Abstract
The aberrant generation or activation of T follicular helper (Tfh) cells contributes to the pathogenesis of systemic lupus erythematosus (SLE), yet little is known about how these cells are regulated. In this study, we demonstrated that the frequency of Tfh cells was increased in lupus-prone B6.MRL-Faslpr (B6.lpr) mice and positively correlated to plasma cell proportions and serum total IgG as well as anti-dsDNA antibody levels. Transplantation of mesenchymal stem cells derived from Wharton's jelly of human umbilical cords (hUC-MSCs) ameliorated lupus symptoms in B6.lpr mice, along with decreased percentages of Tfh cells. In vitro studies showed that the differentiation and proliferation of Tfh cells were markedly suppressed by hUC-MSCs. The production of inducible nitric oxide synthase (iNOS) was dramatically upregulated in hUC-MSCs when cocultured with CD4+ T cells directly, while adding the specific inhibitor of iNOS into the coculture system significantly reversed the inhibitory effect of hUC-MSCs on Tfh cell generation. Interestingly, the efficacy of hUC-MSCs in inhibiting Tfh cells was impaired in the Transwell system, with the reduction of iNOS in both mRNA and protein levels. Taken together, our findings suggest that hUC-MSCs could effectively inhibit Tfh cell expansion through the activation of iNOS in lupus-prone B6.lpr mice, which is highly dependent on cell-to-cell contacts.
Keywords: T follicular helper cells, Systemic lupus erythematosus (SLE), Mesenchymal stem cells (MSCs), Inducible nitric oxide synthase (iNOS)
Introduction
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease characterized by the production of autoantibodies and multiorgan damage1,2. Although the pathogenesis is still unclear, much attention has recently focused on the role of T follicular helper (Tfh) cells in this disease3,4.
With the discovery of B-cell lymphoma-6 (Bcl-6) as the essential transcription factor in 2009, Tfh cells are considered to be a novel subtype of CD4+ T helper cells5. They are mainly localized to B-cell follicles in secondary lymphoid tissues and express phenotypic molecules, such as C-X-C chemokine receptor 5 (CXCR5), programmed cell death-1 (PD-1), inducible costimulatory molecule (ICOS), CD40 ligand, and interleukin-21 (IL-21), to mediate their function in assisting B cells and controlling their differentiation6–8. Overactivation of Tfh cells is likely to result in autoimmunity3,9. As suggested by our study and others, circulating Tfh cell proportions are elevated in SLE patients and are correlated with disease symptoms and antinuclear antibody levels10,11. Interestingly, therapies targeting Tfh cells have been shown to dramatically ameliorate lupus syndrome in a lot of autoimmune murine models12–16, making Tfh cells a promising treatment target for SLE patients.
Mesenchymal stem cells (MSCs) are pluripotent stem cells with a wide array of immunomodulatory effects on T and B lymphocytes, natural killer (NK) cells, and antigen-presenting cells, which can be isolated from bone marrow (BM) and a variety of other tissues, such as umbilical cord or adipose tissue17,18. Previously, human umbilical cord-derived MSCs (hUC-MSCs) have been applied to treat lupus mice and refractory SLE patients, with significant improvement in both disease activity and autoantibody levels19,20. However, despite the remarkable therapeutic effect of MSCs in SLE, the mechanisms by which MSCs exert their immunomodulatory and reparative effects are still not completely understood.
Recently, we demonstrated that MSCs suppressed the differentiation of circulating Tfh cells in patients with primary Sjogren's syndrome (pSS), which was mediated by indoleamine 2,3-dioxygenase (IDO)21. To find out whether MSCs could also modulate Tfh cells in lupus through the same pathway, in this study the frequency of Tfh cells in lupus-prone B6.MRL-Faslpr (B6.lpr) mice was examined and the effect and possible mechanism of hUC-MSCs on mouse Tfh cells was also investigated.
Materials and Methods
Mice
Female B6.lpr mice and C57BL/6 (B6) mice were purchased from the Laboratory Animal Center, Academy of Military Medical Sciences (Beijing, P.R. China). Mice were housed under specific pathogen-free conditions in the animal center of the Affiliated Drum Tower Hospital of Nanjing University Medical School. All experimental animal protocols were approved by the Committee of Experimental Animal Administration of the Affiliated Drum Tower Hospital of Nanjing University Medical School.
Isolation, Culture, and Identification of hUC-MSCs and Synovial Fibroblasts (FLSs)
The study on human subjects was approved by the ethics committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School, and written informed consent was obtained from all subjects. hUC-MSCs and FLSs were prepared as previously described22,23 and cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin (all from Gibco, Life Technologies, Grand Island, NY, USA) until confluent. The adherent cells were detached by 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco) and reseeded into new flasks for further expansion. All cell cultures were maintained at 37°C in a 5% CO2 humidified atmosphere. Flow cytometry analysis showed hUC-MSCs used in this study were positive for the surface staining of CD73, CD90, and CD105, but lacked CD34, CD45, CD14, CD19, and human leukocyte antigen D-related (HLA-DR) expression. The cells possessed the capacity of osteogenic and adipogenic differentiation.
Isolation, Culture, and Identification of Mouse BM-MSCs
BM cells were flushed out of the tibia and femoral marrow compartments of 6- to 8-week-old B6 mice, and then cultivated in plastic dishes according to the protocol for isolation and culture of MSCs from mouse BM developed by Soleimani and Nadri24. Briefly, BM cells were cultured in DMEM/F12 supplemented with 15% FBS for 3 h at 37°C in a 5% CO2 humidified atmosphere. Then nonadherent cells were removed carefully, and fresh medium was replaced. When primary cultures became confluent, the cells were treated with 0.5 ml of 0.25% trypsin-EDTA and reseeded into new dishes for further expansion. Flow cytometry analysis showed mouse BM-MSCs expanded in culture were with positive surface staining for stem cell antigen-1 (Sca-1), CD29, CD44, and CD73, but negative for major histocompatibility complex (MHC) class II (I-A), CD11b, CD19, and CD45. The cells preserved the capacity of osteogenic and adipogenic differentiation.
hUC-MSC Transplantation
Female B6.lpr mice were randomly divided into three groups [MSCs, FLSs, and phosphate-buffered saline (PBS) treatment group] according to proteinuria levels and transfused with 1 × 106 hUC-MSCs, 1 × 106 FLSs, or PBS, respectively, via the tail vein at the age of 6 months. After 1 month, all treated mice were sacrificed for further analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum levels of IL-21, immunoglobulin G (IgG), and anti-double stranded (ds)DNA were measured using mouse IL-21 and IgG ELISA Ready-SET-Go!® kits (eBioscience, San Diego, CA, USA) and mouse anti-dsDNA ELISA kit (Shibayagi, Gunma, Japan), respectively, according to the manufacturer's instructions.
