Abstract
Islet allotransplantation results in increasing success in treating type 1 diabetes, but the shortage of deceased human donor pancreata limits progress. Islet xenotransplantation, using pigs as a source of islets, is a promising approach to overcome this limitation. The greatest obstacle is the primate immune/inflammatory response to the porcine (pig) islets, which may take the form of rapid early graft rejection (the instant blood-mediated inflammatory reaction) or T-cell-mediated rejection. These problems are being resolved by the genetic engineering of the source pigs combined with improved immunosuppressive therapy. The results of pig-to-diabetic nonhuman primate islet xenotransplantation are steadily improving, with insulin independence being achieved for periods >1 year. An alternative approach is to isolate islets within a micro- or macroencapsulation device aimed at protecting them from the human recipient's immune response. Clinical trials using this approach are currently underway. This review focuses on the major aspects of pig-to-primate islet xenotransplantation and its potential for treatment of type 1 diabetes.
Keywords: Type 1 diabetes (T1D), Encapsulation, Instant blood-mediated inflammatory reaction (IBMIR), Islets, Porcine, Xenotransplantation
Introduction
Islet allotransplantation provides a potential cure for type 1 diabetes (T1D)1, but the shortage of islets from deceased human donors limits progress. Currently, diabetic patients may require islets from two or more donors to become normoglycemic2,3. A significant number of islets (perhaps up to 70%) may be lost when transplanted into the portal vein as a result of what is known as the instant blood-mediated inflammatory reaction (IBMIR)4 and/or from a delay in revascularization of the graft5–11.
Because of these limitations, alternative sources of insulin-producing cells are being investigated. Developments in stem cell research have allowed the transformation of embryonic stem cells (ESCs) into pancreatic β-cells12. The in vitro generation of functional β-cells from human induced pluripotent stem cells (hiPSCs) derived from patients with T1D can correct hyperglycemia in mice13. In adult mice, exocrine cells have been directly reprogrammed into cells that closely resemble β-cells14. However, stem cells may possibly continue to proliferate in an uncontrolled manner after implantation in patients. Furthermore, stem cell-derived insulin-secreting cells have not yet been demonstrated to produce long-term normoglycemia in diabetic nonhuman primates (NHPs) or patients.
Islet xenotransplantation is an alternative promising approach to the treatment of T1D15,16. Pigs have anatomical and physiological similarities to humans. Additionally, the porcine pancreas can be easily excised, and successful islet isolation procedures have been developed17. Furthermore, in contrast to when a deceased human is the source, unlimited neonatal as well as adult porcine (pig) islets are obtainable. When transplanted into streptozotocin-induced diabetic NHPs, several studies reported long-term normoglycemia18–26. Use of pigs also has the great advantage of being readily genetically modifiable to provide some protection against the human immune and inflammatory responses. Porcine islet xenotransplantation has therefore become an attractive option for treating patients with T1D.
Here we focus on the major aspects of pig-to-primate islet xenotransplantation, including islet-source pig choice (optimal age, strain, and genetic modification), porcine endogenous retrovirus potential risks, free or encapsulated (immunoisolated) islet transplantation, combined islet and mesenchymal stromal cell or Sertoli cell (SC) transplantation, immunological tolerance induction, previous clinical trials on islet xenotransplantation, criteria for future clinical trials, and future perspectives.
Choice of Islet-Source Pig
Pig age, strain, genetic modifications, insulin secretion, and porcine endogenous retroviruses (PERVs) need to be considered27,28.
Choice of Pig Based on Age
Adult pigs can provide a large number of islets of large size and mature structure and function (Table 1)17,27,29,30. Following transplantation, the porcine islets have the potential to secrete insulin within minutes or hours31,32. However, the cost of housing the pig for a long period before pancreas excision, fragility of islets (making isolation difficult), and high cost of islet isolation are significant disadvantages31. In contrast, neonatal islet-like cell clusters (NICCs) and fetal porcine islet-like cell clusters (FICCs) are easy and inexpensive to isolate. They also have the potential for islet proliferation following transplantation33,34. Embryonic porcine islets, with their reduced immunogenicity, proliferative potential, and revascularization by host endothelium might provide a further advantage35. The main disadvantages of embryonic, fetal, and neonatal islet cell clusters is their delay in in vivo functioning after transplantation and the high expression of oligosaccharide galactose-α1,3-galactose (Gal), the major antigenic target for primate anti-pig antibodies. Expression of this antigen is much lower in adult islets36.
Table 1.
Comparison of Islets Isolated From Pigs of Different Ages*
| Embryonic | Fetal | Neonatal | Adult | |
|---|---|---|---|---|
| Isolation procedure | Simple | Simple | Simple | Difficult |
| Proliferation in vivo | Yes | Yes | Yes | Little |
| Insulin production | Delayed >3 months | Delayed >2 months | Delayed >1 month | Immediate |
| Gal expression | High | High | High | Low |
| Islet yield/pancreas (IEQs) | N/A | ~8,000 | 25,000––50,000 | 200,000––500,000 |
| β-Cells (% of islet cells) | N/A | 10% | 25% | >70% |
| Tumorigenicity | Possible | Low | Low | None |
| Risk of pathogen transmission | Low | Low | Low | Low |
| Cost | Low | Low | Low | High |
Choice of Pig Based on Strain
Islet quantity and function may vary with pig breed37. Despite several studies on the yield of high-quality islets from different strains, there is no consensus regarding the optimal pig strain for preclinical/clinical islet xenotransplantation.
High expression of extracellular matrix (ECM) proteins in islet capsules may make islet isolation easier, thus retaining healthier islets for transplantation. Expression of these proteins is higher in German Landrace pigs than in Deutsches Edelschwein pigs38. Hampshire and Duroc pigs have lower expression than Landrace and Pietrain pigs39. Older pigs express more ECM proteins than younger pigs38,40. How important this would be in moving toward clinical trials remains uncertain. Since the number of potentially transplantable islets is unlimited, this could compensate for any loss of islets during isolation, though clearly this would be less efficient and more expensive.
A high yield of islets was obtained from Chicago Medical School miniature pigs (now Seoul National University miniature pigs, Seoul, South Korea); the yield was higher than from other miniature pigs41. Islet yields from Landrace pigs were higher than German Landrace and Pietrain pigs42,43. Wuzhishan miniature pigs were considered to be a feasible source of islets as a much higher yield was obtained from this strain than from some market pigs27,44.
Choice of Pig Based on Genetic Engineering
In an attempt to protect islets from IBMIR and the primate innate and adaptive immune responses, various genetically engineered pigs have been developed (Table 2)27,30,45,46. These modifications have included gene knockout or gene knockdown, for example, knockout of the gene for the enzyme α1,3-galactosyltransferase (which adds Gal to the underlying oligosaccharides on the surface of the pig vascular endothelium) (GTKO pigs)47, knockout of the enzyme cytidine monophosphate-N-acetylneuraminic acid hydroxylase (which adds N-glycolylneuraminic acid)48,49, and knockout of the enzyme β1,4 N-acetylgalactosaminyltransferase (which adds N-acetylgalactosamine)50,51. Whether knockout of all of these genes will prove beneficial in porcine islet transplantation remains uncertain. These modifications have also included insertion of a human transgene, for example, for a human complement-regulatory protein (e.g., CD4624,52, CD5553–57, CD5958–60) or a human coagulation-regulatory protein (e.g., thrombomodulin61, endothelial protein C receptor62, tissue factor pathway inhibitor63, asialoglycoprotein receptor-164,65, CD3959, CD7366) to provide some protection from primate complement injury and coagulation dysfunction26,67. Manipulations have also aimed toward providing a local immunosuppressive effect by introducing a molecule that:
Table 2A.
Experience With the Xenotransplantation of Islets From Wild-Type Pigs in Immunosuppressed NHPs*
| Reference | Donor/Recipient | Immunosuppressive Therapy | Maximal Graft Survival (Days) |
|---|---|---|---|
| Hering et al., 200619 | Adult/CM | FTY720 + rapamycin + anti-IL-2R + anti-CD154 | >187 |
| Cardona et al., 200620 | Neonatal/rhesus monkey | CTLA4-Ig + rapamycin + anti-IL-2R + anti-CD154 | >260 |
| Thompson et al., 2011166 | Neonatal/rhesus monkey | CTLA4-Ig + rapamycin + anti-IL-2R + anti-CD40 | >203 |
| Thompson et al., 201222 | Neonatal/rhesus monkey | MMF + CTLA4-Ig + LFA-3-Ig + anti-IL-2R + anti-LFA-1 | 114 |
| Shin et al., 201523 | Adult/rhesus monkey | ATG + CVF + rapamycin + anti-TNF + anti-CD154 (+Treg) | >603 |
Table modified from Park et al.30.
-
1.
provides a T-cell costimulation blockade [e.g., cytotoxic T-lymphocyte antigen-4 immunoglobulin (CTLA4-Ig), LEA29Y (belatacept, a high-affinity variant of CTLA4-Ig)]68,69;
-
2.
suppresses the cellular immune response [e.g., major histocompatibility complex (MHC) class II transactivator knockdown (CIITA-DN)]70; MHC class 1 or class 2 knockout or knockdown71; insertion of human leukocyte antigen class I histocompatibility antigen, α chain E (HLA-E)72,73, HLA-G74,75, human leukocyte antigen Cw3 (HLA-Cw3)76, human β-D-mannoside β-1,4-N-acetylglucosaminyltransferase III (GnT-III)77, or human TNF-related apoptosis-inducing ligand (TRAIL)78;
-
3.
provides a local anti-inflammatory effect [e.g., by the introduction of a transgene for hemeoxygenase-1 (HO-1), A2079,80, or CD4781].
Recently, pigs with one or more genetic manipulations were produced with transgene expression being driven by an insulin promoter to specifically target pancreatic β-cells28. In a humanized mouse model, islets from insLEA29Y transgenic pigs demonstrated the potential to normalize glucose homeostasis and inhibit cellular rejection68. Transgenic expression of human complement-regulatory proteins (e.g., hCD46, hCD59, hCD5524,26,52,55,56,58,59) has been shown to provide significant protection against the primate humoral response. Expression of HO-1 can reduce islet apoptosis79. Knockout of pig tissue factor or overexpression of the human “anti-thrombotic” gene, CD39, reduces the effect of IBMIR and coagulation dysfunction82. Genetically “humanized” pigs exclusively expressing human insulin have been generated83.
Genetic Engineering to Increase Pig Insulin Production
Casu et al. reported that the metabolic demands on porcine islets in their natural host are significantly less than after their transplantation into a primate, particularly if the new host is a monkey rather than a human84. Nondiabetic cynomolgus monkeys show lower levels of fasting and stimulated blood glucose but higher levels of C-peptide and insulin than nondiabetic pigs. The reported levels in humans lie between those of monkeys and pigs85–88. Graham et al. reported that species incompatibilities in the pig-to-macaque islet xenotransplant model affect the translational and predictive value of pig-to-NHP islet transplant studies with regard to pig-to-human islet transplantation86,87. Developing approaches to improve insulin secretion may be beneficial or even necessary.
Genetic modification of the pig, even if this involves transgenes with an insulin promoter, does not appear to reduce porcine islet function further89,90. Increased insulin production by genetic modification may be a direction for the future. In vitro perifusion assays have shown that porcine islets exhibit a biphasic pattern of glucose-stimulated insulin secretion91–94, but isolated porcine islets secrete six to three times less insulin than human islets during the first and second phases after stimulation with 15 mM glucose. Insulin granule exocytosis triggered by glucose metabolism, and the ensuing rise in cytosolic calcium concentration, is regulated by two major amplifying pathways95,96. The first is a cyclic adenosine monophosphate (cAMP)-dependent pathway, which is activated physiologically by binding of glucagon-like peptide-1 (GLP-1) to its G protein-coupled receptor on β-cells. The second is a cholinergic pathway, which is activated by binding of acetylcholine or cholecystokinin to a type 3 muscarinic receptor. Both of these pathways, if efficiently activated, increase the number of readily releasable insulin granules in β-cells97 and result in a greater secretory response to glucose stimulation. Cooper et al. have demonstrated that islets coexpressing GLP-1 and activated muscarinic receptor type 3 have significantly improved insulin secretion98. The authors have suggested that permanently inducing these changes in porcine β-cells by means of genetic engineering might be a novel approach to increase insulin secretion from isolated porcine islets, bringing their secretory function closer to that of human islets and rendering them more efficient in controlling host glycemia in both preclinical and clinical trials without the need to transplant extremely high numbers of islets98.
The Potential Risk of Porcine Endogenous Retroviruses (PERVs)
The potential risk of the presence of PERVs in islets has long been discussed99–102. Even the use of islets from designated/specific pathogen-free (DPF) pigs will not eradicate PERVs, but their presence is currently not thought to be a major problem that would prevent clinical application99,100. No evidence of PERV transmission was found in patients with T1D after long-term follow-up after porcine islet transplantation27,103–106. The existence of PERV-C-free Auckland pigs that have been used in preclinical and clinical trials may possibly offer a solution107. If necessary, PERV activation could be suppressed by genetic manipulation108–110, and there is also the potential to knock out PERV111, though neither approach may be essential, as no PERV transmission has been documented in clinical trials of porcine islet transplantation112–117.
When all of these data are considered together, there is evidence that the ideal sources of islets might be those isolated from neonatal DPF pigs with specific genetic modifications to protect islets from IBMIR and innate and adaptive immune and inflammatory responses. However, exact choices of strain and genetic manipulation have not yet been conclusively identified.
Free or Encapsulated Islet Transplantation
Two major approaches have been explored in islet xenotransplantation, namely, (i) free (or naked) porcine islet transplantation, in which islets are transplanted without physical protection around them, and (ii) encapsulated islet transplantation, where islets are encased in some form of protective capsule or device. The purpose of encapsulation is to protect islets from the recipient's immune and inflammatory responses, yet allow insulin to be released. When free islets are transplanted, some form of exogenous pharmacologic immunosuppressive therapy is required to prevent rejection (unless this could be achieved entirely by genetic modification of islets, which is not currently possible).
Free Islet Transplantation
In current clinical practice, free allo-islets are delivered into the portal vasculature, and the liver has been proven to be a site associated with clinical success2,118. Islets are infused through a catheter placed into the portal vein under ultrasound or fluoroscopic guidance119,120. One disadvantage of the hepatic site is the low oxygen tension121, as hypoxia is an apoptosis-inducing signal in β-cells122. Furthermore, after infusion into the portal vein, IBMIR has been proven a major hurdle123,124.
