Abstract
Flow cytometric analysis of cell surface antigens is a powerful tool for the isolation and characterization of stem cells residing in adult tissues. In contrast to the collection of hematopoietic stem cells, the process of enzymatic digestion is usually necessary to prepare mesenchymal stem cells (MSCs) suspensions, which can influence the expression of cell surface markers. In this study, we examined the effects of various cell-detaching reagents and digestion times on the expression of stem cell-related surface antigens and MSC functions. Human MSCs were detached from dishes using four different reagents: trypsin, TrypLE, collagenase, and a non enzymatic cell dissociation reagent (C5789; Sigma-Aldrich). Following dissociation reagent incubations ranging from 5 to 120 min, cell surface markers were analyzed by flow cytometry. Trypsin and TrypLE quickly dissociated the cells within 5 min, while collagenase and C5789 required 60 min to obtain maximum cell yields. C5789 significantly decreased cell viability at 120 min. Trypsin treatment significantly reduced CD44+, CD55+, CD73+, CD105+, CD140a+, CD140b+, and CD201+ cell numbers within 30 min. Collagenase treatment reduced CD140a expression by 30 min. In contrast, TrypLE treatment did not affect the expression of any cell surface antigens tested by 30 min. Despite the significant loss of surface antigen expression after 60 min of treatment with trypsin, adverse effects of enzymatic digestion on multipotency of MSCs were limited. Overall, our data indicated that TrypLE is advantageous over other cell dissociation reagents tested for the rapid preparation of viable MSC suspensions.
Keywords: Mesenchymal stem cells (MSCs), Surface antigen, Cell-detaching method, Viability, Multipotency
Introduction
Analysis of cell surface antigen expression is a powerful tool to identify and isolate stem cells residing in adult tissues. Recent advancements in the field of hematopoietic stem cell (HSC) research are greatly indebted to the development of fluorescence-activated cell sorting (FACS) techniques and to the systematization of the cluster of differentiation (CD) nomenclature1,2. In addition to these milestones in HSC research, recent studies have identified the mesenchymal stem/stromal cells (MSCs) that reside in adult tissues and play crucial roles in adult tissue homeostasis3–5. The International Society for Cellular Therapy (ISCT) has defined the criteria for a bone marrow cell to be considered an MSC: (1) MSCs are plastic-adherent cells when maintained in standard culture conditions; (2) MSCs express CD73, CD90, and CD105 surface antigens; (3) MSCs lack surface antigens observed in various hematopoietic lineage cells such as CD45, CD34, CD14 or CD11b, CD79a or CD19, and human leukocyte antigen-antigen D related (HLA-DR); and (4) MSCs have the ability to differentiate into multiple cell types of mesenchymal lineage, such as osteoblasts, adipocytes, and chondroblasts, in vitro6.
The synovial membrane is a thin layer of connective tissue that lines the joint cavity, tendon sheaths, and bursae7. It is considered to play central roles in synovial joint homeostasis since it produces synovial fluid, which is important for lubrication, shock absorption, nutrient supplementation, and waste transportation in the joint7. In addition, the synovial membrane is considered to include multipotent cells that contribute to tissue regeneration after injury3. It is reported that cells migrate from the synovial membrane after meniscal injury to regenerate the damaged tissue8,9.
We have analyzed cultured fibroblastic cells isolated from the suprapatella synovial membrane in the knee joint. We regard these cells as MSCs (synovial MSCs) since they have characteristics that satisfy the definition of MSCs described by ISCT10. We have reported that intra-articular administration of synovial MSCs in suspension significantly enhanced both articular cartilage and meniscus regeneration in experimental animals11–18. Based on these results, we have started clinical trials for the regeneration of both articular cartilage and meniscus by the administration of autologous synovial MSC suspensions19. In these clinical trials, MSCs are isolated from autologous tissues obtained by synovial biopsies and expanded in our university hospital's Cell Processing Center (ISO9001 certified). To conduct safe, effective, and reproducible synovial MSC transplantation therapy, we consider it quite important to control both quantity (cell number) and quality (purity and cell viability) of the transplanted cells. Since MSCs are adherent, fibroblast-like cells, enzymatic digestion is usually required for the preparation of cell suspensions for transplantation. A major concern is that inappropriate cell-detaching procedures may greatly abolish not only cell viability but also stem cell functions in transplanted MSCs, altering cell surface antigen expression profiles. Therefore, we consider it important to establish a standardized procedure for detaching adherent MSCs from culture dishes. However, few articles carefully detail the cell-detaching procedures utilized prior to flow cytometric analyses and cell transplantation experiments.
In this study, we examined the effects of different methods of detaching adherent cells (both enzymatic and nonenzymatic) on the viability and expression of cell surface antigens in cultured synovial MSCs. Here we showed that trypsin and TrypLE quickly dissociated the cells within 5 min at 37°C, while collagenase and C5789 required 60 min to obtain maximum cell yields. C5789 significantly decreased cell viability at 120 min. Trypsinization of cultured synovial MSCs significantly altered various surface antigens reported to be expressed in MSCs such as CD44, CD49c, CD55, CD58, CD73, CD105, CD140a, CD140b, and CD201. Collagenase treatment reduced CD140a expression by 30 min. In contrast, TrypLE treatment did not affect any cell surface antigen expression tested by 30 min. Despite the significant loss of surface antigens after trypsin treatment for 60 min, adverse effects of enzymatic digestion on MSCs were limited. Based on these data, we concluded that within 30 min of incubation, TrypLE was the most appropriate method for cell detachment among all other methods employed in this study.
Materials and Methods
Synovial MSCs
This study was approved by the ethics committee of Tokyo Medical and Dental University. All patients included in this study gave their full written, informed consent for participation. Primary human synovial cells were isolated from synovial membranes obtained from patients who underwent total knee arthroplasty (TKA) as described previously with modification19. Briefly, nucleated cells were dispersed from 0.5 to 1 g of synovial membrane dissected from suprapatellar bursae by collagenase digestion (C9263; Sigma-Aldrich, St. Louis, MO, USA) for 3 h at 37°C. Cells were seeded onto 15-cm-diameter dishes at 5.7 × 102 cells/cm2 and maintained for 14 days in modified Eagle's medium-α (MEM-α) (Gibco 12561056; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco 12483040; Thermo Fisher Scientific) and antibiotic-antimycotic (penicillin, streptomycin, and amphotericin B; Gibco 15240062; Thermo Fisher Scientific). Cells were detached from the dishes using TrypLE (Gibco 12563–011; Thermo Fisher Scientific) and replated twice to obtain enough numbers for the experiments.
