Abstract
Disease or trauma-induced loss or dysfunction of neurons in any central nervous system (CNS) tissue will have a significant impact on the health of the affected patient. The retina is a multilayered tissue that originates from the neuroectoderm, much like the brain and spinal cord. While sight is not required for life, neurodegeneration-related loss of vision not only affects the quality of life for the patient but also has societal implications in terms of health care expenditure. Thus, it is essential to develop effective strategies to repair the retina and prevent disease symptoms. To address this need, multiple techniques have been investigated for their efficacy in treating retinal degeneration. Recent advances in cell transplantation (CT) techniques in preclinical, animal, and in vitro culture studies, including further evaluation of endogenous retinal stem cells and the differentiation of exogenous adult stem cells into various retinal cell types, suggest that this may be the most appropriate option to replace lost retinal neurons. Unfortunately, the various limitations of CT, such as immune rejection or aberrant cell behavior, have largely prevented this technique from becoming a widely used clinical treatment option. In parallel with the advances in CT methodology, the use of electrical stimulation (ES) to treat retinal degeneration has also been recently evaluated with promising results. In this review, we propose that ES could be used to enhance CT therapy, whereby electrical impulses can be applied to the retina to control both native and transplanted stem cell behavior/survival in order to circumvent the limitations associated with retinal CT. To highlight the benefits of this dual treatment, we have briefly outlined the recent developments and limitations of CT with regard to its use in the ocular environment, followed by a brief description of retinal ES, as well as described their combined use in other CNS tissues.
Keywords: Neurodegeneration, Retina, Electrical stimulation (ES), Combination therapy
Introduction
The vast majority of cells in the human body are programmed to perform specialized functions, having unique morphological, functional, and electrical properties. This is also true for cells in the ocular environment, including those of the retina. The mammalian retina contains five groups of neurons responsible for transmitting light signals to the brain: photoreceptor cells (cones and rods), bipolar cells, horizontal cells, amacrine cells, and retinal ganglion cells (RGCs). These cells are supported by the Mμller cells, which are the primary immune cells in the retinal environment along with retinal microglia. Furthermore, the retinal pigmented epithelium (RPE), a monolayer of cells located between the photoreceptor cells and Bruch's membrane, forms the blood–retinal barrier in addition to metabolically supporting other processes, including phagocytosis of photoreceptor outer segments and secretion of various trophic factors1. During disease or following traumatic injury, any number of these specialized cells of the retina can be damaged or lost, resulting in varying levels of retinal degeneration and decreased visual acuity.
Although the mechanisms underlying retinal degeneration are distinct for each disease, they invariably involve the irreversible loss of retinal neurons, neuronal connections, and/or supporting glia2. For example, glaucoma involves the selective loss of RGCs, while photoreceptor loss is common during retinitis pigmentosa (RP)3. Age-related macular degeneration (AMD), on the other hand, involves degeneration of the RPE as well as photoreceptor cell death4. To date, there are currently no singularly reliable clinical treatment options to completely halt or reverse the loss of vision associated with these diseases. However, a number of novel techniques are currently being evaluated for their potential therapeutic use in the treatment of retinal degeneration, including gene therapy, optogenetics, electrical stimulation (ES), cell transplantation (CT), etc.
In this review, we have focused on the treatment of several prominent retinal diseases, including AMD, RP, traumatic optic neuropathy (TON), nonarterial ischemic optic neuropathy (NAION), and retinal artery occlusion (RAO). Basic descriptions of these diseases can be found in Figure 1. Once neuronal degradation has occurred, it is essential that these cells and synaptic connections be replaced in order to restore vision to the patient, making CT one of the most attractive treatment options. However, the application of CT methods in the retina comes with a number of limitations. Thus, we hypothesize that a dual therapeutic approach should be adopted utilizing ES to augment CT to treat retinal degeneration. While this theory has never been tested in the retina, it is supported by various studies in other tissues, highlighting the additive benefits of the two techniques, whereby CT can be used to replace lost cells and/or provide trophic support, while ES can be utilized to stimulate and protect the remaining endogenous cells in addition to facilitating function in the transplanted cells. Together, we believe these treatments provide the highest possibility for improvement in visualacuity following retinal degeneration or trauma. In this review, we begin our analysis by first discussing current CT techniques and their limitations along with a brief history of ES, followed by an evaluation of their combined therapy in other tissues emphasizing the potential applications in the retina.
Figure 1.
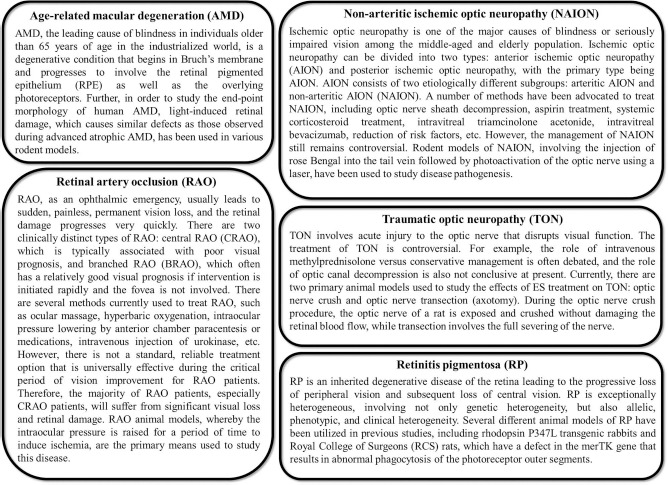
General descriptions of various retinal degenerative diseases, including age-related macular degeneration (AMD), retinitis pigmentosa (RP), traumatic optic neuropathy (TON), nonarterial ischemic optic neuropathy (NAION), and retinal artery occlusion (RAO).
Evaluation of Current Retinal Cell Transplantation Methodologies
CT involves the replacement of dead or abnormal cells with healthy cells that mimic their normal function and behavior. Thus, in retinal degenerative diseases, cells must be replaced with the specific cell type lost, namely, the photoreceptor cells, RPE, and/or RGCs. While transplantation of sheets or suspensions/aggregates of retinal cells is feasible to some extent, the level of integration of these cells appears to be largely dependent on the age of the donor/host, quality of the transplanted cells, and the type of retinal degradation involved, as highlighted by experiments performed in various disease models5–21, thus limiting their effectiveness. Further, developing fetal retinal cells have also been investigated for their use in retinal CT techniques with some success8. However, a large quantity of these postmitotic cells is required, the collection of which also poses ethical issues. In fact, many research groups have turned to use of adult stem cells in clinical and laboratory settings in order to avoid these ethical concerns and enhance the level of cellular integration into the retina.
Stem cells are cells that have the potential to develop into many other cell types and can be used to replace cells that are lost, damaged, or altered during disease or trauma. Although the term “stem cell” has been used variably since 1906 when Alexander Maximow developed his theory of hematopoiesis22,23, it was not until 1981 that murine embryonic stem cells (ESCs) were isolated24,25. Further, it was another 17 years before Thomson et al.26 isolated human ESCs and developed the first human embryonic culture stem cell lines. Since their discovery, researchers worldwide have sought to understand stem cell behavior, morphology, and function. Notably, the three essential characteristics of these cells are that they are unspecialized, self-renewing, and potent. The level of stem cell potency, or the range of cell types they can differentiate into, may vary depending on the age of the organism at isolation. For example, totipotent stem cells, which can differentiate into all cell types found in both embryonic and extraembryonic tissues, are typically derived from cells in the first 4 days following fertilization of the egg in mammals (up to approximately the 16-cell stage), while pluripotent (giving rise to all embryonic cell types) and multipotent stem cells (giving rise to cells of a specific germ layer) can be found later in development as well as in some adult tissues, including the eye27.
Endogenous Retinal Stem Cells for Transplantation
The retina develops from the neural tube and, while morphologically unique, is classified as a central nervous system (CNS) tissue. Although somatic stem cells are present in most tissues, being largely found in clusters known as niches, endogenous adult retinal stem cells have been difficult to identify. A large proportion of the ocular regeneration literature is focused on fish and amphibian models28–30, which utilize a type of intrinsic retinal regeneration that does not occur in mammals. However, these studies have led to the identification of three primary types of endogenous adult retinal cells that have been evaluated for transplantation in various species: Mμller cells31–38, RPE cells39,40, and ciliary pigmented epithelial (CPE) cells41–43 (Fig. 2A). While there is some controversy as to the specific stem cell nature of these cells44,45, there is evidence indicating that they can differentiate into retinal cell types under certain conditions. In fact, Mμller cells appear to primarily differentiate into cone and rod photoreceptors31–33,46–48 as well as RGCs27, while CPE and RPE cells have the potential to differentiate into rod or cone photoreceptor-like cells, respectively45,49–54. During the transplantation/retinal regeneration process, these cell types would need to undergo several cellular actions, including dedifferentiation, proliferation, migration, neural differentiation, and functional integration55. Interestingly, subretinal or intravitreal injection of Mμller cells derived from humans leads to migration of the transplanted cells into the retinal parenchyma and expression of neuronal cell markers31, while transplantation of CPE or RPE cells has been performed with varying levels of success56.
Figure 2.
