Abstract
We investigated the effects of transplantation of CXCR4-overexpressing adipose tissue-derived stem cells (ADSCs) into a mouse diabetic hindlimb ischemia model on homing and engraftment as early as 48 h after transplant. CXCR4-overexpressing ADSCs were intramuscularly or intravenously injected into diabetic mice with hindlimb ischemia. After 48 h, muscle tissues in the femur and tibia were collected, and the CXCR4 expression pattern was analyzed by immunofluorescence staining. The homing and engraftment of transplanted CXCR4-overexpressing ADSCs into the ischemic area were significantly increased, and intravenous (systemic) injection resulted in the more effective delivery of stem cells to the target site 48 h posttransplantation. Furthermore, CXCR4-overexpressing ADSCs more efficiently contributed to long-term engraftment and muscle tissue regeneration than normal ADSCs in a limb ischemia model. In addition, the homing and engraftment of ADSCs were correlated with the CXCR4 transfection efficiency. These results demonstrated that enhanced CXCR4 signaling could significantly improve the early homing and engraftment of ADSCs into ischemic areas as well as the long-term engraftment and ultimate muscle tissue regeneration.
Keywords: Adipose tissue-derived stem cells (ADSCs), C-X-C chemokine receptor type 4 (CXCR4), Homing and engraftment, Diabetic limb ischemia, Muscle tissue regeneration
Introduction
Diabetes mellitus (DM) is a major cause of a variety of severe complications, such as cardiovascular-, renal-, nervous system-, and ischemic-related diseases1. Improperly controlled or long-term DM may cause problems in blood vessel-related tissues or organs, leading to premature vascular disease (PVD)2.
Diabetic hindlimb ischemia involves muscle infarction resulting from poorly controlled and long-term DM. Critical hindlimb ischemia originated from DM causes arterial occlusive disease and reduces the formation of new blood vessels. Therefore, ischemic tissue may undergo necrosis because of poor nutrient supply at the injured site3. Many approaches have been attempted to treat diabetic ischemic disease, including angioplasty, femorodistal bypass4, application of vascular endothelial cell growth factor (VEGF) and hepatocyte growth factor (HGF)5 gene transfer, and stem cell transplantation, such as with hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and adipose tissue-derived stem cells (ADSCs)6–8. Nevertheless, no effective therapeutic method has been developed that can completely cure diabetic hindlimb ischemic disease.
When a tissue or an organ is damaged by disease or injury, signaling is induced throughout the area to initiate the repair of the damage. This signaling may recruit preexisting stem cells or various precursor cells such as HSCs, MSCs, and endothelial precursor cells (EPCs) or involve chemokines. Chemokine stromal cell-derived factor-1α (SDF-1α), a chemotaxic recruiting agent, is the main factor that induces the migration of stem cells to repair damaged tissue9–11.
C-X-C chemokine receptor type 4 (CXCR4) is the receptor for SDF-1, and SDF-1α/CXCR4 signaling plays an important role in the migration of preexisting or externally transplanted stem cells to the damaged site12–16. Recent studies have reported that only a small population of stem cells expresses CXCR4 and that the transplantation of CXCR4+ stem cells enhanced angiogenesis in ischemic disease17–19.
Among the population of stem cells, only a small number express CXCR420,21. In addition, when numerous stem cells are transplanted into the ischemic tissue, therapeutic expectancy is limited due to the diminution of stem cell viability in the poor conditions of the ischemic tissue22,23. To overcome these obstacles, we enhanced CXCR4 expression in ADSCs using lentiviral infection techniques and then determined whether CXCR4-overexpressing ADSCs could improve homing and engraftment in a diabetic hindlimb ischemia model.
Previously, we demonstrated the effects of human ADSCs on the healing of ischemic wounds in a diabetic nude mouse model24. In the current study, we used modified ADSCs that were transfected with CXCR4 to improve engraftment as well as ischemic wound healing in limb ischemia. We also compared local, direct intramuscular (IM) injection and systemic, intravenous (IV) injection as ADSC transplantation techniques and evaluated the effects on the site-specific homing of CXCR4-overexpressing ADSCs.
Materials and Methods
Induction of Hindlimb Ischemia, DM, and DM Hindlimb Ischemia
Animal procedures were approved by the Asan Medical Center Animal Care Committee (University of Ulsan College of Medicine, Seoul, South Korea). BALB/c-nu mice (male, 8 weeks old; Orient Bio Inc., Seongnam, South Korea) were used in this study. All animals were fed a standard pellet laboratory diet, provided water ad libitum, and maintained in the laboratory of animal research at the Asan Institute for Life Sciences, Seoul, South Korea. The animals were kept in a clean conventional environment using microisolator cages and racks. All animals were given sterilized drinking water and autoclaved bedding, maintained on a 7:00 am–7:00 pm light–dark cycle, and acclimatized to laboratory conditions for 1 week before use.