Detaching the Cells from Dishes
To dissociate the cells from culture dishes, 0.25% trypsin (Gibco 25200072; Thermo Fisher Scientific), TrypLE, collagenase (3 mg/ml in MEM-α), and nonenzymatic cell dissociation reagent (C5789; Sigma-Aldrich) were employed for comparison in this study. Of note, among these reagents, only TrypLE is accepted for use in clinical studies since it is derived from an animal-free source. Important information regarding the cell-detaching reagents is summarized in Table 1. Cells were treated with these reagents at 37°C for 5, 30, 60, and 120 min to prepare cell suspensions. Total cell numbers were measured using an automatic cell counter (TC-20; Bio-Rad, Hercules, CA, USA).
Table 1.
Cell-Detaching Reagent Employed in This Experiment
| Reagent | Cat. No. | Origin | Substrate Specificity |
|---|---|---|---|
| Trypsin | Gibco #25200-072 | Porcine pancreas | Cleaves peptides on the C-terminal side of lysine and arginine amino acid residues, contains EDTA |
| TrypLE | Gibco #12563-011 | Nondisclosure | Trypsin-like |
| Collagenase | Sigma #C9263 | Clostridium histolyticum | Cleaves peptide bonds between Gly and Pro of Pro-X-Gly-Pro sequence |
| C5789 | Sigma #C5789 | Mixture of EDTA and nitrilotriacetic acid | Divalent cation chelator |
EDTA, ethylenediaminetetraacetic acid; Gly, glycine; Pro, proline.
Cell Viability and Surface Antigen Expression of Detached MSCs
To detect dead cells, detached cells were stained with 7-aminoactinomycin D (7AAD; BD Biosciences, San Jose, CA, USA) for 10 min at 4°C and analyzed by a flow cytometer (FACSVerse; BD Biosciences). To analyze surface antigen expression, detached cells were stained with antibodies against surface antigens according to the manufacturers' manual, and positive cell populations were analyzed by flow cytometry. Surface antibodies analyzed in this study were purchased from BD Biosciences and are listed in Table 2; all of the markers have been reported to be expressed in MSCs previously4,6,20–24. Numbers of predicted trypsin recognition sequences in each antigen were also indicated in Table 2 (PeptideCutter; Wilkins et al.25).
Table 2.
Surface Antigens Analyzed in This Study
| CD No. | Aliases | Accession No. | Molecular Function | No. of Trypsin Cleavage Sites |
|---|---|---|---|---|
| CD9 | TSPAN29 | NP_001760 | A tetraspanin family molecule, cell adhesion | 21 |
| CD13 | APN | NP_001141 | Aminopeptidase | 81 |
| CD26 | DPPIV | NP_001926 | Dipeptidyl peptidase | 69 |
| CD29 | ITGB1 | NP_000880 | An integrin family molecule, cell adhesion | 70 |
| CD44 | Pgp1 | NP_000601 | A receptor for hyaluronic acid (HA), osteopontin, collagens, and matrix metalloproteinases (MMPs) | 51 |
| CD45 | LCA | NP_002829 | Protein tyrosine phosphatase | 132 |
| CD49c | ITGA3 | NP_002195 | Cell adhesion | 90 |
| CD51 | ITGAV | NP_006608 | Cell adhesion | 149 |
| CD61 | ITGB3 | NP_000203.2 | Cell adhesion | 75 |
| CD55 | DAF | NP_001287831 | Complement control protein | 48 |
| CD58 | LFA3 | NP_001770 | Adhesion and activation of T cells | 20 |
| CD59 | MAC-IP | NP_000602 | Complement control protein | 9 |
| CD73 | E5NT | NP_002517 | Ecto-5′-nucleotidase | 57 |
| CD90 | Thy1 | NP 937839 | Cell adhesion | 48 |
| CD105 | Endoglin | NP_114452 | A component of the transforming growth factor-β receptor complex | 63 |
| CD140a | PDGFRA | NP_006197 | A receptor for PDGF | 105 |
| CD140b | PDGFRB | NP_002600 | A receptor for PDGF | 90 |
| CD201 | EPCR | NP_006395 | A receptor for activated protein C, a serine protease activated by and involved in the blood coagulation pathway | 16 |
TSPAN29, tetraspanin 29; APN, aminopeptidase N; DPPIV, depeptidyl peptidase IV; ITGB1, integrin β 1; Pgp1, phagocytic glycoprotein-1; LCA, leukocyte common antigen; ITGA3, integrin α 3; ITGAV, integrin α V; ITGB3, integrin beta 3; DAF, decay accelerating factor; LFA3, lymphocyte function-associated antigen 3; MAC-IP, membrane attack complex-inhibitory protein; E5NT, ecto-5¢-nucleotidase; Thy1, thymocyte antigen 1; PDGFRA, platelet-derived growth factor receptor α; PDGFRB, platelet-derived growth factor receptor β; EPCR, endothelial protein C receptor.
