Abstract
CD34+ progenitor cells are growing in use for vascular repair. However, in diabetic individuals with cardiovascular diseases, these cells have dysfunctional engraftment capabilities, which compromise their use for autologous cell therapy. The thrombospondin-1-derived peptide RFYVVMWK has previously been reported to stimulate cell adhesiveness through CD47 and integrin activation pathways. Our aim was to test whether RFYVVMWK preconditioning could modulate CD34+ cell phenotype and enhance its proadhesive properties in diabetic patients. Peripheral blood mononuclear CD34+ cells isolated from 40 atherosclerotic patients with type 2 diabetes (T2D; n = 20) or without (non-T2D; n = 20) were preconditioned with 30 μM RFYVVMWK or truncated peptide RFYVVM. CD34+ cell adhesion was assessed on a vitronectin–collagen matrix and on TNF-α or IL-1β-stimulated HUVEC monolayers. Adhesion receptors, platelet/CD34+ cell conjugates, and cell viability were analyzed by flow cytometry and confocal microscopy. RFYVVMWK increased the adhesion of T2D CD34+ cells by eightfold to the vitronectin–collagen matrix (p < 0.001) corresponding to a threefold increase compared to unstimulated non-T2D CD34+ cells. The peptide induced the formation of platelet/CD34+ conjugates and increased the expression of TSP-1, CD29, CD51/CD61, and CD62P in both T2D and non-T2D cells. However, RFYVVMWK treatment did not affect the viability/apoptosis of CD34+ progenitor cells. In conclusion, priming CD34+ cells with RFYVVMWK may enhance their vascular engraftment during autologous proangiogenic cell therapy.
Keywords: Thrombospondin-1 (TSP-1), Atherosclerosis, Type 2 diabetes (T2D), CD34, CD47
Introduction
CD34 is a marker of hematopoietic stem cells (HSCs) that is also expressed on several non-HSCs including endothelial and epithelial progenitors, embryonic fibroblasts, multipotent mesenchymal stromal cells (MSCs), and interstitial dendritic cells. The plasticity of CD34+ cells and their paracrine-stimulating properties on the endothelium during hypoxia make these cells potential candidates for cell transplant therapies combating ischemic diseases such as cardiac failure1.
Circulating progenitor cells are known to contribute to neoangiogenesis during numerous processes including healing, lower limb ischemia, vascular graft endothelialization, atherosclerosis, post-myocardial infarction, lymphoid organ neovascularization, and tumoral growth2. Clinical trials have demonstrated that intracoronary administration of bone marrow-derived mononuclear cells (BM-MNCs) from autologous progenitor cells or peripheral blood (PB) CD34+ cells mobilized by granulocyte colony-stimulating factor improves the left ventricular ejection fraction and reduces the size of the infarcted area3,4. However, the benefit of cell therapy is dampened by the negative impact of cardiovascular risk factors, such as atherosclerosis, obesity, and type 2 diabetes (T2D), on the number and function of progenitor cells, thereby jeopardizing their use for autologous treatment5,6. Interestingly, a reduced progenitor cell adhesion capacity has been reported in T2D5,7. Moreover, strategies aiming at preconditioning CD34+ cells or endothelial progenitor cells prior to injection in animal models of ischemia were reported to improve revascularization in vivo, together with increased adhesion capacity of cells in in vitro models8.
The ability of CD34+ cells to adhere and engraft onto damaged vessel walls is crucial to initiate neovascularization9. During this process, activated platelets are instrumental in targeting CD34+ cell recruitment to injured vessels via stromal cell-derived factor-1 (SDF-1α) secretion and chemotaxism10. Platelets also stimulate the “homing” of CD34+ cells, via CD62–CD162 interaction11 and their differentiation into mature endothelial cells8. One of the most abundant proteins secreted by activated platelets is thrombospondin-1 (TSP-1), which is a multifunctional matricellular glycoprotein bearing proatherogenic, prothrombotic, as well as both pro- and antiangiogenic properties12. The interaction of TSP-1, through its COOH terminal RFYVVMWK sequence, with the transmembrane protein CD47 [integrin-associated protein (IAP)] occurs following a conformational reorganization of the C-terminal domain of TSP-113. This interaction positively modulates the function of several integrins including CD51/CD61, CD41/CD61, and CD49b/CD29, thereby modulating cellular functions including platelet activation and adhesion, leukocyte adhesion, migration, and phagocytosis through heterotrimeric Gi protein signaling14–17.