IBMIR encompasses complement activation, coagulation activation, platelet activation and aggregation, proinflammatory cytokine/chemokine production, and infiltration of leukocytes (Fig. 1)125. Interventions directed against the various components of IBMIR reduce early graft loss but are far from completely successful in this respect124,126–128. Complement activation can be partially controlled by agents such as cobra venom factor or soluble complement receptor 1 (CR1)4,129 or C5a-blocking peptide130,131. Coagulation can be reduced by heparin infusion4,132,133, low-molecular-weight dextran134,135, melagatran136, or an anti-tissue factor antibody (CNTO859)137. Antiplatelet agents, such as tirofiban, can inhibit platelet activation/aggregation138. Developmental endothelial locus-1 (Del-1) downregulates the interaction between platelets and monocytes, thus reducing aggregation139. By decreasing tissue factor expression, pretreatment of por cine islets with nicotinamide can ameliorate IBMIR140,141.
Figure 1.
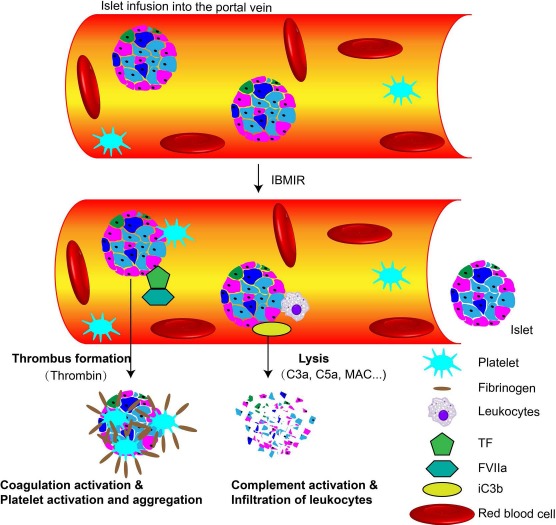
Overview of instant blood-mediated inflammatory reaction (IBMIR). The contact between blood and islets triggers the activation of coagulation that is mediated through tissue factor (TF). As a result, thrombin is generated, leading to fibrinogen deposition. Attachment of platelets to islets further increases the procoagulant. Complement (iC3b) is deposited on the islet surface, and C3a and C5a are activated, attract leukocytes, and promote formation of the membrane attack complex (MAC), which mediates the lysis of islets. FVIIa, activated coagulation factor VII.
However, several of these experimental agents cannot be used clinically, and so alternative agents (e.g., agents specifically targeting complement, such as compstatin) are under investigation142,143. The combination of antiplatelet and anticoagulant agents can, of course, increase the risk of bleeding, and therefore the patients (or NHPs) would require close monitoring.
Other strategies include the transplantation of islets from pigs overexpressing CD39144,145, human tissue factor pathway inhibitor146, human thrombomodulin147, or the knockout (or knockdown) of tissue factor148,149. Hawthorne et al. prevented IBMIR by using pig NICCs transgenic for human complement-regulatory proteins transplanted into baboons150.
Site of Implantation of Free Islets
Because of the loss of islets after their transplantation into the portal vein, other potential sites are being explored, though a perfectly hospitable site remains elusive (Table 3)151. Because the native pancreatic bed is relatively inaccessible, attempts have been made to deliver islet grafts at several other sites. Transplantation into the splenic vasculature resulted in significant morbidity, including infarction, rupture, and gastric perforation152. Although transplantation into the renal subcapsular space has become the gold standard for experimental purposes and for islet quality control in rodents, this site has not proved entirely successful in large animals or clinical studies, possibly because of a relative ischemia until revascularization takes place153,154. The intramuscular transplantation of pig NICCs into diabetic mice has been successful but has not yet been proven successful in large animal models155.
Table 2B.
Experience With the Xenotransplantation of Islets From Wild-Type Pigs in Nonimmunosuppressed NHPs*
| Reference | Donor/ Recipient | Immunoisolation/Site of Transplantation | Maximal Graft Survival (Days) |
|---|---|---|---|
| Sun et al., 199618 | Adult/CM | Alginate encapsulated/intraperitoneal | 804 |
| Dufrane et al., 201021 | Adult/CM | Alginate encapsulated/subcutaneous with monolayer device | >180 |
Table modified from Park et al.30.
The omental pouch is a viable site that offers a safe, convenient, and efficacious alternative to islet transplantation into the renal subcapsular in rodents156. The omentum offers good vascularization and drainage of the produced insulin into the portal vein for direct utilization in the liver151. The results of allotransplantation in NHP models using the omental pouch have been reported157. There is a multicenter trial ongoing with the BioHub system, using omentum as an alternative transplant site158. Compared with islet transplantation into the portal vein, islets in the omentum have to secrete insulin and release it into the portal vein, but the omentum is anatomically more similar to the pancreas151,159.
Transplantation into the submucosal space of the gastrointestinal tract can be achieved by endoscopy and offers the advantage of subsequent biopsy160–162, but clinical testing has been very limited to date163.
Bone marrow is currently being considered as an alternative site for islet transplantation. Studies in mice demonstrated that syngeneic islets could survive in bone marrow indefinitely with greater success in inducing normoglycemia compared to islets transplanted into the liver164,165.
Immunosuppressive Therapy Following the Transplantation of Free Islets
Free islet transplantation requires the administration of significant exogenous immunosuppressive therapy to prevent rejection, especially in the xenotransplant setting. Modulation of the CD40–CD154 pathway has been associated with encouraging results19,20,23–26,166. However, anti-CD154mAb is currently unlikely to be administered clinically in view of the associated risk of thromboembolic complications. The anti-CD40mAb Chi-220 has been reported to be effective in pig-to-NHP islet xenotransplantation, but it is a depleting antibody166. Another anti-CD40mAb, 2C10R4, is a nondepleting antibody that has shown success in preclinical islet allotransplantation167 and pig-to-NHP organ transplantation168–171, and so there is great potential for this mAb in clinical practice.
Other agents that might also contribute to successful suppression of the immune response are also being investigated, but efalizumab [anti-lymphocyte function-associated antigen-1 monoclonal antibody (anti-LFA-1mAb)] was also withdrawn from the market because of the occurrence of three cases of progressive multifocal leukoencephalopathy following a trial in patients with psoriasis172.
Transplantation of Immunoisolated Islets
Lifelong immunosuppressive therapy, as would be required after the transplantation of free porcine islets (unless immunological tolerance can be induced), might be accompanied by significant side effects or complications, and so islet transplantation that might not require such therapy is being explored. Cell immunoisolation by encapsulation in a semipermeable matrix to protect islets from immune cells is one such approach173,174. Encapsulated porcine islets have been transplanted to nonimmunosuppressed NHPs (Table 4)175,176.
Table 2C.
Experience With the Xenotransplantation of Islets From Genetically Engineered Pigs in NHPs (±Immunosuppressive Therapy)*
| Reference | Donor/Recipient | Immunosuppressive Therapy | Maximal Graft Survival (Days) |
|---|---|---|---|
| Mandel et al., 199756 | hCD55 fetal/CM | Cyclosporine + steroids + cyclophosphamide or brequinar | >40 |
| Komoda et al., 2005269 | GnT-III adult/CM | None | 5 |
| Van der Windt et al., 200924 | hCD46 adult/CM | MMF + ATG + anti-CD154mAb | >396 |
| Thompson et al., 201125 | GTKO neonatal/rhesus monkey | MMF + anti-CD154mAb + anti-LFA-lmAb + CTLA4-Ig | 249 |
| Chen et al., 201458 | GTKO/hCD55/hCD59/hHT neonatal/baboon | MMF + ATG + tacrolimus | 28 |
| Bottino et al., 201426 | Multitransgenic adult/CM | MMF + ATG + anti-CD154mAb | >365 |
Anti-LFA-1, anti-lymphocyte function-associated antigen-1 monoclonal antibody; ATG, antithymocyte globulin; CM, cynomolgus monkey; GnT-III, N-acetylglucosaminyltransferase III; hHT, human α(1,2)fucosyltransferase; MMF, mycophenolate mofetil
Table modified from Zhu et al.27.
Encapsulation entails coating cells or tissue in a semipermeable biocompatible material that allows for the entry of nutrients, oxygen, and hormones while blocking the entry of immune cells and, ideally, immune molecules (e.g., antibody, complement, cytokines, chemokines) that might recognize and destroy the islets177. Islet encapsulation requires the encapsulating material to have several properties, including (i) biocompatibility, (ii) immunoprotection (yet allowing insulin to be released through the capsule wall), and (iii) the ability to allow oxygen and nutrient diffusion into the capsule for islet survival178.
There are three main types of encapsulation systems: intravascular devices, microcapsules, and macrocapsules179, and there is also a relatively new technique—conformal coating (Table 5)175,180.
Table 3.
Comparison of Different Sites for Islet Transplantation*
| Liver | Renal Capsule | Spleen | Skin | Omentum | Gastric Submucosa | Pancreas | Muscle | |
|---|---|---|---|---|---|---|---|---|
| Efficacy of clinical trial | Good | Poor | Not reported | Poor | Not reported | Limited experience | Not reported | Limited experience |
| Patient safety | Safe | Safe | Safe | Safe | Safe | Safe | Possible pancreatitis | Safe |
| Oxygen tension | Low | Not reported | High | Low | Not reported | High | Not reported | Not reported |
| Vasculature | Rich | Poor | Not reported but probably rich | Poor | Rich | Rich | Not reported | Rich |
| Site of insulin released by the graft | Liver | Not reported | Portal vein | Systemic circulation | Portal vein | Portal vein | Not reported | Systemic circulation |
| Surgery | Invasive, some complications | Invasive | Invasive | Easy | Easy | Easy (endoscopy) | Difficult | Easy |
| IBMIR | Yes | Not reported | Yes | Not reported | Not reported | Not reported | Not reported | Not reported |
IBMIR, does IBMIR occur.
Table modified from van der Windt et al.151.
Table 5.
Devices for Encapsulation of Porcine Islets*
| ntravascular | Microcapsule | Macrocapsule | Conformal Coating | |
|---|---|---|---|---|
| Encapsulation material | PAN-PVC | Alginate with/without PLL/PLO coating, agarose, NaCS | Alginate, PTFE, acrylic copolymer, agarose, PSU, APCN membrane | PEG, alginate, heparin, or multilayer of biomaterials |
| Implantation site | Iliac vessels, APF, CV | Peritoneal cavity, subcutaneous, kidney subcapsular | Peritoneal cavity, subcutaneous | Intraportal, peritoneal cavity |
| Oxygen and nutrient supply | Good | Good | Poor | Good |
| Efficiency of insulin use | Rapid | Slow | Slow | Slow |
| Implantation surgery | Difficult | Easy | Easy | Easy |
| Retrieval surgery | Difficult | Difficult | Easy | Difficult |
| Graft volume | Large | Small | Large | Small |
APCN, amphiphilic conetwork; APF, arteria profunda femoris; CV, cubital vein; MCD, monolayer cellular device; NaCS, sodium cellulose sulfate; PAN-PVC, polyacrylonitrile-polyvinylchloride copolymer; PEG, polyethylene glycol; PLL, poly-L-lysine; PLO, poly-L-ornithine; PSU, polysulfone; PTFE, polytetrafluoroethylene.
Table modified from Zhu et al.175.
Intravascular and Extravascular Devices
Intravascular devices are islet-containing perfusion devices anastomosed to the vascular system as arteriovenous shunts179,181. Although this device ensures a rapid exchange of insulin and glucose, complications (e.g., thrombus formation, bleeding) associated with vascular prosthetic surgery potentially limit the therapeutic potential of this approach.
Extravascular devices can be categorized by their size into (i) microcapsules (150–1,000 μm) and (ii) macrocapsules (3 cm × 8 cm)182,183.
Microencapsulation
Microcapsules (Fig. 2A) offer better oxygen and nutrient transport because of higher surface area-to-volume ratio184,185. They require less complex manufacturing procedures and can be simply injected but are difficult to remove completely, if this becomes necessary 186. Alginate is commonly used to entrap islets187,188.
Figure 2.
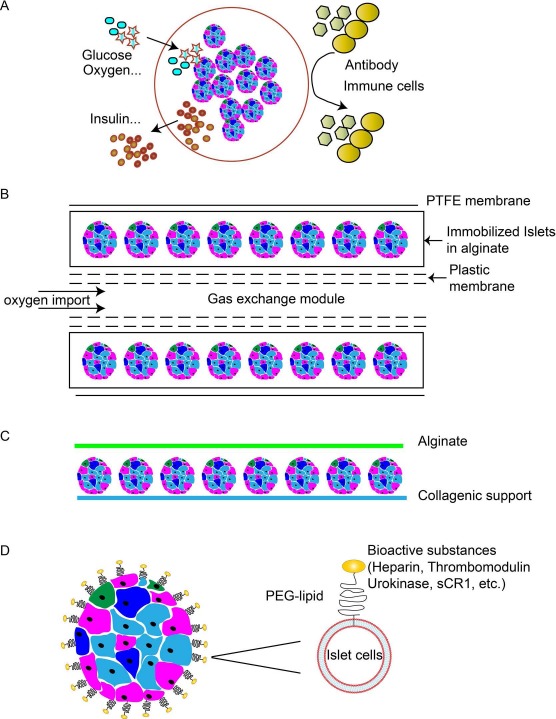
Examples of encapsulation systems and modification of islets. (A) Overview of microencapsulation. Microcapsules incorporate individual or small groups of islets in a spherical hydrogel polymer with a stable structure. (B) Beta-O2 macroencapsulation system. Islets immobilized within the alginate compartment, which is covered by a polytetrafluoroethylene (PTFE) membrane. Alginate and PTFE provide immune protection and facilitate neovascularization. The double-chambered bioreactor is connected to subcutaneous refueling ports through which an oxygen–CO2 mixture is delivered by daily injection. (C) Monolayer cellular device. The collagen support is covered by a mono/bilayer of porcine islets and embedded with alginate. (D) Conformal coating. Cell surfaces can be modified with amphiphilic polymers that interact both with the lipid membrane and the bioactive substance.
Xenotransplantation of microencapsulated porcine islets in an alginate matrix confirmed their biocompatibility and safety and reduced the insulin requirement in NHPs189,190. There has been one report of long-term survival (>9.5years) of some alginate-based microencapsulated FICCs transplanted into the peritoneal cavity in a T1D patient in 1996, though the patient's glucose level was not controlled191. A clinical trial of a microencapsulated porcine islet system (“Diabecell”) conducted by Living Cell Technologies Limited (Auckland, New Zealand) has been carried out (see below)192,193.
A nationally regulated clinical trial of intraperitoneal alginate-poly-L-ornithine-alginate (APA)-encapsulated NICCs (obtained from Diatranz Otsuka) was carried out in 14 nonimmunosuppressed diabetic patients in New Zealand194. The results, though not yet fully reported, suggest only partial graft function, with little impact on the clinical status of the patients98. A second clinical trial conducted in Argentina, although again not fully reported yet, demonstrated improved diabetic status of patients for more than 2 years98 (A. Abalovich and S. Matsumoto, unpublished data, personal communication). These pilot clinical trials have largely confirmed the safety of microencapsulated islets but have not yet convincingly confirmed their efficacy. Improvements in microcapsule design and fabrication, optimization of biomaterials and implantation site to facilitate oxygen transport, coupled with sufficient or renewable islets of high quality and low antigenicity may help to provide favorable results175.