Immunohistochemical Analyses
Sections were deparaffinized in xylene (Wako Pure Chemical Industries, Tokyo, Japan), rehydrated in graded alcohol, and saturated with phosphate-buffered saline (PBS). For type II collagen staining, samples were pre-treated with 0.4 mg/ml proteinase K (Dako, Agilent Technologies, Santa Clara, CA, USA) in Tris-HCl buffer (Wako Pure Chemical Industries) for 10 min. We did not perform proteinase K digestion for type I collagen staining. Next, endogenous peroxidases were quenched using 0.3% hydrogen peroxide in methanol for 30 min. Primary antibodies (human anti-type I collagen, 1:500 dilution; human anti-type II collagen, 1:500 dilution; Daiichi Fine Chemical Co. Ltd., Takaoka, Japan) were applied to sections and incubated at 4°C overnight. The sections were rinsed with 0.1% PBS–Triton X-100 (MP Biomedicals Inc., Solon, OH, USA), and then incubated in a 1:200 dilution of the secondary antibody [biotinylated horse anti-mouse immunoglobulin G (IgG) for type I and II collagen; Vector Laboratories, Burlingame, CA, USA] for 30 min at room temperature. After sections were rinsed, signals were visualized using Vectastain ABC reagent (Vector Laboratories) followed by 3,3′-diaminobenzidine (DAB) staining (Vector Laboratories). The sections were counterstained with hematoxylin (Muto Pure Chemicals, Tokyo, Japan).
Immunocytochemical Analysis
MSCs at passage 4 were detached by incubating with trypsin for 60 min and reseeded on cell culture slides (354118; BD Falcon, Bedford, MA, USA). On either day 1, 3, or 5 of culture, cells were fixed with 4% formaldehyde for 5 min, incubated in 1% bovine serum albumin (BSA; Sigma-Aldrich)/10% normal goat serum (Vector Laboratories) in 0.1% PBS-Triton X-100 (MP Biomedicals Inc.) for 30 min, and then incubated with rabbit anti-human CD73 (Ab175396; Abcam, Cambridge, MA, USA), rabbit anti-human CD105 (Ab49228; Abcam), or rabbit anti-human CD140b (Ab107169; Abcam). After rinsing, slides were incubated with Alexa Fluor® 555 goat anti-rabbit IgG antibody (A-31570; Life Technologies, Woburn, MA, USA) for CD105, or Alexa Fluor® 488 goat anti-rabbit IgG antibody (S-32354) for CD73 and CD140b, and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI; D-21490; Invitrogen, Carlsbad, CA, USA) and coverslipped. Fluorescent images were taken with a fluorescence microscope (BX53; Olympus, Tokyo, Japan).
In Vitro Differentiation Assay
An in vitro differentiation assay was performed according to the method described by Colter et al. with small modifications26.
For chondrogenesis, 2.5 × 105 cells were placed in a 15-ml polypropylene tube (BD Falcon), centrifuged at 450 × g for 10 min, and cultured in chondrogenesis medium containing 1,000 ng/ml recombinant human bone morphogenetic protein 7 (rhBMP-7) (a gift from Stryker Biotech, Hopkinton, MA, USA), 10 ng/ml transforming growth factor-β3 (TGF-β3; R&D Systems, Minneapolis, MN, USA), and 100 nM dexamethasone (Sigma-Aldrich) for 14 days.
For adipogenesis, cells were cultured in MEM-α supplemented with 10% FBS, 100 nM dexamethasone (Sigma-Aldrich), 0.5 mM isobutyl-methylxanthine (IBMX; Sigma-Aldrich), and 50 μM indomethacin (Wako Pure Chemical Industries) for 21 days. The adipogenic cultures were fixed in 4% paraformaldehyde (PFA) and then stained with fresh Oil red O solution (Sigma-Aldrich).
For calcification, cells were cultured in MEM-α supplemented with 10% FBS, antibiotics, 1 nM dexamethasone, 20 mM β-glycerol phosphate (Wako Pure Chemical Industries), and 50 μg/ml ascorbate-2-phosphate (Sigma-Aldrich) for 21 days. The calcified nodules were visualized by 0.5% Alizarin red staining (Sigma-Aldrich).
Statistical Analysis
The Kruskal-Wallis test followed by the Steel-Dwass test or Mann-Whitney U-test was employed for statistical analyses. Values of p < 0.05 were considered as significant (StatView+5.0 Software Package; SAS Institute Inc., Cary, NC, USA).
Results
Effect of Different Cell-Detaching Procedures on Cell Recovery and Cell Viability
Trypsin and TrypLE quickly dissociated the cells within 5 min at 37°C, while collagenase required 60 min to obtain the maximum cell yields (Fig. 1A). The average cell yields at 5 min were quite comparable between trypsin and TrypLE (trypsin: 1.84 ± 0.74 × 105 cells/dish, TrypLE: 1.61 ± 0.59 × 105 cells/dish) (Fig. 1B). In contrast, the average cell yield at 5 min by collagenase digestion was almost one third of that of trypsin digestion (0.51 ± 0.62 × 105 cells/dish) (Fig. 1B). We did not observe any significant difference in cell recovery between the incubation periods with C5789 (Fig. 1A). Average cell yield at 5 min by C5789 incubation was 0.62 ± 0.51 × 105 cells/dish (Fig. 1B). Cell viability was not significantly altered by enzymatic digestion; however, nonenzymatic C5789 treatment significantly reduced the live cell population at 120 min (Fig. 1C). In addition, viability of the cells detached by C5789 for 30 min was significantly low if compared to that of trypsin (Fig. 1D).
Figure 1.

Effect of different cell-detaching procedures on cell recovery and cell viability. One hundred thousand cells were seeded on 15-cm dishes and maintained for 2 weeks. (A) Number of the cells recovered by each cell-detaching reagent and incubation time indicated. (B) Number of cells recovered by each cell-detaching reagent at 5 min. (C) Detached cells were stained with 7-aminoactinomycin D (7AAD), and living cell populations were calculated by flow cytometry. (D) Survival rate of the detached mesenchymal stem cells (MSCs) by each reagent at 30 min. Data are represented as the average and standard deviation of six donors. *p < 0.05. N.E., not examined; n.s., not significant.
