culture medium14,15 or by using osteoblast-specific fluorescent markers16 have been reported. However, these methods are not directly clinically applicable because of the use of fluorescent markers for monitoring. We previously reported a method for measuring osteocalcin secreted into the culture medium by cultivating cells using an enzyme-linked immunosorbent assay (ELISA) system17. In addition, we have demonstrated that secreted osteocalcin can be used as a nondestructive marker for selecting tissue-engineered constructs with high osteogenic capacity18. Although the ELISA is a useful nondestructive method, it is inherently time-consuming. Therefore, the development of a more rapid, simple, and nondestructive technique to assess osteogenesis is essential.
In the present study, we measured the calcium concentration in the culture medium because calcium deposition is a reliable marker of osteoblastic differentiation in vitro19. The aim of this study was to determine whether calcium levels in the medium could be used as a nondestructive osteogenic marker for selecting tissue-engineered constructs. We evaluated the correlation between the calcium concentration and osteocalcin secretion, which has previously been demonstrated to be useful for assessing osteogenic potential in a nondestructive manner.
Materials and Methods
Cell Culture
Approval was obtained from the animal experimental review board of Nara Medical University (Nara, Japan) before the start of the experiments. Rat BM-MSCs were isolated and maintained in primary culture as described previously7,11,20,21. Briefly, BM cells were obtained from both femur bone shafts of two 7-week-old male Fischer 344 rats purchased from Japan SLC (Shizuoka, Japan) and seeded into 75-cm2 culture flasks (Falcon/BD Biosciences, Franklin Lanes, NJ, USA). The culture flasks contained 15 ml of standard medium comprising Eagle's minimal essential medium (MEM; Nacalai Tesque, Kyoto, Japan) supplemented with 15% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and a mixture of antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Nacalai Tesque). The cell cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The medium was changed every 48 h to remove nonadherent cells and replenish nutrients; thus, only BM-MSCs adhering to the bottom of the T-75 flasks were cultured. This method has been established and used in previous studies7,11,20,21. After reaching confluence (approximately 14 days), the cells were released from the substratum using trypsin-ethylenediaminetetraacetic acid (EDTA) solution (0.25% trypsin, 0.53 mM EDTA-4Na; Nacalai Tesque). The cell suspension was used for sub-culturing to prepare passage 2 (P2) and P3 cells. Briefly, the released cells were seeded into 75-cm2 culture flasks (Falcon) at a density of 1×104 cells/cm2 for 14 days to harvest the P2 cells. This subculture step was repeated to obtain P3 cells. The cells of the three different passages had different osteogenic potentials and were used for subsequent experiments.
Preparation and Implantation of the β-TCP/BM-MSC Constructs
Sterile, porous β-TCP ceramic scaffolds (SuperPore; 5 mm in diameter and 2 mm thick, 75% porosity) were purchased from Hoya (Tokyo, Japan). The scaffolds were soaked in MEM containing 106 BM-MSCs/ml until the air within the scaffolds was replaced with MEM (no more air bubbles were formed)22. Then the β-TCP scaffolds were incubated in the cell suspension under atmospheric pressure in 5% CO2 at 37°C for 1 h, resulting in the formation of β-TCP/BM-MSC constructs. We prepared three types of constructs containing P1, P2, and P3 BM-MSCs (β-TCP/P1, β-TCP/P2, and β-TCP/P3, respectively). The constructs were placed into a 12-well culture plate (one construct per well) and further subcultured in 1 ml of standard medium with the osteoinductive supplements dexamethasone (Dex; 10 nM; Sigma-Aldrich), ascorbic acid-2-phosphate (Asap; 0.28 mM; Wako, Osaka, Japan), and β-glycerophosphate (β-GP; 10 mM; Calbiochem, La Jolla, CA, USA). β-TCP/BM-MSCs were transferred to a new culture plate with fresh medium on day 1—to remove the influence of BM-MSCs that flowed out from the β-TCP/BM-MSCs and attached to the plate surface—and, subsequently, every 48 h throughout the cultivation period of 15 days. The conditioned medium was collected for measurements. Fourteen constructs each of β-TCP/P1, β-TCP/P2, and β-TCP/P3 were prepared. Of these, four constructs of each type were used for the quantification of total DNA in the disk and four for real-time quantitative polymerase chain reaction (qPCR) at day 15 of subculture, as described below. Four cell-free β-TCPs were included as controls. The remaining six constructs per type were implanted subcutaneously into the back of a syngeneic 7-week-old male rat, as described previously23, and used for histology (n = 2) and measurements of osteocalcin and alkaline phosphatase (ALP) activity (n = 4). The experiment was conducted in triplicate. Thus, we used a total of 15 rats (2 rats for primary culture and 3 rats as recipients for study).