Macroencapsulation
Macrocapsules can be implanted and removed with minimal risk, but oxygen and nutrient transport are limited185,195. Research on macrocapsules is focused on promoting neovascularization and providing sufficient oxygen and nutrition21,186,196–198. However, revascularization might be associated with the risk of rejection of islets.
A commercially available form of a macrocapsule is TheraCyte, which is made of bilayered polytetrafluoroethylene (PTFE)199–201. Either free or microencapsulated islets are placed in the membrane. NICCs in a TheraCyte system have reversed diabetes for up to 10 weeks in diabetic mice189. The TheraCyte system is impermeable to immune cells but permeable to antibodies and complement, which is a major limiting factor220. Furthermore, the beta-O2 implantable chamber has been created to offer an adequate oxygen supply to islets (Fig. 2B)196,202–203,204. Oxygen is supplied to islets via two ports connected to a gas reservoir integrated into the device202. Following structural improvements and successful application in large animals204,205, clinical evaluation of the beta-O2 device was initiated in eight patients by Beta-O2 Technologies in 2014. However, to ensure that adequate oxygen reaches the islets, the cell density within this device needs to be quite low and thus may be inadequate to sustain normoglycemia.
A monolayer made of alginate (adult porcine islets seeded as a monolayer on a human decellularized collagen matrix) (Fig. 2C)174 implanted subcutaneously demonstrated an ability to correct hyperglycemia for up to 6 months in diabetic monkeys without the need for immunosuppressive therapy21. However, no recent studies appear to have been published by this group.
Conformal Coating
Conformal coating (Fig. 2D)206 is a new approach to overcome the diffusion limitations associated with capsules of large size (>600 μm) by modification of the islet surface with polymerization [e.g., polyethylene glycol (PEG)] to form a thin coat (<50 μm)186,207–211. This approach allows transplantation into the portal vein, but the normoglycemia obtained has been very transient212–214. Improvements in the technology may allow coating of islets by molecules such as heparin, urokinase, or thrombomodulin132,186,209,215–219. The main drawback is the possible cytotoxicity of the compounds used. This strategy offers an opportunity to combine the inherent advantage of microencapsulation with conformal coating.
Site of Implantation of Encapsulated Islets
The microenvironment of the implant site plays a major role in the survival of encapsulated porcine islet xenografts. The intraperitoneal cavity has been the site of implantation most often, as there is less restriction of the volume of the grafts that can be implanted18,189,191,197,220–225. However, transplantation into the peritoneal cavity may aggravate hypoxia and inhibit the insulin-secretory response226. Furthermore, macrophages and lymphocytes in the peritoneum may be involved in the rapid degradation of the capsule223,227,228.
By contrast, the subcapsular kidney space (only suitable for microcapsules) and subcutaneous tissue (suitable for different encapsulation devices) have been associated with a weaker cellular response, better islet viability, and fewer broken capsules21,190,228. The subcutaneous space with prevascularization or cotransplantation of porcine islets and mesenchymal stem cells (MSCs) was reported to be associated with the promotion of neovascularization and reduced hypoxic stress198,229,230.
With the emergence of conformal coating and surface modification, the liver is being investigated as a potential site132,209,210,218. Other sites for microencapsulated or conformal-coated islet transplantation, such as muscle and bone marrow, have the advantage of good vascularization and relatively easy access164,231,232, but further studies are required.
Materials
The emergence of novel biocompatible encapsulation materials may promote progress in encapsulated islet transplantation. Such a membrane should be biocompatible and nondegradable and should allow the passage of insulin and glucose while preventing that of antibodies, lymphocytes, and toxic cytokines/chemokines. Materials such as alginate have resulted in various successful clinical applications and biocompatibility studies. A recent report indicated that a silicon nanopore membrane, designed with 7-nm-wide slit pores, protected encapsulated islets from cytokines, retained islet viability over 6 h, and islets remained responsive to changes in glucose levels233.
However, our opinion is that it will be difficult to develop a system that allows nutrients and oxygen to reach islets sufficiently and prevents islets from being damaged by antibodies, complement, and/or cytokines and chemokines.
Combined Islet and Mesenchymal Stem Cell And/Or Sertoli Cell Transplantation
Mesenchymal Stem Cells (MSCs)
MSCs are known to have regenerative, anti-inflammatory, and immunodulatory effects. There are extensive indicators that MSCs function satisfactorily across species234. MSCs might have considerable therapeutic potential in islet xenotransplantation. The cotransplantation of MSCs with adult porcine islets significantly improved islet vascularization and oxygenation198.
Porcine MSCs (pMSCs) express surface markers of MSCs but very low levels of swine leukocyte antigens (SLAs) and costimulatory molecules. pMSCs downregulate the human T-cell response to pig antigens as efficiently as do human MSCs235,236. They also have the ability to differentiate and help rebuild the vascular system after islet xenotransplantation237,238. In summary, pig or human MSCs have considerable potential in xenotransplantation. The ability to obtain pMSCs in very large numbers from adult pigs (from the adipose tissue or bone marrow) may prove a significant advantage over human MSCs.
Sertoli Cells (SCs)
Testicular SCs can secrete immunosuppressive factors, such as transforming growth factor-β1 (TGF-β1), which can inhibit lymphocyte proliferation239,240, prolong survival of transplanted islets241, promote β-cell replication242,243, and accelerate functional maturation and differentiation of neonatal porcine islets244. SCs exert a global immunosuppressive effect that extends across species barriers245. Cotransplantation of islets with SCs prolonged fish, rat, and porcine islet survival in mice246–248. Moreover, some islet survival might have been achieved following the cotransplantation of SCs with neonatal porcine islets in humans, though this study was not conclusive249.
In summary, SCs can prolong the survival time of islets during in vitro culture and promote vascularization of islets. The intravenous infusion of SCs can inhibit rejection of islet transplants. The combined transplantation of islets and SCs might attenuate both short-term and long-term loss of islet grafts250.
Induction of Immunological Tolerance
The induction of tolerance, if it could be achieved, would be particularly important after islet transplantation, as many young patients with T1D may require exogenous immunosuppressive drug therapy for decades if they are to remain normoglycemic. Tolerance induction could possibly have a dual protective effect, with the potential for inducing both tolerance to the islet graft and restoration of self-tolerance to prevent recurrence of autoimmunity251–254. Attempts have been made to induce a stable hematopoietic cell chimerism by the infusion of bone marrow to prolong islet graft survival252,255–257.
Previous Clinical Trials of Islet Xenotransplantation (Table 6)
Table 4.
Experience With the Xenotransplantation of Encapsulated Porcine Islets in Nonimmunosuppressed NHPs*
| Reference | Donor/Recipient | Device | Implant Site | Maximal Graft Survival | Clinical Outcome |
|---|---|---|---|---|---|
| Sun et al., 199618 | Adult/diabetic CM | Alginate-PLL-alginate | Peritoneal cavity | 120––803 days | 7/9 recipients achieved normoglycemia |
| Elliott et al., 2005189 | NICCs/nondiabetic CM | Alginate-PLO-alginate | Peritoneal cavity | >8 weeks | Insulin+ islets in retrieved capsules |
| Elliott et al., 2005224 | NICCs/diabetic CM | Alginate-PLO-alginate | Peritoneal cavity | >36 weeks | Reduced 16% insulin requirement |
| Dufrane et al., 2006190 | Adult/nondiabetic CM | High-M alginate | Kidney capsule | >180 days | Urine porcine C-peptide+ |
| Dufrane et al., 201021 | Adult/diabetic CM | Alginate MCD | Subcutaneous | >6 months | Diabetes correction >6 months |
| Veriter et al., 2014198 | Adult + pig MSCs/diabetic CM | Alginate MCD | Subcutaneous | >32 weeks | Diabetes correction >32 weeks |
CM, cynomolgus monkey; MCD, monolayer cellular device; MSC, mesenchymal stromal cells; PLL, poly-L-lysine; PLO, poly-L-ornithine; NICCs, neonatal islet-like clusters.
Table modified from Zhu et al.175.
Groth et al. first transplanted FICCs into the kidney subcapsular space of T1D patients in 1994258. NICCs were cotransplanted with SCs in a stainless steel chamber under the skin of patients in a study in Mexico; results showed some decrease in insulin requirement103,249. Some function of the transplanted cells, a low frequency of chronic complications, and no evidence of PERV activation were also reported by the same group in long-term follow-up of 23 patients with T1D after NICC transplantation in a device without exogenous immunosuppressive therapy104,105. The patient with long-term survival (>9.5 years) of microencapsulated FICCs into the peritoneal cavity, but without insulin independence, has been mentioned above191. In P.R. China, Wang et al. reported that NICCs were transplanted into the hepatic artery in 22 T1D patients who received clinically relevant immunosuppressive therapy; they provided evidence that some NICCs survived in 20 patients259.
Table 6.
Experience With Clinical Porcine Islet Transplantation*
| Reference | Donor | Implantation Site | Immunosuppressive Therapy | Maximal Graft Survival | Clinical Outcome |
|---|---|---|---|---|---|
| Groth et al., 1994258 | FICCs | Kidney capsule | CsA+prednisolone | 21 days | Plasma porcine C-peptide negative |
| Portal vein | CsA+prednisolone + ATG+ 15-deoxyspergualin | >460 days | Urine porcine C-peptide positive | ||
| Valdes-Gonzalez et al., 2005249 | NICCs + SCs (encapsulated) | Subcutaneous | None | >4 years | Insulin requirement reduced by 50% (in 50% of patients) |
| Elliott et al., 2007191 | FICCs (encapsulated) | Peritoneal cavity | None | >9 years | Insulin requirement reduced by 30% |
| Valdes-Gonzalez et al., 2007103 | NICCs + SCs (encapsulated) | Subcutaneous | None | >3 years | Insulin requirement reduced from 19––28 IU/day to 6 IU/day |
| Valdes-Gonzalez et al., 2010104 | NICCs (encapsulated) | Subcutaneous | None | >7.7 years | Insulin requirement reduced by 33% (in >50% of patients) |
| Wangetal., 2011259 | NICCs | Hepatic artery | CsA+MMF + prednisolone | >1 year | Insulin requirement reduced by 33%––62% |
| OKT-3 + tacrolimus + sirolimus + prednisolone | >1 year | Insulin requirement reduced 33%––62% | |||
| CsA+MMF None | Not available | Not available | |||
| Matsumoto et al., 2014194 | NICCs (encapsulated) | Peritoneal cavity | >52 weeks | 1/14 showed full graft function for a period of time |
ATG, anti-thymocyte globulin; CsA, cyclosporine; FICCs, fetal islet-like cell clusters; MMF, mycophenolate mofetil; NICCs, neonatal islet-like clusters; SCs, Sertoli cells.
Table modified from Rood et al.270.
Studies have been undertaken by Living Cell Technologies, a company that has carried out phase I/II clinical trials in Russia, Argentina, and New Zealand under approval from the local government health authorities. NICCs encapsulated with alginate and ornithine were transplanted into various groups of diabetic patients in each of those countries, but reports of the results have been less than comprehensive.
At this time, there have been no clinical islet xenotransplantation trials in which the protocols have been proven to be effective. This underpins the urgent need for preclinical studies in NHPs to prove the effectiveness and safety of the porcine islets and the treatment protocols.
Criteria for Future Clinical Trials
Suggested criteria to be fulfilled in clinical trials of islet xenotransplantation have been published by the International Xenotransplantation Association260,261. The World Health Organization (WHO) urged its members to embark on clinical trials only when the national health authority, in the country where the trial takes place, establishes effective national regulatory control and surveillance mechanisms. Subsequently, the WHO convened a WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials in Changsha, P.R. China, in 2008, and again in Geneva, Switzerland, in 2011262.
It was suggested that the pigs should be DPF and PERV-C negative. The porcine islet products should be isolated under current good manufacturing practice (cGMP) conditions using standard operating procedures (SOPs) with strict quality control263–265. Successful reversal of diabetes in four of six (or five of eight) consecutive NHPs with a minimum follow-up of 6 months was considered to be sufficient to indicate potential success of a clinical trial266. Prior analyses of microorganisms, recipient monitoring, and a response plan for preventing disease transmission needed to be well organized267.
Patient selection for a pilot clinical trial should be restricted to those with T1D or T2D complicated by impaired awareness of hypoglycemia and/or end-stage renal failure268. Informed consent should be obtained after informing the patients of the benefit–risk determination and postprotocol subject responsibilities261. The absence to date of reported in vivo transmission of PERV provides some confidence that well-planned pilot clinical trials could be safely undertaken.
Perspectives and Conclusions
The availability of organs and cells from deceased humans for transplantation does not meet the demand. Even though several obstacles remain before clinical islet xenotransplantation can become a therapeutic reality, significant progress has been made in the development of genetically engineered pigs, effective immunosuppressive regimens, immunoisolation techniques, and the establishment of guidelines for the conduct of clinical trials. Diabetic monkeys receiving exogenous immunosuppressive therapy in the form of T-cell costimulation blockade have remained normoglycemic and insulin independent after transplantation with porcine islets for >1 year. Novel genetically engineered pigs including those with manipulations to increase insulin production, the identification of sites for islet implantation where loss from IBMIR is reduced, and the cotransplantation of MSCs and/or SCs may advance the field further. Porcine islet xenotransplantation has been demonstrated to be a potentially successful strategy to achieve normoglycemia and prevent some of the complications of diabetes and may open a new avenue for the treatment of T1D.
Acknowledgments
Some of the authors of this work were supported in part by grants from Sanming Projects of Medicine in Shenzhen, Fund for High Level Medical Discipline Construction of Shenzhen (2016031638), Shenzhen Foundation of Science and Technology (Grant No. JCYJ20160229204849975), the Project of Shenzhen Engineering Center (GCZX2015043017281705), Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (2016A030306051), China Post doctoral Science Foundation (2015M580755), China Postdoctoral Science Special Foundation (2016T90813), Clinical Doctor-Basic Scientist Combination Foundation of Shenzhen Second People's Hospital, and Key Laboratory Project of Shenzhen Second People's Hospital. The authors declare no conflicts of interest.