Effect of Different Cell-Detaching Procedures on the Expression of CD73, CD90, and CD105 Mesenchymal Cell Markers
To examine the effects of different cell-detaching reagents and incubation times for cell detachment on the expression of synovial MSC surface antigens, we analyzed the expression of CD73, CD90, and CD105 by flow cytometry, as they are representative stem cell markers for MSCs. Trypsin treatment significantly reduced the population of CD73+ cells within 60 min (Fig. 2 and Table 3). In addition, trypsin treatment reduced median fluorochrome intensity within 30 min (Fig. 2A, upper left, compare red and orange lines), indicating that expression levels of CD73 on each CD73+ cell were also decreased after trypsin treatment. Another MSC marker, CD105, seemed to be more severely affected by trypsin treatment, as both the positive cell population and median fluorochrome intensity were quickly and significantly reduced within 30 min (Fig. 2 and Table 3). In contrast, subtle effects on CD90 expression were observed in MSCs by trypsin treatment, although fluorochrome intensity was slightly reduced by 30 min (Fig. 2A). Among the other surface antigens examined, CD44+, CD55+, CD140b+, and CD201+ cell populations were significantly reduced by trypsin treatment (Table 3). The population of CD140a+ cells did not decrease significantly during the period of trypsin treatment; however, the positive cell population was already decreased by trypsin treatment for 5 min if compared with that of TrypLE treatment for 5 min, indicating that CD140a may be quite sensitive to trypsin treatment (Fig. 3A and Table 3). TrypLE treatment had subtle effects on MSC surface antigen expression by 30 min, but CD44+, CD49c+, CD73+, CD140a+, and CD140b+ cell numbers were significantly reduced at 60 min (Fig. 2 and Table 3). Collagenase treatment significantly reduced CD58+, CD105+, and CD140b+ cell populations at 120 min (Table 3). Similar to trypsin treatment, median fluorochrome intensity for CD105 on MSCs was significantly reduced with time during collagenase treatment (Fig. 2). In addition, CD140a+ cell populations were significantly reduced by collagenase treatment for 30 min (Fig. 3B). C5789 had the mildest effect on MSC surface antigen expression by 120 min, where only the numbers of CD73+ cells were significantly reduced at 120 min (Fig. 2 and Table 3).
Figure 2.
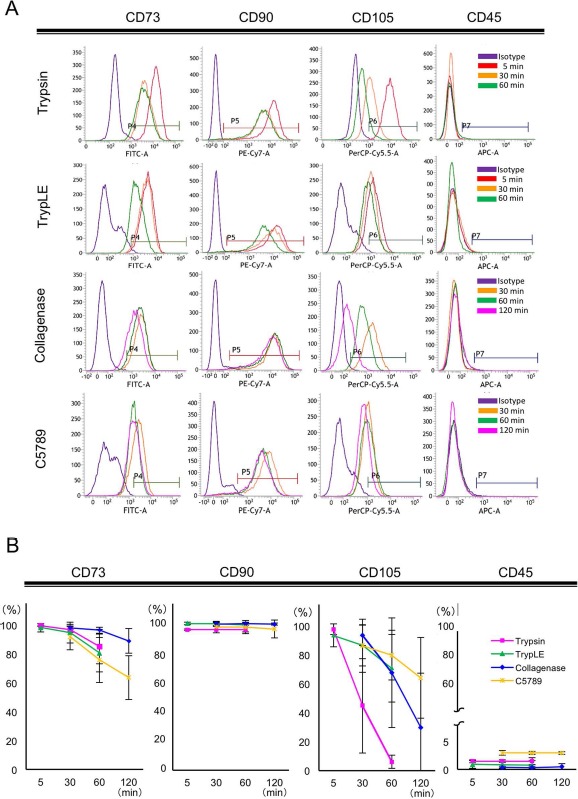
Surface antigen expression of detached MSCs. One hundred thousand cells were seeded on 15-cm dishes and maintained for 2 weeks. Cell suspensions were prepared using detaching reagents and incubation times indicated. Detached cells were stained with antibodies listed in Table 1, and each antigen+ population was calculated by flow cytometry. (A) A representative result of flow cytometric analysis. (B) Time course effects of each cell-detaching reagent on the positive population of each cell surface antigen. Data are presented as the average and standard deviation of six donors. FITC, fluorescein isothiocyanate; PE-Cy7, phycoerythrin-cyanin 7; PerCP-Cy5.5, peridinin chlorophyll protein–cyanin 5.5.
Table 3.
Positive Cell Populations for Each Surface Antigen Expression After Treatment With Different Cell-Detaching Reagents
| Trypsin |
TrypLE |
Collagenase |
C5789 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 min | 30 min | 60 min | 5 min | 30 min | 60 min | 30 min | 60 min | 120 min | 30 min | 60 min | 120 min | |
| CD9 | 94.50±6.10 | 93.75±5.22 | 91.59±8.03 | 90.85±5.65 | 87.47±6.44 | 81.87±8.79 | 87.10±21.30 | 78.64±36.06 | 74.38±37.39 | 89.90±4.89 | 84.36±9.29 | 81.77±8.42 |
| CD13 | 99.95±0.03 | 99.95±0.03 | 99.91±0.06 | 99.92±0.06 | 99.91±0.16 | 99.93±0.05 | 97.80±3.19 | 97.43 ±5.97 | 96.43±7.06 | 99.93±0.03 | 99.88±0.10 | 99.88±0.12 |
| CD26 | 23.90±14.89 | 18.64±11.92 | 13.67±7.30 | 21.80±13.35 | 19.99±10.57 | 8.72±5.23 | 16.22±12.51 | 14.54±5.95 | 14.35±10.39 | 18.35±15.50 | 12.41±11.45 | 9.73±7.34 |