Secretory Osteocalcin and Calcium Concentration Measurement
Conditioned medium from four constructs of each group was used for the measurement of secretory osteocalcin and the calcium concentration during the cultivation period. The amount of osteocalcin secreted into the medium was measured using the rat osteocalcin ELISA system (DS Pharma Biomedical, Osaka, Japan) as described previously17.
The calcium concentration was determined using an X-ray fluorescence spectrometer. Briefly, 50 μl of sample solution taken directly from the culture medium was placed on filter paper (MicroCarry; Rigaku, Tokyo, Japan), dried overnight at room temperature, and transferred to a wavelength-dispersive X-ray fluorescence spectrometer (ZSX Primus; Rigaku) under vacuum. The amount of calcium deposited on the filter paper was measured by detecting the X-ray fluorescence intensity and interpolation with a calibration curve generated using calcium chloride standard solutions. In addition, we estimated calcium reduction in the medium as follows: calcium reduction = (calcium concentration of original medium – calcium concentration of medium after culture for the selected number of days) × culture volume (1 ml), and defined the total estimated calcium reduction as the cumulative estimated calcium reduction over the selected culture period. We assumed that the reduction in calcium in the medium (with an initial concentration of 80 μg/ml) was inversely affected by the calcium deposition in the scaffolds. Therefore, the amount of calcium deposit in the scaffold during cultivation was considered as the reduction in calcium concentration in the culture medium.
Additionally, we decided to measure the calcium concentration using a simple and rapid commercially available kit, as the protocol described above relies on the use of expensive equipment, such as the X-ray fluorescence spectrometer, that is not readily available to most laboratories. Therefore, the calcium concentration was also determined using the methyl xylenol blue method (Calcium E-test; Wako) according to the manufacturer's instructions.
Quantification of Total DNA in the β-TCP/BM-MSC Constructs After Subculture
Total DNA in the β-TCP/P1, β-TCP/P2, and β-TCP/P3 constructs was quantified at day 15 of subculture using a DNA Quantity Kit (Cosmo Bio Co., Ltd., Tokyo Japan). Briefly, β-TCP/BM-MSC constructs at day 15 were crushed and homogenized in 1 ml of sterile distilled water, on ice, using a microhomogenizer, followed by centrifugation at 700×g at 25°C for 10 min. For each construct, 50 μl of supernatant was mixed with 1 ml of buffer solution for fluorescence measurement.
qPCR
The mRNA expression of ALP and osteocalcin was measured to confirm osteogenesis in the β-TCP/BM-MSC constructs after subculture. Total RNA was isolated from four disks of each group using an Isogen RNA extraction kit (Nippon Gene, Toyama, Japan). Briefly, each sample was placed in Isogen solution containing matrix beads and disrupted using a FastPrep FP24 Cell Disrupter (Qbiogene; Carlsbad, CA, USA)24. RNA was extracted according to the manufacturer's instructions. cDNA was prepared by reverse transcription (High Capacity cDNA Reverse Transcription Kits; Applied Biosystems, Waltham, MA, USA). qPCR was carried out on a StepOnePlus instrument (Applied Biosystems) using primers for ALP, osteocalcin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as an internal standard), as previously described25. ALP (Rn00564931 ml), osteocalcin (Rn01455285 gl), and GAPDH (Rn99999916 sl) primer and probes sets were purchased from Applied Biosystems. Thermal cycle conditions were 20 s at 95°C for activation of the TaqMan Fast Universal PCR Master Mix, followed by 40 cycles of 1 s at 95°C for denaturation and 20 s at 60°C for annealing and extension.
Histology
The implanted constructs were harvested 4 weeks after implantation. Two constructs per group were fixed for 3 days in 10% buffered formalin and decalcified with K-CX solution (Falma, Tokyo, Japan). The constructs were embedded in paraffin, cut into three equal sections parallel to the round surface of the β-TCP disk, and stained with hematoxylin and eosin (H&E; Merck, Tokyo, Japan) and Sirius Red (Sigma-Aldrich), which include stain in Fast green (Wako) solution under light shielding before Sirius Red staining.