Splenomegaly Assessment and Renal Histopathologic Analysis
When mice were sacrificed, spleens and kidneys were collected. The spleen index (ratio of spleen weight to body weight) was calculated. One kidney was fixed in 4% paraformaldehyde (PFA), embedded in paraffin, sectioned at 3 μm, and stained with hematoxylin and eosin (H&E; Sinopharm Chemical Reagent, Shanghai, P.R. China). The other one was snap frozen in liquid nitrogen and placed in optimal cutting temperature (OCT) embedding matrix (Leica Biosystems, Nussloch, Germany). Frozen sections (3 μm) were stained with fluorescein isothiocyanate (FITC)–anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Histological scores of renal lesions and the intensities of IgG deposits were determined as described previously25. Briefly, the severity of glomerulonephritis was graded on a 1–4 scale as follows: 1, focal, mild, or early proliferative; 2, multifocal proliferative with increased matrix; 3, diffuse proliferative; 4, extensive sclerosis/crescents. Interstitial and vascular lesions were also graded on a 1–4 scale according to the number/foci of mononuclear cells around tubules and vessels. Glomerular IgG staining was graded according to intensity on a scale of 0 to 3 as follows: 0, absent; 1, faint; 2, intense; 3, very intense.
Flow Cytometry
For surface staining, human-derived cells were labeled with FITC-anti-CD19, anti-CD34, and anti-CD73, or phycoerythrin (PE)-anti-CD14, anti-CD45, anti-CD90, anti-CD105, and anti-HLA-DR. Mouse-derived cells were labeled with the following antibodies: FITC-anti-CD11b, anti-CD19, anti-CD45, anti-CD34 anti-CD4, and anti-B220, or PE-anti-CD73, anti-CD29, anti-MHC II (I-A), anti-CD138, and anti-PD-1, or allophycocyanin (APC)-anti-Sca-1 and anti-PD-1. CXCR5 was stained with either APC-anti-CXCR5 directly or biotin-anti-CXCR5 for 1 h on ice, followed by PE-streptavidin. Secondary controls (PE-streptavidin only) without primary antibodies were applied to confirm staining specificity. For intracellular cytokine staining, cells were stimulated with 20 ng/ml phorbol-12-myristate-13-acetate (PMA) plus 1 μg/ml ionomycin at 37°C for 4–5 h in the presence of 5 mg/ml brefeldin A (all from Enzo Life Science, Farmingdale, NY, USA). Then cells were fixed and permeabilized with a fixation/permeabilization kit (Nordic-MUbio, Maastricht, Limburg, the Netherlands), followed by staining with APC-anti-interferon-γ (anti-IFN-γ), PE-anti-IL-4, or APC-anti-IL-17A. All antibodies were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany) or eBioscience. Data were acquired by a fluorescence-activated cell sorting (FACS) Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Coculture of Mouse B Cells and Tfh Cells
B cells were purified from spleens of 6- to 8-week-old female B6 mice by a B-cell isolation kit (Miltenyi Biotec). To obtain CD4+CXCR5+ T cells, purified CD4+ T cells were labeled with PE-anti-CXCR5 and anti-PE microbeads, and then positively selected by magnetic separation (Miltenyi Biotec).
B cells (3 × 105 cells/well) were cocultured with CD4+ CXCR5+ T cells (1×105 cells/well) from either B6.lpr mice or B6 mice in the presence of 2.5 μg/ml anti-CD3, 1 μg/ml anti-CD28 (eBioscience), 2.5 μM CpG oligodeoxynucleotide 2395 (Invitrogen, Camarillo, CA, USA), 50 ng/ml IL-4 (PeproTech, Rocky Hill, NJ, USA), 5 μg/ml anti-IgM (Jackson ImmunoResearch, West Grove, PA, USA), and 5 μg/ml anti-CD40 (eBioscience) in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco) with 10% FBS for 5 days.
Coculture of Mouse CD4+ T Cells and MSCs
Total CD4+ T cells were isolated from spleens of 6- to 8-month-old female B6.lpr mice and purified by negative selection (CD4+ T-cell isolation kit; Miltenyi Biotec). hUC-MSCs (2.5×104) or BM-derived MSCs from B6 mice (B6BM-MSCs) were seeded in flat-bottomed well plates and allowed to adhere overnight. Mouse CD4+ T cells (5×105) were added to adherent hUC-MSCs or B6BM-MSCs in RPMI-1640 supplemented with 10% FBS and 100 U/ml penicillin/streptomycin and incubated in the presence of 2.5 μg/ml anti-CD3 and 2.5 μg/ml anti-CD28 (eBioscience) for 3 days. In some experiments, 1 mM NG-methyl-L-arginine acetate salt (L-NMMA; Sigma-Aldrich, St. Louis, MO, USA), 10 μg/ml anti-human hepatocyte growth factor (HGF) (R&D Systems, Minneapolis, MN, USA), or 20 μM AH23848 (Cayman Chemical, Ann Arbor, MI, USA) was added into hUC-MSC-CD4+ T-cell cocultures to neutralize the effects of inducible nitric oxide synthase (iNOS), HGF, or prostaglandin (PG) E2. To block direct cell-to-cell contact, a Transwell system with 0.4-μm pores (Millipore, Billerica, MA, USA) was used as previously described23. In this Transwell system, hUC-MSCs were preseeded into the upper chamber overnight to allow adherence, and then mouse CD4+ T cells were added into the lower chamber for further coculture.