| CD29 | 99.96±0.03 | 99.96±0.02 | 99.79±0.15 | 99.79±0.41 | 99.69±3.16 | 99.82±0.11 | 98.97±2.30 | 99.94±0.02 | 99.77±0.39 | 99.93±0.04 | 99.81±0.17 | 99.72±0.40 |
| CD44 | 99.74±0.23 | 98.86±2.09 | 95.49±3.57 * † | 99.92±0.06 | 99.95±0.03 * | 99.64±0.21 * † | 99.52±0.36 | 99.52±0.86 | 97.06±6.40 | 99.88±0.09 | 99.82±0.14 | 99.72±0.30 |
| CD45 | 0.55±0.27 | 0.42±0.25 | 0.81±0.61 | 0.71±0.55 | 0.70±0.45 | 0.59±0.64 | 0.48±0.33 | 0.55±0.66 | 0.69±0.72 | 0.40±0.40 | 0.30±0.28 | 0.30±0.23 |
| CD49c | 66.00±23.06 | 54.01±16.83 | 37.77±13.84 | 59.93±18.35 | 48.51±14.54 | 27.72±6.06 * † | 62.24±30.82 | 57.53±28.85 | 48.65±30.62 | 50.29±22.43 | 36.44±18.48 | 25.76±12.05 |
| CD51/61 | 16.52±10.45 | 11.95±10.38 | 9.38±6.49 | 16.20±12.64 | 15.36±12.32 | 4.81±3.72 | 7.41±5.18 | 7.10±3.39 | 4.50±2.58 | 8.23±7.72 | 5.72±7.06 | 3.80±2.91 |
| CD55 | 88.95±7.12 | 62.03±12.40 * | 30.24±12.40 * † | 86.85±8.48 | 80.6±11.11 | 68.11±18.39 | 77.72±26.14 | 67.45±28.56 | 45.41±23.17 | 89.60±12.69 | 84.20±20.38 | 82.21±18.58 |
| CD58 | 22.54±20.14 | 5.31±5.30 | 3.59±3.70 | 22.57±27.19 | 14.26±16.14 | 8.18±7.38 | 14.32±5.46 | 11.24±5.51 | 4.85±1.67 * † | 12.47±11.29 | 9.29±7.99 | 8.87±7.38 |
| CD59 | 98.78±2.83 | 99.85±0.29 | 99.86±0.19 | 99.93±0.03 | 99.93±0.09 | 99.91±0.06 | 99.52±1.02 | 99.92±0.06 | 99.94±0.03 | 99.88±0.07 | 99.79±0.16 | 99.79±0.20 |
| CD73 | 99.56±0.46 | 96.93±2.03 | 85.19±11.88 * † | 98.21±2.98 | 94.83±7.16 | 80.65±13.10 * † | 97.73±1.65 | 96.32±2.35 | 91.23±9.47 | 91.48±8.63 | 75.83±15.71 | 63.72±15.12 * † |
| CD90 | 99.76±0.17 | 96.28±0.83 | 99.25±0.60 | 99.64±0.33 | 99.54±0.48 | 99.26±0.66 | 99.40±0.40 | 99.49±0.32 | 99.45±0.38 | 83.20±37.85 | 98.65±1.79 | 98.16±2.39 |
| CD 105 | 97.77±3.70 | 44.77±32.73* | 5.98±4.60* | 93.55±7.93 | 86.76±14.31 | 70.81±23.43 | 94.05±7.13 | 69.60±37.16 | 18.67±17.90 * † | 86.43±18.41 | 79.91±17.26 | 64.17±27.93 |
| CD 140a | 2.97±1.78 | 1.76±1.14 | 1.91±1.15 | 63.92±18.65 | 29.67±25.30 | 7.40±13.19 * † | 1.64±1.46 | 1.05±0.81 | 0.76±0.57 | 0.40±29.12 | 0.30±27.24 | 0.30±11.43 |
| CD 140b | 99.30±0.72 | 87.63±11.41* | 11.69±9.21 * † | 99.44±0.33 | 98.68±0.83 | 91.61±10.47 * † | 96.14±4.37 | 91.72±10.54 | 76.98±23.70 * † | 49.09±5.15 | 31.04±15.00 | 18.00±23.06 |
| CD201 | 77.25±17.77 | 63.2±14.65 | 48.46±4.61 * † | 68.81±18.09 | 57.34±15.39 | 41.31±12.12 | 67.47±19.62 | 62.70±26.67 | 53.18±29.37 | 40.25±12.99 | 26.52±11.63 | 24.50±6.91 |
The mesenchymal stem cells (MSCs) at passage 4 were subjected to the flow cytometric analysis. Positive cell populations for each surface antigen expression were presented as mean ± standard deviation of six donors.
Statistical analysis was performed using Kruskal-Wallis test followed by Steel-Dwass test.
The difference was statistically significant (p<0.05) if compared to that of the shortest incubation time (5 min for trypsin and TrypLE or 30 min for collagenase and C5789).
The difference between 30 and 60 min (trypsin and TrypLE) or 60 and 120 min (collagenase and C5789) was statistically significant (p<0.05).
Figure 3.
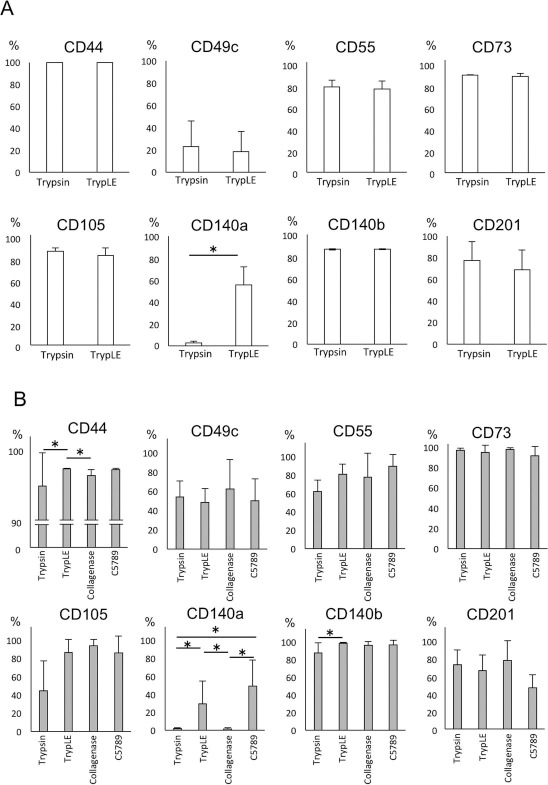
Surface antigen expression of detached MSCs. One hundred thousand cells were seeded on 15-cm dishes and maintained for 2 weeks. Cell suspensions were prepared using detaching reagents indicated. Detached cells were stained with antibodies as listed in the figure. Each antigen-positive population was calculated by a flow cytometer. (A) Positive cell population of each surface antigen after trypsin or TrypLE treatment for 5 min. CD140a+ cell population was quickly and significantly reduced by trypsin treatment. (B) Positive cell population of each surface antigen after four different cell-detaching reagent treatments for 30 min. CD140a+ cell population was significantly reduced by trypsin, collagenase, and C5789 treatment. Data are presented as the average and standard deviation of six donors. *p < 0.05.