The animal model of hindlimb ischemia was developed in nude mice, and the model of diabetic hindlimb ischemia was developed in male nude mice (BALB/c-nu, 8 weeks old; Orient Bio Inc.). All animal procedures were approved by the Asan Medical Center Animal Care Committee. DM was induced by a single peritoneal injection of 150 mg/kg streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA) in 0.01 M sodium citrate (pH 4.5; Sigma-Aldrich). Blood glucose levels were checked daily and kept between 400 and 500 mg/dl. After 3 weeks, hindlimb ischemia was induced in the diabetic mice model. Anesthesia was induced by peritoneal injection using a 3:2 mixture of tiletamine/zolazepam (Zoletil; Virbac, Carros, France) and xylazine (Rompun; Bayer Korea, Seoul, South Korea) with a dose of 5 mg/kg Zoletil25. Skin incision at the femoral region was performed by surgical blade, and the femoral artery and branch (near external iliac artery) were then ligated twice using titanium clips (Ligaclip Small; Ethicon Inc., Somerville, NJ, USA), and skin was sutured by 4-0 nylon suture (Ethiolon; Ethicon Inc.).
Mixed-breed canines were purchased from Orient Bio Inc., with approximately 25 kg of body weight. Canine ischemic models were generated as follows. Under general anesthesia, the femoral arteries in both lower limbs were dissected and encircled with an ameroid constrictor (Research Instruments NW, Inc., Lebanon, OR, USA) with an inner diameter of 1.5 mm. Cefazolin (20 mg/kg; Chong Kun Dang Pharmaceutical Corp., Seoul, South Korea) was subsequently injected intramuscularly twice a day for 3 days. After 4 weeks, we confirmed the complete occlusion of the femoral artery by Doppler examination. Stem cells were injected intramuscularly in the right limbs of the canines, while an equal volume of normal saline was injected into the same muscle location in the control (left) ischemic limbs. A total of 1 × 107 ADSCs (RNL Bio, Seoul, South Korea) were injected. Eight weeks after cell transplantation, the canines were reanesthetized. Ischemic muscles that underwent cell injection were harvested for histologic analysis.
Cell Preparation
ADSCs were obtained from RNL Bio. Cells were maintained in minimum essential medium-α (MEM-α) (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco) and 1% antibiotic/antimycotic solution (Gibco). ADSCs were maintained at 37°C in a humidified atmosphere containing 5% CO2 in basal culture medium (Gibco), which was changed every 3 days.
Construction and Production of Lentiviral Vectors
The genetic manipulation of cells was approved by Korea University (Seoul, South Korea), and the handling facility for lentivirus was assigned at Hana Science Building, Room #616, Korea University. The hCXCR4 complementary DNA (cDNA; NdeI–MluI 1.0-kb fragment) was cloned into the pWPI lentiviral vector (Fig. 1), which was a gift from Dr. Trono (Université de Genève, Switzerland), between the elongation factor 1-α (EF1-a) promoter and the encephalomyocarditis virus (EMCV) internal ribosomal entry site (IRES)-green fluorescent protein (GFP). The same vector carrying a GFP gene, pWPT-GFP (plasmid #12255; Addgene, Cambridge, MA, USA), was used as a negative control (with comparable results). Lentiviral particles were produced by transient transfection of 293T cells using a calcium phosphate transfection technique. The following plasmids were used: a packaging plasmid (psPAX2; plasmid #12260; Addgene), an envelope plasmid (pMD2.G; plasmid #12259; Addgene) from the vesicular stomatitis virus glycoprotein envelope (VSV-G), and transfer vectors (pWPI and pWPT) as described previously26,27. Briefly, 5–6 × 106 293FT cells were seeded onto 100-mm tissue culture dishes 24 h before transfection. The three-plasmid mixture consisted of 15 μg of packaging plasmid, 6 μg of envelope plasmid, and 20 μg of vector plasmid, proportions that had been empirically shown to maximize vector particle production. The medium conditioned by vector-producing cells was harvested 48 h later, cleared by centrifugation, and filtered through a 0.45-μm filter. The medium was then ultracentrifuged at 100,000 × g for 2 h at 4°C in a Beckman Optima X series ultracentrifuge. After the spin supernatant was discarded, the virus was resuspended in the desired volume of phosphate-buffered saline (PBS)–1% bovine serum albumin (BSA) (Sigma-Aldrich), and aliquots were stored at −70°C for further analysis. Determination of the titer of each viral supernatant was performed by assessing GFP expression by flow cytometry.
Figure 1.