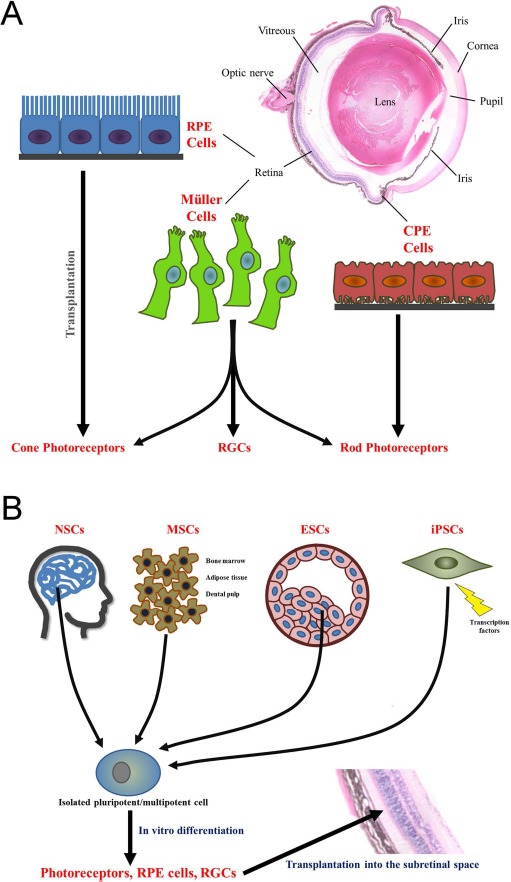
Cell transplantation cell types and basic approaches. (A) Endogenous retinal stem cells: Mμller cells, retinal pigmented epithelial (RPE) cells, and ciliary pigmented epithelial (CPE) cells. Following transplantation, Mμller cells have been shown to differentiate into both rod and cone photoreceptors as well as retinal ganglion cells (RGCs), while RPE and CPE cells differentiate into cone and rod photoreceptors, respectively. (B) Exogenous retinal stem cells: neural stem cells (NSCs; isolated from the brain), mesenchymal stem cells (MSCs; isolated from bone marrow, adipose tissue, and dental pulp), embryonic stem cells (ESCs; isolated from the inner cell mass at the blastocyst stage), and induced pluripotent stem cells (iPSCs; isolated adult fibroblasts/somatic cells that are reprogrammed using retroviral transduction). Stem cells are isolated and cultured in vitro. While these multipotent/pluripotent cells have been directly transplanted into the eye, higher levels of integration and functional restoration have been observed when the cells are differentiated into a retinal cell type prior to transplantation. This is particularly important if the stem cells are being used to replace degraded photoreceptors, RGCs, or RPE cells. The image of the hematoxylin and eosin-stained mouse eye was obtained using a light microscope (Carl Zeiss, Jena, Germany) using a 10× objective.
Notably, the use of CPE or similar progenitor cells in the iris57–60 could, in theory, be isolated from the affected patient, limiting issues with immune rejection. However, caution should be exercised if the patient's particular retinal disease has a genetic component. In contrast, autologous CT using Mμller glia from the patient's retina is not feasible, necessitating the use of donor cells47. Preparation and transplantation of postmitotic RPE cells or sheets from a donor, while possible, involve a significant amount of tissue and may also be associated with a higher level of surgical risk61. To circumvent these issues, recent research has described methodologies using nonretinal stem cell-derived RPE cells, photoreceptors, and RGCs that may, in some aspects, provide additional advantages over endogenous ocular cells, such as lowering the chances of immune rejection and providing an unlimited number of donor cells62–67.
Exogenous Stem Cells for Retinal Transplantation
Various types of nonretinal stem cells have been studied for their use in retinal CT (Fig. 2B). This includes neural stem cells (NSCs), which have been employed for neural and glial cell replacement in spinal cord and brain injuries68–70 and function to protect the photoreceptors in the damaged retina via their phagocytotic capabilities to eliminate the photoreceptor outer segments—a role typically performed by the RPE—and the secretion of neurotrophic factors71–73. Mesenchymal stem cells (MSCs), which can be derived from bone marrow (BM-MSCs)74,75, adipose tissue (ADSCs)76, or dental pulp (DPSCs)77, appear to play a trophic support role via the secretion of factors that stimulate endogenous cells78–80. Finally, ESCs81,82, from the inner cell mass of the blastocyst, and induced pluripotent stem cells (iPSCs), which can be reprogrammed from adult fibroblasts and somatic cells via retroviral transduction62,83, have been used to replace photoreceptor cells, RPE cells, and all other cells of the retina15,84–86.
Recent research suggests that in order to increase integration and function of retinal cells derived from nonretinal stem cells, regardless of origin, they should first be differentiated in vitro prior to transplantation8. BM-MSCs, for example, have been observed to differentiate into both RPE cells and photoreceptors when cocultured with RPE cells in vitro87, but show no evidence of differentiation into mature retinal cells after direct transplantation despite integration into the retina74. Thus, it seems that while BM-MSCs may play a neuroprotective role when transplanted, differentiation into retinal cell types before transplantation is essential if these cells are being used to replace the lost or abnormal cells. Interestingly, human ESCs can be differentiated into retinal cells in serum-free culture media supplemented with a mixture of noggin, dickkopfrelated protein 1 (DKK-1), insulin-like growth factor 1 (IGF-1), and basic fibroblast growth factor (bFGF)82. Similar techniques also work for iPSCs using specific combinations of growth factors63,86. Differentiation into retinal neurons was also enhanced when cocultured with mouse retinas from photoreceptor-degenerated mice. For a full review of ESC/iPSC reprogramming and retinal differentiation/transplantation protocols, please see Reynolds and Lamba88, Borooah et al.85, and Wright et al.89.
Importantly, the transcriptome/proteome of these cells should ideally mimic that of the native cells they are replacing. In doing so, the likelihood of these cells interacting appropriately with the neighboring healthy cells may increase. To this end, Lamba and Reh90 have published genomic profiling data comparing human ESC-derived retinal cells to fetal human retinal cells and showed that the expression of retinal genes between the two subsets of cells are strongly correlated. Thus, it appears that these ESC-derived cells adopt similar gene expression as that utilized during retinal development. iPSCs, on the other hand, have their own distinct expression profile, highlighting some potential differences91. However, regardless of gene expression, the transplanted cells ultimately need to behave as the native cells would. This includes integrating into the correct retinal layer and forming functional synapses, which are essential to improving visual acuity. This has been shown to occur when using human ESC-derived retinal cells following subretinal transplantation in adult wild-type mice, whereby the transplanted photoreceptor cells migrated into the outer nuclear layer and extended outer segments15. In this same study, there was also evidence of neuroprotection on the surrounding host cells when transplanted into a light-damage model as well as functional responses to light using electroretinography (ERG) after transplantation in blind Crx–/– mice15, which display photoreceptor degeneration92. iPSC-derived photoreceptor cells transplanted into the subretinal space also integrated into the correct retinal layer and increased visual function86.
While further research is necessary to determine the optimal transplantation conditions, the use of CT to treat retinal degenerative diseases, using endogenous or exogenous stem cells, is an interesting avenue of regenerative medicine. Notably, a number of clinical trials, as previously highlighted by Mead et al.93, and animal studies using exogenous stem cells to treat retinal diseases are ongoing.
Limitations of Cell Transplantation-Based Retinal Therapies
Although various cell types have been tried for retinal CT, very few have had reliable success. Furthermore, the methods and analyses used in retinal CT studies are often variable and require optimization. The limitations of each cell type, whether endogenous or exogenous, as well as the quality of these cells before transfer also need to be weighed when determining what cell type and transplantation method (e.g., intravitreal injection, subretinal transplantation) to apply. In terms of results, ESCs seem to be a feasible option for retinal CT; however, the ethical issues surrounding their isolation hinder their clinical use. Thus, the use of differentiated iPSCs may be the most advantageous nonretinal donor source for CT to replace neurons following retinal degeneration as well as for disease modeling, testing therapeutic efficacy, and drug discovery64.
An additional limitation to consider when assessing the cell type for retinal CT is the possible effects of using autologous stem cells from a subject with a genetic retinal disease. While using cells from the patient may limit immune system-related complications, it may also be necessary to “correct” the genetic defects in the cells prior to transplantation or risk the possible reoccurrence of the disease. This type of genetic correction has been performed previously to repair the mutation responsible for gyrate atrophy, which affects the RPE and leads to photoreceptor cell loss94. However, the cost and effort required to produce and test gene-corrected cells are currently high. Alternatively, donor stem cells with a similar human leukocyte antigen (HLA) type can be used95. In some diseases involving multiple genetic risk factors and/or cellular aging specific to retinal tissue (e.g., AMD), it may be unnecessary to correct these genetic defects as the reprogramming process may restore a level of cellular youth.
Other limitations concerning the use of retinal CT stem from the plasticity of the transplanted cells (including the level of differentiation prior to and/or after transplantation) as well as the habitability of the host environment. For example, in the ocular environment, while Mμller cell proliferation is required during retinal regeneration, this process of reactive gliosis can also result in scar tissue formation that may hinder integration of the transplanted cells55. To circumvent this issue, it is possible to disrupt the outer limiting membrane formed by the activated Mμller cells in order to increase cellular integration following transplantation96. Indeed, West et al.97 demonstrated that immune suppression (using cyclosporine A treatment to reduce the T-cell-mediated immune response) increased the number of transplanted photoreceptor cells from 76 ± 20 cells per eye in the unsuppressed animals to 275 ± 89 cells per eye at 4 months posttransplantation, highlighting a significant change in the long-term survival of these cells. However, it would be ideal to avoid the need for pharmacological disruption of the already fragile diseased retina.