Measurement of Tfh Cell Differentiation
Naive CD4+ T cells were isolated from the spleens of 6- to 8-month-old female B6.lpr mice with the use of a naive CD4+ T-cell isolation kit (Miltenyi Biotec). Then cells (5×105/well) were cultured alone or cocultured with preseeded hUC-MSCs at a ratio of 10:1 in RPMI-1640 supplemented with 10% FBS and 100 U/ml penicillin/streptomycin under Tfh cell-skewing condition [2.5 μg/ml anti-CD3, 2.5 μg/ml anti-CD28, 100 ng/ml IL-6 (PeproTech), and 50 ng/ml IL-21 (R&D Systems), 10 μg/ml anti-IL-4 antibody (eBioscience), 10 μg/ml anti-IFN-γ antibody (eBioscience), and 20 μg/ml anti-transforming growth factor-β (anti-TGF-β) antibody (R&D Systems)] for 4 days.
Proliferation and Apoptosis Assays
CD4+CXCR5+ T cells (2×105/well) were cultured alone or cocultured with hUC-MSCs at a ratio of 10:1 in the presence of 2.5 μg/ml anti-CD3 and anti-CD28. Four days later, 60 μM 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) was added into the cultures and incubated for 3 h. Subsequently, suspension cells were collected and fixed overnight with 1% PFA in PBS containing 0.1% Tween 20 (Biosharp, Hefei, Anhui, P.R. China). After fixation, cells were treated with 100 Kunitz U/ml DNase I (Invitrogen) and stained with FITC-anti-BrdU (eBioscience). The incorporation of BrdU in CD4+CXCR5+ T cells was detected by flow cytometry.
For the detection of apoptosis, CD4+CXCR5+ T cells (2×105/well) were cultured alone or cocultured with hUC-MSCs at a ratio of 10:1 in the presence of 2.5 μg/ml anti-CD3 and anti-CD28. Three days later, CD4+ CXCR5+ T cells were collected, resuspended in 1× annexin V binding buffer, and stained with FITC-annexin V (BD Biosciences). Annexin V+ cells were detected by flow cytometry.
Real-Time Polymerase Chain Reaction (PCR) and Western Blot
Total RNA was extracted from hUC-MSCs using TRIzol (Takara, Dalian, Liaoning, PR. China), and cDNA was synthesized using reverse transcription by PrimeScript RT Master Mix (Takara). Quantitative real-time PCRs containing the SYBR Premix EX Taq™, ROX Reference Dye, cDNA, and specific gene primers (all from Takara) were run on the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative expressions of each gene were determined and normalized to the expression of housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated using the 2–ΔΔCT method. Gene primers (Takara) are listed in Table 1.
Table 1.
Gene Primers Used for Real-Time PCR
| Genes | Primers |
|---|---|
| GAPDH | Forward 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse 5′-TGGTGAAGACGCCAGTGGA-3′ | |
| MMP-1 | Forward 5′-TTGTGGCCAGAAAACAGAAA-3′ |
| Reverse 5′-TTCGGGGAGAAGTGATGTTC-3′ | |
| Adrenomedullin | Forward 5′-ACGGAAACCAGCTTCATCC-3′ |
| Reverse 5′-GCCAGTGGGACGTCTGAG-3′ | |
| iNOS | Forward 5′-CATGTCTGGCAGGACGAGAA-3′ |
| Reverse 5′-TCAGCATACAGGCAAAGAGCA-3′ | |
| HGF | Forward 5′-GTCAGCCCTGGAGTTCCATGATA-3′ |
| Reverse 5′-AGCGTACCTCTGGATTGCTTGTG-3′ | |
| CCL2 | Forward 5′-GCTCATAGCAGCCACCTTCATTC-3′ |
| Reverse 5′-GGACACTTGCTGCTGGTGATTC-3′ | |
| TGF-β1 | Forward 5′-AGCGACTCGCCAGAGTGGTTA-3′ |
| Reverse 5′-GCAGTGTGTTATCCCTGCTGTCA-3′ | |
| sHLA-G | Forward 5′-CCTTGCAGCTGTAGTCACTGGA-3′ |
| Reverse 5′-CACACAGGGCAGCTGTTTCA-3′ | |
| IDO | Forward 5′-GAATGGCACACGCTATGGAA-3′ |
| Reverse 5′-CAGACTCTATGAGATCAGGCAGATG-3′ | |
| IL-10 | Forward 5′-GAGATGCCTTCAGCAGAGTGAAGA-3′ |
| Reverse 5′-AGTTCACATGCGCCTTGATGTC-3′ | |
| COX2 | Forward 5′-TGACCAGAGCAGGCAGATGAA-3′ |
| Reverse 5′-CCACAGCATCGATGTCACCATAG-3′ |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP-1, matrix metalloproteinase-1; iNOS, inducible nitric oxide synthase; HGF, hepatocyte growth factor; CCL2, chemokine (C-C motif) ligand 2; TGF-β1, transforming growth factor-β1; sHLA-G, soluble human leukocyte antigen-G; IDO, indoleamine 2,3-dioxygenase; IL-10, interleukin-10; COX2, cyclooxygenase 2.
For Western blot, a mouse antibody to iNOS (Novus Biologicals, Littleton, CO, USA), rabbit antibodies to protein kinase B (Akt), p65, IκBα, signal transducer and activator of transcription (Stat)1, Stat3, Stat5 and their phosphorylated forms [Cell Signaling Technology (CST), Danvers, MA, USA], and rabbit antibody to GAPDH (CST) were used as primary antibodies. Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (H + L) and anti-mouse IgG (H + L) (both from Proteintech, Wuhan, Hubei, PR. China) were used as secondary antibodies, respectively. hUC-MSCs were washed twice with PBS and lysed on ice for 30 min with 1× radioimmunopre-cipitation assay (RIPA) buffer (CST) containing 1% 100× protease/phosphatase inhibitor cocktail (CST). Lysates were centrifuged at 12,000×g at 4°C for 20 min, and the supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was then transferred to polyvinylidene fluoride membranes (Millipore), blocked for 1 h in 5% nonfat milk [in 10 mM Tris (Biosharp) (pH 7), 150 mM NaCl, 0.1% Tween 20; TBST], and then immunoblotted with the above-listed primary antibodies and appropriate HRP-conjugated secondary antibodies. Chemiluminescence HRP substrate (Millipore) was used to detect the specific proteins, and the bands were visualized by a G:BOX gel imaging system (Syngene, Cambridge, UK).