The cloning map for green fluorescent protein (GFP) and human C-X-C chemokine receptor type 4 (hCXCR4) into pWPI lentiviral vector. The hCXCR4 complementary DNA (cDNA) (NdeI–MluI 1.0-kb fragment) was cloned into the pWPI lentiviral vector between the EF1-α promoter and the EMCV IRES-GFP. Abbreviations: Ampr, ampicillin resistance gene; F1 ori, origin of replication from f1 phage; pGEM, plasmid for cloning CXCR4 gene; Bgl2, restriction endonuclease from Bacillus globigii; Spe1, restriction endonuclease from Sphaerotilus species; LTR, long terminal repeat; pWPI-MCS, plasmid from Addgene (plasmid #12254) with multicloning site (MCS); Mlu, restriction endonuclease from Micorcoccus luteus; Nde1, restriction endonuclease from Neisseria denitrficans; BamH1, restriction endonuclease H1 from Bacillus amyloliquefaciens; EF1-α, elongation factor 1-α; EMCV IRES, encephalomyocarditis virus internal ribosomal entry site.
Delivering the CXCR4/GFP Genes Using the Lentiviral Vector
ADSCs were infected with the lentiviral vector carrying the GFP or GFP/CXCR4 genes. The lentiviral vector included the pWPT-GFP plasmid only or a combination of the pWPT-GFP and pWPI-CXCR4 plasmids. For lentiviral transduction, 1 × 106 cells were cultured in 100-mm culture dishes. Cells were then mixed with conditioned medium containing the viral vector and harvested after 72 h in culture. To determine the efficiency of viral infection, more than 10,000 labeled cells were acquired, and the GFP and CXCR4 expression pattern was analyzed using a FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA).
Transplantation of Cells
ADSCs in Hank's balanced salt solution (HBSS; Gibco) were transplanted 24 h after induction of diabetic hindlimb ischemia at a density of 1 × 106 cells per mouse by IM or IV injection.
Experimental Groups
Set 1. A total of 24 mice were divided into the following six groups: (1) DM only, (2) DM ischemia, (3) DM ischemia and GFP-hADSC (IM injection), (4) DM ischemia and GFP-hADSC (IV injection), (5) DM ischemia and CXCR4-hADSC (IM injection), and (6) DM ischemia and CXCR4-hADSC (IV injection). Then 48 h after cell transplantation, muscle tissues that underwent cell injection were harvested for histologic analysis.
Set 2. A total of 20 mice were divided into the following five groups: (1) ischemia only, (2) ischemia and GFP-hADSC (IM injection), (3) ischemia and GFP-hADSC (IV injection), (4) ischemia and CXCR4-hADSC (IM injection), and (5) ischemia and CXCR4-hADSC (IV injection). Then 96 h after cell transplantation, muscle tissues that underwent cell injection were harvested for histologic analysis.
Set 3. Two canines (four legs) were used for an ischemia model and were divided into four groups: two legs for ischemia only, one leg for ischemia and GFP-hADSC (IM injection), and one leg for ischemia and CXCR4-hADSC (IM injection). Then 8 weeks after cell transplantation, muscle tissues that underwent cell injection were harvested for histologic analysis.
Histological Analysis
Animals were sacrificed at different time points after transplantation by CO2 inhalation. Muscle tissues from the femur and tibia were obtained at autopsy. The samples were embedded in optimum cutting temperature (OCT) compound and stored at −80°C. Frozen tissues were sectioned into 4-μm-thick slices, placed on slides, and stained with Masson's trichrome (MT) (Sigma-Aldrich).
Immunofluorescence Analysis
Frozen sections were fixed in acetone for 15 min at room temperature (RT). Fixed slides were washed in PBS and incubated for 1 h with PBS including 20% normal goat serum (NGS) (Sigma-Aldrich) and 1% BSA at RT. Sections were incubated with mouse anti-human GFP primary antibody (Chemicon, Temecula, CA, USA) for 1 h 30 min at RT. After rinsing with PBS (0.1% Tween 20; Sigma-Aldrich), fluorescein isothiocyanate (FITC)-labeled secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was applied for 2 h 30 min at RT. After rinsing with PBS (0.1% Tween 20), secondary blocking was performed with PBS including 20% donkey serum (Sigma-Aldrich) and 1% BSA. Sections were then incubated with CXCR4 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc.) for 1 h 30 min at RT, followed by rinsing with PBS (0.1% Tween 20). The sections were incubated with cyanine3 (Cy3)-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA, USA) for 2 h 30 min at RT and then incubated with 4′,6-diamidino-2 phenylindole dihydrochloride (DAPI) (Sigma-Aldrich) for 5 min at RT. After rinsing with PBS (0.1% Tween 20), sections were mounted with 50% glycerol–PBS including 0.5 mM ascorbic acid (Sigma-Aldrich) and examined with an Olympus 1X71 inverted fluorescence microscope (Olympus Corp., Tokyo, Japan). Fluorescence intensity was quantified using ImageJ software (ImageJ for Windows, Version 1.50i; NIH, Bethesda, MD, USA; available in the public domain at http://rsb.info.nih.gov/ij/index.html), and statistical significance was analyzed using SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA).