Furthermore, cell death-induced activation of retinal microglia is another common feature observed in genetic models of various retinal diseases98,99 as well as injury-induced retinal degeneration100,101, and this activation may lead to the secretion of various cytokines and neurotoxins that can further injure the surrounding neurons102–104. Protection of the retinal neurons appears to be largely modulated by the expression of neurotrophic growth factors secreted by microglia/Mμller cells, such as IGF-1, ciliary neurotrophic factor (CNTF), etc.100,105, many of which appear to be altered after injury106. Although the eye, including the subretinal space, is considered an immune-privileged site, when ocular integrity is compromised, macrophage invasion and immune activation can result in rejection of the transplanted cells107. Furthermore, it is currently unclear if an internal retinal microglial-mediated inflammatory response could act to hinder integration or function of the transplanted cells. These issues with immune rejection are common following CT in a number of tissues. In fact, it is important to avoid xenogenic factors (including animal serum, pathogens, and non-human-derived reagents) during culture and differentiation that may cause immune rejection after transplantation. While a chemical-based culture system108 as well as animal serum/contamination-free culture conditions109–112 have been implemented for generating retinal cells from human ESCs and iPSCs, immune rejection still poses a significant issue during retinal CT. Even if the process is successful and the cells are not rejected by the host, there are limited data indicating that the transplanted cells establish the functional synapses required for the restoration of visual function112. Notably, recent literature has suggested that a more habitable native cell environment, allowing the transplanted cells to integrate and form synapses, can be facilitated using ES.
History and Types of Electrical Stimulation in the Retina
ES is a technique that uses an electrical current to activate nerves and restore function. Recent applications of ES in the field of neuroscience include both high-intensity and low-intensity ES currents, otherwise known as “functional ES,” and have been used to treat Parkinson's disease113,114 and other neurodegenerative diseases115. Historically, the application of ES can be traced to 1791, when Galvani discovered that the leg muscles of a dead frog twitched when treated with an electrical spark. In ophthalmology, ES has a long history dating back as early as 1873, when Dor used complicated machines to treat “amblyopia and amauroses,” “retinochoroiditis with pigment infiltration,” and “glaucoma and white optic atrophy”116. Furthermore, during normal ocular development, electrical waves are transmitted across the retina via neuronal action potentials. A study in 2002 by Morimoto et al.117, which demonstrated improved survival of transected RGCs in rats following ES, appears to be the first report that ES can mediate neuroprotection. Since then, various studies have been performed to further evaluate the neuroprotective effect of ES both in vitro and in vivo, using cell culture, human trials, and animal models. Please see Sehic et al.118 for an excellent review of the most recent animal and clinical studies using ES.
Types of Ocular Electrical Stimulation
There are four types of ES commonly used in ophthalmology: subretinal ES (SES), optic nerve stump ES, transcorneal ES (TcES), and whole-eye ES (WES) (Fig. 3). In SES, a microphotodiode array is implanted in the subretinal space, and stimulation is applied directly to the retina. This type of ES has been widely used with retinal implants, which provide the patient with low-resolution images by stimulating the surviving retinal cells119,120. Alternatively, ES of the optic nerve stump uses a pair of electrodes attached to the end of the nerve to stimulate function, while TcES utilizes a contact lens containing both the active and reference electrodes directly on the cornea, making it much less invasive. WES generates an electrical current that goes through the entire eye, whereby one electrode is placed at either the cornea or the orbit, while the reference electrode is placed on the skin, in the mouth, or elsewhere in the body. The electrical current then passes through other tissues before reaching and stimulating the retina. Thus, the intensity may need to be higher for WES compared to other types of ES that come in direct contact with the ocular environment. WES includes techniques such as transorbital ES and transcranial alternating current stimulation (tACS). A number of studies have been conducted utilizing these various types of ES, with TcES currently being the most common118.
Figure 3.
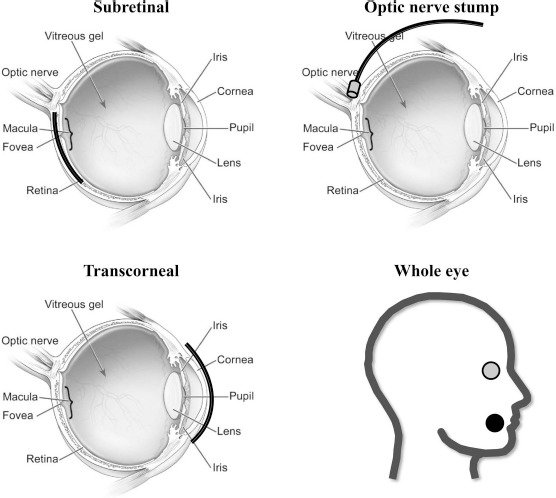
Schematic diagrams highlighting various types of electrical stimulation (ES) in the ocular environment, including subretinal ES, optic nerve stump ES, transcorneal ES, and whole-eye ES. Subretinal ES involves implantation of a microphotodiode array in the subretinal space, whereby the electrical current is directly applied to the retina. Optic nerve stump ES utilizes a pair of electrodes attached to the end of the optic nerve to stimulate function. Transcorneal ES is performed via a contact lens electrode along the surface of the cornea. In general, whole-eye ES involves the placement of one electrode at the cornea or orbit (gray dot) and the reference electrode elsewhere on the skin, body, or in the mouth, as shown here (black dot). Eye diagram obtained and edited from the National Eye Institute at the National Institutes of Health.
Notably, while the published ES studies discussed in the present review primarily focus on stimulation of endogenous retinal function, retinal prostheses that function to fully or partially replace the native retina and/or other segments of the visual system, including the optic nerve, lateral geniculate nucleus, and visual cortex, have also been evaluated. Ultimately these devices all seek to restore vision by artificially activating the electrical responses in each of these locations. In the retina, a number of epiretinal, subretinal, and suprachoroidal prostheses have been developed, with varying levels of success. Furthermore, optogenetics, which utilizes gene transfer of light-sensitive proteins into retinal neurons, is another emerging avenue of study regarding vision loss. While an in-depth discussion of these techniques and devices is beyond the scope of this review, please refer to these recent publications by Yue et al.121, Fernandez and Normann122, and Lewis et al.123 for an all-encompassing assessment of the current progress in these fields.
Regardless of the type of ES utilized, whether SES, optic nerve stump ES, TcES, or WES, very few, if any, detrimental side effects have been reported for the subject during or after treatment. In general, it appears that ES treatment does in fact enhance the survival of neuronal retinal cells in various ophthalmic disease models through various mechanisms or, at the very least, does not hinder vision further. Moreover, the direct and indirect effects of ES on the retinal cells could also similarly affect transplanted cells in the ocular environment. We therefore propose a combination approach utilizing ES to enhance CT techniques to treat disease- or trauma-related visual deficits.
Application of Electrical Stimulation to Enhance Cell Transplantation Therapies for Retinal Diseases
Loss of vision not only affects the quality of life for the patient but also has societal implications in regard to their level of productivity and overall health care expenditure. Thus, it is essential to develop effective strategies for retinal cell survival, repair, and replacement for various neurodegenerative diseases affecting the retina. Fundamentally, our proposed combination of ES and CT techniques focuses on the use of electrical manipulation to control both native and transplanted stem cell behavior in order to bypass some of the common limitations associated with CT. For use in treating retinal degenerative diseases, ES-CT treatment addresses four main issues: native cell survival, transplanted cell survival, transplanted cell integration, and functional synapse formation/axon regeneration (Fig. 4).
Figure 4.
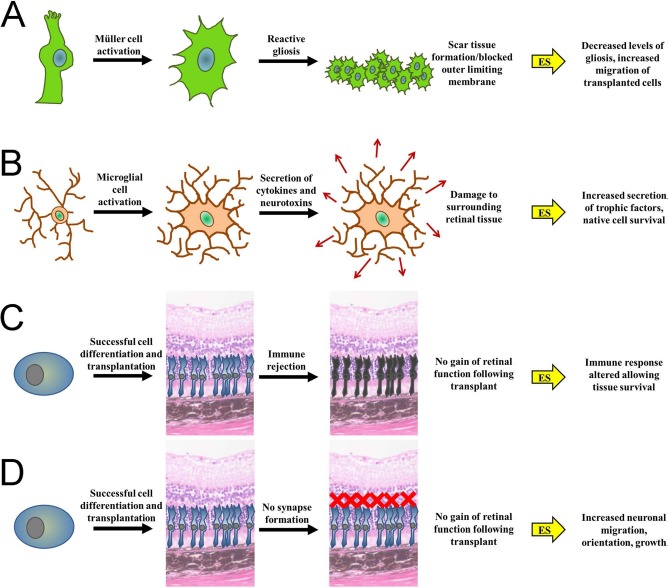
Barriers to the integration and functionality of transplanted retinal stem cells and their possible mediation via subsequent treatment with ES. (A) Activation of Mμller cells can result in reactive gliosis, whereby cells will proliferate and form scar tissue blocking the outer limiting membrane, hindering cellular integration beyond this layer. Application of ES after cell transplantation may decrease the levels of aberrant glial cell proliferation and immune function, thus allowing the transplanted cells to migrate to the correct layer in the retina. (B) Activation of retinal microglial cells can lead to the secretion of various cytokines and neurotoxins that can further injure the surrounding cells, both endogenous and transplanted. ES could be used to limit the detrimental effects of the microglia cells by stimulating them to secrete trophic factors that will support cell survival. (C) Even if transplantation is successful and the cells migrate and integrate properly, it is still possible that the transplanted cells will be rejected by the tissue and attacked by the immune system in an attempt to expel the “foreign” cells. The chance of immune rejection is increased if xenogeneic factors are used to cultivate the transplanted cells or if ocular integrity, and therefore the immune privileged nature of the eye, is compromised. The use of ES prior to immune rejection could help increase the chances of transplanted cell survival. (D) Finally, following successful transplantation, functional synapses are also required if vision is to be restored. It is possible that migration, orientation, and growth, including synapse formation, of the transplanted cells could be enhanced using subsequent ES. The image of the hematoxylin and eosin-stained murine retinal section was obtained using a light microscope (Carl Zeiss) using a 40× objective.