Reduction of Surface Antigen Expression by Long-Term Trypsin Treatment Was Reversed After Recovery Culture
To examine if the reduction of CD73, CD105, and CD140b expression by long-term trypsin treatment altered stem cell character irreversibly, cells subjected to detachment by trypsinization for 60 min were reseeded and cultured for another 14 days under normal conditions (Fig. 4A), and the expression of these antigens was analyzed. As shown in Figure 4B, the reduced expression of CD73, CD105, and CD140b by long-term trypsin treatment (60 min) was completely reversed by recovery culture. Immunohistochemical (Fig. 4C, left) and flow cytometric analyses (Fig. 4C, right) indicated that the expression of CD73, CD105, and CD140b quickly reversed by day 3 (CD73 and CD105) or by day 5 (CD140b). These data indicate that the loss of the expression of these surface antigens by long-term trypsin digestion might not induce an irreversible surface antigen expression profile on synovial MSCs.
Figure 4.
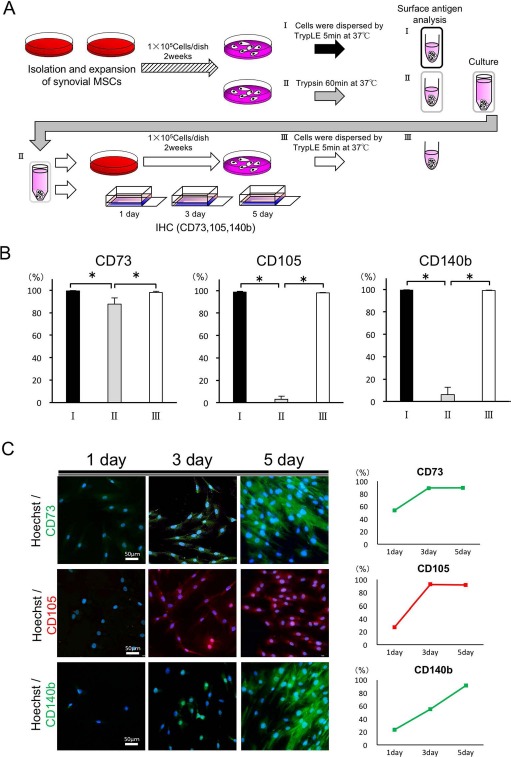
Reduction of surface antigen expression by long-term trypsin treatment was reversed after recovery culture. (A) Schematic diagrams of the experiment. One hundred thousand cells were seeded on 15-cm dishes and maintained for 2 weeks. Cell suspensions were prepared by TrypLE for 5 min (as a control, I) or trypsin treatment for 60 min (II). Cells treated with trypsin for 60 min were reseeded on 15-cm dishes and maintained for another 2 weeks (III). After the culture, cells were detached with TrypLE for 5 min and subjected to flow cytometric analysis (III). (B) One hundred thousand cells were stained against CD73, CD105, or CD140b antibody and subjected to flow cytometric analysis (I, II, and III). (C) Cells treated with trypsin for 60 min were seeded on a slide chamber, and time course changes in CD73, CD105, or CD140b expressions were analyzed by immunostaining (left). Positive cell populations at each time point were calculated by flow cytometer (right). (B) The average with standard deviation of four donors, and (C) the representative results from one donor. *p < 0.05. Scale bars: 50 μm.
Reduction of Surface Antigen Expression by Long-Term Trypsin Treatment Had Limited Effects on Multipotency of Synovial MSCs
To examine if long-term trypsin treatment reduced multipotency of synovial MSCs, we conducted in vitro differentiation assays using the cells detached by trypsin treatment for 60 min (group II) or by TrypLE treatment for 5 min (group I in Fig. 5A). To compare the chondrogenic potential of the detached cells, cells were centrifuged to form pellets and cultured for 2 weeks in chondrogenic medium. As shown in Figure 5B, average wet weight of the pellets was comparable between the groups. Histological analysis suggested that cell density decreased in the group II pellets. However, chondrogenic potential remained since we observed subtle differences in type II collagen expression between group I and group II. In addition, these changes appeared reversible since the average wet volumes of the pellets in group III, in which cells were maintained for another 2 weeks to recover from damage by long-term trypsinization, were quite comparable to those in group I (Fig. 5B). We observed subtle differences with regard to the osteogenic and adipogenic differentiation potential among groups I to III (Fig. 5B).
Figure 5.

Reduction of surface antigen expression by long-term trypsin treatment had limited effects on multipotency of synovial MSCs. (A) Schematic diagrams of the experiment. One hundred thousand cells were seeded on 15-cm dishes and maintained for 2 weeks. Cell suspensions were prepared by TrypLE for 5 min (as a control, I) or trypsin treatment for 60 min (II). Cells (2.5 × 105) were placed in a 15-ml polypropylene tube, centrifuged at 450 × g for 10 min, and cultured in chondrogenesis medium for 2 weeks. The rest of the cells were replated and maintained for another 2 weeks to recover surface antigen expression, detached by TrypLE for 5 min, and then chondrogenic differentiation assay was performed (III). To analyze the osteogenic and adipogenic potential of MSCs, 100 cells detached by three different methods (I, II, and III) were seeded on 10-cm dishes and maintained for 3 weeks in the differentiation medium. Mineralized nodule formation was detected by Alizarin red staining, and fat accumulation in the cell was visualized by Oil red O staining. (B) Macroscopic and histological/immunohistochemical analysis of in vitro chondrogenesis, osteogenesis, or adipogenesis assays. Scale bars: 50 μm (B), 200 μm (C). H.E., hematoxylin and eosin; Col, collagen.