Measurement of ALP Activity and Osteocalcin Content
The remaining four harvested constructs of each group were used for biochemical assay of the ALP activity and measurement of the osteocalcin content as described previously14. Briefly, the harvested implants were immediately crushed and homogenized on ice in 1 ml of 0.2% Nonidet P-40 (Nacalai Tesque) containing 50 mM Tris-HCl (Nacalai Tesque) and 1 mM MgCl2 (Wako) using a microhomogenizer, followed by centrifugation at 10,000 × g at 4°C for 10 min. Ten microliters of each supernatant was added to 1 ml of ALP substrate buffer containing 0.056 M 2-amino-2-methylpropanediol (AMP; Nacalai Tesque) buffer (pH 9.8), 10 mM p-nitrophenyl phosphate (Nacalai Tesque), and 1 mM MgCl2, and the mixtures were incubated at 37°C for 30 min. Enzymatic activity was determined by measuring the increase in absorbance at 410 nm caused by the release of p-nitrophenol using a SpectraMax M2 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The activity was represented as μmol p-nitrophenol/implant released during 30 min of incubation at 37°C.
Subsequently, the osteocalcin content of the harvested constructs was measured7. Osteocalcin was extracted by incubation with 0.2% Nonidet P-40 in 4 ml of 20% formic acid (Wako) at 4°C for 2 weeks. An aliquot (0.5 ml) of the formic acid extract was applied to a prepacked Sephadex G-25 column (NAP-5 column; Amersham Pharmacia Biotech AB, Uppsala, Sweden) and eluted with 10% formic acid. Protein fractions were pooled and evaporated to dryness. After dissolution in 0.5 ml of ELISA sample buffer, the osteocalcin content was determined using the rat osteocalcin ELISA system (DS Pharma Biomedical). Since these four constructs for biochemical assays were used for measuring secretory osteocalcin and calcium concentrations in vitro, the correlation between calcium concentrations in vitro and that of in vivo osteogenic markers (ALP activity and osteocalcin content) could be calculated.
Statistical Analysis
All data were expressed as the mean ± standard deviation (SD). Overall differences between group means were analyzed using analysis of variance (ANOVA). Post hoc tests were performed to assess the differences between two groups. Values of p < 0.05 were considered statistically significant. Pearson's correlation coefficient was used to assess the correlation between the level of osteocalcin secretion and the calcium concentration in the medium, and between the cumulative calcium reduction (from day 3 to day 15) and the ALP activity and osteocalcin content of the harvested grafts. Pearson's correlation coefficient was also used to assess the correlation between calcium concentration by X-ray fluorescence analysis and the methyl xylenol blue method (Calcium E-test). Statistical analysis was performed using SPSS software (version 22; IBM, Chicago, IL, USA).
Results
Osteocalcin Secretion and Calcium Concentration in the Medium
Changes in osteocalcin secretion, an early marker for in vitro osteogenic differentiation, and the calcium concentration in the medium are shown in Figure 1. Osteocalcin secretion from the β-TCP/P1 constructs began at day 5 of culture and increased throughout the culture period, reaching a plateau after day 11. The secretion from the β-TCP/P2 and β-TCP/P3 constructs started later and was significantly lower than that from β-TCP/P1. No osteocalcin secretion was detected from the control (Fig. 1A).
Figure 1.
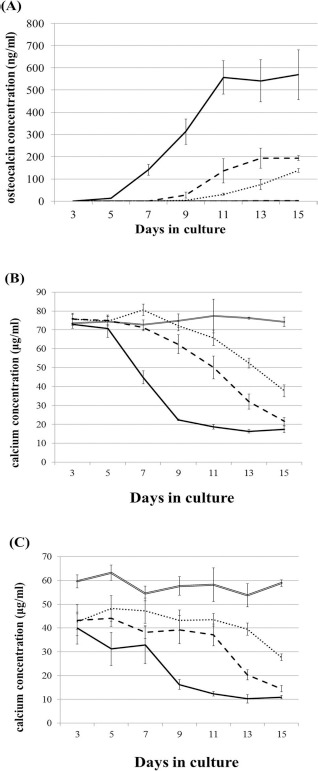
Changes in osteocalcin secretion (A) and calcium concentration measured by X-ray fluorescence analysis method (B) and the commercially available kit (C) in the medium. Black, double broken, broken, dotted, and double lines indicate the data obtained from constructs of β-tricalcium phosphate (β-TCP)/passage 1 (P1), β-TCP/P2, β-TCP/P3, and control (β-TCP alone), respectively. Data are shown as the mean ± standard deviation (SD).