The recent advances in the field of retinal ES imply that this technique could be used as a pretreatment to modulate retinal cell function, essentially priming the host environment for the introduction of transplanted cells. In order to elucidate the comprehensive mechanism of ES on the retina, a whole genome-wide expression profile of TcES-treated wild-type Brown Norway rats was performed using quantitative real-time PCR124. The results indicate that 490 genes were differentially expressed in TcES-treated rats compared to untreated rats 4 h after application and included changes in various genes associated with potential neuroprotective cell functions. For example, the authors observed a significant downregulation in the expression of various proapoptotic genes such as B-cell lymphoma 2-associated X (Bax) and members of the tumor necrosis factor (TNF) family. Furthermore, no negative effects on the structure and function of the retina were observed in the rats up to 35 h after TcES treatment. Ciavatta et al.125, on the other hand, focused their investigation on the specific expression of growth factors in the retinas of Royal College of Surgeons (RCS) rats, a widely used model of RP, after SES using a microphotodiode array. In doing so, they found a consistent, significant elevation in the expression of FGF2 (also known as bFGF), suggesting that this particular growth factor may participate in the local neuroprotection induced by ES.
Focusing on the effects of ES on retinal neuronal cells, Henrich-Noack et al.126 used an optic nerve crush animal model to test the effects of TcES treatment immediately after injury and on postinjury day 11. They found that compared to sham-treated controls, there was a higher percentage of surviving RGCs in the TcES group. TcES also delayed posttraumatic cell death significantly and decreased optic nerve crush-associated neuronal swelling as well as shrinkage, especially in RGCs. It is likely that this ES-mediated protection of the retinal neurons would apply to both endogenous and transplanted neuronal cells as it appears to be primarily related to the expression of neurotrophins, including brain-derived neurotrophic factor (BDNF), IGF-1, CNTF, etc., and injection of these factors appears to increase neuronal survival in several retinal injury models127,128.
Various effects have also been observed following ES treatment via the modulation of Mμller and microglial cell changes. For example, it has been demonstrated that ES regulates neurotrophic factors and the levels of various enzymes in retinal Mμller cells. In fact, in a study evaluating the neuroprotective effect of TcES on an animal model of ischemic retinal damage, Wang et al.129 quantified glutamine synthetase (GS) expression, which appears to be significantly increased following treatment. The morphology and function of the retina were also preserved in treated animals, indicating that the protection against ischemic insults mediated by TcES is possibly related to attenuated glutamate excitotoxicity in Mμller cells. Sato et al.130 also found that after applying ES to cultured retinal Mμller cells, the mRNA level of IGF-1 increased significantly and largely depended on the Ca2+ influx through L-type voltage-dependent calcium channels (L-VDCCs). Upregulation of IGF-1 was also found in optic nerve crush and optic nerve transection models after TcES131,132. In a previous study, ES was observed to increase the mRNA and intracellular protein levels of BDNF in cultured retinal Müller cells, while its extracellular expression remained unchanged133. Moreover, the L-VDCC blocker, nifedipine, has been shown to suppress this ES-induced increase in BDNF mRNA expression. These results indicate that ES of Mμller cells could upregulate endogenous BDNF levels in the retina, and this upregulation may be dependent on L-VDCCs. In support of this theory, Zhou et al.134 found a positive regulatory effect of ES on the production of BDNF and CNTF in Mμller cells, and also provided evidence that BDNF rather than CNTF may play a major role in the promotion of Mμller cell-induced photoreceptor cell survival. Finally, in a recent proteomic study135, TcES performed on wild-type adult Wistar rats was shown to significantly alter the expression of 25 proteins. These proteins appear to play various functions (e.g., cellular signaling, synaptic transmission, metabolism, immune function, and structural maintenance) and included several suspected retinal regeneration factors, such as epidermal growth factor receptor (EGFR), prohibitin, tenascin-X, etc. Interestingly, IGF-1, BDNF, and FGF-2 were surprisingly absent from this list, a discrepancy the authors believe is related to their experiments being conducted on wildtype retinas versus a degenerative model135, similar to a previous study reporting changes in the transcriptome124. Thus, it can be hypothesized that ES treatment may alter the secretion of these neurotrophic factors, resulting in a more balanced and less hostile environment.
In addition to changes in neuronal and Mμller cells, ES has also been shown to influence retinal microglia. In fact, Zhou et al.134 found that ES can significantly decrease the number of activated microglia cells with ameboid shapes when cocultured with light-damaged 661W cells (light-reared cone-derived cells). This decrease was also associated with changes in the secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and TNF-α by the microglial cells. In this same study, it was also apparent that ES treatment had no direct effect, positive or negative, on the survival of the photoreceptor cells themselves, but rather the functional changes are solely mediated by the effects of ES on surrounding microglia and Mμller cells. Previous research also suggests that interactions between microglia and Mμller glial cells in vivo may contribute to the overall effects of ES on the retina100.
The expression of essential neurotrophic factors and altered Mμller/microglial cell function not only appear to help keep the native retinal cells (and possibly the transplanted retinal stem cells) alive but also promote varying levels of axon growth136. In this study, Goldberg et al. showed that increasing electrical activity and prolonging cellular depolarization potentiate the observed BDNF-induced axon growth via increasing cAMP levels. It is therefore possible that ES can enhance axonal function as well as regulate the immune environment in the native retinal cells. Similar effects on axon formation could also occur in the transplanted cells themselves.
The direct effects of ES performed before, during, or after CT on the transplanted cells have not been evaluated in the retina but have been investigated in studies performed using this type of combination therapy in other CNS-derived tissue injuries, such as brain and spinal cord traumas. For example, in a study using a spinal cord injury model in rats, transplanted BM-MSCs were subsequently subjected to ES, which resulted in significantly enhanced locomotor function, increased survival (31.3 ± 4.6% compared to 26.7 ± 2.5% in unstimulated transplants), differentiation, and integration of the transplanted cells as well as increased axonal regeneration, as determined by enhanced somatosensory-evoked potentials137. It should also be noted that this previous study utilized an implantable ES device, which can be equated to similar devices used for retinal implantation. Furthermore, Matsumoto et al.138 have demonstrated that pretreating BM-MSCs with ES in vitro prior to transplantation resulted in an increase in neural markers at day 7 [nestin and paired box protein 6 (Pax6)] as well as day 14 [neurofilament heavy (NF-H) and microtubule-associated protein 2 (MAP2)] in culture. Furthermore, the expression of neurogenin 2 (Ngn2), a protein associated with neural differentiation, and β-catenin, a mediator of cell survival, proliferation, and differentiation, was also increased on culture day 7 in the ES-treated cells. When these 7-day-old stimulated cells were transplanted into C57BL/6 mice with traumatic brain injury, the authors observed a higher percentage of transplanted cells expressing neural markers after stimulation along with enhanced neuronal differentiation and significantly improved motor function, as highlighted by rotarod (performed on days 14 and 28) and beam walking (performed on day 28) tests (p < 0.01 in both compared to the control animals). The mammalian retina also expresses both Ngn2139 and β-catenin140. Ngn2 appears to regulate the expression of the atonal homolog 5 (ATH5) and, thus, the downstream expression of the β3 subunit of a neuronal nicotinic acetycholine receptor141, which is a specific marker of RGC specification during retinogenesis142. β-Catenin signaling, on the other hand, plays an essential role in modulating the effects of various growth factors and cytokines during retinal regeneration after injury143,144 as well as during development, affecting the expression of a number of genes involved in chromatin organization, neurogenesis, and cell motion/migration145. It is, therefore, possible that treating cells with ES prior to or during retinal transplantation could also affect the expression of these genes in a manner that would aid survival, differentiation, and function after CT.
Similar to these results concerning spinal cord and traumatic brain injury, ES-enhanced CT has also been observed during muscle reinnervation after sciatic injury using transplanted neurons146 and myocardial regeneration following ischemic heart failure147, the latter of which appears to occur via stimulation and production of exosomes containing cardioprotective molecules148. Notably, exosomes are also utilized for cell–cell communication in the normal and diseased retina149–152 and confer different biological effects depending on their host cell. For example, using a laser-induced choroidal neovascularization model, Hajrasouliha et al.149 demonstrated that retinal astrocytes produce exosomes that contain factors that suppress vessel leakage and inhibit neovascularization, while exosomes released from the RPE do not mediate these changes. In fact, it is possible that exosomes may play a critical role in ES-induced changes in chorioretinal blood flow observed after treatment153. In this study by Kurimoto et al.153, which investigated the chorioretinal blood flow in 10 healthy subjects before and after TcES in different retinal areas using laser speckle flowgraphy, TcES was shown to induce an increase in chorioretinal blood flow in the area around the macula and midway between the optic disc and macula, but not around the optic disc itself. Indeed, blood flow rapidly increased in the first 30 min after TcES, peaked at 24 h, and decreased gradually by 40 h after treatment. This is consistent with the observed increase in IGF-1, a known effector of vasodilation154–157, in retinal Mμller cells130–132, which are themselves known to regulate retinal blood flow via the secretion of growth factors158. While the full mechanism underlying how TcES leads to this increase in chorioretinal blood flow remains unclear, it is possible that endosomes play a significant role. It also seems plausible that treatment with ES may not only alter the contents of these exosomes but may also induce their production in both the transplanted cells as well as the remaining retinal cells. These changes could have a significant effect on cell communication and the transfer of materials (e.g., proteins, mRNA, miRNA, etc.), leading to greater integration of the exogenous cells, protection of the surviving endogenous cells, and enhanced visual function. Thus, additional research investigating exosome function after ES-CT treatment is warranted.