References
- 1.Reichart B., Niemann H., Chavakis T., Denner J., Jaeckel E., Ludwig B., Marckmann G., Schnieke A., Schwinzer R., Seissler J., Tonjes R.R., Klymiuk N., Wolf E., Bornstein S.R. Xenotransplantation of porcine islet cells as a potential option for the treatment of type 1 diabetes in the future. Horm Metab Res. 2015; 47(1): 31–5. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L., Kneteman N.M., Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343(4): 230–8. [DOI] [PubMed] [Google Scholar]
- 3.Hering B.J., Clarke W.R., Bridges N.D., Eggerman T.L., Alejandro R., Bellin M.D., Chaloner K., Czarniecki C.W., Goldstein J.S., Hunsicker L.G., Kaufman D.B., Korsgren O., Larsen C.P., Luo X., Markmann J.F., Naji A., Oberholzer J., Posselt A.M., Rickels M.R., Ricordi C., Robien M.A., Senior P.A., Shapiro A.M., Stock P.G., Turgeon N.A., Clinical Islet Transplantation Consortium. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39(7): 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennet W., Sundberg B., Groth C.G., Brendel M.D., Brandhorst D., Brandhorst H., Bretzel R.G., Elgue G., Larsson R., Nilsson B., Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 1999; 48(10): 1907–14. [DOI] [PubMed] [Google Scholar]
- 5.Samols E., Stagner J.I., Ewart R.B., Marks V. The order of islet microvascular cellular perfusion is B—A—D in the perfused rat pancreas. J Clin Invest. 1988; 82(1): 350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stagner J.I., Samols E. Altered microcirculation and secretion in transplanted islets. Transplant Proc. 1994; 26(3): 1100–2. [PubMed] [Google Scholar]
- 7.Furuya H., Kimura T., Murakami M., Katayama K., Hirose K., Yamaguchi A. Revascularization and function of pancreatic islet isografts in diabetic rats following transplantation. Cell Transplant. 2003; 12(5): 537–44. [DOI] [PubMed] [Google Scholar]
- 8.Nyqvist D., Speier S., Rodriguez-Diaz R., Molano R.D., Lipovsek S., Rupnik M., Dicker A., Ilegems E., Zahr-Akrawi E., Molina J., Lopez-Cabeza M., Villate S., Abdulreda M.H., Ricordi C., Caicedo A., Pileggi A., Berggren P.O. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes 2011; 60(10): 2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narang A.S., Mahato R.I. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006; 58(2): 194–243. [DOI] [PubMed] [Google Scholar]
- 10.Pepper A.R., Gala-Lopez B., Ziff O., Shapiro A.M. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol. 2013; 2013: 352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Toro-Arreola A., Robles-Murillo A.K., Daneri-Navarro A., Rivas-Carrillo J.D. The role of endothelial cells on islet function and revascularization after islet transplantation. Organogenesis 2016; 12(1): 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2): 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millman J.R., Xie C., Van Dervort A., Gurtler M., Pagliuca F.W., Melton D.A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016; 7: 11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008; 455(7213): 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper D.K., Ezzelarab M.B., Hara H., Iwase H., Lee W., Wijkstrom M., Bottino R. The pathobiology of pig-to-primate xenotransplantation: A historical review. Xenotransplantation 2016; 23(2): 83–105. [DOI] [PubMed] [Google Scholar]
- 16.van der Windt D.J., Bottino R., Kumar G., Wijkstrom M., Hara H., Ezzelarab M., Ekser B., Phelps C., Murase N., Casu A and others. Clinical islet xenotransplantation: How close are we? Diabetes 2012; 61(12): 3046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottino R., Balamurugan A.N., Smetanka C., Bertera S., He J., Rood P.P., Cooper D.K., Trucco M. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation 2007; 14(1): 74–82. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Ma X., Zhou D., Vacek I., Sun A.M. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996; 98(6): 1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hering B.J., Wijkstrom M., Graham M.L., Hardstedt M., Aasheim T.C., Jie T., Ansite J.D., Nakano M., Cheng J., Li W and others. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006; 12(3): 301–3. [DOI] [PubMed] [Google Scholar]
- 20.Cardona K., Korbutt G.S., Milas Z., Lyon J., Cano J., Jiang W., Bello-Laborn H., Hacquoil B., Strobert E., Gangappa S and others. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006; 12(3): 304–6. [DOI] [PubMed] [Google Scholar]
- 21.Dufrane D., Goebbels R.M., Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 2010; 90(10): 1054–62. [DOI] [PubMed] [Google Scholar]
- 22.Thompson P., Badell I.R., Lowe M., Turner A., Cano J., Avila J., Azimzadeh A., Cheng X., Pierson R.N. 3rd, Johnson B., Robertson J., Song M., Leopardi F., Strobert E., Korbutt G., Rayat G., Rajotte R., Larsen C.P., Kirk A.D. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am J Transplant. 2012; 12(7): 1765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin J.S., Kim J.M., Kim J.S., Min B.H., Kim Y.H., Kim H.J., Jang J.Y., Yoon I.H., Kang H.J., Kim J., Hwang E.S., Lim D.G., Lee W.W., Ha J., Jung K.C., Park S.H., Kim S.J., Park C.G. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015; 15(11): 2837–50. [DOI] [PubMed] [Google Scholar]
- 24.van der Windt D.J., Bottino R., Casu A., Campanile N., Smetanka C., He J., Murase N., Hara H., Ball S., Loveland B.E., Ayares D., Lakkis F.G., Cooper D.K., Trucco M. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009; 9(12): 2716–26. [DOI] [PubMed] [Google Scholar]
- 25.Thompson P., Badell I.R., Lowe M., Cano J., Song M., Leopardi F., Avila J., Ruhil R., Strobert E., Korbutt G., Rayat G., Rajotte R., Iwakoshi N., Larsen C.P., Kirk A.D. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011; 11(12): 2593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottino R., Wijkstrom M., van der Windt D.J., Hara H., Ezzelarab M., Murase N., Bertera S., He J., Phelps C., Ayares D., Cooper D.K., Trucco M. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014; 14(10): 2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H.T., Yu L., Lyu Y., Wang B. Optimal pig donor selection in islet xenotransplantation: Current status and future perspectives. J Zhejiang Univ Sci B 2014; 15(8): 681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaraju S., Bottino R., Wijkstrom M., Hara H., Trucco M., Cooper D.K. Islet xenotransplantation from genetically engineered pigs. Curr Opin Organ Transplant. 2013; 18(6): 695–702. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraju S., Bottino R., Wijkstrom M., Trucco M., Cooper D.K. Islet xenotransplantation: What is the optimal age of the islet-source pig? Xenotransplantation 2015; 22(1): 7–19. [DOI] [PubMed] [Google Scholar]
- 30.Park C.G., Bottino R., Hawthorne W.J. Current status of islet xenotransplantation. Int J Surg. 2015; 23(Pt B): 261–6. [DOI] [PubMed] [Google Scholar]
- 31.Dufrane D., D'Hoore W., Goebbels R.M., Saliez A., Guiot Y., Gianello P. Parameters favouring successful adult pig islet isolations for xenotransplantation in pig-to-primate models. Xenotransplantation 2006; 13(3): 204–14. [DOI] [PubMed] [Google Scholar]
- 32.O'Neil J.J., Stegemann J.P., Nicholson D.T., Gagnon K.A., Solomon B.A., Mullon C.J. The isolation and function of porcine islets from market weight pigs. Cell Transplant. 2001; 10(3): 235–46. [DOI] [PubMed] [Google Scholar]
- 33.Rajotte R.V. Isolation and assessment of islet quality. Xenotransplantation 2008; 15(2): 93–5. [DOI] [PubMed] [Google Scholar]
- 34.Korbutt G.S., Elliott J.F., Ao Z., Smith D.K., Warnock G.L., Rajotte R.V. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996; 97(9): 2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht G., Eventov-Friedman S., Rosen C., Shezen E., Tchorsh D., Aronovich A., Freud E., Golan H., El-Hasid R., Katchman H., Hering B.J., Zung A., Kra-Oz Z., Shaked-Mishan P., Yusim A., Shtabsky A., Idelevitch P., Tobar A., Harmelin A., Bachar-Lustig E., Reisner Y. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci USA 2009; 106(21): 8659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayat G.R., Rajotte R.V., Hering B.J., Binette T.M., Korbutt G.S. In vitro and in vivo expression of Galalpha-(1,3) Gal on porcine islet cells is age dependent. J Endocrinol. 2003; 177(1): 127–35. [DOI] [PubMed] [Google Scholar]
- 37.Prabhakaran S., Hering B.J. What strain of pig should be used? Xenotransplantation 2008; 15(2): 83–6. [DOI] [PubMed] [Google Scholar]
- 38.Meyer T., Buhler C., Czub S., Beutner U., Otto C., Thiede A., Ulrichs K. Selection of donor pigs for pancreatic islet transplantation may depend on the expression level of connective tissue proteins in the islet capsule. Transplant Proc. 1998; 30(5): 2471–3. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhof N., Hering B.J., Geiss V., Federlin K., Bretzel R.G. Evidence for breed-dependent differences in porcine islets of Langerhans. Transplant Proc. 1994; 26(2): 616–7. [PubMed] [Google Scholar]
- 40.Meyer T., Czub S., Chodnewska I., Beutner U., Hamelmann W., Klock G., Zimmermann U., Thiede A., Ulrichs K. Expression pattern of extracellular matrix proteins in the pancreas of various domestic pig breeds, the Goettingen minipig and the wild boar. Ann Transplant. 1997; 2(3): 17–26. [PubMed] [Google Scholar]
- 41.Kim H.I., Lee S.Y., Jin S.M., Kim K.S., Yu J.E., Yeom S.C., Yoon T.W., Kim J.H., Ha J., Park C.G., Kim S.J. Parameters for successful pig islet isolation as determined using specific-pathogen-free miniature pigs. Xenotransplantation 2009; 16(1): 11–8. [DOI] [PubMed] [Google Scholar]
- 42.Cavanagh T.J., Lakey J.R., Wright M.J., Albertson T., Wile K., Fetterhoff T.J. Identification of a pig strain with maximal islet mass. Transplant Proc. 1998; 30(2): 368. [DOI] [PubMed] [Google Scholar]
- 43.Heiser A., Ulrichs K., Muller-Ruchholtz W. Influence of porcine strain, age, and pH of the isolation medium on porcine pancreatic islet isolation success. Transplant Proc. 1994; 26(2): 618–20. [PubMed] [Google Scholar]
- 44.Jiang X., Qian T., Linn T., Cao L., Xiang G., Wang Y., Peng H., Xue P., Zhang L., Chen D., Yang X. Islet isolation and purification from inbred Wuzhishan miniature pigs. Xenotransplantation 2012; 19(3): 159–65. [DOI] [PubMed] [Google Scholar]
- 45.Cooper D.K., Ekser B., Ramsoondar J., Phelps C., Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016; 238(2): 288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozzi E., White D.J. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995; 1(9): 964–6. [DOI] [PubMed] [Google Scholar]
- 47.Phelps C.J., Koike C., Vaught T.D., Boone J., Wells K.D., Chen S.H., Ball S., Specht S.M., Polejaeva I.A., Monahan J.A., Jobst P.M., Sharma S.B., Lamborn A.E., Garst A.S., Moore M., Demetris A.J., Rudert W.A., Bottino R., Bertera S., Trucco M., Starzl T.E., Dai Y., Ayares D.L. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003; 299(5605): 411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padler-Karavani V., Varki A. Potential impact of the nonhuman sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011; 18(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutz A.J., Li P., Estrada J.L., Sidner R.A., Chihara R.K., Downey S.M., Burlak C., Wang Z.Y., Reyes L.M., Ivary B., Yin F., Blakenship R.L., Paris L.L., Tector A.J. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013; 20(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 50.Estrada J.L., Martens G., Li P., Adams A., Newell K.A., Ford M.L., Butler J.R., Sidner R., Tector M., Tector J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 2015; 22(3): 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrne G.W., Du Z., Stalboerger P., Kogelberg H., McGregor C.G. Cloning and expression of porcine beta1, 4 N-acetylgalactosaminyl transferase encoding a new xeno-reactive antigen. Xenotransplantation 2014; 21(6): 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diamond L.E., Quinn C.M., Martin M.J., Lawson J., Platt J.L., Logan J.S. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation 2001; 71(1): 132–42. [DOI] [PubMed] [Google Scholar]
- 53.Rosengard A.M., Cary N.R., Langford G.A., Tucker A.W., Wallwork J., White D.J. Tissue expression of human complement inhibitor, decay-accelerating factor, in transgenic pigs. A potential approach for preventing xenograft rejection. Transplantation 1995; 59(9): 1325–33. [PubMed] [Google Scholar]
- 54.Cary N., Moody J., Yannoutsos N., Wallwork J., White D. Tissue expression of human decay accelerating factor, a regulator of complement activation expressed in mice: A potential approach to inhibition of hyperacute xenograft rejection. Transplant Proc. 1993; 25(1 Pt 1): 400–1. [PubMed] [Google Scholar]
- 55.Liu D., Kobayashi T., Onishi A., Furusawa T., Iwamoto M., Suzuki S., Miwa Y., Nagasaka T., Maruyama S., Kadomatsu K., Uchida K., Nakao A. Relation between human decay-accelerating factor (hDAF) expression in pig cells and inhibition of human serum anti-pig cytotoxicity: Value of highly expressed hDAF for xenotransplantation. Xenotransplantation 2007; 14(1): 67–73. [DOI] [PubMed] [Google Scholar]
- 56.Mandel T.E., Koulmanda M., Cozzi E., Waterworth P., Tolan M., Langford G., White D.J. Transplantation of normal and DAF-transgenic fetal pig pancreas into cynomolgus monkeys. Transplant Proc. 1997; 29(1–2/01): 940. [DOI] [PubMed] [Google Scholar]