Angiography
The canines were reanesthetized 8 weeks after cell transplantation. After exposure of the abdominal aorta, a contrast dye (Ultravist 370; Schering, South Korea) was injected into the abdominal aorta and the angiograms of the limbs were obtained. After angiography, the ischemic muscles that underwent cell injection were harvested for histological analysis.
Results
Flow Cytometric Analysis of GFP and CXCR4 Expression in Modified ADSCs
Lentiviral-mediated CXCR4 overexpression in ADSCs was verified by fluorescence-activated cell sorting (FACS) analysis. The FACS data showed that CXCR4 expression was significantly higher (99.92%) in CXCR4-overexpressing ADSCs than in ADSCs transfected with the GFP vector only (3.36%) (Fig. 2).
Figure 2.
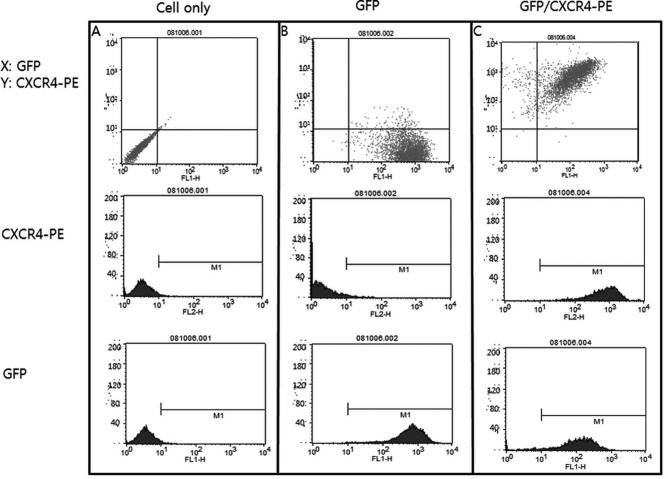
Flow cytometric analysis of GFP and CXCR4 expression in transfected adipose tissue-derived stem cells (ADSCs). Each group of ADSCs was modified with the GFP vector (A) or GFP/CXCR4 vector (B, C). Modified ADSCs were stained with PE-conjugated anti-CXCR4 antibody and analyzed. ADSCs with only the GFP vector (B) were positive for GFP (99.83%), and CXCR4-overexpressing ADSCs (C) were positive for GFP (96.04%) and CXCR4 (99.92%).
Transfection Efficiency of CXCR4 Analyzed by FACS
Although the transfection method had been preoptimized previously, the transfection efficiency was different in each ADSC batch and experiment. In the set 1 experiment, both GFP and CXCR4 expression levels were very high, at over 98% of transfected cells (Fig. 2 and Table 1). In the set 2 experiment, GFP+ cells represented about 65.4% and CXCR4+ cells represented about 16.5% of total transfected cells, respectively, in each group. In the set 3 experiment, GFP+ cells represented over 98% but CXCR4+ cells represented around 66.9% of total transfected cells (Table 1). Interestingly, the engrafting pattern seems to be correlated with CXCR4 transfection efficiency (Figs. 3–8).
Table 1.
Transfection Efficiency and Constitutive Expression Level of CXCR4 in ADSCs Were Analyzed by FACS (Constitutive Expression Level of CXCR4 Was Determined Without Transfection)
| Transfection Efficiency of GFP (%) |
Transfection Efficiency of CXCR4 (%) | |
|---|---|---|
| Experiment | (CXCR4+ Cells Without Transfection) | |
| Set 1 | 99.83 (>3.36) | 99.92 |
| Set 2 | 65.43 (>0.1) | 16.57 |
| Set 3 | 98.16 (>5.65) | 66.94 |
Figure 3.

Detection of CXCR4+ and GFP+ by immunofluorescent (IF) staining in diabetic mouse ischemic muscle after 48 h posttransplantation. IF staining was performed for GFP (FITC, green) and CXCR4 [cyanine3 (Cy3), red], and nuclei were stained with DNA-binding dye [4′,6-diamidino-2 phenylindole dihydrochloride (DAPI), blue]. GFP and CXCR4 expression levels were increased significantly in the intravenous (IV) group of CXCR4-overexpressing ADSCs compared to other experimental groups after 48 h. Overall CXCR4-overexpressing ADSCs showed much higher engraftment than GFP groups. Damaged site in the diabetic ischemic muscle was analyzed by Masson's trichrome (MT) staining. DM, diabetes mellitus; i.m., intramuscular; i.v., intravenous. Original magnification: 200×, scale bar: 50 μM.
Figure 8.

Quantification of fluorescence intensity of Figure 7 was done using ImageJ software (ImageJ for Windows, Version 1.50i), and statistical significance was analyzed using SPSS Statistics for Windows, Version 23.0 (IBM Corp.). All results were statistically significant (∗p < 0.05, ∗∗p < 0.01).