It should be noted that the basis of the direct behavioral response of the stem cells to ES is not altogether surprising as neurons are known for their quick depolarization in response to electrical changes159. In fact, ES has been shown to promote neurite outgrowth during normal developmental differentiation160–165 as well as guide the growth direction of regenerating nerve fibers166–168. ES also appears to be capable of inducing the differentiation of ESCs169, BM-MSCs138, and ADSCs170 into neurons in culture with or without simultaneous gene therapy. Thus, the dual application of stem cell therapy and a controllable electrical current may provide a novel mechanism to realize the full advantages of CT therapies for retinal repair.
Taken together, it is likely that the utilization of ES-CT combination therapy to treat retinal degenerative diseases will have two primary benefits: (1) increased trophic support by the remaining endogenous retinal cells resulting in improved habitability of the host environment and (2) enhanced differentiation, integration, and synapse formation of the transplanted stem cells and remaining endogenous neurons. It is also possible that increased cell–cell communication via exosome production could function as a feedback loop between the indirect and direct effects on the immune response and transplanted cells, respectively. Optimization of ES-CT combination therapy in future studies should focus on the type and timing of ES (e.g., as a pretreatment, simultaneous, or post-CT) as well as the ideal cell type for transplantation under these conditions. Additional investigations should also be performed to evaluate other experimental and evaluation parameters.
Conclusion
The eye has various characteristics that make it an ideal organ system to study regeneration, including unique immune properties, isolation from other organs, easy surgical accessibility, well-understood anatomy and physiology, and convenience of imaging and functional monitoring. Further, while vision is one of our most important senses, it is not essential to life, permitting continued study of the tissue following complications and/or failed treatment. The eye is also a paired structure in vertebrates, which allows comparison to the contralateral eye during treatment in a single organism.
The retina is an essential component of the visual axis and is also susceptible to a number of degenerative diseases involving photoreceptor, RGC, and/or RPE cell death or dysfunction. It is imperative that effective treatment options to replace and stimulate these cells be developed for clinical use. While both ES and CT have beneficial effects when used as individual courses of treatment, studies in a number of other CNS tissues suggest that they may have additive advantages when ES is used to augment CT.
In the present review, we have outlined the basic concepts, mechanisms, and limitations of CT as well as a brief history of ES in the hopes that future research into their optimization and combined use will enhance their effectiveness in treating retinal degenerative diseases. The current literature describing ES-CT combination methods in other cellular contexts provides an excellent foundation for future investigations in the retina. Ongoing research in this field will be paramount to the prevention and cure of retinal degenerative diseases.
Acknowledgments
This work was supported by a grant from the Seed Funding Program for Basic Research at the University of Hong Kong, as well as funding from Neurotech Ltd. The authors declare no conflicts of interest.
References
- 1.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85(3): 845–81. [DOI] [PubMed] [Google Scholar]
- 2.Berry M., Ahmed Z., Lorber B., Douglas M., Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008; 26(2–3): 147–74. [PubMed] [Google Scholar]
- 3.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet 2006; 368(9549): 1795–809. [DOI] [PubMed] [Google Scholar]
- 4.Rattner A., Nathans J. Macular degeneration: Recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006; 7(11): 860–72. [DOI] [PubMed] [Google Scholar]
- 5.Mohand-Said S., Hicks D., Simonutti M., Tran-Minh D., Deudon-Combe A., Dreyfus H., Silverman M.S., Ogilvie J.M., Tenkova T., Sahel J. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997; 29(5): 290–7. [DOI] [PubMed] [Google Scholar]
- 6.Seiler M.J., Aramant R.B., Seeliger M.W., Bragadottir R., Mahoney M., Narfstrom K. Functional and structural assessment of retinal sheet allograft transplantation in feline hereditary retinal degeneration. Vet Ophthalmol. 2009; 12(3): 158–69. [DOI] [PubMed] [Google Scholar]
- 7.Thomas B.B., Seiler M.J., Sadda S.R., Aramant R.B. Superior colliculus responses to light—Preserved by transplantation in a slow degeneration rat model. Exp Eye Res. 2004; 79(1): 29–39. [DOI] [PubMed] [Google Scholar]
- 8.MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature 2006; 444(7116): 203–7. [DOI] [PubMed] [Google Scholar]
- 9.Gust J., Reh T.A. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011; 52(8): 5266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber A.C., Hippert C., Duran Y., West E.L., Bainbridge J.W., Warre-Cornish K., Luhmann U.F., Lakowski J., Sowden J.C., Ali R.R., Pearson R.A. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci USA 2013; 110(1): 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aramant R., Seiler M., Ehinger B., Bergstrom A., Gustavii B., Brundin P., Adolph A.R. Transplantation of human embryonic retina to adult rat retina. Restor Neurol Neurosci. 1990; 2(1): 9–22. [DOI] [PubMed] [Google Scholar]
- 12.Seiler M.J., Aramant R.B. Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function. Semin Ophthalmol. 2005; 20(1): 31–42. [DOI] [PubMed] [Google Scholar]
- 13.Eberle D., Kurth T., Santos-Ferreira T., Wilson J., Corbeil D., Ader M. Outer segment formation of transplanted photoreceptor precursor cells. PLoS One 2012; 7(9): e46305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos-Ferreira T., Postel K., Stutzki H., Kurth T., Zeck G., Ader M. Daylight vision repair by cell transplantation. Stem Cells 2015; 33(1): 79–90. [DOI] [PubMed] [Google Scholar]
- 15.Lamba D.A., Gust J., Reh T.A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 2009; 4(1): 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Q., Thomas B.B., Aramant R.B., Chen Z., Sadda S.R., Seiler M.J. Structure and function of embryonic rat retinal sheet transplants. Curr Eye Res. 2007; 32(9): 781–9. [DOI] [PubMed] [Google Scholar]
- 17.Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y., Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2014; 2(5): 662–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aramant R., Seiler M., Turner J.E. Donor age influences on the success of retinal grafts to adult rat retina. Invest Ophthalmol Vis Sci. 1988; 29(3): 498–503. [PubMed] [Google Scholar]
- 19.Seiler M.J., Aramant R.B., Thomas B.B., Peng Q., Sadda S.R., Keirstead H.S. Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur J Neurosci. 2010; 31(3): 508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivert L., Gouras P., Naeser P., Narfstrom K. Photoreceptor allografts in a feline model of retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 1998; 236(11): 844–52. [DOI] [PubMed] [Google Scholar]
- 21.Bartsch U., Oriyakhel W., Kenna P.F., Linke S., Richard G., Petrowitz B., Humphries P., Farrar G.J., Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008; 86(4): 691–700. [DOI] [PubMed] [Google Scholar]
- 22.Maximov A. Über experimentelle erzeugung von knochenmarks-gewebe. Anatomischer Anzeiger 1906; 28: 24–38. [Google Scholar]
- 23.Konstantinov I.E. In search of Alexander A. Maximow: The man behind the unitarian theory of hematopoiesis. Perspect Biol Med. 2000; 43(2): 269–76. [DOI] [PubMed] [Google Scholar]
- 24.Evans M.J., Kaufman M.H. Establishment in culture of pluri-potential cells from mouse embryos. Nature 1981; 292(5819): 154–6. [DOI] [PubMed] [Google Scholar]
- 25.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981; 78(12): 7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282(5391): 1145–7. [DOI] [PubMed] [Google Scholar]
- 27.Singhal S., Bhatia B., Jayaram H., Becker S., Jones M.F., Cottrill P.B., Khaw P.T., Salt T.E., Limb G.A. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012; 1(3): 188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez Alvarado A., Tsonis P.A. Bridging the regeneration gap: Genetic insights from diverse animal models. Nat Rev Genet. 2006; 7(11): 873–84. [DOI] [PubMed] [Google Scholar]
- 29.Tsonis P.A. Regeneration in vertebrates. Dev Biol. 2000; 221(2): 273–84. [DOI] [PubMed] [Google Scholar]
- 30.Tsonis P.A., Del Rio-Tsonis K. Lens and retina regeneration: Transdifferentiation, stem cells and clinical applications. Exp Eye Res. 2004; 78(2): 161–72. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence J.M., Singhal S., Bhatia B., Keegan D.J., Reh T.A., Luthert P.J., Khaw P.T., Limb G.A. MIO-M1 cells and similar Muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 2007; 25(8): 2033–43. [DOI] [PubMed] [Google Scholar]
- 32.Das A.V., Mallya K.B., Zhao X., Ahmad F., Bhattacharya S., Thoreson W.B., Hegde G.V., Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev Biol. 2006; 299(1): 283–302. [DOI] [PubMed] [Google Scholar]
- 33.Ooto S., Akagi T., Kageyama R., Akita J., Mandai M., Honda Y., Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA 2004; 101(37): 13654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karl M.O., Hayes S., Nelson B.R., Tan K., Buckingham B., Reh T.A. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA 2008; 105(49): 19508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan J., Zheng H., Chen Z.L., Xiao H.L., Shen Z.J., Zhou G.M. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 2008; 48(2): 223–34. [DOI] [PubMed] [Google Scholar]