Nitric Oxide (NO) Production Assay
NO is quickly converted to NO2– and NO3– in the culture medium. Since there is a large amount of NO3– in the RPMI-1640 media, NO2– concentration was measured to represent NO production using a Griess reagent kit (Beyotime Biotechnology, Shanghai, P.R. China).
Statistical Analysis
Data are shown as mean ± standard deviation (SD). Student's t-tests were applied when two groups were compared for statistical differences. One-way analysis of variance (ANOVA) followed by the Newman–Keuls test was used for the comparison of more than two groups. Correlations were determined by Pearson correlation coefficients. Data were analyzed by the SPSS 19.0 software (IBM, Armonk, NY, USA), and a value of p < 0.05 was considered to be statistically significant.
Results
Increased Tfh Cell Proportion Contributed to Abnormal B-Cell Activation in B6.lpr Mice
Similar to a previous report26, lupus nephritis began to develop in 6- to 8-month-old female B6.lpr mice, characterized by enhanced cellular proliferation and IgG deposition in the glomeruli (Fig. 1A). Enlarged spleens, elevated levels of serum total IgG and anti-dsDNA antibody, as well as increased splenic CD138hi plasma cell proportions were detected in B6.lpr mice (Fig. 1B), indicating there was aberrant B-cell activation in this kind of lupus model.
Figure 1.
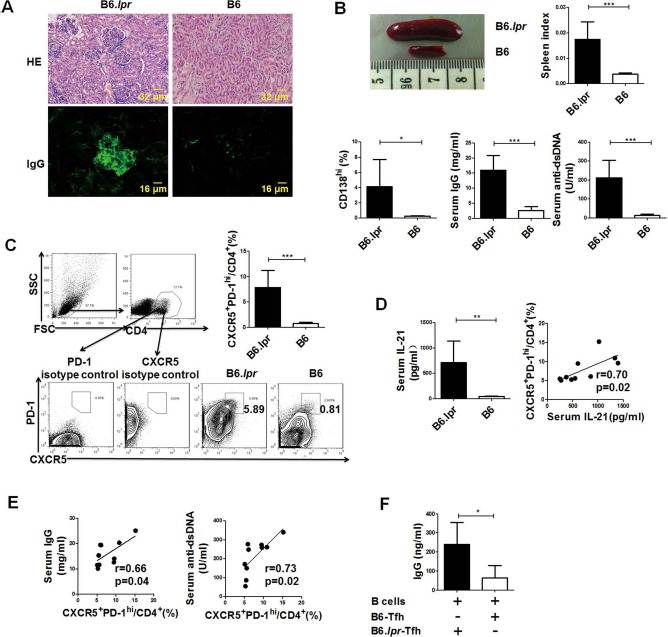
Increased T follicular helper (Tfh) cell proportion contributed to the abnormal B-cell response in B6.lpr mice. (A–E) Female B6.lpr mice (6–8 months old; n = 10) and age- and sex-matched B6 mice (n = 5) were sacrificed. (A) Representative images of kidney hematoxylin and eosin (H&E) sections and immunoglobulin G (IgG) immunostaining. (B) Representative image of spleen, spleen index, frequencies of splenic plasma cells, serum total IgG levels, and anti-double stranded (ds)DNA antibody levels. (C) Gating strategy of C-X-C chemokine receptor 5-positive (CXCR5+) programmed cell death-1 (PD-1)hi Tfh cells and the frequencies of splenic Tfh cells. (D) Serum interleukin-21 (IL-21) concentrations and correlation between the serum IL-21 concentrations and the frequencies of splenic Tfh cells in B6.lpr mice. (E) Correlations of the frequencies of splenic Tfh cells with serum IgG and serum anti-dsDNA levels in B6.lpr mice. (F) B cells isolated from 6- to 8-week-old female B6 mice were cocultured with Tfh cells isolated from either B6.lpr mice (n = 5) or B6 mice (n = 5) for 5 days, and then the concentrations of IgG in the supernatants were detected. *p < 0.05; **p < 0.01; ***p < 0.001. FSC, forward-scattered light; SSC, side-scattered light. Data were pooled from two independent experiments.
Next, we compared the percentages of CD4+CXCR5+ PD-1hi Tfh cells, the main T helper cell subset that provides help to B cells, between B6.lpr mice and age-matched B6 mice. As expected, proportions of splenic Tfh cells were significantly higher in B6.lpr mice (Fig. 1C). IL-21, the signature cytokine of Tfh cells, was also markedly elevated in the serum of B6.lpr mice and positively correlated with the proportions of Tfh cells (Fig. 1D).
Our data showed that the proportions of Tfh cells were positively correlated with both IgG and anti-dsDNA levels (Fig. 1E), suggesting that Tfh cells were related to aberrant B-cell response in B6.lpr mice. To confirm this notion, CD4+CXCR5+ T cells isolated from either B6.lpr mice or B6 mice were cocultured with B cells from B6 mice. As shown in Figure 1F, higher levels of IgG were detected when B cells were treated by Tfh-like cells from B6.lpr mice.
hUC-MSC Transplantation Decreased Tfh Cell Proportions in B6.lpr Mice
To find out whether hUC-MSCs had an impact on Tfh cells in lupus-prone mice, 1 × 106 hUC-MSCs were transfused into B6.lpr mice intravenously, compared with the two control groups given FLSs or PBS, respectively. To distinguish FLSs from MSCs, the mRNA levels of two molecular markers previously reported27, matrix metalloproteinase-1 (MMP-1) and adrenomedullin, are measured by real-time PCR. Our data show that the expression levels of these two genes in FLSs are comparable to those in human embryonic lung fibroblasts (HLFs), a fibroblast cell line, and are much higher than those in MSCs (Fig. 2A), suggesting that FLSs and MSCs are two different cell types in this study.
Figure 2.