CXCR4+ and GFP+ Cells Were Detected by Immunofluorescent (IF) Staining in Mouse Diabetic Hindlimb Ischemic Muscle 48 h Posttransplantation
Homing and engraftment of CXCR4-overexpressing ADSCs in diabetic hindlimb ischemia were assessed 48 h posttransplantation. In the set 1 experiment, the transfection rate was as high as 99.83% for GFP and 99.92% for CXCR4 (Fig. 2 and Table 1). The level of CXCR4 expression was investigated by IF staining. GFP and CXCR4 were predominantly expressed in the diabetic ischemic hindlimb muscle in GFP/CXCR4-transfected ADSCs. CXCR4-overexpressing ADSCs migrated and engrafted to a higher degree in the damaged muscle site than ADSCs expressing GFP alone at 48 h posttransplantation in both the IM and IV injection groups. In the GFP-alone group with direct IM injection of cells into the damaged hindlimb site, almost no cells were detected 48 h posttransplantation (Fig. 3). Fluorescence intensity was quantified using ImageJ software (ImageJ for Windows, Version 1.50; NIH), and statistical significance was analyzed using SPSS. All results were statistically significant (p < 0.01, p < 0.001) (Fig. 4).
Figure 4.

Quantification of fluorescence intensity of Figure 3 was done using ImageJ software (ImageJ for Windows, Version 1.50i), and statistical significance was analyzed using SPSS Statistics for Windows, Version 23.0 (IBM Corp.). All results were statistically significant (∗∗p < 0.01, ∗∗∗p < 0.001).
CXCR4+ and GFP+ Cells Were Detected by IF Staining in Mouse Hindlimb Ischemic Muscle 96 h Posttransplantation
Homing and engraftment of CXCR4-overexpressing ADSCs in hindlimb ischemia were assessed 96 h posttransplantation. In the set 2 experiment, the transfection rate was as low as 65.43% for GFP and 16.57% for CXCR4 (Table 1). CXCR4 transfection efficiency is almost one fourth of GFP (Table 1), but CXCR4-transfected cells are still good for homing into ischemic muscle as GFP-transfected cells (Figs. 5 and 6).
Figure 5.

Detection of CXCR4+ and GFP+ cells by IF staining in mouse ischemic muscle after 96 h posttransplantation. IF staining was performed for GFP (green) and CXCR4 (Cy3, red), and nuclei were stained with DNA-binding dye (DAPI, blue). GFP and CXCR4 expression levels were similar both in the IM and IV group of GFP- and CXCR4-overexpressing ADSCs. In this set of experiment, CXCR4 transfection efficiency was less than one fourth of GFP's. Because of the low transfection efficiencies, the CXCR4-transfected cells were not dominant in the damaged muscle site. The damaged site in the diabetic hindlimb ischemic muscle was analyzed by MT staining. i.m., intramuscular; i.v., intravenous. Original magnification: 200×, scale bar: 50 μM.
Figure 6.

Quantification of fluorescence intensity of Figure 5 was done using ImageJ software (ImageJ for Windows, Version 1.50i), and statistical significance was analyzed using SPSS Statistics for Windows, Version 23.0 (IBM Corp.). All results were statistically significant (∗p < 0.05, ∗∗∗p < 0.001).
CXCR4+ and GFP+ Cells Were Detected by IF Staining in Canine Hindlimb Ischemic Muscle 8 Weeks Posttransplantation
Homing and engraftment of CXCR4-overexpressing ADSCs in canine hindlimb ischemia were assessed 8 weeks posttransplantation. In the set 3 experiment, long-term engrafting and muscle regeneration were studied using a canine ischemia model. The transfection rate was 98.2% for GFP and 66.94% for CXCR4 (Table 1). CXCR4-transfected cells were engrafted successfully into the damaged muscle site and significantly improved muscle regeneration (Figs. 7 and 8). Of the control ADSCs (untreated cells) only 5.6% expressed CXCR4, whereas the CXCR4-overexpressing group had an expression level of 66.9% (Table 1). Homing and engraftment rate were critically correlated with CXCR4 expression levels. Higher homing and engraftment as well as ultimate long-term muscle tissue regeneration were observed in the CXCR4-overexpressing group as expected. All results were statistically significant (p < 0.05, p < 0.01).
Figure 7.

Detection of CXCR4+ and GFP+ cells by IF staining in canine ischemic muscle after 8 weeks posttransplantation. IF staining was performed for GFP (green) and CXCR4 (Cy3, red), and nuclei were stained with DNA-binding dye (DAPI, blue). After 8 weeks, GFP and CXCR4 expression levels were detected in the IM injection group of CXCR4-overexpressing ADSCs. The damaged site in the canine hindlimb ischemic muscle was analyzed by MT staining. i.m., intramuscular. Original magnification: 200×, scale bar: 50 μM.