- 57.Storck M., Abendroth D., Prestel R., Pino-Chavez G., Muller-Hoker J., White D.J., Hammer C. Morphology of hDAF (CD55) transgenic pig kidneys following ex-vivo hemoperfusion with human blood. Transplantation 1997; 63(2): 304–10. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y., Stewart J.M., Gunthart M., Hawthorne W.J., Salvaris E.J., O'Connell P.J., Nottle M.B., d'Apice A.J., Cowan P.J., Kearns-Jonker M. Xenoantibody response to porcine islet cell transplantation using GTKO, CD55, CD59, and fucosyltransferase multiple transgenic donors. Xenotransplantation 2014; 21(3): 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Bas-Bernardet S., Tillou X., Poirier N., Dilek N., Chatelais M., Devalliere J., Charreau B., Minault D., Hervouet J., Renaudin K., Crossan C., Scobie L., Cowan P.J., d'Apice A.J., Galli C., Cozzi E., Soulillou J.P., Vanhove B., Blancho G. Xenotransplantation of galactosyl-transferase knockout, CD55, CD59, CD39, and fucosyl-transferase transgenic pig kidneys into baboons. Transplant Proc. 2011; 43(9): 3426–30. [DOI] [PubMed] [Google Scholar]
- 60.Kroshus T.J., Bolman R.M. 3rd, Dalmasso A.P., Rollins S.A., Guilmette E.R., Williams B.L., Squinto S.P., Fodor W.L. Expression of human CD59 in transgenic pig organs enhances organ survival in an ex vivo xenogeneic perfusion model. Transplantation 1996; 61(10): 1513–21. [DOI] [PubMed] [Google Scholar]
- 61.Miwa Y., Yamamoto K., Onishi A., Iwamoto M., Yazaki S., Haneda M., Iwasaki K., Liu D., Ogawa H., Nagasaka T., Uchida K., Nakao A., Kadomatsu K., Kobayashi T. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation 2010; 17(1): 26–37. [DOI] [PubMed] [Google Scholar]
- 62.Petersen B., Ramackers W., Tiede A., Lucas-Hahn A., Herrmann D., Barg-Kues B., Schuettler W., Friedrich L., Schwinzer R., Winkler M., Niemann H. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation 2009; 16(6): 486–95. [DOI] [PubMed] [Google Scholar]
- 63.Lee K.F., Salvaris E.J., Roussel J.C., Robson S.C., d'Apice A.J., Cowan P.J. Recombinant pig TFPI efficiently regulates human tissue factor pathways. Xenotransplantation 2008; 15(3): 191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paris L.L., Estrada J.L., Li P., Blankenship R.L., Sidner R.A., Reyes L.M., Montgomery J.B., Burlak C., Butler J.R., Downey S.M., Wang Z.Y., Tector M., Tector A.J. Reduced human platelet uptake by pig livers deficient in the asialoglycoprotein receptor 1 protein. Xenotransplantation 2015; 22(3): 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bongoni A.K., Kiermeir D., Denoyelle J., Jenni H., Burlak C., Seebach J.D., Vogelin E., Constantinescu M.A., Rieben R. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplantation 2015; 99(4): 693–701. [DOI] [PubMed] [Google Scholar]
- 66.Kaniewska E., Sielicka A., Sarathchandra P., Pelikant-Malecka I., Olkowicz M., Slominska E.M., Chester A.H., Yacoub M.H., Smolenski R.T. Immunohistochemical and functional analysis of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and ecto-5′-nucleotidase (CD73) in pig aortic valves. Nucleosides Nucleotides Nucleic Acids 2014; 33(4–6): 305–12. [DOI] [PubMed] [Google Scholar]
- 67.Bottino R., Nagaraju S., Satyananda V., Hara H., Wijkstrom M., Massimo T., Cooper D.K. Potential for clinical pancreatic islet xenotransplantation. Transplant Res Risk Manag. 2014; 6: 79–86. [Google Scholar]
- 68.Klymiuk N., van Buerck L., Bahr A., Offers M., Kessler B., Wuensch A., Kurome M., Thormann M., Lochner K., Nagashima H., Herbach N., Wanke R., Seissler J., Wolf E. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 2012; 61(6): 1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelps C.J., Ball S.F., Vaught T.D., Vance A.M., Mendicino M., Monahan J.A., Walters A.H., Wells K.D., Dandro A.S., Ramsoondar J.J., Cooper D.K., Ayares D.L. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 2009; 16(6): 477–85. [DOI] [PubMed] [Google Scholar]
- 70.Hara H., Witt W., Crossley T., Long C., Isse K., Fan L., Phelps C.J., Ayares D., Cooper D.K., Dai Y., Starzl T.E. Human dominant-negative class II transactivator transgenic pigs—Effect on the human anti-pig T-cell immune response and immune status. Immunology 2013; 140(1): 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reyes L.M., Estrada J.L., Wang Z.Y., Blosser R.J., Smith R.F., Sidner R.A., Paris L.L., Blankenship R.L., Ray C.N., Miner A.C., Tector M., Tector A.J. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014; 193(11): 5751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forte P., Baumann B.C., Weiss E.H., Seebach J.D. HLA-E expression on porcine cells: Protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005; 5(9): 2085–93. [DOI] [PubMed] [Google Scholar]
- 73.Weiss E.H., Lilienfeld B.G., Muller S., Muller E., Herbach N., Kessler B., Wanke R., Schwinzer R., Seebach J.D., Wolf E., Brem G. HLA-E/human beta2-microglobulin transgenic pigs: Protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 2009; 87(1): 35–43. [DOI] [PubMed] [Google Scholar]
- 74.Esquivel E.L., Maeda A., Eguchi H., Asada M., Sugiyama M., Manabe C., Sakai R., Matsuura R., Nakahata K., Okuyama H., Miyagawa S. Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl Immunol. 2015; 32(2): 109–15. [DOI] [PubMed] [Google Scholar]
- 75.Forte P., Pazmany L., Matter-Reissmann U.B., Stussi G., Schneider M.K., Seebach J.D. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001; 167(10): 6002–8. [DOI] [PubMed] [Google Scholar]
- 76.Seebach J.D., Comrack C., Germana S., LeGuern C., Sachs D.H., DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997; 159(7): 3655–61. [PubMed] [Google Scholar]
- 77.Miyagawa S., Murakami H., Takahagi Y., Nakai R., Yamada M., Murase A., Koyota S., Koma M., Matsunami K., Fukuta D., Fujimura T., Shigehisa T., Okabe M., Nagashima H., Shirakura R., Taniguchi N. Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. J Biol Chem. 2001; 276(42): 39310–9. [DOI] [PubMed] [Google Scholar]
- 78.Klose R., Kemter E., Bedke T., Bittmann I., Kelsser B., Endres R., Pfeffer K., Schwinzer R., Wolf E. Expression of biologically active human TRAIL in transgenic pigs. Transplantation 2005; 80(2): 222–30. [DOI] [PubMed] [Google Scholar]
- 79.Yeom H.J., Koo O.J., Yang J., Cho B., Hwang J.I., Park S.J., Hurh S., Kim H., Lee E.M., Ro H., Kang J.T., Kim S.J., Won J.K., O'Connell P.J., Kim H., Surh C.D., Lee B.C., Ahn C. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS One 2012; 7(10): e46646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oropeza M., Petersen B., Carnwath J.W., Lucas-Hahn A., Lemme E., Hassel P., Herrmann D., Barg-Kues B., Holler S., Queisser A.L., Schwinzer R., Hinkel R., Kupatt C., Niemann H. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation 2009; 16(6): 522–34. [DOI] [PubMed] [Google Scholar]
- 81.Tena A., Kurtz J., Leonard D.A., Dobrinsky J.R., Terlouw S.L., Mtango N., Verstegen J., Germana S., Mallard C., Arn J.S., Sachs D.H., Hawley R.J. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014; 14(12): 2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekser B., Cooper D.K. Overcoming the barriers to xenotransplantation: Prospects for the future. Expert Rev Clin Immunol. 2010; 6(2): 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y., Wang K., Wu H., Jin Q., Ruan D., Ouyang Z., Zhao B., Liu Z., Zhao Y., Zhang Q., Fan N., Liu Q., Guo S., Bu L., Fan Y., Sun X., Li X., Lai L. Genetically humanized pigs exclusively expressing human insulin are generated through custom endonuclease-mediated seamless engineering. J Mol Cell Biol. 2016; 8(2): 174–7. [DOI] [PubMed] [Google Scholar]
- 84.Casu A., Bottino R., Balamurugan A.N., Hara H., van der Windt D.J., Campanile N., Smetanka C., Cooper D.K., Trucco M. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: Implications for translation into clinical practice. Diabetologia 2008; 51(1): 120–9. [DOI] [PubMed] [Google Scholar]
- 85.Greenspan F.S., Gardner D.G. Normal hormone reference ranges. In: Greenspan F.S., Gardner D.G., editors. Basic and clinical endocrinology, 7th ed. New York (NY): McGraw-Hill; 2006. p. 920–38. [Google Scholar]
- 86.Graham M.L., Bellin M.D., Papas K.K., Hering B.J., Schuurman H.J. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: A comparison with allotransplantation. Xenotransplantation 2011; 18(6): 328–42. [DOI] [PubMed] [Google Scholar]
- 87.Graham M.L., Schuurman H.J. The usefulness and limitations of the diabetic macaque model in evaluating long-term porcine islet xenograft survival. Xenotransplantation 2013; 20(1): 5–17. [DOI] [PubMed] [Google Scholar]
- 88.Mueller K.R., Balamurugan A.N., Cline G.W., Pongratz R.L., Hooper R.L., Weegman B.P., Kitzmann J.P., Taylor M.J., Graham M.L., Schuurman H.J., Papas K.K. Differences in glucose-stimulated insulin secretion in vitro of islets from human, nonhuman primate, and porcine origin. Xenotransplantation 2013; 20(2): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Casu A., Echeverri G.J., Bottino R., van der Windt D.J., He J., Ekser B., Ball S., Ayares D., Cooper D.K. Insulin secretion and glucose metabolism in alpha 1,3-galactosyltransferase knock-out pigs compared to wild-type pigs. Xenotransplantation 2010; 17(2): 131–9. [DOI] [PubMed] [Google Scholar]
- 90.Wijkstrom M., Bottino R., Iwase H., Hara H., Ekser B., van der Windt D., Long C., Toledo F.G., Phelps C.J., Trucco M., Cooper D.K., Ayares D. Glucose metabolism in pigs expressing human genes under an insulin promoter. Xenotransplantation 2015; 22(1): 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bertuzzi F., Zacchetti D., Berra C., Socci C., Pozza G., Pontiroli A.E., Grohovaz F. Intercellular Ca2+ waves sustain coordinate insulin secretion in pig islets of Langerhans. FEBS Lett. 1996; 379(1): 21–5. [DOI] [PubMed] [Google Scholar]
- 92.Krickhahn M., Meyer T., Buhler C., Thiede A., Ulrichs K. Highly efficient isolation of porcine islets of Langerhans for xenotransplantation: Numbers, purity, yield and in vitro function. Ann Transplant. 2001; 6(3): 48–54. [PubMed] [Google Scholar]
- 93.Dufrane D., Nenquin M., Henquin J.C. Nutrient control of insulin secretion in perifused adult pig islets. Diabetes Metab. 2007; 33(6): 430–8. [DOI] [PubMed] [Google Scholar]
- 94.Mueller K.R., Balamurugan A.N., Cline G.W., Pongratz R.L., Hooper R.L., Weegman B.P., Kitzmann J.P., Taylor M.J., Graham M.L., Schuurman H.J., Papas K.K. Differences in glucose-stimulated insulin secretion in vitro of islets from human, nonhuman primate, and porcine origin. Xenotransplantation 2013; 20(2): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mourad N.I., Nenquin M., Henquin J.C. cAMP-mediated and metabolic amplification of insulin secretion are distinct pathways sharing independence of beta-cell microfilaments. Endocrinology 2012; 153(10): 4644–54. [DOI] [PubMed] [Google Scholar]
- 96.Mourad N.I., Nenquin M., Henquin J.C. Amplification of insulin secretion by acetylcholine or phorbol ester is independent of beta-cell microfilaments and distinct from metabolic amplification. Mol Cell Endocrinol. 2013; 367(1–2): 11–20. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y., Gillis K.D. A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol. 2004; 124(6): 641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper D.K., Matsumoto S., Abalovich A., Itoh T., Mourad N., Gianello P.R., Wolf E., Cozzi E. Progress in clinical encapsulated islet xenotransplantation. Transplantation 2016; 100(11): 2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Laan L.J., Lockey C., Griffeth B.C., Frasier F.S., Wilson C.A., Onions D.E., Hering B.J., Long Z., Otto E., Torbett B.E., Salomon D.R. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 2000; 407(6800): 90–4. [DOI] [PubMed] [Google Scholar]
- 100.Mueller N.J., Takeuchi Y., Mattiuzzo G., Scobie L. Microbial safety in xenotransplantation. Curr Opin Organ Transplant. 2011; 16(2): 201–6. [DOI] [PubMed] [Google Scholar]
- 101.Paradis K., Langford G., Long Z., Heneine W., Sandstrom P., Switzer W.M., Chapman L.E., Lockey C., Onions D., Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science 1999; 285(5431): 1236–41. [DOI] [PubMed] [Google Scholar]
- 102.Blusch J.H., Patience C., Martin U. Pig endogenous retroviruses and xenotransplantation. Xenotransplantation 2002; 9(4): 242–51. [DOI] [PubMed] [Google Scholar]
- 103.Valdes-Gonzalez R.A., White D.J., Dorantes L.M., Teran L., Garibay-Nieto G.N., Bracho-Blanchet E., Davila-Perez R., Evia-Viscarra L., Ormsby C.E., Ayala-Sumuano J.T., Silva-Torres M.L., Ramirez-Gonzalez B. Three-yr follow-up of a type 1 diabetes mellitus patient with an islet xenotransplant. Clin Transplant. 2007; 21(3): 352–7. [DOI] [PubMed] [Google Scholar]
- 104.Valdes-Gonzalez R., Rodriguez-Ventura A.L., White D.J., Bracho-Blanchet E., Castillo A., Ramirez-Gonzalez B., Lopez-Santos M.G., Leon-Mancilla B.H., Dorantes L.M. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010; 162(3): 537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valdes-Gonzalez R., Dorantes L.M., Bracho-Blanchet E., Rodriguez-Ventura A., White D.J. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long-term porcine islet xenotransplantation. J Med Virol. 2010; 82(2): 331–4. [DOI] [PubMed] [Google Scholar]
- 106.Wynyard S., Nathu D., Garkavenko O., Denner J., Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation 2014; 21(4): 309–23. [DOI] [PubMed] [Google Scholar]