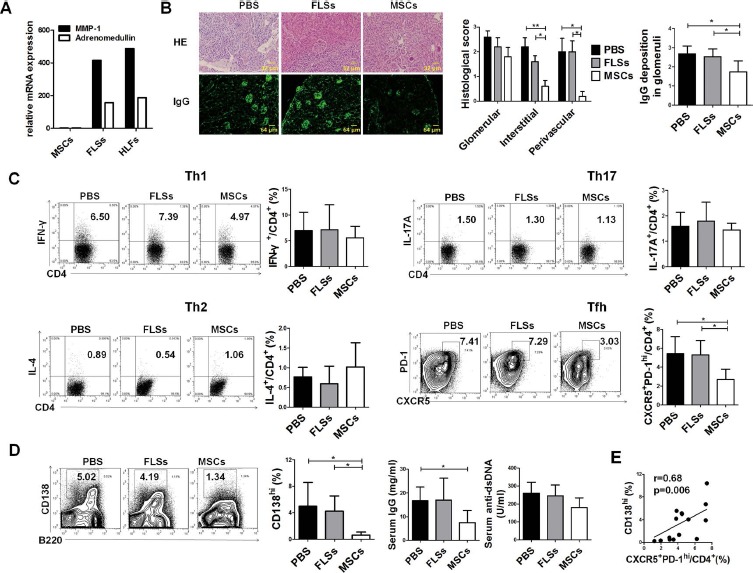
Human umbilical cord mesenchymal stem cell (hUC-MSC) transplantation decreased Tfh-cell proportion in B6.lpr mice. B6.lpr mice were treated with phosphate-buffered saline (PBS), synovial fibroblasts (FLSs), or hUC-MSCs and were sacrificed 1 month after administration. (A) Relative gene expressions of matrix metalloproteinase-1 (MMP-1) and adrenomedullin in MSCs, FLSs, and human embryonic lung fibroblasts (HLFs). (B) Representative images of kidney H&E sections and IgG immunostaining, and histological scores for glomerular, interstitial, and perivascular lesions, according to H&E staining and fluorescence intensity of IgG deposition in glomeruli. (C) The frequencies of splenic Th1, Th2, Th17, and Tfh cells. (D) The frequencies of splenic plasma cells and levels of serum total IgG and anti-double stranded (ds)DNA antibody. (E) The correlation of the frequencies of splenic plasma cells with Tfh cells (n = 5 for each group). *p<0.05; **p<0.01. IFN-γ, interferon-γ; IL-4, interleukin-4.
One month after treatment, mice were sacrificed, and histopathology examinations revealed that renal interstitial and perivascular lesions were greatly improved in hUC-MSC-treated mice, as characterized by diminished mononuclear cell infiltration around tubules and vessels. Meanwhile, IgG depositions in glomeruli were greatly reduced in hUC-MSC-treated mice (Fig. 2B), indicating that hUC-MSC transplantation effectively ameliorated disease progression in B6.lpr mice.
Next, we measured the frequencies of four main CD4+ T helper cell subsets, including Th1, Th2, Th17, and Tfh cells, after the treatment. As shown in Figure 2C, only Tfh cell proportions were significantly decreased in hUC-MSC-treated mice, while Th1, Th2, and Th17 cells remained unchanged. Decreased CD138hi splenic plasma cell proportions and serum IgG levels were observed after hUC-MSC transplantation (Fig. 2D). The level of anti-dsDNA antibody also showed a declining trend after MSC transplantation (180.2 ± 53.8 U/ml) compared with either PBS (260.5 ± 60.0 U/ml) or FLS (245.7 ± 60.0 U/ml) treatment (Fig. 2D). Additionally, there was a strong correlation between Tfh cells and plasma cells among the groups (Fig. 2E), suggesting that hUC-MSCs could act through the regulation of Tfh cells to restore balance to B cells.
hUC-MSCs Suppressed Tfh Cell Expansion Through the Inhibition of Cell Differentiation and Proliferation
To confirm the effect of hUC-MSCs on Tfh cells, splenic CD4+ T cells isolated from B6.lpr mice were cocultured with hUC-MSCs in the presence of anti-CD3 and anti-CD28. After coculturing for 3 days, the frequency of CXCR5+PD-1hi Tfh cells was significantly downregulated (Fig. 3A).
Figure 3.
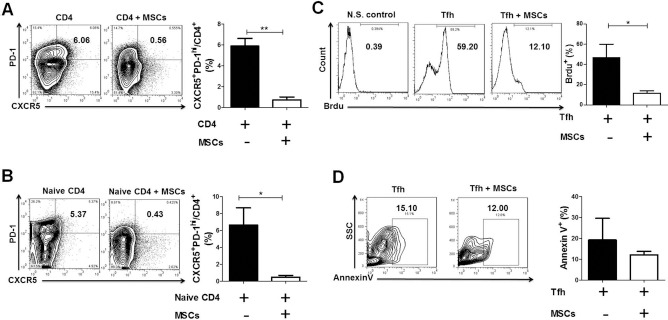
hUC-MSCs suppressed mouse Tfh cell expansion in vitro. (A) Splenic CD4+ T cells from of B6.lpr mice were cultured in the absence or presence of hUC-MSCs. hUC-MSCs downregulated the frequency of CXCR5+ PD-1hi Tfh cells (n = 4). (B) Naive CD4+ T cells from spleens of B6.lpr mice were cultured under a Tfh cell-skewing condition in the absence or presence of hUC-MSCs. hUC-MSCs suppressed naive CD4+ T cells differentiating into Tfh cells (n = 3). (C, D) Mouse CD4+CXCR5+ T cells were cultured in the absence or presence of hUC-MSCs (n = 4). (C) Histograms showed the proliferation of CD4+CXCR5+ T cells, indicated by the percentages of 5-bromo-2-deoxyuridine-positive (BrdU+) cells. (D) The apoptosis of CD4+CXCR5+ T cells was shown as the frequency of annexin V+ cells. *p < 0.05; **p< 0.01; N.S., not stimulated; SSC, side-scattered light. Data were pooled from three independent experiments.
To explore how hUC-MSCs act on Tfh cells, experiments of cell differentiation, proliferation, and apoptosis were performed. Naive CD4+ T cells from B6.lpr mice were cultured under a Tfh cell-skewing condition in the absence or presence of hUC-MSCs. As shown in Figure 3B, hUC-MSCs remarkably inhibited naive CD4+ T cells from differentiating into Tfh cells. Next, CD4+CXCR5+ T cells were isolated as Tfh cells and cocultured with hUC-MSCs to investigate the effects of hUC-MSCs on Tfh cell proliferation and apoptosis. Our data showed that the proliferation of Tfh cells was downregulated by hUC-MSCs (Fig. 3C), while the frequency of annexin V+ apoptotic Tfh cells was not significantly altered between the two groups (Fig. 3D). Taken together, these data imply that hUC-MSCs could effectively suppress Tfh cell expansion through the inhibition of both cell differentiation and proliferation.