The calcium concentration in the culture medium of β-TCP/P1, which was measured by X-ray fluorescence analysis, began to decrease at day 5 of culture and decreased further throughout the culture period. Similarly, the calcium concentration in the β-TCP/P2 and β-TCP/P3 conditioned media decreased from day 5; however, the rate of decline decreased with increasing BM-MSC passages. There was no decrease in calcium concentration in the control (Fig. 1B). The calcium concentration in the culture medium measured by the methyl xylenol blue method (Calcium E-test) showed very similar values (Fig. 1C), confirming the strong correlation between both analytical methods (Pearson's correlation coefficient was 0.90).
Estimated Cumulative Calcium Reduction
The cumulative estimated calcium reduction in the scaffolds is shown for each group in Figure 2. The estimated cumulative reduction in the β-TCP/P1 constructs was the highest at all time points, followed by that in the β-TCP/P2 and β-TCP/P3 constructs. The differences were significant.
Figure 2.
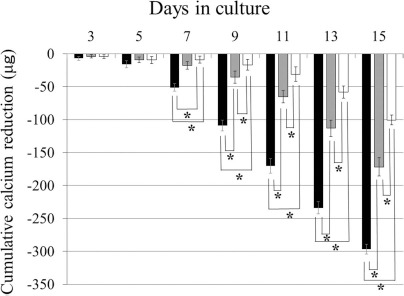
Cumulative calcium reduction. The amount of calcium reduction in the β-TCP/P1 constructs (black columns) was significantly higher than that in the β-TCP/P2 (gray columns) and β-TCP/P3 (white columns) constructs. Data are shown as the mean+standard deviation SD. *p < 0.05.
Quantification of DNA, ALP mRNA, and Osteocalcin mRNA in β-TCP/BM-MSC Constructs After Subculture
The quantity of DNA in the β-TCP/BM-MSCs on day 15 (immediately after the subculture) decreased as cell passage increased (Fig. 3A). The mRNA expression of ALP in the β-TCP/P3 constructs was significantly lower than that in the β-TCP/P1 and β-TCP/P2 constructs (Fig. 3B). Osteocalcin mRNA expression showed similar trends (Fig. 3C).
Figure 3.
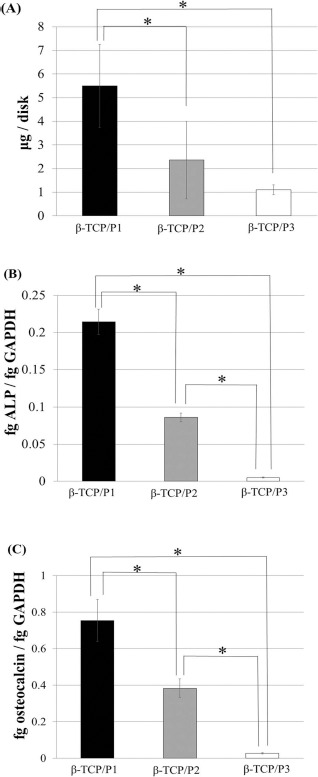
Total DNA, alkaline phosphatase (ALP) mRNA, and osteocalcin mRNA in β-TCP/bone marrow-derived mesenchymal stromal cell (BM-MSC) constructs after subculture. The amount of total DNA in the β-TCP/BM-MSCs decreased with increasing cell passages (A). The expression of ALP (B) and osteocalcin (C) mRNA decreased with increasing cell passages. ALP expression showed similar trends. Data are shown as the mean ± standard deviation (SD). *p < 0.05.
Correlation Between Osteocalcin Secretion and Calcium Concentration In Vitro
Scatter plots of the osteocalcin secretion versus the calcium concentration in vitro are shown in Figure 4. For each group, a strong correlation was found between osteocalcin secretion and calcium concentration in the medium. β-TCP/P1 showed a biphasic Pearson correlation, with a breaking point at an osteocalcin concen tration of 300 ng/ml: r = −0.99 at osteocalcin concentrations below 300 ng/ml, r = −0.70 at osteocalcin concentrations above 300 ng/ml (Fig. 4). The Pearson correlation coefficient was −0.95 for β-TCP/P2 (Fig. 4B) and −0.96 for β-TCP/P3 (Fig. 4C), respectively.
Figure 4.