We have previously observed that TSP-1-deficient mice exhibit a significant drop in the vessel wall recruitment of BM-MNCs in a FeCl3-induced intravascular thrombosis mouse model18. We also found that ex vivo RFYVVMWK preconditioning of mouse BM-MNCs stimulates their recruitment to sites of intravascular thrombosis induced by FeCl319. Indeed, RFYVVMWK increased BM-MNC-to-vessel wall interactions and decreased their rolling speeds to the damaged vessel wall, leading to a 12-fold increase in permanent cell engraftment19.
The goal of the present study was to analyze the proadhesive effects of RFYVVMWK preconditioning on CD34+ progenitor cells isolated from PB of atherosclerotic patients with T2D. We first explored their “proengraftment” phenotype through the measurement of a panel of biomarkers including cell adhesion receptors, platelet/CD34+ conjugates, and apoptotic markers. We next investigated whether this preconditioning could improve their capacity to adhere to stimulated endothelial cells and subendothelial components.
Material and Methods
Patients
Blood samples were drawn from participants after obtaining informed consent as part of a protocol approved by the ethics committee of the Montreal Heart Institute and in accordance with the recommendations of the Helsinki Declaration. A total of 40 adult males (>18 years old) with stable coronary artery disease or stable angina documented by angiography, all treated with antiplatelet agents and statins, were included in the study. Among these patients, 20 had T2D (T2D group) and 20 were nondiabetic (non-T2D group). The patients were predominantly hypertensive (n = 27), dyslipidemic (n = 38), overweight (n = 25), and with a smoking history (n = 27). Diabetic patients received biguanide (metformin) mono-therapy (n = 10), biguanide + sulfonylureas (glyburide or glimepiride) bitherapy (n = 6), biguanide + sulfonylureas (glyburide) + DPP-4 inhibitor (gliptin) tritherapy (n = 2), or no medication (diabetes was controlled by diet). Exclusion criteria were acute coronary syndrome (ACS) or stroke within the past 6 months, treatment with insulin, treatment with peroxisome proliferator-activated receptors (PPARs; pioglitazone and rosiglitazone), extra cardiac inflammatory syndromes, surgery within the last 8 weeks, kidney or liver failure, use of systemic corticosteroids, cancer in the last 5 years, chronic anticoagulation, heart failure [NYHA class 3 or 4 and/or left ventricular ejection fraction (LVEF) <40%], and hemoglobin <100 g/L. Six healthy adult males [healthy donors (HD)] who showed no cardiovascular disease or known T2D were also recruited if they had not taken any medication during the past 15 days before blood sampling. All samples were analyzed in a single-blind manner with respect to the group (T2D or non-T2D).
Isolation of CD34+ and CD34− Peripheral Blood Mononuclear Cells (PBMCs)
One hundred milliliters of blood was collected by venipuncture into syringes containing ethylenediaminetetraacetic acid (EDTA; 1.8 mg/ml of blood) (Sigma-Aldrich, St. Louis, MO, USA), dispensed into 50-ml conical tubes, and centrifuged at 400 × g for 15 min at 20°C, to remove a maximum quantity of platelets while minimizing PBMC loss. EDTA was used throughout the isolation process to avoid platelet binding to CD34+ cells. The platelet-rich plasma (PRP; upper phase) was removed, and the remaining blood components were diluted 1:1 in phosphate-buffered saline (PBS) containing 2 mM EDTA and 0.5% fetal bovine serum (FBS) (Sigma-Aldrich) (PBS/EDTA/FBS). Ficoll at a density of 1.077 g/ml (Amersham Biosciences, Little Chalfont, UK) was added to samples in a ratio of 1:3 and centrifuged at 400 × g for 40 min at 20°C (without brakes). The resulting mononuclear cell ring was collected at the Ficoll/plasma interface. Cells were then washed twice with PBS/EDTA/FBS and incubated for 10 min at 4°C with 100 μl of FcR blocking reagent (Miltenyi Biotec, Bergisch Gladbach, Germany) to remove FcR-specific binding antibodies. Cells were then incubated for 30 min at 4°C with 100 μl of magnetic beads bearing anti-CD34 monoclonal antibodies (Microbead; Miltenyi Biotec). After washing with PBS/EDTA/FBS, cells were filtered (30-μm nylon cell strainer; Miltenyi Biotec) to remove cell aggregates or other large contaminants and loaded on a MACS magnetic column (Miltenyi Biotec). Unbound CD34− cells were collected, while CD34+ PBMCs were retained on the column. After three washes with PBS/EDTA/FBS, CD34+ cells were recovered in 1 ml of PBS/EDTA/FBS. To increase the purity of CD34+ cells, this step was repeated once on a new column with the retained fraction. Finally, cell viability was measured with trypan blue (Sigma-Aldrich).