The Inhibitory Effects of hUC-MSCs on Tfh Cells We re Mediated by iNOS
Previous studies have suggested that MSCs inhibited T-cell proliferation mainly through the secretion of soluble factors, including iNOS, HGF, chemokine (C-C motif) ligand 2 (CCL2), TGF-β1, soluble human leukocyte antigen-G (sHLA-G), IDO, cyclooxygenase 2 (COX2)/PGE2, and IL-1028. To find out the specific factors participating in regulating Tfh cells in B6.lpr mice, mRNA expression levels of the above eight factors in hUC-MSCs were measured after coculturing the cells with mouse CD4+ T cells for 3 days. As shown in Figure 4A, cocultured hUC-MSCs expressed extremely higher levels of iNOS and HGF. Meanwhile, the levels of COX2, TGF-β1, and sHLA-G were slightly increased, and the levels of CCL2, IDO, and IL-10 remained unchanged (Fig. 4A).
Figure 4.
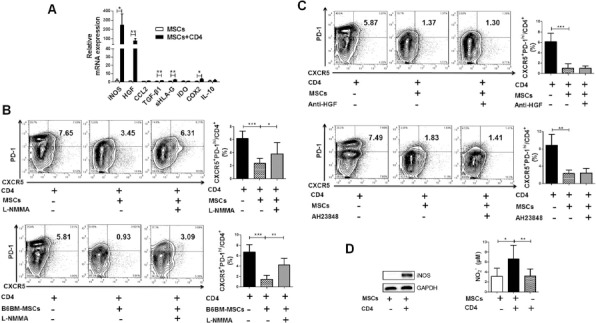
The inhibitor of inducible nitric oxide synthase (iNOS) partly restored the generation of mouse Tfh cells. (A) Real-time polymerase chain reaction (RT-PCR) showed that the expression of iNOS in hUC-MSCs was significantly increased after coculturing with mouse CD4+ T cells. (B) NG-methyl-L-arginine acetate salt (L-NMMA), the inhibitor of iNOS, reversed the suppression of hUC-MSCs (n = 4, top) and bone marrow MSCs of B6 mice (B6BM-MSCs) (n = 3, bottom) on Tfh cell generation. (C) Anti-hepatocyte growth factor (HGF) treatment (n = 1, top) or prostaglandin (PG) E2 blockade (AH23848, n = 4, bottom) could not recover the generation of mouse Tfh cells. (D) Western blot analysis confirms the activation of iNOS in hUC-MSCs being cocultured with CD4+ T cells and nitric oxide (NO) production in the culture supernatant of hUC-MSCs-CD4+ T-cell cocultures elevated markedly than that of hUC-MSCs or CD4+ T cells cultured alone. *p<0.05; **p<0.01; ***p< 0.001. Western blot data were representatives of three independent experiments, and other data were pooled from three independent experiments. CCL2, chemokine (C-C motif) ligand 2; TGF-β1, transforming growth factor-β1; sHLA-G, soluble human leukocyte antigen-G; IDO, indoleamine 2,3-dioxygenase; COX2, cyclooxygenase 2; IL, interleukin; CXCR5, C-X-C chemokine receptor 5; PD-1, programmed cell death-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To verify whether these differentially elevated factors were truly involved in the regulation of MSC function, an iNOS inhibitor, a PGE2 antagonist, and a neutralizing antibody against HGF were added into the hUC-MSCs-CD4+ T-cell coculture system, respectively. Our data showed that only L-NMMA, a specific inhibitor of iNOS, significantly restored the generation of Tfh cells (Fig. 4B), while neither anti-HGF treatment nor PGE2 blockade had an impact on Tfh cell production (Fig. 4C). Consistently, the inhibitory effect of mouse MSCs on Tfh cells was also reversed by L-NMMA (Fig. 4B). When culturing with mouse CD4+ T cells, hUC-MSCs produced more iNOS protein and higher levels of NO production were detected in coculture supernatants (Fig. 4D). Collectively, these data suggest that iNOS is an important mediator in MSC–Tfh cell regulation.
Cell-to-Cell Contacts Were Required for High iNOS Production in hUC-MSCs
Recently, it has been suggested that cell-to-cell contact was important for MSC-mediated inhibition of Tfh-cell differentiation29. To verify this phenomenon, CD4+ T cells collected from the spleens of B6.lpr mice were cocultured with hUC-MSCs directly or in the Transwell system. Our data showed that hUC-MSCs did have decreased efficacy in inhibiting Tfh cell production in the Transwell system (Fig. 5A). Interestingly, both the mRNA expression and protein level of iNOS were reduced when cultured in the Transwell system compared to those in direct coculture (Fig. 5B), implying that increased iNOS level may account for improved Tfh cell balance in the cell-to-cell contact system.
Figure 5.
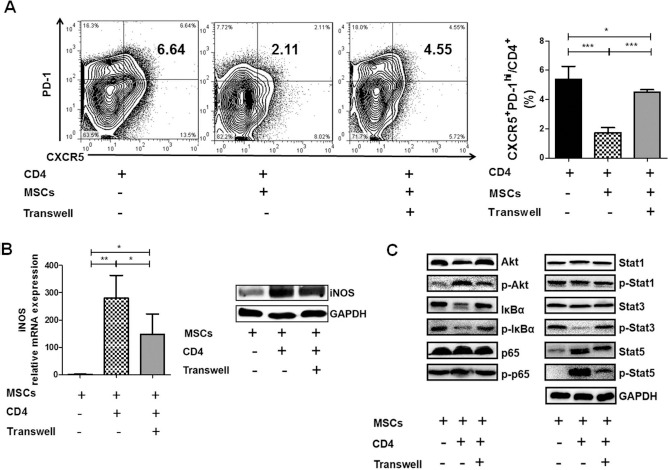
Complete inhibition of Tfh cells and full induction of iNOS in hUC-MSCs were dependent on direct cell-to-cell contact with CD4+ cells. CD4+ T cells from spleens of B6.lpr mice were cocultured with hUC-MSCs directly or in a Transwell system. (A) The frequency of CXCR5+ PD-1hi Tfh cells (n = 4). (B) The mRNA expression (n = 4) and protein level of iNOS in hUC-MSCs. (C) Western blot analysis of protein kinase B (Akt), p65, IκBα, signal transducer and activator of transcription (Stat)1, Stat3, Stat5, and their phosphorylation forms. *p < 0.05; **p < 0.01; ***p < 0.001. Western blot data were representative of three independent experiments, and other data were pooled from three independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To further explore the mechanisms involved in iNOS production, key proteins of PI3K/Akt, nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and Janus kinase (Jak)/Stat signaling pathways were measured by Western blot. As shown in Figure 5C, the levels of phosphorylated Akt and Stat5 were increased, while the levels of phosphorylated IκBα and Stat3 were decreased in hUC-MSCs after coculturing with mouse CD4+ T cells.