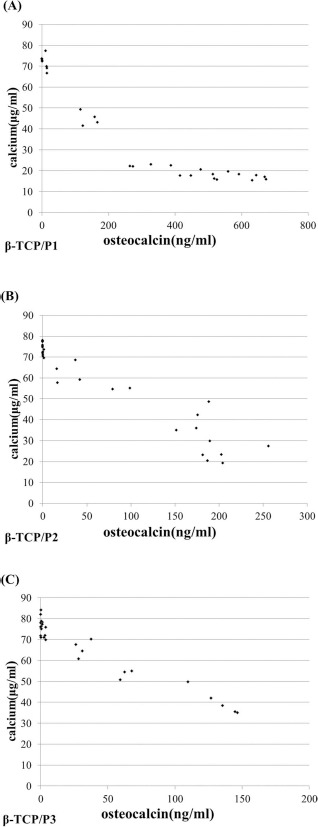
Scatter diagrams of the calcium concentration versus osteocalcin secretion in vitro. A strong inverse correlation between both parameters was observed for the β-TCP/P1 (A), β-TCP/P2 (B), and β-TCP/P3 (C) constructs.
ALP Activity and Osteocalcin Content of Harvested Constructs
The ALP activity and osteocalcin content, which were used as in vivo markers for osteogenic activity of the harvested constructs, are shown in Figure 5. The ALP activity of the β-TCP/P1 construct was the highest and significantly differed from that in the two other constructs (Fig. 5A). The osteocalcin content of the constructs showed a similar pattern (Fig. 5B).
Figure 5.
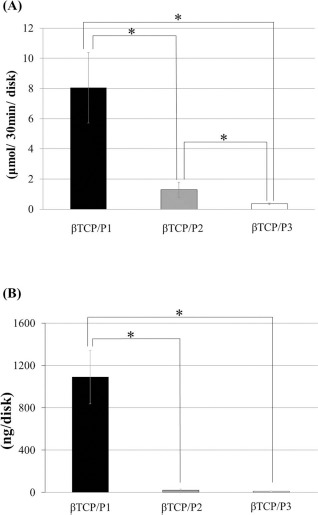
ALP activity (A) and osteocalcin content (B) of harvested constructs at 4 weeks after implantation. ALP activity and osteocalcin content of the β-TCP/P1 construct were significantly higher than those of the β-TCP/P2 and β-TCP/P3 constructs. Data are shown as the mean±standard deviation (SD). *p < 0.05.
Correlation Between Estimated Cumulative Calcium Reduction and Osteogenesis of the Harvested Constructs
We observed a strong inverse correlation between the estimated cumulative calcium reduction and the level of in vivo osteogenic markers from day 7 onward in the 15-day culture period. The Pearson correlation coefficients for the estimated cumulative calcium reduction and the ALP activity and osteocalcin content were consistently higher than 0.90 during this period.
Histology
Representative histological sections of the harvested constructs are shown in Figure 6. H&E and Sirius Red staining revealed bone formation in all constructs. However, the amount of newly formed bone in the β-TCP/P1 constructs seemed to be higher than that in the β-TCP/P2 and β-TCP/P3 constructs.
Figure 6.
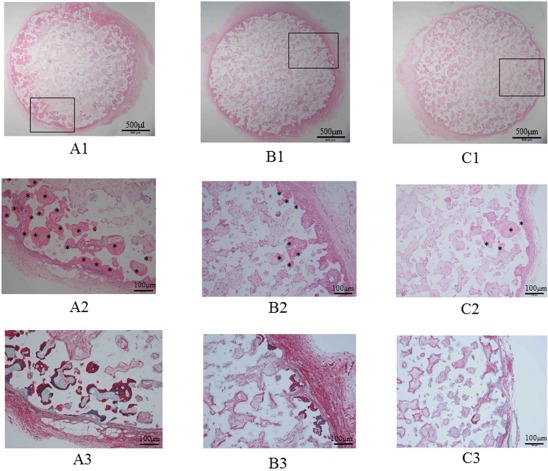
Histology of hematoxylin and eosin (H&E)- and Sirius Red-stained graft sections. The amount of newly formed bone in the β-TCP/P1 construct (A) seems higher than that in the β-TCP/P2 (B) and β-TCP/P3 (C) constructs. The images in (A2), (B2), and (C2) are magnifications of the rectangular areas in (A1), (B1), and (C1), respectively. (A3), (B3), and (C3) show Sirius Red-stained sections. Asterisks indicate newly formed bone area.