- 107.Garkavenko O., Wynyard S., Nathu D., Simond D., Muzina M., Muzina Z., Scobie L., Hector R.D., Croxson M.C., Tan P., Elliot B.R. Porcine endogenous retrovirus (PERV) and its transmission characteristics: A study of the New Zealand designated pathogen-free herd. Cell Transplant. 2008; 17(12): 1381–8. [DOI] [PubMed] [Google Scholar]
- 108.Ramsoondar J., Vaught T., Ball S., Mendicino M., Monahan J., Jobst P., Vance A., Duncan J., Wells K., Ayares D. Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation 2009; 16(3): 164–80. [DOI] [PubMed] [Google Scholar]
- 109.Dieckhoff B., Karlas A., Hofmann A., Kues W.A., Petersen B., Pfeifer A., Niemann H., Kurth R., Denner J. Inhibition of porcine endogenous retroviruses (PERVs) in primary porcine cells by RNA interference using lentiviral vectors. Arch Virol. 2007; 152(3): 629–34. [DOI] [PubMed] [Google Scholar]
- 110.Dieckhoff B., Petersen B., Kues W.A., Kurth R., Niemann H., Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 2008; 15(1): 36–45. [DOI] [PubMed] [Google Scholar]
- 111.Yang L., Guell M., Niu D., George H., Lesha E., Grishin D., Aach J., Shrock E., Xu W., Poci J., Cortazio R., Wilkinson R.A., Fishman J.A., Church G. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015; 350(6264): 1101–4. [DOI] [PubMed] [Google Scholar]
- 112.Morozov V.A., Wynyard S., Matsumoto S., Abalovich A., Denner J., Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2016; 227: 34–40. [DOI] [PubMed] [Google Scholar]
- 113.Denner J. Is porcine endogenous retrovirus (PERV) transmission still relevant? Transplant Proc. 2008; 40(2): 587–9. [DOI] [PubMed] [Google Scholar]
- 114.Hermida-Prieto M., Domenech N., Moscoso I., Diaz T., Ishii J., Salomon D.R., Manez R. Lack of cross-species transmission of porcine endogenous retrovirus (PERV) to transplant recipients and abattoir workers in contact with pigs. Transplantation 2007; 84(4): 548–50. [DOI] [PubMed] [Google Scholar]
- 115.Loss M., Arends H., Winkler M., Przemeck M., Steinhoff G., Rensing S., Kaup F.J., Hedrich H.J., Winkler M.E., Martin U. Analysis of potential porcine endogenous retrovirus (PERV) transmission in a whole-organ xenotransplantation model without interfering microchimerism. Transpl Int. 2001; 14(1): 31–7. [DOI] [PubMed] [Google Scholar]
- 116.Nyberg S. Cross-species transmission of PERV appears unlikely. Liver Transpl. 2000; 6(1): 115–17. [DOI] [PubMed] [Google Scholar]
- 117.Birmingham K. FDA subcommittee finds no evidence of PERV transmission. Nat Med. 1999; 5(8): 855. [DOI] [PubMed] [Google Scholar]
- 118.Ryan E.A., Lakey J.R., Paty B.W., Imes S., Korbutt G.S., Kneteman N.M., Bigam D., Rajotte R.V., Shapiro A.M. Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes 2002; 51(7): 2148–57. [DOI] [PubMed] [Google Scholar]
- 119.Goss J.A., Soltes G., Goodpastor S.E., Barth M., Lam R., Brunicardi F.C., Froud T., Alejandro R., Ricordi C. Pancreatic islet transplantation: The radiographic approach. Transplantation 2003; 76(1): 199–203. [DOI] [PubMed] [Google Scholar]
- 120.Owen R.J., Ryan E.A., O'Kelly K., Lakey J.R., McCarthy M.C., Paty B.W., Bigam D.L., Kneteman N.M., Korbutt G.S., Rajotte R.V., Shapiro A.M. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: Radiologic aspects. Radiology 2003; 229(1): 165–70. [DOI] [PubMed] [Google Scholar]
- 121.Merani S., Shapiro A.M. Current status of pancreatic islet transplantation. Clin Sci (Lond) 2006; 110(6): 611–25. [DOI] [PubMed] [Google Scholar]
- 122.Emamaullee J.A., Rajotte R.V., Liston P., Korneluk R.G., Lakey J.R., Shapiro A.M., Elliott J.F. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes 2005; 54(9): 2541–8. [DOI] [PubMed] [Google Scholar]
- 123.Korsgren O., Nilsson B., Berne C., Felldin M., Foss A., Kallen R., Lundgren T., Salmela K., Tibell A., Tufveson G. Current status of clinical islet transplantation. Transplantation 2005; 79(10): 1289–93. [DOI] [PubMed] [Google Scholar]
- 124.Rood P.P., Bottino R., Balamurugan A.N., Smetanka C., Ayares D., Groth C.G., Murase N., Cooper D.K., Trucco M. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation 2007; 83(2): 202–10. [DOI] [PubMed] [Google Scholar]
- 125.Kourtzelis I., Magnusson P.U., Kotlabova K., Lambris J.D., Chavakis T. Regulation of instant blood mediated inflammatory reaction (IBMIR) in pancreatic islet xenotransplantation: Points for therapeutic interventions. Adv Exp Med Biol. 2015; 865: 171–88. [DOI] [PubMed] [Google Scholar]
- 126.van der Windt D.J., Bottino R., Casu A., Campanile N., Cooper D.K. Rapid loss of intraportally transplanted islets: An overview of pathophysiology and preventive strategies. Xenotransplantation 2007; 14(4): 288–97. [DOI] [PubMed] [Google Scholar]
- 127.van der Windt D.J., Marigliano M., He J., Votyakova T.V., Echeverri G.J., Ekser B., Ayares D., Lakkis F.G., Cooper D.K., Trucco M., Bottino R. Early islet damage after direct exposure of pig islets to blood: Has humoral immunity been underestimated? Cell Transplant. 2012; 21(8): 1791–802. [DOI] [PubMed] [Google Scholar]
- 128.Nagaraju S., Bertera S., Tanaka T., Hara H., Rayat G.R., Wijkstrom M., Ayares D., Trucco M., Cooper D.K., Bottino R. In vitro exposure of pig neonatal isletlike cell clusters to human blood. Xenotransplantation 2015; 22(4): 317–24. [DOI] [PubMed] [Google Scholar]
- 129.Bennet W., Sundberg B., Lundgren T., Tibell A., Groth C.G., Richards A., White D.J., Elgue G., Larsson R., Nilsson B., Korsgren O. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: Protective effects of sCR1 and heparin. Transplantation 2000; 69(5): 711–9. [DOI] [PubMed] [Google Scholar]
- 130.Tokodai K., Goto M., Inagaki A., Nakanishi W., Okada N., Okada H., Satomi S. C5a-inhibitory peptide combined with gabexate mesilate prevents the instant blood-mediated inflammatory reaction in a rat model of islet transplantation. Transplant Proc. 2010; 42(6): 2102–3. [DOI] [PubMed] [Google Scholar]
- 131.Tokodai K., Goto M., Inagaki A., Nakanishi W., Ogawa N., Satoh K., Kawagishi N., Sekiguchi S., Nilsson B., Okada N., Okada H., Satomi S. Attenuation of cross-talk between the complement and coagulation cascades by C5a blockade improves early outcomes after intraportal islet transplantation. Transplantation 2010; 90(12): 1358–65. [DOI] [PubMed] [Google Scholar]
- 132.Cabric S., Sanchez J., Lundgren T., Foss A., Felldin M., Kallen R., Salmela K., Tibell A., Tufveson G., Larsson R., Korsgren O., Nilsson B. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes 2007; 56(8): 2008–15. [DOI] [PubMed] [Google Scholar]
- 133.Cabric S., Eich T., Sanchez J., Nilsson B., Korsgren O., Larsson R. A new method for incorporating functional heparin onto the surface of islets of Langerhans. Tissue Eng Part C Methods 2008; 14(2): 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johansson H., Goto M., Dufrane D., Siegbahn A., Elgue G., Gianello P., Korsgren O., Nilsson B. Low molecular weight dextran sulfate: A strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006; 6(2): 305–12. [DOI] [PubMed] [Google Scholar]
- 135.Goto M., Johansson H., Maeda A., Elgue G., Korsgren O., Nilsson B. Low-molecular weight dextran sulfate abrogates the instant blood-mediated inflammatory reaction induced by adult porcine islets both in vitro and in vivo. Transplant Proc. 2004; 36(4): 1186–7. [DOI] [PubMed] [Google Scholar]
- 136.Ozmen L., Ekdahl K.N., Elgue G., Larsson R., Korsgren O., Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: Possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002; 51(6): 1779–84. [DOI] [PubMed] [Google Scholar]
- 137.Berman D.M., Cabrera O., Kenyon N.M., Miller J., Tam S.H., Khandekar V.S., Picha K.M., Soderman A.R., Jordan R.E., Bugelski P.J., Horninger D., Lark M., Davis J.E., Alejandro R., Berggren P.O., Zimmerman M., O'Neil J.J., Ricordi C., Kenyon N.S. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation 2007; 84(3): 308–15. [DOI] [PubMed] [Google Scholar]
- 138.Akima S., Hawthorne W.J., Favaloro E., Patel A., Blyth K., Mudaliar Y., Chapman J.R., O'Connell P.J. Tirofiban and activated protein C synergistically inhibit the instant blood mediated inflammatory reaction (IBMIR) from allogeneic islet cells exposure to human blood. Am J Transplant. 2009; 9(7): 1533–40. [DOI] [PubMed] [Google Scholar]
- 139.Kourtzelis I., Kotlabova K., Lim J.H., Mitroulis I., Ferreira A., Chen L.S., Gercken B., Steffen A., Kemter E., Klotzschevon Ameln A., Waskow C., Hosur K., Chatzigeorgiou A., Ludwig B., Wolf E., Hajishengallis G., Chavakis T. Developmental endothelial locus-1 modulates platelet-monocyte interactions and instant blood-mediated inflammatory reaction in islet transplantation. Thromb Haemost. 2016; 115(4): 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moberg L., Olsson A., Berne C., Felldin M., Foss A., Kallen R., Salmela K., Tibell A., Tufveson G., Nilsson B., Korsgren O. Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: Implications for clinical islet transplantation. Transplantation 2003; 76(9): 1285–8. [DOI] [PubMed] [Google Scholar]
- 141.Jung D.Y., Park J.B., Joo S.Y., Joh J.W., Kwon C.H., Kwon G.Y., Kim S.J. Effect of nicotinamide on early graft failure following intraportal islet transplantation. Exp Mol Med. 2009; 41(11): 782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goto M., Tjernberg J., Dufrane D., Elgue G., Brandhorst D., Ekdahl K.N., Brandhorst H., Wennberg L., Kurokawa Y., Satomi S., Lambris J.D., Gianello P., Korsgren O., Nilsson B. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation 2008; 15(4): 225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qu H., Ricklin D., Bai H., Chen H., Reis E.S., Maciejewski M., Tzekou A., DeAngelis R.A., Resuello R.R., Lupu F., Barlow P.N., Lambris J.D. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immuno biology 2013; 218(4): 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanthi Y.M., Sutton N.R., Pinsky D.J. CD39: Interface between vascular thrombosis and inflammation. Curr Atheroscler Rep. 2014; 16(7): 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dwyer K.M., Mysore T.B., Crikis S., Robson S.C., Nandurkar H., Cowan P.J., D'Apice A.J. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation 2006; 82(3): 428–32. [DOI] [PubMed] [Google Scholar]
- 146.Ayares D., Phelps C., Vaught T., Ball S., Monahan J., Walters A., Giraldo A., Bertera S., van der Windt D., Wijkstrom M., Cooper D.K.C., Bottino R., Trucco M. Multi-transgenic pigs for xenoislet transplantation. Xenotransplantation 2013; 20(1): 46. [Google Scholar]
- 147.Wuensch A., Baehr A., Bongoni A.K., Kemter E., Blutke A., Baars W., Haertle S., Zakhartchenko V., Kurome M., Kessler B., Faber C., Abicht J.M., Reichart B., Wanke R., Schwinzer R., Nagashima H., Rieben R., Ayares D., Wolf E., Klymiuk N. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplantation 2014; 97(2): 138–47. [DOI] [PubMed] [Google Scholar]
- 148.Ji M., Yi S., Smith-Hurst H., Phillips P., Wu J., Hawthorne W., O'Connell P. The importance of tissue factor expression by porcine NICC in triggering IBMIR in the xenograft setting. Transplantation 2011; 91(8): 841–6. [DOI] [PubMed] [Google Scholar]
- 149.Ma X., Ye B., Gao F., Liang Q., Dong Q., Liu Y., Rong P., Wang W., Yi S. Tissue factor knockdown in porcine islets: An effective approach to suppressing the instant blood-mediated inflammatory reaction. Cell Transplant. 2012; 21(1): 61–71. [DOI] [PubMed] [Google Scholar]
- 150.Hawthorne W.J., Salvaris E.J., Phillips P., Hawkes J., Liuwantara D., Burns H., Barlow H., Stewart A.B., Peirce S.B., Hu M., Lew A.M., Robson S.C., Nottle M.B., D'Apice A.J., O'Connell P.J., Cowan P.J. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am J Transplant. 2014; 14(6): 1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van der Windt D.J., Echeverri G.J., Ijzermans J.N., Cooper D.K. The choice of anatomical site for islet transplantation. Cell Transplant. 2008; 17(9): 1005–14. [DOI] [PubMed] [Google Scholar]
- 152.White S.A., London N.J., Johnson P.R., Davies J.E., Pollard C., Contractor H.H., Hughes D.P., Robertson G.S., Musto P.P., Dennison A.R. The risks of total pancreatectomy and splenic islet autotransplantation. Cell Transplant. 2000; 9(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 153.Hesse U.J., Sutherland D.E., Gores P.F., Sitges-Serra A., Najarian J.S. Comparison of splenic and renal subcapsular islet autografting in dogs. Transplantation 1986; 41(2): 271–4. [DOI] [PubMed] [Google Scholar]
- 154.Kaufman D.B., Morel P., Field M.J., Munn S.R., Sutherland D.E. Purified canine islet autografts. Functional outcome as influenced by islet number and implantation site. Transplantation 1990; 50(3): 385–91. [PubMed] [Google Scholar]
- 155.Wolf-van Buerck L., Schuster M., Baehr A., Mayr T., Guethoff S., Abicht J., Reichart B., Nam-Apostolopoulos Y.C., Klymiuk N., Wolf E., Seissler J. Engraftment and reversal of diabetes after intramuscular transplantation of neonatal porcine islet-like clusters. Xenotransplantation 2015; 22(6): 443–50. [DOI] [PubMed] [Google Scholar]
- 156.Kin T., Korbutt G.S., Rajotte R.V. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003; 3(3): 281–5. [DOI] [PubMed] [Google Scholar]
- 157.Berman D.M., O'Neil J.J., Coffey L.C., Chaffanjon P.C., Kenyon N.M., Ruiz P. Jr, Pileggi A., Ricordi C., Kenyon N.S. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009; 9(1): 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Diabetes Research Institute Foundation. Hollywood (FL). A ‘mini-organ’ delivering real insulin in real time; 2017. Available from https://www.diabetesresearch.org/BioHub.