Angiography Results for Confirmation of Angiogenesis Effect in the Canine Ischemia Model
GFP- and CXCR4-overexpressing ADSC-treated limbs showed improved vascularity compared to the control ischemia limbs after 8 weeks of transplantation through angiographic analysis (Fig. 9). The CXCR4-overexpressing treated limbs exhibited the most abundant vascularity. Though the ischemic muscle tissue regeneration rate is lower in the GFP group, angiogenesis seems to be promoted at a similar rate as the CXCR4-overexpressing group (Fig. 9). We injected about 1 × 107 human ADSCs into a canine ischemia model, but no immunological responses were detected.
Figure 9.

Angiography results for confirmation of angiogenesis effect. Eight weeks after cell transplantation, the angiograms of the limbs were obtained. GFP- and CXCR4-overexpressing ADSC-treated limbs showed improved vascularity compared to the control ischemia limbs. The CXCR4-overexpressing treated limbs exhibited the most abundant vascularity. Ischemia only: normal saline, GFP: 1 × 107 ADSCs, CXCR4: 1 × 107 CXCR4-overexpressing ADSCs. Scale bar is marked at the right side.
Discussion
Methods to improve angiogenesis and muscle regeneration following diabetic hindlimb ischemia by transplantation of proangiogenic factors and stem or progenitor cells have been widely investigated28–30. CXCR4/SDF-1α signaling is a crucial factor for the homing, migration, and engraftment of stem cells31–33. The upregulated expression of SDF-1α, CXCR4, and VEGF in the ischemic tissue is the first step in the process of stem cell homing. In addition, because only a limited number of stem cells among all those transplanted reach and contribute to regeneration in the ischemic tissue at the initial phase of transplantation34,35, it is necessary to enhance the viability and homing ability of transplanted stem cells.
SDF-1α, CXCR4, and Akt signaling are homing- and survival-related factors, and the presence of this signaling at the cell transplantation site can improve stem cell homing and survival36. Pasha et al.37 suggested that preconditioning with SDF-1α enhanced the survival, proliferation, and engraftment of MSCs via SDF-1α/CXCR4 signaling in infarcted myocardium. Moreover, Zhang et al.38 demonstrated that overexpression of CXCR4 in MSCs enhanced in vivo mobilization and neomyoangiogenesis in the ischemic area.
Tissues under ischemic conditions release stem cell-recruiting factors such as SDF-1α and proangiogenic factors to promote vasculogenesis to repair the ischemic area13,39–41. On the other hand, DM interferes with these self-recovering processes39, so diabetic ischemia is more likely to worsen than general cases of ischemic disease. In cases of DM-complicated ischemia, the enhancement of stem cell homing efficiency or viability may be more necessary than in non-DM-related cases to improve the chances of obtaining an appropriate number of transplanted stem cells in the damaged area and achieving a good therapeutic effect.
In the present study, we transfected CXCR4 into ADSCs using a lentiviral-mediated infection technique, and the efficiency of ADSC CXCR4 viral infection reached 99.92% (Fig. 2). These data suggested that our CXCR4-overexpressing ADSCs could reach the site of ischemic muscle more efficiently than pure normal ADSCs. In fact, we demonstrated that the homing of CXCR4-overexpressing ADSCs significantly increased at the ischemic muscle site compared with ADSCs containing the GFP control vector (Fig. 3).
We also showed enhanced homing and engraftment at the diabetic ischemic injury site in the early stage using systemic (IV) and local (IM) injection of CXCR4-overexpressing ADSCs. As early as 48 h after transplantation, CXCR4 expression seems to be critical for homing and engrafting at the damaged site (Table 1 and Figs. 3 and 4). When the CXCR4 transfection rate was low (as low as one fourth the transfection efficiency of the GFP group), the homing and engrafting patterns were similar in both groups (Table 1 and Figs. 5 and 6). Furthermore, when evaluating long-term engraftment and muscle regeneration using a canine ischemia model, the CXCR4-transfected ADSC group not only engrafted successfully into the damaged muscle site but also significantly improved muscle tissue regeneration (Figs. 7 and 8).
Regarding cell transplantation systems, Yoshida et al.42 suggested that the conditions in ischemic muscle may sometimes be too severe for directly transplanting stem cells. In addition, they demonstrated that intra-arterial administration offered similar angiogenic effects as IM injection in a rat hindlimb ischemia model42. Although many other studies used IM injection techniques for transplantation in ischemic disease7,43,44, in our system the muscles were exposed to DM for a long time prior to ischemic induction, so the ischemic tissues in our model might have been more severely impaired by DM than in general cases of ischemic muscle. We hypothesized that the systemic transplantation via IV injection might enhance the homing of ADSCs due to avoiding direct contact with the severe conditions in the diabetic ischemic tissue. For severely damaged tissue, as in diabetic limb ischemia, systemic IV injection of stem cells is much more effective than direct IM injection (Figs. 3 and 4).