Discussion
Several methods for the evaluation of reliable osteogenic markers, such as the measurement of ALP activity26,27, calcium deposition28, osteocalcin deposition29, and mRNA expression of ALP and osteocalcin30, have been reported. However, these methods are destructive, which is not desirable for reconstructive surgery. Recently, various nondestructive methods such as the measurement of secretory osteocalcin19 and the use of fluorimetric image analysis have been reported. Kuhn et al.16 reported quantification of the distribution of intensities and the area percentage of green fluorescent protein-positive osteoblasts in carbonated HA scaffolds by fluorimetry and fluorescence microscopy image analyses. However, these methods might not be suitable for clinical application because they rely on the treatment of the BM-MSCs with the calcium-chelating agent calcein, which might harm the BM-MSCs. To avoid this problem, parallel cell cultures for monitoring osteogenesis might be used with the above-mentioned methods. However, the assessment of samples taken directly from BM-MSCs or cultivated bone grafts is more desirable than methods using parallel cell cultures, which do not allow direct evaluation of osteogenesis of the BM-MSCs. In this regard, conditioned medium might be a good sample source for the evaluation of osteogenesis. We previously reported that secretory osteocalcin in the conditioned medium of β-TCP/BM-MSC constructs is a suitable nondestructive marker of osteogenesis and relates to bone formation after implantation18. However, quantitative analysis of secretory osteocalcin requires ELISA, which is often troublesome to perform and is time-consuming. Therefore, the development of a reliable, rapid, easy, and nondestructive method is imperative. During cultivation of the construct, the calcium deposited in the matrix is supplied from the culture medium and regulated by the osteogenic activity of the osteoblasts31–34. Thus, we hypothesized that decreased calcium in the medium would reflect the amount of calcium deposited.
Our results showed a biphasic correlation between osteocalcin and calcium concentrations in the conditioned medium with a breaking point at an osteocalcin concentration of 300 ng/ml, which indicates that this method might lose its utility for very osteogenic specimens. However, the correlation coefficients for the estimated cumulative calcium reduction and the osteogenesis in vivo (ALP activity and osteocalcin content) were very high. Therefore, our results indicate that the calcium concentration in the culture medium can be used as a nondestructive marker of osteogenesis in tissue-engineered bone. Tissue-engineered bone with high osteogenesis can be selected before transplantation and, subsequently, a high level of bone formation can be achieved after transplantation.
We used serially passaged cells for the construction of β-TCP/BM-MSCs in the present study, since osteogenesis decreases with cell passaging34. To verify whether calcium concentration can be a nondestructive marker of osteogenesis, we needed a simple experimental model in which BM-MSC osteogenesis decreases obviously. The proliferation and osteoblastic differentiation ability immediately after subculture in the serially passaged β-TCP/BM-MSC constructs decreased, and our proposed marker effectively indicated the osteogenic level of the implanted constructs. Evaluating osteogenesis in conditioned medium is a reasonable and attractive technique since the culture medium is replaced and discarded generally, allowing nondestructive and direct evaluation of osteogenesis in the BM-MSCs or cultivated bone grafts (BM-MSC-loaded scaffolds). Our results clearly indicated that our method can be useful for hard tissue reconstructions in a clinical setting.
Our assessment of the calcium concentration for the prediction of osteogenesis after implantation was based on X-ray fluorescence analysis and clearly indicated that calcium concentration in the medium can be used as a reliable and nondestructive marker. However, measuring calcium concentrations by X-ray fluorescence requires expensive equipment and troublesome techniques. Therefore, we tested a simple, commercially available kit based on the methyl xylenol blue method (Calcium E-test; Wako), which yielded results very similar to that of the X-ray method. Our results showed that calcium concentration in the culture medium could be employed as a marker of osteogenesis in tissue-engineered bones using a simple, rapid, and nondestructive standard calcium titration assay kit.
Limitations
A few limitations of our study should be mentioned. First, we used rat BM-MSCs; experiments with human BM-MSCs should be conducted to test their utility for clinical applications. Second, the decrease in calcium in the medium might not be a reliable marker of osteogenesis under certain conditions; for example, in subculture without the use of an osteogenic differentiation factor such as β-GP, calcium deposition does not occur and, consequently, the calcium concentration in the medium does not decrease even with the advancement of osteoblastic differentiation of MSCs. Third, we measured the calcium concentration using X-ray fluorescence and a methyl xylenol blue-based commercial kit. However, simpler methods such as the ion electrode method should be considered. Fourth, we used serially passaged cells to compare the osteogenesis of BM-MSCs since serial passage generally decreases the osteogenesis. Further study using BM-MSCs with various osteogenic potential such as BM-MSCs from young and elderly rats or from different rat species will be required to confirm the present result. Finally, our findings will have to be confirmed for other scaffolds such as HA.