- 159.Rajab A. Islet transplantation: Alternative sites. Curr Diab Rep. 2010; 10(5): 332–7. [DOI] [PubMed] [Google Scholar]
- 160.Echeverri G.J., McGrath K., Bottino R., Hara H., Dons E.M., van der Windt D.J., Ekser B., Casu A., Houser S., Ezzelarab M., Wagner R., Trucco M., Lakkis F.G., Cooper D.K. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): Technique and initial results in diabetic pigs. Am J Transplant. 2009; 9(11): 2485–96. [DOI] [PubMed] [Google Scholar]
- 161.Fujita M., McGrath K.M., Bottino R., Dons E.M., Long C., Kumar G., Ekser B., Echeverri G.J., Hata J., Haruma K., Cooper D.K., Hara H. Technique of endoscopic biopsy of islet allografts transplanted into the gastric submucosal space in pigs. Cell Transplant. 2013; 22(12): 2335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tanaka T., Fujita M., Bottino R., Piganelli J.D., McGrath K., Li J., Lee W., Iwase H., Wijkstrom M., Bertera S., Long C., Landsittel D., Haruma K., Cooper D.K., Hara H. Endoscopic biopsy of islet transplants in the gastric submucosal space provides evidence of islet graft rejection in diabetic pigs. Islets 2016; 8(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tian X.H., Xue W.J., Ding X.M., Pang X.L., Teng Y., Tian P.X., Feng X.S. Small intestinal submucosa improves islet survival and function during in vitro culture. World J Gastroenterol. 2005; 11(46): 7378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meier R.P., Seebach J.D., Morel P., Mahou R., Borot S., Giovannoni L., Parnaud G., Montanari E., Bosco D., Wandrey C., Berney T., Buhler L.H., Muller Y.D. Survival of free and encapsulated human and rat islet xenografts transplanted into the mouse bone marrow. PLoS One 2014; 9(3): e91268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Meier R.P.H., Sun P., Gerber-Lemaire S., Berney T., Muller Y.D. Pancreatic islet transplantation into the bone marrow. CellR4 2016; 4(3). [Google Scholar]
- 166.Thompson P., Cardona K., Russell M., Badell I.R., Shaffer V., Korbutt G., Rayat G.R., Cano J., Song M., Jiang W., Strobert E., Rajotte R., Pearson T., Kirk A.D., Larsen C.P. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011; 11(5): 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lowe M., Badell I.R., Thompson P., Martin B., Leopardi F., Strobert E., Price A.A., Abdulkerim H.S., Wang R., Iwakoshi N.N., Adams A.B., Kirk A.D., Larsen C.P., Reimann K.A. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012; 12(8): 2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mohiuddin M.M., Singh A.K., Corcoran P.C., Hoyt R.F., Thomas M.L. 3rd, Lewis B.G., Eckhaus M., Reimann K.A., Klymiuk N., Wolf E., Ayares D., Horvath K.A. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014; 14(2): 488–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iwase H., Ekser B., Satyananda V., Bhama J., Hara H., Ezzelarab M., Klein E., Wagner R., Long C., Thacker J., Li J., Zhou H., Jiang M., Nagaraju S., Zhou H., Veroux M., Bajona P., Wijkstrom M., Wang Y., Phelps C., Klymiuk N., Wolf E., Ayares D., Cooper D.K. Pig-to-baboon heterotopic heart transplantation—Exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015; 22(3): 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iwase H., Liu H., Wijkstrom M., Zhou H., Singh J., Hara H., Ezzelarab M., Long C., Klein E., Wagner R., Phelps C., Ayares D., Shapiro R., Humar A., Cooper D.K. Pig kidney graft survival in a baboon for 136 days: Longest life-supporting organ graft survival to date. Xenotransplantation 2015; 22(4): 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iwase H., Kobayashi T. Current status of pig kidney xenotransplantation. Int J Surg. 2015; 23(Pt B): 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gadzia J., Turner J. Progressive multifocal leukoencephalopathy in two psoriasis patients treated with efalizumab. J Drugs Dermatol. 2010; 9(8): 1005–9. [PubMed] [Google Scholar]
- 173.Klomp G.F., Ronel S.H., Hashiguchi H., D'Andrea M., Dobelle W.H. Hydrogels for encapsulation of pancreatic islet cells. Trans Am Soc Artif Intern Organs 1979; 25: 74–6. [DOI] [PubMed] [Google Scholar]
- 174.Gianello P. Macroencapsulated pig islets correct induced diabetes in primates up to 6 months. Adv Exp Med Biol. 2015; 865: 157–70. [DOI] [PubMed] [Google Scholar]
- 175.Zhu H.T., Lu L., Liu X.Y., Yu L., Lyu Y., Wang B. Treatment of diabetes with encapsulated pig islets: An update on current developments. J Zhejiang Univ Sci B 2015; 16(5): 329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Markmann J.F., Bartlett S.T., Johnson P., Korsgren O., Hering B.J., Scharp D., Kay T.W., Bromberg J., Odorico J.S., Weir G.C., Bridges N., Kandaswamy R., Stock P., Friend P., Gotoh M., Cooper D.K., Park C.G., O'Connell P.J., Stabler C., Matsumoto S., Ludwig B., Choudhary P., Khovatchev B., Rickels M.R., Sykes M., Wood K., Kraemer K., Hwa A., Stanley E., Ricordi C., Zimmerman M., Greenstein J., Montanya E., Otonkoski T. Executive summary of IPITA-TTS opinion leaders report on the future of beta-cell replacement. Transplantation 2016; 100(7): e25–31. [DOI] [PubMed] [Google Scholar]
- 177.Krishnan R., Alexander M., Robles L., Foster C.E. 3rd, Lakey J.R. Islet and stem cell encapsulation for clinical transplantation. Rev Diabet Stud. 2014; 11(1): 84–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Veriter S., Mergen J., Goebbels R.M., Aouassar N., Gregoire C., Jordan B., Leveque P., Gallez B., Gianello P., Dufrane D. In vivo selection of biocompatible alginates for islet encapsulation and subcutaneous transplantation. Tissue Eng Part A 2010; 16(5): 1503–13. [DOI] [PubMed] [Google Scholar]
- 179.Lanza R.P., Hayes J.L., Chick W.L. Encapsulated cell technology. Nat Biotechnol. 1996; 14(9): 1107–11. [DOI] [PubMed] [Google Scholar]
- 180.Bartlett S.T., Markmann J.F., Johnson P., Korsgren O., Hering B.J., Scharp D., Kay T.W., Bromberg J., Odorico J.S., Weir G.C., Bridges N., Kandaswamy R., Stock P., Friend P., Gotoh M., Cooper D.K., Park C.G., O'Connell P., Stabler C., Matsumoto S., Ludwig B., Choudhary P., Kovatchev B., Rickels M.R., Sykes M., Wood K., Kraemer K., Hwa A., Stanley E., Ricordi C., Zimmerman M., Greenstein J., Montanya E., Otonkoski T. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(Suppl 2): S1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Borg D.J., Bonifacio E. The use of biomaterials in islet transplantation. Curr Diab Rep. 2011; 11(5): 434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buder B., Alexander M., Krishnan R., Chapman D.W., Lakey J.R. Encapsulated islet transplantation: Strategies and clinical trials. Immune Netw. 2013; 13(6): 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.de Vos P., Spasojevic M., Faas M.M. Treatment of diabetes with encapsulated islets. Adv Exp Med Biol. 2010; 670: 38–53. [DOI] [PubMed] [Google Scholar]
- 184.van Schilfgaarde R., de Vos P. Factors influencing the properties and performance of microcapsules for immunoprotection of pancreatic islets. J Mol Med. (Berl) 1999; 77(1): 199–205. [DOI] [PubMed] [Google Scholar]
- 185.Beck J., Angus R., Madsen B., Britt D., Vernon B., Nguyen K.T. Islet encapsulation: Strategies to enhance islet cell functions. Tissue Eng. 2007; 13(3): 589–99. [DOI] [PubMed] [Google Scholar]
- 186.Scharp D.W., Marchetti P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014; 67-68: 35–73. [DOI] [PubMed] [Google Scholar]
- 187.de Vos P., Lazarjani H.A., Poncelet D., Faas M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014; 67-68: 15–34. [DOI] [PubMed] [Google Scholar]
- 188.Orlando G., Gianello P., Salvatori M., Stratta R.J., Soker S., Ricordi C., Dominguez-Bendala J. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes 2014; 63(5): 1433–44. [DOI] [PubMed] [Google Scholar]
- 189.Elliott R.B., Escobar L., Calafiore R., Basta G., Garkavenko O., Vasconcellos A., Bambra C. Transplantation of micro-and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005; 37(1): 466–9. [DOI] [PubMed] [Google Scholar]
- 190.Dufrane D., Goebbels R.M., Saliez A., Guiot Y., Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: Proof of concept. Transplantation 2006; 81(9): 1345–53. [DOI] [PubMed] [Google Scholar]
- 191.Elliott R.B., Escobar L., Tan P.L., Muzina M., Zwain S., Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007; 14(2): 157–61. [DOI] [PubMed] [Google Scholar]
- 192.Tan P.L. Company profile: Tissue regeneration for diabetes and neurological diseases at Living Cell Technologies. Regen Med. 2010; 5(2): 181–7. [DOI] [PubMed] [Google Scholar]
- 193.Living Cell Technologies. Development to date: DIABECELL is currently in late-stage clinical trials; 2017. Available from http://www.lctglobal.com/products/diabecell/development-to-date.
- 194.Matsumoto S., Tan P., Baker J., Durbin K., Tomiya M., Azuma K., Doi M., Elliott R.B. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc. 2014; 46(6): 1992–5. [DOI] [PubMed] [Google Scholar]
- 195.Weir G.C. Islet encapsulation: Advances and obstacles. Diabetologia 2013; 56(7): 1458–61. [DOI] [PubMed] [Google Scholar]
- 196.Barkai U., Weir G.C., Colton C.K., Ludwig B., Bornstein S.R., Brendel M.D., Neufeld T., Bremer C., Leon A., Evron Y., Yavriyants K., Azarov D., Zimermann B., Maimon S., Shabtay N., Balyura M., Rozenshtein T., Vardi P., Bloch K., de Vos P., Rotem A. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013; 22(8): 1463–76. [DOI] [PubMed] [Google Scholar]
- 197.Grundfest-Broniatowski S.F., Tellioglu G., Rosenthal K.S., Kang J., Erdodi G., Yalcin B., Cakmak M., Drazba J., Bennett A., Lu L., Kennedy J.P. A new bioartificial pancreas utilizing amphiphilic membranes for the immunoisolation of porcine islets: A pilot study in the canine. ASAIO J. 2009; 55(4): 400–5. [DOI] [PubMed] [Google Scholar]
- 198.Veriter S., Gianello P., Igarashi Y., Beaurin G., Ghyselinck A., Aouassar N., Jordan B., Gallez B., Dufrane D. Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates. Cell Transplant. 2014; 23(11): 1349–64. [DOI] [PubMed] [Google Scholar]
- 199.Sorenby A.K., Kumagai-Braesch M., Sharma A., Hultenby K.R., Wernerson A.M., Tibell A.B. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: Studies in a rodent model. Transplantation 2008; 86(2): 364–6. [DOI] [PubMed] [Google Scholar]
- 200.Malavasi N.V., Rodrigues D.B., Chammas R., Chura-Chambi R.M., Barbuto J.A., Balduino K., Nonogaki S., Morganti L. Continuous and high-level in vivo delivery of endostatin from recombinant cells encapsulated in TheraCyte immunoisolation devices. Cell Transplant. 2010; 19(3): 269–77. [DOI] [PubMed] [Google Scholar]
- 201.Kirk K., Hao E., Lahmy R., Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014; 12(3): 807–14. [DOI] [PubMed] [Google Scholar]
- 202.Ludwig B., Zimerman B., Steffen A., Yavriants K., Azarov D., Reichel A., Vardi P., German T., Shabtay N., Rotem A., Evron Y., Neufeld T., Mimon S., Ludwig S., Brendel M.D., Bornstein S.R., Barkai U. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010; 42(13): 918–22. [DOI] [PubMed] [Google Scholar]
- 203.Reichart B., Guethoff S., Brenner P., Poettinger T., Wolf E., Ludwig B., Kind A., Mayr T., Abicht J.M. Xenotransplantation of cells, tissues, organs and the German Research Foundation Transregio Collaborative Research Centre 127. Adv Exp Med Biol. 2015; 865: 143–55. [DOI] [PubMed] [Google Scholar]
- 204.Ludwig B., Reichel A., Steffen A., Zimerman B., Schally A.V., Block N.L., Colton C.K., Ludwig S., Kersting S., Bonifacio E., Solimena M., Gendler Z., Rotem A., Barkai U., Bornstein S.R. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA 2013; 110(47): 19054–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neufeld T., Ludwig B., Barkai U., Weir G.C., Colton C.K., Evron Y., Balyura M., Yavriyants K., Zimermann B., Azarov D., Maimon S., Shabtay N., Rozenshtein T., Lorber D., Steffen A., Willenz U., Bloch K., Vardi P., Taube R., de Vos P., Lewis E.C., Bornstein S.R., Rotem A. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One 2013; 8(8): e70150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Teramura Y., Asif S., Ekdahl K.N., Nilsson B. Cell surface engineering for regulation of immune reactions in cell therapy. Adv Exp Med Biol. 2015; 865: 189–209. [DOI] [PubMed] [Google Scholar]
- 207.Cruise G.M., Hegre O.D., Scharp D.S., Hubbell J.A. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998; 57(6): 655–65. [DOI] [PubMed] [Google Scholar]
- 208.Sefton M.V., May M.H., Lahooti S., Babensee J.E. Making microencapsulation work: Conformal coating, immobilization gels and in vivo performance. J Control Release 2000; 65(1–2): 173–86. [DOI] [PubMed] [Google Scholar]
- 209.Teramura Y., Iwata H. Islets surface modification prevents blood-mediated inflammatory responses. Bioconjug Chem. 2008; 19(7): 1389–95. [DOI] [PubMed] [Google Scholar]
- 210.Teramura Y., Iwata H. Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation. Transplantation 2009; 88(5): 624–30. [DOI] [PubMed] [Google Scholar]
- 211.Tomei A.A., Manzoli V., Fraker C.A., Giraldo J., Velluto D., Najjar M., Pileggi A., Molano R.D., Ricordi C., Stabler C.L., Hubbell J.A. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci USA 2014; 111(29): 10514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jeong J.H., Yook S., Hwang J.W., Jung M.J., Moon H.T., Lee D.Y., Byun Y. Synergistic effect of surface modification with poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat. Transplant Proc. 2013; 45(2): 585–90. [DOI] [PubMed] [Google Scholar]
- 213.Lee D.Y., Park S.J., Nam J.H., Byun Y. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: Long-functioning PEGylated islets in vivo. Tissue Eng. 2006; 12(3): 615–23. [DOI] [PubMed] [Google Scholar]
- 214.Jung Y.S., Jeong J.H., Yook S., Im B.H., Seo J., Hong S.W., Park J.B., Yang V.C., Lee D.Y., Byun Y. Surface modification of pancreatic islets using heparin-DOPA conjugate and anti-CD154 mAb for the prolonged survival of intrahepatic transplanted islets in a xenograft model. Biomaterials 2012; 33(1): 295–303. [DOI] [PubMed] [Google Scholar]