- 36.Bernardos R.L., Barthel L.K., Meyers J.R., Raymond P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007; 27(26): 7028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A.J., Reh T.A. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001; 4(3): 247–52. [DOI] [PubMed] [Google Scholar]
- 38.Raymond P.A., Barthel L.K., Bernardos R.L., Perkowski J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L.X., Turner J.E. Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988; 47(6): 911–7. [DOI] [PubMed] [Google Scholar]
- 40.Lopez R., Gouras P., Kjeldbye H., Sullivan B., Reppucci V., Brittis M., Wapner F., Goluboff E. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci. 1989; 30(3): 586–8. [PubMed] [Google Scholar]
- 41.Ahmad I., Tang L., Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000; 270(2): 517–21. [DOI] [PubMed] [Google Scholar]
- 42.Tropepe V., Coles B.L., Chiasson B.J., Horsford D.J., Elia A.J., McInnes R.R., van der Kooy D. Retinal stem cells in the adult mammalian eye. Science 2000; 287(5460): 2032–6. [DOI] [PubMed] [Google Scholar]
- 43.Xu H., Sta Iglesia D.D., Kielczewski J.L., Valenta D.F., Pease M.E., Zack D.J., Quigley H.A. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 2007; 48(4): 1674–82. [DOI] [PubMed] [Google Scholar]
- 44.Cicero S.A., Johnson D., Reyntjens S., Frase S., Connell S., Chow L.M., Baker S.J., Sorrentino B.P., Dyer M.A. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci USA 2009; 106(16): 6685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballios B.G., Clarke L., Coles B.L., Shoichet M.S., Van Der Kooy D. The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol Open 2012; 1(3): 237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B., Hunter D.J., Rooker S., Chan A., Paulus Y.M., Leucht P., Nusse Y., Nomoto H., Helms J.A. Wnt signaling promotes Muller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2013; 54(1): 444–53. [DOI] [PubMed] [Google Scholar]
- 47.Osakada F., Ooto S., Akagi T., Mandai M., Akaike A., Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007; 27(15): 4210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jayaram H., Jones M.F., Eastlake K., Cottrill P.B., Becker S., Wiseman J., Khaw P.T., Limb G.A. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014; 3(3): 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke L., Ballios B.G., van der Kooy D. Generation and clonal isolation of retinal stem cells from human embryonic stem cells. Eur J Neurosci. 2012; 36(1): 1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Debbio C.B., Peng X., Xiong H., Ahmad I. Adult ciliary epithelial stem cells generate functional neurons and differentiate into both early and late born retinal neurons under non-cell autonomous influences. BMC Neurosci. 2013; 14: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan R.T., Li X., Huang J., Guidry C., Wang S.Z. Photoreceptor-like cells from reprogramming cultured mammalian RPE cells. Mol Vis. 2013; 19: 1178–87. [PMC free article] [PubMed] [Google Scholar]
- 52.Carr A.J., Vugler A.A., Yu L., Semo M., Coffey P., Moss S.E., Greenwood J. The expression of retinal cell markers in human retinal pigment epithelial cells and their augmentation by the synthetic retinoid fenretinide. Mol Vis. 2011; 17: 1701–15. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao S., Thornquist S.C., Barnstable C.J. In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res. 1995; 677(2): 300–10. [DOI] [PubMed] [Google Scholar]
- 54.Salero E., Blenkinsop T.A., Corneo B., Harris A., Rabin D., Stern J.H., Temple S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 2012; 10(1): 88–95. [DOI] [PubMed] [Google Scholar]
- 55.Gallina D., Todd L., Fischer A.J. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014; 123: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeon S., Oh I.H. Regeneration of the retina: Toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015; 48(4): 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haruta M., Kosaka M., Kanegae Y., Saito I., Inoue T., Kageyama R., Nishida A., Honda Y., Takahashi M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001; 4(12): 1163–4. [DOI] [PubMed] [Google Scholar]
- 58.Akagi T., Akita J., Haruta M., Suzuki T., Honda Y., Inoue T., Yoshiura S., Kageyama R., Yatsu T., Yamada M., Takahashi M. Iris-derived cells from adult rodents and primates adopt photoreceptor-specific phenotypes. Invest Ophthalmol Vis Sci. 2005; 46(9): 3411–9. [DOI] [PubMed] [Google Scholar]
- 59.Asami M., Sun G., Yamaguchi M., Kosaka M. Multipotent cells from mammalian iris pigment epithelium. Dev Biol. 2007; 304(1): 433–46. [DOI] [PubMed] [Google Scholar]
- 60.MacNeil A., Pearson R.A., MacLaren R.E., Smith A.J., Sowden J.C., Ali R.R. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells 2007; 25(10): 2430–8. [DOI] [PubMed] [Google Scholar]
- 61.Joussen A.M., Heussen F.M., Joeres S., Llacer H., Prinz B., Rohrschneider K., Maaijwee K.J., van Meurs J., Kirchhof B. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006; 142(1): 17–30. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–76. [DOI] [PubMed] [Google Scholar]
- 63.Hirami Y., Osakada F., Takahashi K., Okita K., Yamanaka S., Ikeda H., Yoshimura N., Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009; 458(3): 126–31. [DOI] [PubMed] [Google Scholar]
- 64.Wiley L.A., Burnight E.R., Songstad A.E., Drack A.V., Mullins R.F., Stone E.M., Tucker B.A. Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2015; 44: 15–35. [DOI] [PubMed] [Google Scholar]
- 65.Haruta M., Sasai Y., Kawasaki H., Amemiya K., Ooto S., Kitada M., Suemori H., Nakatsuji N., Ide C., Honda Y., Takahashi M. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004; 45(3): 1020–5. [DOI] [PubMed] [Google Scholar]
- 66.Klimanskaya I., Hipp J., Rezai K.A., West M., Atala A., Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 2004; 6(3): 217–45. [DOI] [PubMed] [Google Scholar]
- 67.Lund R.D., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauve Y., Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 2006; 8(3): 189–99. [DOI] [PubMed] [Google Scholar]
- 68.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., Zheng B., Conner J.M., Marsala M., Tuszynski M.H. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012; 150(6): 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong S.W., Chu K., Jung K.H., Kim S.U., Kim M., Roh J.K. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 2003; 34(9): 2258–63. [DOI] [PubMed] [Google Scholar]
- 70.Chu K., Kim M., Jeong S.W., Kim S.U., Yoon B.W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003; 343(2): 129–33. [DOI] [PubMed] [Google Scholar]
- 71.Jung G., Sun J., Petrowitz B., Riecken K., Kruszewski K., Jankowiak W., Kunst F., Skevas C., Richard G., Fehse B., Bartsch U. Genetically modified neural stem cells for a local and sustained delivery of neuroprotective factors to the dystrophic mouse retina. Stem Cells Transl Med. 2013; 2(12): 1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu B., Morgans C.W., Girman S., Luo J., Zhao J., Du H., Lim S., Ding S., Svendsen C., Zhang K., Wang S. Neural stem cells derived by small molecules preserve vision. Transl Vis Sci Technol. 2013; 2(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGill T.J., Cottam B., Lu B., Wang S., Girman S., Tian C., Huhn S.L., Lund R.D., Capela A. Transplantation of human central nervous system stem cells—Neuroprotection in retinal degeneration. Eur J Neurosci. 2012; 35(3): 468–77. [DOI] [PubMed] [Google Scholar]
- 74.Yu S., Tanabe T., Dezawa M., Ishikawa H., Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006; 344(4): 1071–9. [DOI] [PubMed] [Google Scholar]
- 75.Johnson T.V., Bull N.D., Hunt D.P., Marina N., Tomarev S.I., Martin K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51(4): 2051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsuruma K., Yamauchi M., Sugitani S., Otsuka T., Ohno Y., Nagahara Y., Ikegame Y., Shimazawa M., Yoshimura S., Iwama T., Hara H. Progranulin, a major secreted protein of mouse adipose-derived stem cells, inhibits light-induced retinal degeneration. Stem Cells Transl Med. 2014; 3(1): 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mead B., Logan A., Berry M., Leadbeater W., Scheven B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013; 54(12): 7544–56. [DOI] [PubMed] [Google Scholar]
- 78.Johnson T.V., DeKorver N.W., Levasseur V.A., Osborne A., Tassoni A., Lorber B., Heller J.P., Villasmil R., Bull N.D., Martin K.R., Tomarev S.I. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014; 137(Pt 2): 503–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mead B., Logan A., Berry M., Leadbeater W., Scheven B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 2014; 9(10): e109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levkovitch-Verbin H., Sadan O., Vander S., Rosner M., Barhum Y., Melamed E., Offen D., Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010; 51(12): 6394–400. [DOI] [PubMed] [Google Scholar]
- 81.Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y., Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008; 26(2): 215–24. [DOI] [PubMed] [Google Scholar]
- 82.Lamba D.A., Karl M.O., Ware C.B., Reh T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA 2006; 103(34): 12769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008; 451(7175): 141–6. [DOI] [PubMed] [Google Scholar]