Conclusions
The results from this study clearly showed that the calcium concentration in the culture medium can be used as a nondestructive marker of osteogenesis in tissue-engineered bone. Tissue-engineered bone with high osteogenesis can be selected before transplantation and, subsequently, a high level of bone formation can be achieved after transplantation. Measurement of calcium can be useful for hard tissue reconstruction cases in a clinical setting.
Acknowledgments
This study was supported by the Magnetic Health Science Foundation (Grant No. 26110). The authors thank Dr. K. Kawate and Dr. T. Shimizu (Nara Medial University) for providing experimental advice. The authors also thank F. Kunda and M. Matsumura (Nara Medial University Faculty of Medicine) for providing technical assistance. The authors declare no conflicts of interest.
References
- 1.Dumic-Cule I., Pecina M., Jelic M., Jankolija M., Popek I., Grgurevic L., Vukicevic S. Biological aspects of segmental bone defects management. Int Orthop. 2015; 39: 1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Mauffrey C., Barlow B.T., Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015; 23: 143–53. [DOI] [PubMed] [Google Scholar]
- 3.Myeroff C., Archdeacon M. Autogenous bone graft: Donor sites and techniques. J Bone Joint Surg Am. 2011; 93: 2227–36. [DOI] [PubMed] [Google Scholar]
- 4.Hinz P., Wolf E., Schwesinger G., Hartelt E., Ekkernkamp A. A new resorbable bone void filler in trauma: Early clinical experience and histologic evaluation. Orthopedics 2002; 25: s597–s600. [DOI] [PubMed] [Google Scholar]
- 5.Holmes R.E., Hagler H.K. Porous hydroxyapatite as a bone graft substitute in cranial reconstruction: A histometric study. Plast Reconstr Surg. 1988; 81: 662–71. [DOI] [PubMed] [Google Scholar]
- 6.Samavedi S., Whittington A.R., Goldstein A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013; 9: 8037–45. [DOI] [PubMed] [Google Scholar]
- 7.Iida J., Yoshikawa T., Akahane M., Ohgushi H., Dohi Y., Takakura Y., Nonomura A. Osteogenic potential of cultured bone/ceramic construct: Comparison with marrow mesenchymal cell/ceramic composite. Cell Transplant. 2004; 13: 357–65. [DOI] [PubMed] [Google Scholar]
- 8.Kawate K., Yajima H., Ohgushi H., Kotobuki N., Sugimoto K., Ohmura T., Kobata Y., Shigematsu K., Kawamura K., Tamai K., Takakura Y. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: Transplantation of autologous mesenchymal stem cells cultured with β-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs 2006; 30: 960–2. [DOI] [PubMed] [Google Scholar]
- 9.Kihara T., Oshima A., Hirose M., Ohgushi H. Three-dimensional visualization analysis of in vitro cultured bone fabricated by rat marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2004; 316: 943–8. [DOI] [PubMed] [Google Scholar]
- 10.Kotobuki N., Ioku K., Kawagoe D., Fujimori H., Goto S., Ohgushi H. Observation of osteogenic differentiation cascade of living mesenchymal stem cells on transparent hydroxyapatite ceramics. Biomaterials 2005; 26: 779–85. [DOI] [PubMed] [Google Scholar]
- 11.Ohgushi H., Dohi Y., Katuda T., Tamai S., Tabata S., Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res. 1996; 32: 333–40. [DOI] [PubMed] [Google Scholar]
- 12.Ueha T., Akahane M., Shimizu T., Uchihara Y., Morita Y., Nitta N., Kido A., Inagaki Y., Kawate K., Tanaka Y. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J Stem Cells 2015; 7: 873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushima A., Kotobuki N., Tadokoro M., Kawate K., Yajima H., Takakura Y., Ohgushi H. In vivo osteogenic capability of human mesenchymal cells cultured on hydroxyapatite and on β-tricalcium phosphate. Artif Organs 2009; 33: 474–81. [DOI] [PubMed] [Google Scholar]
- 14.Maeda M., Hirose M., Ohgushi H., Kirita T. In vitro mineralization by mesenchymal stem cells cultured on titanium scaffolds. J Biochem. 2007; 141: 729–36. [DOI] [PubMed] [Google Scholar]