- 215.Wilson J.T., Cui W., Chaikof E.L. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008; 8(7): 1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wilson J.T., Cui W., Kozlovskaya V., Kharlampieva E., Pan D., Qu Z., Krishnamurthy V.R., Mets J., Kumar V., Wen J., Song Y., Tsukruk W., Chaikof E.L. Cell surface engineering with polyelectrolyte multilayer thin films. J Am Chem Soc. 2011; 133(18): 7054–64. [DOI] [PubMed] [Google Scholar]
- 217.Gattas-Asfura K.M., Stabler C.L. Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyper-branched polymers. ACS Appl Mater Interfaces 2013; 5(20): 9964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Luan N.M., Iwata H. Inhibition of instant blood-mediated inflammatory responses by co-immobilization of sCR1 and heparin on islets. Biomaterials 2013; 34(21): 5019–24. [DOI] [PubMed] [Google Scholar]
- 219.Chen H., Teramura Y., Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid. J Control Release 2011; 150(2): 229–34. [DOI] [PubMed] [Google Scholar]
- 220.Veriter S., Gianello P., Dufrane D. Bioengineered sites for islet cell transplantation. Curr Diab Rep. 2013; 13(5): 745–55. [DOI] [PubMed] [Google Scholar]
- 221.Lanza R.P., Butler D.H., Borland K.M., Staruk J.E., Faustman D.L., Solomon B.A., Muller T.E., Rupp R.G., Maki T., Monaco A.P., Chick W.L. Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppression. Proc Natl Acad Sci USA 1991; 88(24): 11100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kin T., Iwata H., Aomatsu Y., Ohyama T., Kanehiro H., Hisanaga M., Nakajima Y. Xenotransplantation of pig islets in diabetic dogs with use of a microcapsule composed of agarose and polystyrene sulfonic acid mixed gel. Pancreas 2002; 25(1): 94–100. [DOI] [PubMed] [Google Scholar]
- 223.Omer A., Keegan M., Czismadia E., De Vos P., Van Rooijen N., Bonner-Weir S., Weir G.C. Macrophage depletion improves survival of porcine neonatal pancreatic cell clusters contained in alginate macrocapsules transplanted into rats. Xenotransplantation 2003; 10(3): 240–51. [DOI] [PubMed] [Google Scholar]
- 224.Elliott R.B., Escobar L., Tan P.L., Garkavenko O., Calafiore R., Basta P., Vasconcellos A.V., Emerich D.F., Thanos C., Bambra C. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc. 2005; 37(8): 3505–8. [DOI] [PubMed] [Google Scholar]
- 225.Vinerean H.V., Gazda L.S., Hall R.D., Rubin A.L., Smith B.H. Improved glucose regulation on a low carbohydrate diet in diabetic rats transplanted with macroencapsulated porcine islets. Cell Transplant. 2008; 17(5): 567–75. [DOI] [PubMed] [Google Scholar]
- 226.de Groot M., Schuurs T.A., van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res. 2004; 121(1): 141–50. [DOI] [PubMed] [Google Scholar]
- 227.Vaithilingam V., Fung C., Ratnapala S., Foster J., Vaghjiani V., Manuelpillai U., Tuch B.E. Characterisation of the xenogeneic immune response to microencapsulated fetal pig islet-like cell clusters transplanted into immunocompetent C57BL/6 mice. PLoS One 2013; 8(3): e59120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dufrane D., Steenberghe M., Goebbels R.M., Saliez A., Guiot Y., Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials 2006; 27(17): 3201–8. [DOI] [PubMed] [Google Scholar]
- 229.Wang W., Gu Y., Tabata Y., Miyamoto M., Hori H., Nagata N., Touma M., Balamurugan A.N., Kawakami Y., Nozawa M., Inoue K. Reversal of diabetes in mice by xenotransplantation of a bioartificial pancreas in a prevascularized subcutaneous site. Transplantation 2002; 73(1): 122–9. [DOI] [PubMed] [Google Scholar]
- 230.Wang W., Gu Y., Hori H., Sakurai T., Hiura A., Sumi S., Tabata Y., Inoue K. Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation 2003; 76(2): 290–6. [DOI] [PubMed] [Google Scholar]
- 231.Christoffersson G., Henriksnas J., Johansson L., Rolny C., Ahlstrom H., Caballero-Corbalan J., Segersvard R., Permert J., Korsgren O., Carlsson P.O., Phillipson M. Clinical and experimental pancreatic islet transplantation to striated muscle: Establishment of a vascular system similar to that in native islets. Diabetes 2010; 59(10): 2569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Espes D., Eriksson O., Lau J., Carlsson P.O. Striated muscle as implantation site for transplanted pancreatic islets. J Transplant. 2011; 2011: 352043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Song S., Faleo G., Yeung R., Kant R., Posselt A.M., Desai T.A., Tang Q., Roy S. Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Sci Rep. 2016; 6: 23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li J., Ezzelarab M.B., Ayares D., Cooper D.K. The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Stem Cell Rev. 2014; 10(1): 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ezzelarab M., Ezzelarab C., Wilhite T., Kumar G., Hara H., Ayares D., Cooper D.K. Genetically-modified pig mesenchymal stromal cells: Xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation 2011; 18(3): 183–95. [DOI] [PubMed] [Google Scholar]
- 236.Kumar G., Hara H., Long C., Shaikh H., Ayares D., Cooper D.K., Ezzelarab M. Adipose-derived mesenchymal stromal cells from genetically modified pigs: Immunogenicity and immune modulatory properties. Cytotherapy 2012; 14(4): 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Silva G.V., Litovsky S., Assad J.A., Sousa A.L., Martin B.J., Vela D., Coulter S.C., Lin J., Ober J., Vaughn W.K., Branco R.V., Oliveira E.M., He R., Geng Y.J., Willerson J.T., Perin E.C. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005; 111(2): 150–6. [DOI] [PubMed] [Google Scholar]
- 238.Janeczek Portalska K., Leferink A., Groen N., Fernandes H., Moroni L., van Blitterswijk C., de Boer J. Endothelial differentiation of mesenchymal stromal cells. PLoS One 2012; 7(10): e46842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Selawry H.P., Kotb M., Herrod H.G., Lu Z.N. Production of a factor, or factors, suppressing IL-2 production and T cell proliferation by Sertoli cell-enriched preparations. A potential role for islet transplantation in an immunologically privileged site. Transplantation 1991; 52(5): 846–50. [DOI] [PubMed] [Google Scholar]
- 240.Suarez-Pinzon W., Korbutt G.S., Power R., Hooton J., Rajotte R.V., Rabinovitch A. Testicular Sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000; 49(11): 1810–8. [DOI] [PubMed] [Google Scholar]
- 241.Selawry H.P., Cameron D.F. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993; 2(2): 123–9. [DOI] [PubMed] [Google Scholar]
- 242.Luca G., Calvitti M., Neri L.M., Becchetti E., Capitani S., Basta G., Angeletti G., Fanelli C., Brunetti P., Calafiore R. Sertoli cell-induced reversal of adult rat pancreatic islet beta-cells into fetal-like status: Potential implications for islet transplantation in type I diabetes mellitus. J Investig Med. 2000; 48(6): 441–8. [PubMed] [Google Scholar]
- 243.Luca G., Calvitti M., Baroni T., Basta G., Angeletti G., Neri L.M., Becchetti E., Capitani S., Brunetti P., Calafiore R. Sertoli cell-induced adult rat islet beta-cell mitogen-esis: Causative pathways. Diabetes Nutr Metab. 2003; 16(1): 1–6. [PubMed] [Google Scholar]
- 244.Mancuso F., Calvitti M., Luca G., Nastruzzi C., Baroni T., Mazzitelli S., Becchetti E., Arato I., Boselli C., Ngo Nselel M.D., Calafiore R. Acceleration of functional maturation and differentiation of neonatal porcine islet cell monolay-ers shortly in vitro cocultured with microencapsulated Sertoli cells. Stem Cells Int. 2010; 2010: 587213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yin Z., Chen D., Hu F., Ruan Y., Li J., Wang L., Xiang Y., Xie L., Wang X., Ichim T.E., Chen S., Chen G. Cotransplantation with xenogenetic neonatal porcine Sertoli cells significantly prolongs islet allograft survival in nonimmunosuppressive rats. Transplantation 2009; 88(3): 339–45. [DOI] [PubMed] [Google Scholar]
- 246.Yang H., Wright J.R. Jr. Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation 1999; 67(6): 815–20. [DOI] [PubMed] [Google Scholar]
- 247.Dufour J.M., Rajotte R.V., Kin T., Korbutt G.S. Immunoprotection of rat islet xenografts by cotransplantation with Sertoli cells and a single injection of antilymphocyte serum. Transplantation 2003; 75(9): 1594–6. [DOI] [PubMed] [Google Scholar]
- 248.Ramji Q.A., Bayrack K., Arefanian H., Marcet-Palacios M., Bleackley R.C., Rajotte R.V., Rayat G.R. Protection of porcine islet xenografts in mice using Sertoli cells and monoclonal antibodies. Transplantation 2011; 92(12): 1309–15. [DOI] [PubMed] [Google Scholar]
- 249.Valdes-Gonzalez R.A., Dorantes L.M., Garibay G.N., Bracho-Blanchet E., Mendez A.J., Davila-Perez R., Elliott R.B., Teran L., White D.J. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: A 4-year study. Eur J Endocrinol. 2005; 153(3): 419–27. [DOI] [PubMed] [Google Scholar]
- 250.Li Y., Xue W., Liu H., Fan P., Wang X., Ding X., Tian X., Feng X., Pan X., Zheng J., Tian P., Ding C., Fan X. Combined strategy of endothelial cells coating, Sertoli cells coculture and infusion improves vascularization and rejection protection of islet graft. PLoS One 2013; 8(2): e56696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ricordi C. Islet transplantation: A brave new world. Diabetes 2003; 52(7): 1595–603. [DOI] [PubMed] [Google Scholar]
- 252.Zeng Y., Ildstad S.T., Wren S.M., Rilo H.L., Bereiter D.R., Carroll P.B., Tzakis A.G., Starzl T.E., Ricordi C. Long-term survival of donor-specific pancreatic islet xenografts in fully xenogeneic chimeras. Transplant Proc. 1992; 24(3): 985. [PMC free article] [PubMed] [Google Scholar]
- 253.Ricordi C., Zeng Y., Carroll P.B., Rilo H.L., Beretier D.R., Starzl T.E., Ildstad S.T. Islet xenografts in fully xenogeneic (rat→mouse) chimeras: Evidence for normal regulation of function in a xenogeneic mouse environment. Surgery 1992; 112(2): 327–32. [PMC free article] [PubMed] [Google Scholar]
- 254.Starzl T.E., Demetris A.J., Murase N., Ildstad S., Ricordi C., Trucco M. Cell migration, chimerism, and graft acceptance. Lancet 1992; 339(8809): 1579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Luo J.Z., Xiong F., Al-Homsi A.S., Ricordi C., Luo L. Allogeneic bone marrow cocultured with human islets significantly improves islet survival and function in vivo. Transplantation 2013; 95(6): 801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pileggi A., Ricordi C. A new home for pancreatic islet transplants: The bone marrow. Diabetes 2013; 62(10): 3333–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Buhler L., Deng S., O'Neil J., Kitamura H., Koulmanda M., Baldi A., Rahier J., Alwayn I.P., Appel J.Z., Awwad M., Sachs D.H., Weir G., Squifflet J.P., Cooper D.K., Morel P. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation 2002; 9(1): 3–13. [DOI] [PubMed] [Google Scholar]
- 258.Groth C.G., Korsgren O., Tibell A., Tollemar J., Moller E., Bolinder J., Ostman J., Reinholt F.P., Hellerstrom C., Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet 1994; 344(8934): 1402–4. [DOI] [PubMed] [Google Scholar]
- 259.Wang W., Mo Z., Ye B., Hu P., Liu S., Yi S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011; 36(12): 1134–40. [DOI] [PubMed] [Google Scholar]
- 260.Cozzi E., Tonjes R.R., Gianello P., Buhler L.H., Rayat G.R., Matsumoto S., Park C.G., Kwon I., Wang W., O'Connell P., Jessamine S., Elliott R.B. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 1: Update on national regulatory frameworks pertinent to clinical islet xenotransplantation. Xenotransplantation 2016; 23(1): 14–24. [DOI] [PubMed] [Google Scholar]
- 261.Vanderpool H.Y. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 7: Informed consent and xenotransplantation clinical trials. Xenotransplantation 2009; 16(4): 255–62. [DOI] [PubMed] [Google Scholar]
- 262.Cooper D.K. Global consultation on regulatory requirements for xenotransplantation in clinical trials. Conference held in Changsha, China, November 19–21, 2008. Xenotransplantation 2009; 16(2): 58–60. [DOI] [PubMed] [Google Scholar]
- 263.Spizzo T., Denner J., Gazda L., Martin M., Nathu D., Scobie L., Takeuchi Y. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 2a: Source pigs-preventing xenozoonoses. Xenotransplantation 2016; 23(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 264.Cowan P.J., Ayares D., Wolf E., Cooper D.K. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 2b: Genetically modified source pigs. Xenotransplantation 2016; 23(1): 32–7. [DOI] [PubMed] [Google Scholar]
- 265.Rayat G.R., Gazda L.S., Hawthorne W.J., Hering B.J., Hosking P., Matsumoto S., Rajotte R.V. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 3: Porcine islet product manufacturing and release testing criteria. Xenotransplantation 2016; 23(1): 38–45. [DOI] [PubMed] [Google Scholar]
- 266.Cooper D.K., Bottino R., Gianello P., Graham M., Hawthorne W.J., Kirk A.D., Korsgren O., Park C.G., Weber C. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 4: Pre-clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation 2016; 23(1): 46–52. [DOI] [PubMed] [Google Scholar]
- 267.Denner J., Tonjes R.R., Takeuchi Y., Fishman J., Scobie L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 5: Recipient monitoring and response plan for preventing disease transmission. Xenotransplantation 2016; 23(1): 53–9. [DOI] [PubMed] [Google Scholar]
- 268.Hering B.J., O'Connell P.J. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes—Chapter 6: Patient selection for pilot clinical trials of islet xenotransplantation. Xenotransplantation 2016; 23(1): 60–76. [DOI] [PubMed] [Google Scholar]
- 269.Komoda H., Miyagawa S., Omori T., Takahagi Y., Murakami H., Shigehisa T., Ito T., Matsuda H., Shirakura R. Survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase-III(GnT-III) in cynomolgus monkeys. Xenotransplantation 2005; 12(3): 209–16. [DOI] [PubMed] [Google Scholar]
- 270.Rood P.P., Cooper D.K. Islet xenotransplantation: Are we really ready for clinical trials? Am J Transplant. 2006; 6(6): 1269–74. [DOI] [PubMed] [Google Scholar]