- 84.Carr A.J., Vugler A.A., Hikita S.T., Lawrence J.M., Gias C., Chen L.L., Buchholz D.E., Ahmado A., Semo M., Smart M.J., Hasan S., da Cruz L., Johnson L.V., Clegg D.O., Coffey P.J. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One 2009; 4(12): e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borooah S., Phillips M.J., Bilican B., Wright A.F., Wilmut I., Chandran S., Gamm D., Dhillon B. Using human induced pluripotent stem cells to treat retinal disease. Prog Retin Eye Res. 2013; 37: 163–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tucker B.A., Park I.H., Qi S.D., Klassen H.J., Jiang C., Yao J., Redenti S., Daley G.Q., Young M.J. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One 2011; 6(4): e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiou S.H., Kao C.L., Peng C.H., Chen S.J., Tarng Y.W., Ku H.H., Chen Y.C., Shyr Y.M., Liu R.S., Hsu C.J., Yang D.M., Hsu W.M., Kuo C.D., Lee C.H. A novel in vitro retinal differentiation model by co-culturing adult human bone marrow stem cells with retinal pigmented epithelium cells. Biochem Biophys Res Commun. 2005; 326(3): 578–85. [DOI] [PubMed] [Google Scholar]
- 88.Reynolds J., Lamba D.A. Human embryonic stem cell applications for retinal degenerations. Exp Eye Res. 2014; 123: 151–60. [DOI] [PubMed] [Google Scholar]
- 89.Wright L.S., Phillips M.J., Pinilla I., Hei D., Gamm D.M. Induced pluripotent stem cells as custom therapeutics for retinal repair: Progress and rationale. Exp Eye Res. 2014; 123: 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamba D.A., Reh T.A. Microarray characterization of human embryonic stem cell-Derived retinal cultures. Invest Ophthalmol Vis Sci. 2011; 52(7): 4897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao J.L., Yu J., Huang K., Hu J., Diemer T., Ma Z., Dvash T., Yang X.J., Travis G.H., Williams D.S., Bok D., Fan G. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010; 19(21): 4229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furukawa T., Morrow E.M., Cepko C.L. Crx, a novel otxlike homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 1997; 91(4): 531–41. [DOI] [PubMed] [Google Scholar]
- 93.Mead B., Berry M., Logan A., Scott R.A., Leadbeater W., Scheven B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015; 14(3): 243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howden S.E., Gore A., Li Z., Fung H.L., Nisler B.S., Nie J., Chen G., McIntosh B.E., Gulbranson D.R., Diol N.R., Taapken S.M., Vereide D.T., Montgomery K.D., Zhang K., Gamm D.M., Thomson J.A. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci USA 2011; 108(16): 6537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor C.J., Bolton E.M., Pocock S., Sharples L.D., Pedersen R.A., Bradley J.A. Banking on human embryonic stem cells: Estimating the number of donor cell lines needed for HLA matching. Lancet 2005; 366(9502): 2019–25. [DOI] [PubMed] [Google Scholar]
- 96.West E.L., Pearson R.A., Tschernutter M., Sowden J.C., MacLaren R.E., Ali R.R. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008; 86(4): 601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.West E.L., Pearson R.A., Barker S.E., Luhmann U.F., Maclaren R.E., Barber A.C., Duran Y., Smith A.J., Sowden J.C., Ali R.R. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells 2010; 28(11): 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roque R.S., Imperial C.J., Caldwell R.B. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1996; 37(1): 196–203. [PubMed] [Google Scholar]
- 99.Hughes E.H., Schlichtenbrede F.C., Murphy C.C., Sarra G.M., Luthert P.J., Ali R.R., Dick A.D. Generation of activated sialoadhesin-positive microglia during retinal degeneration. Invest Ophthalmol Vis Sci. 2003; 44(5): 2229–34. [DOI] [PubMed] [Google Scholar]
- 100.Harada T., Harada C., Kohsaka S., Wada E., Yoshida K., Ohno S., Mamada H., Tanaka K., Parada L.F., Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002; 22(21): 9228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C., Lam T.T., Tso M.O. Heterogeneous populations of microglia/macrophages in the retina and their activation after retinal ischemia and reperfusion injury. Exp Eye Res. 2005; 81(6): 700–9. [DOI] [PubMed] [Google Scholar]
- 102.Joly S., Francke M., Ulbricht E., Beck S., Seeliger M., Hirrlinger P., Hirrlinger J., Lang K.S., Zinkernagel M., Odermatt B., Samardja M., Reichenbach A., Grimm C., Remé C.E. Cooperative phagocytes: Resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am J Pathol. 2009; 174(6): 2310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci. 2007; 8(1): 57–69. [DOI] [PubMed] [Google Scholar]
- 104.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007; 81(6): 1345–51. [DOI] [PubMed] [Google Scholar]
- 105.West E.L., Pearson R.A., Duran Y., Gonzalez-Cordero A., MacLaren R.E., Smith A.J., Sowden J.C., Ali R.R. Manipulation of the recipient retinal environment by ectopic expression of neurotrophic growth factors can improve transplanted photoreceptor integration and survival. Cell Transplant. 2012; 21(5): 871–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao W., Li F., Steinberg R.H., Lavail M.M. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. Exp Eye Res. 2001; 72(5): 591–604. [DOI] [PubMed] [Google Scholar]
- 107.Streilein J.W., Ma N., Wenkel H., Ng T.F., Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002; 42(4): 487–95. [DOI] [PubMed] [Google Scholar]
- 108.Osakada F., Jin Z.B., Hirami Y., Ikeda H., Danjyo T., Watanabe K., Sasai Y., Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009; 122(Pt 17): 3169–79. [DOI] [PubMed] [Google Scholar]
- 109.Amit M., Shariki C., Margulets V., Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004; 70(3): 837–45. [DOI] [PubMed] [Google Scholar]
- 110.Xu C., Inokuma M.S., Denham J., Golds K., Kundu P., Gold J.D., Carpenter M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001; 19(10): 971–4. [DOI] [PubMed] [Google Scholar]
- 111.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., Crandall L.J., Daigh C.A., Conard K.R., Piekarczyk M.S., Llanas R.A., Thomson J.A. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006; 24(2): 185–7. [DOI] [PubMed] [Google Scholar]
- 112.Barnea-Cramer A.O., Wang W., Lu S.J., Singh M.S., Luo C., Huo H., McClements M.E., Barnard A.R., MacLaren R.E., Lanza R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016; 6: 29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Volkmann J. Deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord. 2007; 13(Suppl 3): S462–5. [DOI] [PubMed] [Google Scholar]
- 114.Castrioto A., Lozano A.M., Poon Y.Y., Lang A.E., Fallis M., Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: A blinded evaluation. Arch Neurol. 2011; 68(12): 1550–6. [DOI] [PubMed] [Google Scholar]
- 115.Thompson D.M., Koppes A.N., Hardy J.G., Schmidt C.E. Electrical stimuli in the central nervous system microenvironment. Annu Rev Biomed Eng. 2014; 16: 397–430. [DOI] [PubMed] [Google Scholar]
- 116.Dor H. Beiträge zur electrotherapie der augenkrankheiten. Graefes Arch Clin Exp Ophthalmol. 1873; 19: 352. [Google Scholar]
- 117.Morimoto T., Miyoshi T., Fujikado T., Tano Y., Fukuda Y. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport 2002; 13(2): 227–30. [DOI] [PubMed] [Google Scholar]
- 118.Sehic A., Guo S., Cho K.S., Corraya R.M., Chen D.F., Utheim T.P. Electrical stimulation as a means for improving vision. Am J Pathol. 2016; 186(11): 2783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fernandes R.A., Diniz B., Ribeiro R., Humayun M. Artificial vision through neuronal stimulation. Neurosci Lett. 2012; 519(2): 122–8. [DOI] [PubMed] [Google Scholar]
- 120.Pardue M.T., Phillips M.J., Yin H., Sippy B.D., Webb-Wood S., Chow A.Y., Ball S.L. Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci. 2005; 46(2): 674–82. [DOI] [PubMed] [Google Scholar]
- 121.Yue L., Weiland J.D., Roska B., Humayun M.S. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog Retin Eye Res. 2016; 53: 21–47. [DOI] [PubMed] [Google Scholar]
- 122.Fernandez E., Normann R. Introduction to visual prostheses. In: Kolb H., Fernandez E., Nelson R., editors. Webvision: The organization of the retina and visual system. Salt Lake City (UT): University of Utah Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK391004/. [Google Scholar]
- 123.Lewis P.M., Ayton L.N., Guymer R.H., Lowery A.J., Blamey P.J., Allen P.J., Luu C.D., Rosenfeld J.V. Advances in implantable bionic devices for blindness: A review. ANZ J Surg. 2016; 86(9): 654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Willmann G., Schaferhoff K., Fischer M.D., Arango-Gonzalez B., Bolz S., Naycheva L., Rock T., Bonin M., Bartz-Schmidt K.U., Zrenner E., Schatz A., Gekeler F. Gene expression profiling of the retina after transcorneal electrical stimulation in wild-type Brown Norway rats. Invest Ophthalmol Vis Sci. 2011; 52(10): 7529–37. [DOI] [PubMed] [Google Scholar]
- 125.Ciavatta V.T., Kim M., Wong P., Nickerson J.M., Shuler R.K. Jr, McLean G.Y., Pardue M.T. Retinal expression of Fgf2 in RCS rats with subretinal microphotodiode array. Invest Ophthalmol Vis Sci. 2009; 50(10): 4523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Henrich-Noack P., Voigt N., Prilloff S., Fedorov A., Sabel B.A. Transcorneal electrical stimulation alters morphology and survival of retinal ganglion cells after optic nerve damage. Neurosci Lett. 2013; 543: 1–6. [DOI] [PubMed] [Google Scholar]
- 127.Mey J., Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993; 602(2): 304–17. [DOI] [PubMed] [Google Scholar]