Cell Preconditioning with TSP-1-Derived Peptides
CD34+ and CD34− cells were diluted either at a concentration of 1,000 cells/μl for adhesion assays or at a concentration of 4,000 cells/μl for flow cytometry assays. Cells were then preincubated with either 30 μM of the CD47 interacting peptide RFYVVMWK (amino acid sequence: Arg-Phe-Tyr-Val-Val-Met-Trp-Lys) (4N1-1; Bachem, Bubendorf, Switzerland), 30 μM of the RFYVVM truncated peptide devoid of CD47-binding activity (Arg-Phe-Tyr-Val-Val-Met) (4N1-2; Bachem), or saline (vehicle) for 30 min at 37°C.
Phenotyping of Preconditioned Cells
The phenotype of preconditioned cells (with TSP-1 peptides or the vehicle, as previously described) was analyzed by flow cytometry using fluorescent-labeled antibodies directed against biomarkers grouped in four panels: panel 1 with CD47 (clone B6H12; R&D Systems, Minneapolis, MN, USA) and TSP-1 (clone A4.1; Santa Cruz Biotechnology, Santa Cruz, CA, USA); panel 2 with the adhesion molecules CD29 (clone TS2/16; eBioscience, San Diego, CA, USA), CD51/CD61 (clone 23C6, eBioscience), and CD162 (clone KPL-1; BD Biosciences, Franklin Lakes, NJ, USA); panel 3 with CD62P (clone P.seK02.22; BD Biosciences); and panel 4 with the apoptosis and cell death markers phosphatidylserine (annexin V labeling), 4′,6′-diamidino-2-phenylindole (DAPI), and propidium iodide (PI) (BD Biosciences). Each panel also included antibodies against CD34 (clone 581; BD Biosciences), CD42b (platelet marker; clone HIP1; BioLegend, San Diego, CA, USA), and DAPI to discriminate living cells.
Cell suspension (4 × 103 cells/μl) was incubated with each antibody panel (previously centrifuged at 2 × 103 × g for 2 min to remove aggregates of antibodies) for 30 min at room temperature in the dark. Immunophenotyping of CD34+ cells was performed on an LSR II flow cytometer (BD Biosciences) and analyzed with Kaluza software (Beckman Coulter, Miami, FL, USA).
Detection of Integrin Polarization and Platelet/CD34+ Conjugates
CD29 and CD51/CD61 distribution on cell surfaces and platelet (CD42b+)/CD34+ cell conjugates was visualized by confocal microscopy (Zeiss Observer Z1 equipped with a Yokogawa CSU-X1 confocal head QuantEM 512SC camera; Intelligent Imaging Innovations, Denver, CO, USA).
Cell Adhesion Onto Collagen–Vitronectin Matrices
Ninety-six-well plates (Sarstedt, Nümbrecht, Germany) were coated overnight at 4°C in PBS containing a mixture of 0.3 μg/ml vitronectin (Sigma-Aldrich) and 1 μg/ml type I collagen (Sigma-Aldrich). The wells were then saturated with 0.1% gelatin [American Type Culture Collection (ATCC), Manassas, VA, USA] for 1 h at room temperature and washed with PBS. Twenty thousand cells in 200 μl of endothelial basal medium-2 (EBM-2; Lonza, Walkersville, MD, USA) were pretreated with either the vehicle, RFYVVMWK, or RFYVVM for 5 min at 150×g at room temperature to quickly spin down the cells onto the matrix. Plates were then incubated for 30 min at 37°C and gently washed with EBM-2. Finally, 100 μl of 2% paraformaldehyde (PFA) and 100 μl of DAPI were sequentially added. Nuclei were counted using an inverted epifluorescence microscope (Axiovert 200M, camera AxioCam MRm; Zeiss, Stockholm, Sweden) coupled with the image analysis software ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA]. Results were expressed as the number of cells adhered per 20 × 103 cells originally loaded per well.