Discussion
Since MSCs are adherent fibroblast-like cells, enzymatic digestion is required to prepare cell suspensions. However, inappropriate cell-detaching procedures may damage the cells, abolishing not only living cell numbers but also cell functions. Thus, it is important to evaluate the effects of different cell-detaching reagents and different incubation times on cell surface antigen expression and stem cell function in order to establish a standardized protocol for preparing MSC suspensions for cell transplantation therapy.
In this study, we compared four different cell-detaching reagents: trypsin, TrypLE, collagenase, and C5789. Among them, trypsin and TrypLE are advantageous for cell dissociation since they quickly detached the cells from the dishes. In contrast, collagenase required almost 60 min to reach maximum cell yields. This may be due to the type of extracellular matrices produced by MSCs to attach to the plastic surfaces. Both collagens and fibronectins are reported to be substrates for trypsins27–30. Collagenases are also reported to be able to degrade fibronectins; however, enzymatic activity is more specific to collagen fibers31,32. Our preliminary data suggest that major extracellular matrix components, which are utilized for MSCs to attach on a plastic surface, might be the fibronectin family molecules, since MSCs highly express integrin subsets for fibronectins (αvβ3) but not for collagens (α1 integrins) (Tsuji et al., manuscript in preparation). We observed the lowest cell yields with C5789 treatment. In addition, cell viability was significantly reduced by C5789 treatment for 120 min. C5789 is a mixture of ethylenediaminetetraacetic acid (EDTA) and nitrilotriacetic acid, which are chelators for divalent cations. C5789 functions to deplete the Mn2+ and Mg2+ ions, which are required for integrin–matrix conjugation33. Since divalent cations are also required for most enzymes prerequisite for basal cell metabolism34, we consider that the reduced cell viability after long-term C5789 treatment may be due to the inhibition of these enzymes rather than inhibition of integrin function.
Flow cytometric analyses indicated that cells positive for MSC surface antigens are deeply influenced by the methods for cell detaching. Among the antigens tested (17 in total), positive populations of 9 antigens, CD44, CD49c, CD55, CD58, CD73, CD105, CD140a, CD140b, and CD201, were significantly reduced by the cell-detaching procedures in a time-dependent manner. Among the four reagents tested, trypsin treatment appeared to be the most harmful for cultured MSCs. Seven out of nine antigens were significantly decreased by trypsin treatment. Among them, MSCs positive for CD140a were quickly decreased and almost disappeared within 5 min of trypsin incubation. CD55, CD105, and CD140b were also severely affected, which quickly and significantly dissociated by trypsin treatment within 30 min. CD44-, CD73-, and CD201-expressing cells were significantly decreased within 60 min. Trypsin is the most commonly used cell-detaching reagent that cleaves peptides on the C-terminal side of lysine and arginine amino acid residues. Interestingly, computer-based analyses indicated that 17 out of 17 antigens had predicted multiple trypsin recognition sequences25; however, we found that only 7 antigens were severely affected by trypsin treatment. This may be due to posttranslational modification, such as glycosylation, or the three-dimensional conformation of each antigen. These data further indicate the significance of our results, as in silico analysis is not powerful enough to predict the change in antigenicity of MSCs by enzymatic digestion. TrypLE is a recombinant enzyme with trypsin-like activity, although detailed information such as the origin of the cDNA and the manufacturing method have not been disclosed by the manufacturer. This enzyme is currently widely employed to detach MSCs from culture dishes for transplantation therapy since this enzyme is certified as an animal-free source. Our results indicated that the substrate specificity between trypsin and TrypLE was similar but not identical to each other. TrypLE had milder effects on CD140a and CD105 antigenicity than trypsin. Of note, we did not observe a significant reduction in any surface antigen expression by TrypLE treatment by 30 min. Collagenase and C5789 had milder effects on surface antigen conservation, and significant reduction in surface antigen expression was observed after 60 min. This suggests that TrypLE, collagenase, and C5789 are more suitable cell-detaching reagents for conservation of cell surface antigens than trypsin. Taken together, we concluded that TrypLE is advantageous over the other cell dissociation reagents tested for the rapid detachment of viable MSC suspensions.
In this study, we found that reduction of surface antigen expression by trypsin treatment for 60 min did not induce irreversible changes in MSC functions since surface antigen expression was recovered by 5 days. In addition, we found that chondrogenic, osteogenic, and adipogenic differentiation abilities of trypsin-treated MSCs were almost comparable with those treated with TrypLE after 5 min. These data suggest that the effects of cell-detaching procedures on stem cell functions were quite limited. This may be due to the hyperplasticity of MSCs, where these cells may have the capacity to endure transient cell surface modifications or environmental changes to maintain stem cell functions.
Cell-detaching procedure cost is another important issue to be considered in cell transplantation therapy. Our calculation indicated that the cost of each enzyme solution per unit volume was as follows: collagenase—about 10-fold of trypsin; C5789—about 3.6-fold of trypsin; and TrypLE—about 1.8-fold of trypsin. These indicate that trypsin and TrypLE are more cost-effective than others.
There are several limitations in this study. We concluded that TrypLE treatment within 30 min is the best procedure to detach the cells based on cell yields, viability, and the preservation of surface antigens. However, we did not examine all of the cell surface antigens expressed on MSCs, and a more comprehensive analysis may result in a different conclusion. MSCs used in this study were passaged four to five times before being subjected to experimentation. For passaging MSCs, we treated the cells with TrypLE for 5 min to detach the cells from the plastic dishes, which may affect the homogeneity of the cells. Currently, many of the autologous cell transplantation therapies inject primary expanded cells19. It is possible that sensitivity to enzymatic digestion of primary cells may differ from passaged cells. An in vitro differentiation assay was conducted using the cells treated with trypsin for 60 min. We have not analyzed the multipotency of trypsin-treated cells in vivo. In addition, we have not checked the multipotency of MSCs after repeated 60-min trypsin treatments. We conclude that an intermittent enzymatic digestion of surface antigens may have adverse effects on MSCs.