The set 2 experiment results came out differently as those of the set 1 experiment. Since GFP gene transfection rate was nearly four times higher than GFP/CXCR4, the homing and engraftment rate of the GFP/CXCR4 group was not as good as in the set 1 experiment. Low fluorescence detection at 96 h posttransplantation may originate from the low transfection rate of ADSCs or low cell viability (after transplantation) compared to 48 h posttransplantation. However, we can still speculate that if transfection rates were equivalent in both groups, the CXCR4-overexpressing group (IV) could have been much higher in homing efficiency than the GFP group (IV) (Fig. 6).
ADSCs have been widely used as a therapeutic cell source in ischemic disease17,45 due to their advantages of being simple to obtain and having similar properties as MSCs46. There have been many studies on cell-based therapeutic approaches for treating hindlimb ischemia or myocardial infarction38,42 and skeletal muscle regeneration41,47, but no successful approach for treating diabetic hindlimb ischemia has been developed.
Overexpression of CXCR4 increases not only migration to the damaged site but also the proliferation of human ADSCs. In this study, we discovered a new therapeutic potential of ADSCs using CXCR4 overexpression techniques and demonstrated that CXCR4-overexpressing ADSCs successfully migrated and engrafted in an animal model of diabetic ischemia. We also showed that CXCR4-overexpressing ADSCs contributed to long-term engraftment and muscle regeneration. Moreover, CXCR4-mediated homing and engrafting were directly correlated with the CXCR4 expression level.
Even in the xenotransplantation setting, there were no signs for human stem cell graft rejection in the canine ischemia model. Since the homing and engraftment rate is critically correlated with the CXCR4 expression level, though ischemic muscle tissue regeneration rate is lower in the GFP group, angiogenesis seems to be similar as that in the CXCR4-overexpressing group (Fig. 9). It could be hypothesized that CXCR4− ADSCs might not directly engraft into muscle tissue but contribute to tissue regeneration by promoting angiogenesis. The detailed mechanism and the reason why only CXCR4-overexpressing human ADSCs can engraft for a long time without rejection in the canine ischemia model need to be investigated further.
In summary, although we did not investigate the detailed mechanism in the present study, the CXCR4 molecule is critical for the early homing and engrafting of ADSCs as well as for long-term engraftment and skeletal muscle regeneration including angiogenesis48,49 via the SDF-1α/CXCR4 signaling pathway12. The systemic transplantation of CXCR4-overexpressing cells may serve as an effective therapeutic technique for regenerating muscle in DM-related severe ischemic injuries.
Acknowledgments
The authors thank Seung A. Kim and Min H. Kim for their technical support and Dr. Ae K. Kim for angiography. This work was supported by grants from KISTEP, South Korea (#SC3280) and Korea University grant to Dr. MiJung Kim, and School of Life Sciences and Biotechnology for BK21 PLUS, Korea University. The authors declare no conflicts of interest.
References
- 1.Naderi AS, Farsian FN, Palmer BF. Diabetic muscle necrosis. J Diabetes Complications 2008; 22: 150–2. [DOI] [PubMed] [Google Scholar]
- 2.Creager MA, Lüscher TF, Cosentino F, Beckman JA,. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003; 108: 1527–32. [DOI] [PubMed] [Google Scholar]
- 3.Lévigne D, Tobalem M, Modarressi A, Pittet-Cuénod B. Hyperglycemia increases susceptibility to ischemic necrosis. Biomed Res Int. 2013; 2013: 490964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007; 3: 65–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: Molecular mechanisms of delayed angiogenesis in diabetes. Circulation 2001; 104: 2344–50. [DOI] [PubMed] [Google Scholar]
- 6.Kirana S, Stratmann B, Lammers D, Negrean M, Stirban A, Minartz P, Koerperich H, Gastens MH, Götting C, Prohaska W, Kleesiek K, Tschoepe D,. Wound therapy with autologous bone marrow stem cells in diabetic patients with ischaemia-induced tissue ulcers affecting the lower limbs. Int J Clin Pract. 2007; 61: 690–2. [DOI] [PubMed] [Google Scholar]
- 7.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006; 17: 279–90. [DOI] [PubMed] [Google Scholar]
- 8.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005; 25: 2542–7. [DOI] [PubMed] [Google Scholar]
- 9.Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013; 45: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation 2010; 122: 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennert RC, Sorkin M, Garg RK, Gurtner GC. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen Med. 2012; 7: 833–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012; 94: 400–7. [DOI] [PubMed] [Google Scholar]
- 13.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004; 35: 233–45. [DOI] [PubMed] [Google Scholar]
- 14.Penn MS. Importance of the SDF-1: CXCR4 axis in myocardial repair. Circ Res. 2009; 104: 1133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Togel FE, Westenfelder C. Role of SDF-1 as a regulatory chemokine in renal regeneration after acute kidney injury. Kidney Int Suppl. 2011; 1: 87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4: From bench to bedside. Clin Cancer Res. 2008; 14: 7975–80. [DOI] [PubMed] [Google Scholar]