Discussion
Tfh cells are essential to the development of germinal center (GC) B-cell responses, and thus may play a critical role in the pathogenesis of lupus3,4,9. Recently, it has been reported that MSCs slowed down the progression of lupus nephritis by suppressing the emergence of the Tfh cells29. However, the underlying mechanism remains unclear. Here we confirmed there was a significantly higher frequency of CXCR5+PD-1hi Tfh cells in lupus-prone B6.lpr mice and, for the first time, demonstrated that hUC-MSCs could suppress Tfh-cell expansion in B6.lpr mice through the activation of iNOS.
In this study, we confirmed that there was an excessive accumulation of Tfh cells and elevated IL-21 expression in lupus-prone B6.lpr mice, consistent with previous results showing that the percentages of Tfh cells were increased in a lot of other lupus models including MRL/lpr mice, Roquinsan/san mice, BXSB-Yaa mice, and BXD2 mice14,30–32. Similar to that in SLE patients10,11,33, Tfh cell proportions were increased in B6.lpr mice and positively related to the levels of plasma cells, serum IgG, as well as anti-dsDNA antibody, and Tfh cells from B6.lpr mice promoted B cells to produce more IgG, suggesting that aberrant Tfh cell production could break down the GC B-cell tolerance and promote autoimmunity in lupus disease.
Tfh cells may serve as a new therapeutic target for lupus, as blocking overactivated signals to target Tfh cells has been shown to alleviate Tfh and GC B-cell accumulation and result in the amelioration of lupus symptoms12–16. Previously we have demonstrated that MSC transplantation remarkably ameliorated lupus symptoms both in SLE patients and lupus mice and led to increased survival rates and improvement of renal function20,34. In this study, we demonstrated that hUC-MSCs restrained the expansion of Tfh cells both in vitro and in vivo, while the decline of Tfh cells was closely correlated with the levels of IgG and anti-dsDNA. Although there was evidence showing MSCs could directly regulate the proliferation and differentiation of B cells35, it is likely that MSCs could also act on Tfh cells to regulate B-cell response in B6.lpr mice.
As suggested by a recent study in NZB/W F1 mice29, the effect of MSCs on Tfh-cell differentiation was dependent on cell-to-cell contacts, yet the underlying mechanism remains unclear. In patients with pSS, we have previously verified that hUC-MSCs inhibited circulating Tfh cell differentiation through the production of IDO21. However, in the present study, hUC-MSCs cocultured with mouse CD4+ T cells did not express high levels of IDO; instead, they produced a large amount of iNOS. Inhibiting iNOS reversed the effects of hUC-MSCs on mouse Tfh cells, suggesting that iNOS is a key factor involved in MSC–Tfh cell regulation in B6.lpr mice. When hUC-MSCs were cocultured with Tfh cells indirectly, the production of iNOS greatly declined, which may lead to weakened regulation of MSCs on Tfh cells.
The pathways involved in the induction of iNOS in MSCs are still not clear. Previously we have shown that the Jak/Stats signaling pathway was involved in the suppression of T cells by MSCs in human SLE23. In macrophage, lipopolysaccharide (LPS)-induced iNOS production has been reported to be dependent on PI3K/Akt and NF-κB signaling pathway36. In this study, we demonstrated that the phosphorylation of Akt and Stat5 was increased and the phosphorylation of IκBα and Stat3 was decreased in hUC-MSCs when they were cocultured with mouse CD4+ T cells, suggesting these three signaling pathways are all involved in the induction of iNOS in hUC-MSCs. Interestingly, the effect was almost diminished when hUC-MSCs were not directly cultured with CD4+ T cells, indicating that direct cell-to-cell contact is critical for the activation of these pathways in hUC-MSCs.
In summary, we show, for the first time, that hUC-MSCs inhibit Tfh cell expansion through the activation of iNOS in lupus-prone B6.lpr mice. These data may help to extend our knowledge of the immunoregulatory function of MSCs in SLE.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (Nos. 81172846, 81373198, 81302558, and 81601414) and the Jiangsu Provincial Science and Technology Project (No. BE2015602). The authors declare no conflicts of interest.