- 15.Uchimura E., Machida H., Kotobuki N., Kihara T., Kitamura S., Ikeuchi M., Hirose M., Miyake J., Ohgushi H. In-situ visualization and quantification of mineralization of cultured osteogenetic cells. Calcif Tissue Int. 2003; 73: 575–83. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn L.T., Liu Y., Advincula M., Wang Y.H., Maye P., Goldberg A.J. A nondestructive method for evaluating in vitro osteoblast differentiation on biomaterials using osteoblast-specific fluorescence. Tissue Eng Part C Methods 2010; 16: 1357–66. [DOI] [PubMed] [Google Scholar]
- 17.Funaoka H., Dohi Y., Ohgushi H., Akahane M., Imamura T. Development of a high-specificity enzyme-linked immunosorbent assay (ELISA) system for the quantification and validation of intact rat osteocalcin. Immunol Invest. 2010; 39: 54–73. [DOI] [PubMed] [Google Scholar]
- 18.Akahane M., Ueha T., Dohi Y., Shimizu T., Tohma Y., Kido A., Kawate K., Imamura T., Tanaka Y. Secretory osteocalcin as a nondestructive osteogenic marker of tissue-engineered bone. J Orthop Sci. 2011; 16: 622–8. [DOI] [PubMed] [Google Scholar]
- 19.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010; 77: 4–12. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A., Akahane M., Shigematsu H., Tadokoro M., Morita Y., Ohgushi H., Dohi Y., Imamura T., Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone 2009; 46: 418–24. [DOI] [PubMed] [Google Scholar]
- 21.Uchihara Y., Akahane M., Shimizu T., Ueha T., Morita Y., Nakasaki S., Kura T., Tohma Y., Kido A., Kawate K., Tanaka Y. Osteogenic matrix cell sheets facilitate osteogenesis in irradiated rat bone. BioMed Res Int. 2015; 6291–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshii T., Sotome S., Torigoe I., Tsuchiya A., Maehara H., Ichinose S., Shinomiya K. Fresh bone marrow introduction into porous scaffolds using a simple low-pressure loading method for effective osteogenesis in a rabbit model. J Orthop Res. 2009; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 23.Akahane M., Nakamura A., Ohgushi H., Shigematsu H., Dohi Y., Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008; 2: 196–201. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura A., Dohi Y., Akahane M., Ohgushi H., Nakajima H., Funaoka H., Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods 2009; 15: 169–80. [DOI] [PubMed] [Google Scholar]
- 25.Akahane M., Shimizu T., Shigematsu H., Kido A., Omokawa S., Kawate K., Imamura T., Tanaka Y. Cell sheet injection as a technique of osteogenic supply. Int J Stem Cells 2010; 3: 138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian J.B., Gundberg C.M. Osteocalcin. Biochemical considerations and clinical applications. Clin Orthop Relat Res. 1998; 226: 267–91. [PubMed] [Google Scholar]
- 27.Sharma U., Pal D., Prasad R. Alkaline phosphatase: An overview. Indian J Clin Biochem. 2014; 29: 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rashidi H., Strohbuecker S., Jackson L., Kalra S., Blake A.J., France L., Tufarelli C., Sottile V. Differences in the pattern and regulation of mineral deposition in human cell lines of osteogenic and non-osteogenic origin. Cells Tissues Organs 2012; 195: 484–94. [DOI] [PubMed] [Google Scholar]
- 29.Hoemann C.D., El-Gabalawy H., McKee M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol. 2009; 57: 318–23. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H., Choong P., McCarthy R., Chou S.T., Martin T.J., Ng K.W. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J Bone Miner Res. 1994; 9: 1489–99. [DOI] [PubMed] [Google Scholar]
- 31.Vacanti V., Kong E., Suzuki G., Sato K., Canty J.M., Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005; 205: 194–201. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura F., Kitoh H., Ishiguro N. Osteogenic potential of rat mesenchymal stem cells after several passages. Biochem Biophys Res Commun. 2004; 26: 233–9. [DOI] [PubMed] [Google Scholar]
- 33.Ter Brugge P.J., Jansen J.A. In vitro osteogenic differentiation of rat bone marrow cells subcultured with and without dexamethasone. Tissue Eng. 2002; 8: 321–31. [DOI] [PubMed] [Google Scholar]
- 34.Akahane M., Ueha T., Shimizu T., Inagaki U., Kido A., Imamura T., Kawate K., Tanaka Y. Increased osteogenesis with hydroxyapatite constructs combined with serially-passaged bone marrow-derived mesenchymal stem cells. Stem Cell Dis. 2012; 2: 133–40. [Google Scholar]