- 128.Parrilla-Reverter G., Agudo M., Sobrado-Calvo P., Salinas-Navarro M., Villegas-Perez M.P., Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp Eye Res. 2009; 89(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 129.Wang X., Mo X., Li D., Wang Y., Fang Y., Rong X., Miao H., Shou T. Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp Eye Res. 2011; 93(5): 753–60. [DOI] [PubMed] [Google Scholar]
- 130.Sato T., Fujikado T., Morimoto T., Matsushita K., Harada T., Tano Y. Effect of electrical stimulation on IGF-1 transcription by L-type calcium channels in cultured retinal Muller cells. Jpn J Ophthalmol. 2008; 52(3): 217–23. [DOI] [PubMed] [Google Scholar]
- 131.Tagami Y., Kurimoto T., Miyoshi T., Morimoto T., Sawai H., Mimura O. Axonal regeneration induced by repetitive electrical stimulation of crushed optic nerve in adult rats. Jpn J Ophthalmol. 2009; 53(3): 257–66. [DOI] [PubMed] [Google Scholar]
- 132.Morimoto T., Miyoshi T., Matsuda S., Tano Y., Fujikado T., Fukuda Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Invest Ophthalmol Vis Sci. 2005; 46(6): 2147–55. [DOI] [PubMed] [Google Scholar]
- 133.Sato T., Fujikado T., Lee T.S., Tano Y. Direct effect of electrical stimulation on induction of brain-derived neurotrophic factor from cultured retinal Muller cells. Invest Ophthalmol Vis Sci. 2008; 49(10): 4641–6. [DOI] [PubMed] [Google Scholar]
- 134.Zhou W.T., Ni Y.Q., Jin Z.B., Zhang M., Wu J.H., Zhu Y., Xu G.Z., Gan D.K. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Muller cells. Exp Neurol. 2012; 238(2): 192–208. [DOI] [PubMed] [Google Scholar]
- 135.Kanamoto T., Souchelnytskyi N., Kurimoto T., Ikeda Y., Sakaue H., Munemasa Y., Kiuchi Y. Proteomic study of retinal proteins associated with transcorneal electric stimulation in rats. J Ophthalmol. 2015; 2015: 492050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goldberg J.L., Espinosa J.S., Xu Y., Davidson N., Kovacs G.T., Barres B.A. Retinal ganglion cells do not extend axons by default: Promotion by neurotrophic signaling and electrical activity. Neuron 2002; 33(5): 689–702. [DOI] [PubMed] [Google Scholar]
- 137.Liu H., Yang K., Xin T., Wu W., Chen Y. Implanted electro-acupuncture electric stimulation improves outcome of stem cells' transplantation in spinal cord injury. Artif Cells Blood Substit Immobil Biotechnol. 2012; 40(5): 331–7. [DOI] [PubMed] [Google Scholar]
- 138.Matsumoto M., Imura T., Fukazawa T., Sun Y., Takeda M., Kajiume T., Kawahara Y., Yuge L. Electrical stimulation enhances neurogenin2 expression through beta-catenin signaling pathway of mouse bone marrow stromal cells and intensifies the effect of cell transplantation on brain injury. Neurosci Lett. 2013; 533: 71–6. [DOI] [PubMed] [Google Scholar]
- 139.Sommer L., Ma Q., Anderson D.J. Neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996; 8(4): 221–41. [DOI] [PubMed] [Google Scholar]
- 140.Liu X., Sarthy V.P., Craft C.M., Honda Y., Ide C. Developmental expression of beta-catenin in mouse retina. Anat Sci Int. 2002; 77(3): 182–8. [DOI] [PubMed] [Google Scholar]
- 141.Skowronska-Krawczyk D., Ballivet M., Dynlacht B.D., Matter J.M. Highly specific interactions between bHLH transcription factors and chromatin during retina development. Development 2004; 131(18): 4447–54. [DOI] [PubMed] [Google Scholar]
- 142.Matter-Sadzinski L., Matter J.M., Ong M.T., Hernandez J., Ballivet M. Specification of neurotransmitter receptor identity in developing retina: The chick ATH5 promoter integrates the positive and negative effects of several bHLH proteins. Development 2001; 128(2): 217–31. [DOI] [PubMed] [Google Scholar]
- 143.Sanges D., Romo N., Simonte G., Di Vicino U., Tahoces A.D., Fernandez E., Cosma M.P. Wnt/beta-catenin signaling triggers neuron reprogramming and regeneration in the mouse retina. Cell Rep. 2013; 4(2): 271–86. [DOI] [PubMed] [Google Scholar]
- 144.Meyers J.R., Hu L., Moses A., Kaboli K., Papandrea A., Raymond P.A. Beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ha A., Perez-Iratxeta C., Liu H., Mears A.J., Wallace V.A. Identification of Wnt/beta-catenin modulated genes in the developing retina. Mol Vis. 2012; 18: 645–56. [PMC free article] [PubMed] [Google Scholar]
- 146.Grumbles R.M., Liu Y., Thomas C.M., Wood P.M., Thomas C.K. Acute stimulation of transplanted neurons improves motoneuron survival, axon growth, and muscle reinnervation. J Neurotrauma 2013; 30(12): 1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang G., Pashmforoush M., Chung B., Saxon L.A. The role of cardiac electrophysiology in myocardial regenerative stem cell therapy. J Cardiovasc Transl Res. 2011; 4(1): 61–5. [DOI] [PubMed] [Google Scholar]
- 148.Campbell C.R., Berman A.E., Weintraub N.L., Tang Y.L. Electrical stimulation to optimize cardioprotective exosomes from cardiac stem cells. Med Hypotheses 2016; 88: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hajrasouliha A.R., Jiang G., Lu Q., Lu H., Kaplan H.J., Zhang H.G., Shao H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem. 2013; 288(39): 28058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Locke C.J., Congrove N.R., Dismuke W.M., Bowen T.J., Stamer W.D., McKay B.S. Controlled exosome release from the retinal pigment epithelium in situ. Exp Eye Res. 2014; 129: 1–4. [DOI] [PubMed] [Google Scholar]
- 151.Atienzar-Aroca S., Flores-Bellver M., Serrano-Heras G., Martinez-Gil N., Barcia J.M., Aparicio S., Perez-Cremades D., Garcia-Verdugo J.M., Diaz-Llopis M., Romero F.J., Sancho-Pelluz J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med. 2016; 20(8): 1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang A.L., Lukas T.J., Yuan M., Du N., Tso M.O., Neufeld A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS One 2009; 4(1): e4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kurimoto T., Oono S., Oku H., Tagami Y., Kashimoto R., Takata M., Okamoto N., Ikeda T., Mimura O. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol. 2010; 4: 1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haylor J., Singh I., el Nahas A.M. Nitric oxide synthesis inhibitor prevents vasodilation by insulin-like growth factor I. Kidney Int. 1991; 39(2): 333–5. [DOI] [PubMed] [Google Scholar]
- 155.Steinberg H.O., Brechtel G., Johnson A., Fineberg N., Baron A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994; 94(3): 1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oltman C.L., Kane N.L., Gutterman D.D., Bar R.S., Dellsperger K.C. Mechanism of coronary vasodilation to insulin and insulin-like growth factor I is dependent on vessel size. Am J Physiol Endocrinol Metab. 2000; 279(1): E176–81. [DOI] [PubMed] [Google Scholar]
- 157.Chen Y.L., Messina E.J. Dilation of isolated skeletal muscle arterioles by insulin is endothelium dependent and nitric oxide mediated. Am J Physiol. 1996; 270(6 Pt 2): H2120–4. [DOI] [PubMed] [Google Scholar]
- 158.Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S.N., Osborne N.N., Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25(4): 397–424. [DOI] [PubMed] [Google Scholar]
- 159.Saygili E., Schauerte P., Kuppers F., Heck L., Weis J., Weber C., Schwinger R.H., Hoffmann R., Schroder J.W., Marx N., Rana O.R. Electrical stimulation of sympathetic neurons induces autocrine/paracrine effects of NGF mediated by TrkA. J Mol Cell Cardiol. 2010; 49(1): 79–87. [DOI] [PubMed] [Google Scholar]
- 160.Kimura K., Yanagida Y., Haruyama T., Kobatake E., Aizawa M. Gene expression in the electrically stimulated differentiation of PC12 cells. J Biotechnol. 1998; 63(1): 55–65. [DOI] [PubMed] [Google Scholar]
- 161.Kimura K., Yanagida Y., Haruyama T., Kobatake E., Aizawa M. Electrically induced neurite outgrowth of PC12 cells on the electrode surface. Med Biol Eng Comput. 1998; 36(4): 493–8. [DOI] [PubMed] [Google Scholar]
- 162.Kerns J.M., Fakhouri A.J., Weinrib H.P., Freeman J.A. Electrical stimulation of nerve regeneration in the rat: The early effects evaluated by a vibrating probe and electron microscopy. Neuroscience 1991; 40(1): 93–107. [DOI] [PubMed] [Google Scholar]
- 163.Wood M., Willits R.K. Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 2006; 27(4): 328–31. [DOI] [PubMed] [Google Scholar]
- 164.Ou Y.T., Lu M.S., Chiao C.C. The effects of electrical stimulation on neurite outgrowth of goldfish retinal explants. Brain Res. 2012; 1480: 22–9. [DOI] [PubMed] [Google Scholar]
- 165.Chang Y.J., Hsu C.M., Lin C.H., Lu M.S., Chen L. Electrical stimulation promotes nerve growth factor-induced neurite outgrowth and signaling. Biochim Biophys Acta 2013; 1830(8): 4130–6. [DOI] [PubMed] [Google Scholar]
- 166.Udina E., Furey M., Busch S., Silver J., Gordon T., Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008; 210(1): 238–47. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Z., Rouabhia M., Wang Z., Roberge C., Shi G., Roche P., Li J., Dao L.H. Electrically conductive biodegradable polymer composite for nerve regeneration: Electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs 2007; 31(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 168.Ariza C.A., Fleury A.T., Tormos C.J., Petruk V., Chawla S., Oh J., Sakaguchi D.S., Mallapragada S.K. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev. 2010; 6(4): 585–600. [DOI] [PubMed] [Google Scholar]
- 169.Yamada M., Tanemura K., Okada S., Iwanami A., Nakamura M., Mizuno H., Ozawa M., Ohyama-Goto R., Kitamura N., Kawano M., Tan-Takeuchi K., Ohtsuka C., Miyawaki A., Takashima A., Ogawa M., Toyama Y., Okano H., Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells 2007; 25(3): 562–70. [DOI] [PubMed] [Google Scholar]
- 170.Yang Y., Ma T., Ge J., Quan X., Yang L., Zhu S., Huang L., Liu Z., Liu L., Geng D., Huang J., Luo Z. Facilitated neural differentiation of adipose tissue-derived stem cells by electrical stimulation and Nurr-1 gene transduction. Cell Transplant. 2016; 25(6): 1177–91. [DOI] [PubMed] [Google Scholar]