References
- 1.Mok C.C., Lau C.S. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003; 56(7): 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos G.C. Systemic lupus erythematosus. N Engl J Med. 2011; 365(22): 2110–21. [DOI] [PubMed] [Google Scholar]
- 3.Ueno H., Banchereau J., Vinuesa C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015; 16(2): 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong W., Zhu P., Wang Y., Wang Z. Follicular helper T cells in systemic lupus erythematosus: A potential therapeutic target. Autoimmun Rev. 2011; 10(6): 299–304. [DOI] [PubMed] [Google Scholar]
- 5.Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. Bcl6 mediates the development of T follicular helper cells. Science 2009; 325(5943): 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasheed A.U., Rahn H.P., Sallusto F., Lipp M., Miiller G. Follicular B helper T cell activity is confined to CXCR5(hi) ICOS(hi)CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006; 36(7): 1892–903. [DOI] [PubMed] [Google Scholar]
- 7.Bryant V.L., Ma C.S., Avery D.T., Li Y., Good K.L., Corcoran L.M., Malefyt R.W., Tangye S.G. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007; 179(12): 8180–90. [DOI] [PubMed] [Google Scholar]
- 8.King C., Tangye S.G., Mackay C.R. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008; 26: 741–66. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41(4): 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X., Wang D., Chen J., Lu L., Hua B., Li X., Tsao B.P., Sun L. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. Plos One 2012; 7(12): e51982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson N., Gatenby P.A., Wilson A., Malik S., Fulcher D.A., Tangye S.G., Manku H., Vyse T.J., Roncador G., Huttley G.A., Goodnow C.C., Vinuesa C.G., Cook M.C. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010; 62(1): 234–44. [DOI] [PubMed] [Google Scholar]
- 12.Herber D., Brown T.P., Liang S., Young D.A., Collins M., Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R. Fc reduces disease progression. J Immunol. 2007; 178(6): 3822–30. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y.L., Metz D.P., Chung J., Siu G., Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009; 182(3): 1421–8. [DOI] [PubMed] [Google Scholar]
- 14.Rankin A.L., Guay H., Herber D., Bertino S.A., Duzanski T.A., Carrier Y., Keegan S., Senices M., Stedman N., Ryan M., Bloom L., Medley Q., Collins M., Nickerson-Nutter C., Craft J., Young D., Dunussi-Joannopoulos K. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012; 188(4): 1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubier J.A., Sproule T.J., Foreman O., Spolski R., Shaffer D.J., Morse H.C. 3rd, Leonard W.J., Roopenian D.C. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA 2009; 106(5): 1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y., Li J., Yang P., Luo B., Wu Q., Zajac A.J., Wildner O., Hsu H.C., Mountz J.D. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014; 66(9): 2601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins E., Gilkeson G. Hematopoetic and mesenchymal stem cell transplantation in the treatment of refractory systemic lupus erythematosus—Where are we now? Clin Immunol. 2013; 148(3): 328–34. [DOI] [PubMed] [Google Scholar]
- 18.Tyndall A. Mesenchymal stem cell treatments in rheumatology: A glass half full? Nat Rev Rheumatol. 2014; 10(2): 117–24. [DOI] [PubMed] [Google Scholar]
- 19.Gu Z., Akiyama K., Ma X., Zhang H., Feng X., Yao G., Hou Y., Lu L., Gilkeson G.S., Silver R.M., Zeng X., Shi S., Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 2010; 19(13): 1502–14. [DOI] [PubMed] [Google Scholar]
- 20.Sun L., Wang D., Liang J., Zhang H., Feng X., Wang H., Hua B., Liu B., Ye S., Hu X., Xu W., Zeng X., Hou Y., Gilkeson G.S., Silver R.M., Lu L., Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010; 62(8): 2467–75. [DOI] [PubMed] [Google Scholar]
- 21.Liu R., Su D., Zhou M., Feng X., Li X., Sun L. Umbilical cord mesenchymal stem cells inhibit the differentiation of circulating T follicular helper cells in patients with primary Sjogren's syndrome through the secretion of indoleamine 2,3-dioxygenase. Rheumatology (Oxford) 2015; 54(2): 332–42. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C., Zhang L., Kong W., Liang J., Xu X., Wu H., Feng X., Hua B., Wang H., Sun L. Umbilical cord-derived mesenchymal stem cells inhibit cadherin-11 expression by fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol Res. 2015; 2015: 137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D., Feng X., Lu L., Konkel J.E., Zhang H., Chen Z., Li X., Gao X., Lu L., Shi S., Chen W., Sun L. A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol. 2014; 66(8): 2234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soleimani M., Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4(1): 102–6. [DOI] [PubMed] [Google Scholar]
- 25.Hou L.F., He S.J., Li X., Yang Y., He P.L., Zhou Y., Zhu F.H., Yang Y.F., Li Y., Tang W., Zuo J.P. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011; 63(8): 2445–55. [DOI] [PubMed] [Google Scholar]
- 26.Lartigue A., Courville P., Auquit I., François A., Arnoult C., Tron F., Gilbert D., Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006; 177(2): 1349–54. [DOI] [PubMed] [Google Scholar]
- 27.Ishii M., Koike C., Igarashi A., Yamanaka K., Pan H., Higashi Y., Kawaguchi H., Sugiyama M., Kamata N., Iwata T., Matsubara T., Nakamura K., Kurihara H., Tsuji K., Kato Y. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005; 332(1): 297–303. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ami E., Berrih-Aknin S., Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011; 10(7): 410–5. [DOI] [PubMed] [Google Scholar]
- 29.Jang E., Jeong M., Kim S., Jang K., Kang B.K., Lee D.Y., Bae S.C., Kim K.S., Youn J. Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T cell development. Cell Transplant. 2016; 25(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 30.Vogel K.U., Edelmann S.L., Jeltsch K.M., Bertossi A., Heger K., Heinz G.A., Zoller J., Warth S.C., Hoefig K.P., Lohs C., Neff F., Kremmer E., Schick J., Repsilber D., Geerlof A., Blum H., Wurst W., Heikenwälder M., Schmidt-Supprian M., Heissmeyer V. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity 2013; 38(4): 655–68. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki K., Spolski R., Ettinger R., Kim H.P., Wang G., Qi C.F., Hwu P., Shaffer D.J., Akilesh S., Roopenian D.C., Morse H.C. 3rd, Lipsky P.E., Leonard W.J. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004; 173(9): 5361–71. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y., Li J., Wu Q., Yang P., Luo B., Xie S., Druey K.M., Zajac A.J., Hsu H.C., Mountz J.D. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol. 2013; 191(4): 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H., Liu J., Cui X., Zuo Y., Zhang Z., Li Y., Tao R., Li Y., Pang J. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 2015; 295(1): 46–51. [DOI] [PubMed] [Google Scholar]
- 34.Sun L., Akiyama K., Zhang H., Yamaza T., Hou Y., Zhao S., Xu T., Le A., Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009; 27(6): 1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Che N., Li X., Zhang L., Liu R., Chen H., Gao X., Shi S., Chen W., Sun L. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 2014; 193(10): 5306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cianciulli A., Calvello R., Porro C., Trotta T., Salvatore R., Panaro M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol. 2016; 36: 282–90. [DOI] [PubMed] [Google Scholar]