Conclusion
In conclusion our results suggest that TrypLE is the most advantageous cell dissociation reagent in the preparation of MSC suspensions.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science (JSPS) to K.T. (Nos. 25293317, 25460716, and 26462287). This study was also supported by “the Project for Realization of Regenerative Medicine” from the Japan Agency for Medical Research and Development to I.S. and T.M. (Nos. 122011A182 and 15ek0610006h0001). We thank Ms. Aiko Yamada, Ms. Risa Tada, and Ms. Haruno Kuroda for their wonderful administrative support to this study. The authors declare no conflicts of interests.
References
- 1.Boumsell L. The international workshops and conferences on human leukocyte differentiation antigens. Birth, current status and future. Tissue Antigens 1996; 48: 238–41. [DOI] [PubMed] [Google Scholar]
- 2.Tung J.W., Heydari K., Tirouvanziam R., Sahaf B., Parks D.R., Herzenberg L.A., Herzenberg L.A. Modern flow cytometry: A practical approach. Clin Lab Med. 2007; 27: 453–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurth T.B., Dell'accio F., Crouch V., Augello A., Sharpe P.T., De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011; 63: 1289–300. [DOI] [PubMed] [Google Scholar]
- 4.Klimczak A., Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: Their role in tissue homeostasis. Stem Cells Int. 2016; 2016: 4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattar P., Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015; 6: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–5. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.D. The normal synovium. Open Rheumatol J. 2011; 5: 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox D.B., Luther J.K., Whitener D. An in vitro model to assess mechanisms and efficacy of a cellular conduit for treatment of avascular meniscal injuries. In Vitro Cell Dev Biol Anim. 2008; 44: 185–8. [DOI] [PubMed] [Google Scholar]
- 9.Arnoczky S.P., Warren R.F. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983; 11: 131–41. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005; 52: 2521–9. [DOI] [PubMed] [Google Scholar]
- 11.Koga H., Muneta T., Ju Y.J., Nagase T., Nimura A., Mochizuki T., Ichinose S., von der Mark K., Sekiya I. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 2007; 25: 689–96. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T., Sekiya I., Muneta T., Hatsushika D., Horie M., Tsuji K., Kawarasaki T., Watanabe A., Hishikawa S., Fujimoto Y., Tanaka H., Kobayashi E. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012; 14: 327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatsushika D., Muneta T., Horie M., Koga H., Tsuji K., Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013; 31: 1354–9. [DOI] [PubMed] [Google Scholar]
- 14.Hatsushika D., Muneta T., Nakamura T., Horie M., Koga H., Nakagawa Y., Tsuji K., Hishikawa S., Kobayashi E., Sekiya I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis Cartilage 2014; 22: 941–50. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa Y., Muneta T., Kondo S., Mizuno M., Takakuda K., Ichinose S., Tabuchi T., Koga H., Tsuji K., Sekiya I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage 2015; 23: 1007–17. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki N., Muneta T., Matsuta S., Koga H., Nakagawa Y., Mizuno M., Tsuji K., Mabuchi Y., Akazawa C., Kobayashi E., Saito T., Sekiya I. Synovial mesenchymal stem cells promote meniscus regeneration augmented by an autologous Achilles tendon graft in a rat partial meniscus defect model. Stem Cells 2015; 33: 1927–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozeki N., Muneta T., Koga H., Nakagawa Y., Mizuno M., Tsuji K., Mabuchi Y., Akazawa C., Kobayashi E., Matsumoto K., Futamura K., Saito T., Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage 2016; 24: 1061–70. [DOI] [PubMed] [Google Scholar]
- 18.Kondo S., Muneta T., Nakagawa Y., Koga H., Watanabe T., Tsuji K., Sotome S., Okawa A., Kiuchi S., Ono H., Mizuno M., Sekiya I. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J Orthop Res. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Sekiya I., Muneta T., Horie M., Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015; 473: 2316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H.J., Choi B.H., Min B.H., Park S.R. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis Rheum. 2009; 60: 2325–32. [DOI] [PubMed] [Google Scholar]
- 21.Krampera M., Galipeau J., Shi Y., Tarte K., Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013; 15: 1054–61. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa E.B., Casado P.L., Moura Neto V., Duarte M.E., Aguiar D.P. Synovial fluid and synovial membrane mesenchymal stem cells: Latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014; 5: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elsaesser A.F., Schwarz S., Joos H., Koerber L., Brenner R.E., Rotter N. Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies. Cell Biosci. 2016; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soland M.A., Bego M., Colletti E., Zanjani E.D., St Jeor S., Porada C.D., Almeida-Porada G. Mesenchymal stem cells engineered to inhibit complement-mediated damage. PLoS One 2013; 8: e60461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins M.R., Gasteiger E., Bairoch A., Sanchez J.C., Williams K.L., Appel R.D., Hochstrasser D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999; 112: 531–52. [DOI] [PubMed] [Google Scholar]
- 26.Colter D.C., Sekiya I., Prockop D.J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA 2001; 98: 7841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen B.R. Electron microscope studies on collagen. III. Tryptic digestion of tropocollagen macromolecules. Z Zellforsch Mikrosk Anat. 1964; 61: 913–9. [DOI] [PubMed] [Google Scholar]
- 28.van Deemter M., Kuijer R., Harm Pas H., Jacoba van der Worp R., Hooymans J.M., Los L.I. Trypsin-mediated enzymatic degradation of type II collagen in the human vitreous. Mol Vis. 2013; 19: 1591–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Schor S.L. The effects of EGTA and trypsin on the serum requirements for cell attachment to collagens. J Cell Sci. 1979; 40: 271–9. [DOI] [PubMed] [Google Scholar]
- 30.Pearlstein E., Waterfield M.D. Metabolic studies on 125i-labeled baby hamster kidney cell plasma membranes. Biochim Biophys Acta. 1974; 362: 1–12. [DOI] [PubMed] [Google Scholar]
- 31.Welgus H.G., Jeffrey J.J., Eisen A.Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981; 256: 9511–5. [PubMed] [Google Scholar]
- 32.Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997; 272: 2446–51. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K., Chen J. The regulation of integrin function by divalent cations. Cell Adh Migr. 2012; 6: 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roskoski R. Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res. 2015; 100: 1–23. [DOI] [PubMed] [Google Scholar]