- 17.Kondo K, Shinatani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009; 29: 61–6. [DOI] [PubMed] [Google Scholar]
- 18.Shiba Y, Takahashi M, Hata T, Murayama H, Morimoto H, Ise H, Nagasawa T, Ikeda U. Bone marrow CXCR4 induction by cultivation enhances therapeutic angiogenesis. Cardiovasc Res. 2009; 81: 169–77. [DOI] [PubMed] [Google Scholar]
- 19.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I,. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004; 104: 2643–5. [DOI] [PubMed] [Google Scholar]
- 20.Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica 2007; 92: 897–904. [DOI] [PubMed] [Google Scholar]
- 21.Thangarajah H, Vial I, Chang E, El-Ftesi S, Januszyk M, Chang EI, Paterno J, Neofytou E, Longaker MT, Gurtner GC,. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 2009; 27: 266–74. [DOI] [PubMed] [Google Scholar]
- 22.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9: 1195–201. [DOI] [PubMed] [Google Scholar]
- 23.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008; 2(3): 205–13. [DOI] [PubMed] [Google Scholar]
- 24.Kim EK, Li G, Lee TJ, Hong JP. The effect of human adipose-derived stem cells on healing of ischemic wounds in a diabetic nude mouse model. Plast Reconstr Surg. 2011; 128: 387–94. [DOI] [PubMed] [Google Scholar]
- 25.Krishna SM, Omer SM, Golledge J. Evaluation of the clinical relevance and limitations of current pre-clinical models of peripheral artery disease. Clin Sci. (Lond) 2016; 130(3): 127–50. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani HR, Cario-André M, Pain C, Ged C, deVerneuil H, Taïeb A. Protection of normal human reconstructed epidermis from UV by catalase overexpression. Cancer Gene Ther. 2007; 14: 174–86. [DOI] [PubMed] [Google Scholar]
- 27.Rezvani HR, Mazurier F, Cario-André M, Pain C, Ged C, Taïeb A, deVerneuil H,. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J Biol Chem. 2006; 281: 17999–18007. [DOI] [PubMed] [Google Scholar]
- 28.Hazarika S, Dokun AO, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: Differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007; 101: 948–56. [DOI] [PubMed] [Google Scholar]
- 29.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA,. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: A double-blind randomized trial. Hum Gene Ther. 2006; 17: 683–91. [DOI] [PubMed] [Google Scholar]
- 30.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999; 154: 355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006; 7: 19–24. [DOI] [PubMed] [Google Scholar]
- 32.Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P. Concise review: Diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl Med. 2014; 3: 949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007; 28: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA 2006; 103: 2494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008; 45: 567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haider HKh, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008; 45: 554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008; 77: 134–42. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. overexpression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008; 44: 281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008; 283: 10930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC, Jung JS. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006; 15: 853–64. [DOI] [PubMed] [Google Scholar]
- 41.Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells. J Cell Sci. 2006; 119: 2945–52. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M, Horimoto H, Mieno S, Nomura Y, Okawa H, Nakahara K, Sasaki S. Intra-arterial bone marrow cell transplantation induces angiogenesis in rat hindlimb ischemia. Eur Surg Res. 2003; 35: 86–91. [DOI] [PubMed] [Google Scholar]
- 43.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells 2006; 24: 1620–6. [DOI] [PubMed] [Google Scholar]
- 44.Sepulveda P, Martinez-Leon J, Garcia-Verdugo JM. Neoangiogenesis with endothelial precursors for the treatment of ischemia. Transplant Proc. 2007; 39: 2089–94. [DOI] [PubMed] [Google Scholar]
- 45.Murohara T. Autologous adipose tissue as a new source of progenitor cells for therapeutic angiogenesis. J Cardiol. 2009; 53: 155–63. [DOI] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH,. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M, Choi YS, Yang SH, Hong HN, Cho SW, Cha SM, Pak JH, Kim CW, Kwon SW, Park CJ. Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem Biophys Res Commun. 2006; 348: 386–92. [DOI] [PubMed] [Google Scholar]
- 48.Kim MH, Hong HN, Hong JP, Park CJ, Kwon SW, Kim SH, Kang G, Kim M. The effect of VEGF on the myogenic differentiation of adipose tissue derived stem cells within thermo-sensitive hydrogel matrices. Biomaterials 2010; 31: 1213–18. [DOI] [PubMed] [Google Scholar]
- 49.Sumi M, Sata M, Toya N, Yanaga K, Ohki T, Nagai R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007; 80: 559–65. [DOI] [PubMed] [Google Scholar]