Cell Adhesion Onto HUVEC Monolayers
Human umbilical vein endothelial cells (HUVECs; PromoCell, Heidelberg, Germany) between passage 4 and 8 were seeded into 96-well plates for 48 h at a density of 25 × 103 cells/well. After 36 h at 37°C, 5% CO2 concentration, and 95% relative humidity, HUVECs were stimulated for 18 h with 1 ng/ml tumor necrosis factor-α (TNF-α; R&D Systems) or 10 ng/ml interleukin-1β (IL-1β; Sigma-Aldrich). Cells were then washed twice with Hank's balanced salt solution (HBSS). To differentiate PBMCs from HUVECs during cell counting, PBMCs were prelabeled with 0.5 μg/ml calcein-AM (Sigma-Aldrich) in EBM-2 for 1 h at 37°C and then washed and resuspended in EBM-2 before seeding onto HUVEC monolayers. As for matrix adhesion assays, microplates were centrifuged for 5 min at 150 × g at room temperature to quickly spin down the cells onto the HUVECs and then incubated for 1 h at 37°C. After two washes with HBSS, the cells were fixed with 2% PFA. Calcein-AM-labeled PBMCs were then counted by fluorescence microscopy and ImageJ software. Results were expressed as the number of adherent cells per 10× 103 cells originally loaded in each well. All experiments were performed in duplicate.
Statistics
Analyses were performed using the GraphPad Prism software v.5.01 (GraphPad Software, San Diego, CA, USA). Data were expressed as mean ± standard error of the mean (SEM). The Kruskal–Wallis nonparametric test was used to compare the three preconditioning treatments (vehicle, RFYVVMWK, and RFYVVM) in cell adhesion assays and the Mann–Whitney nonparametric test to compare biomarkers. Values of p < 0.05 were considered statistically significant.
Results
Patient Characteristics
Both T2D and non-T2D had similar demographic characteristics (Table 1). Almost all participants (95%) had dyslipidemia and high cholesterol levels. The majority was also overweight (BMI > 27 kg/m2) with a smoking history (n = 31). Beside a hypoglycemic therapy in T2D, there were no significant differences in drug regimen, with all patients being treated with antiplatelet drugs and statins. As expected, blood glucose (+40%; p < 0.001) and glycated hemoglobin (+19%; p < 0.001) were significantly higher in T2D participants. T2D also had higher triglyceride levels compared to non-T2D participants (+70%; p < 0.002).
Table 1.
Patient Characteristics
| Characteristics | Nondiabetics (n = 20) | Type 2 Diabetics (n = 20) | p |
|---|---|---|---|
| Age (years) | 70.1 ± 1.9 | 69 ± 1.6 | 0.34 |
| BMI (kg/m2) | 29 ± 1.1 | 30 ± 1.6 | 0.2 |
| Hypertension (%) | 15 (75) | 12 (60) | 0.5 |
| Dyslipidemia (%) | 19 (95) | 19 (95) | 1 |
| Former smoking (%) | 12 (60) | 15 (75) | 0.46 |
| Active smoking (%) | 2 (10) | 2 (10) | 1 |
| Blood glucose (mmol/L) | 5.61 ± 0.1 | 7.9 ± 0.5 | <0.001 |
| HbAIc (mmol/mol) | 39 ± 0.9 | 51 ± 3.6 | <0.001 |
| Total cholesterol (mmol/L) | 3.8 ± 0.2 | 3.7 ± 0.2 | 0.7 |
| LDL cholesterol (mmol/L) | 2 ± 0.2 | 1.8 ± 0.2 | 0.08 |
| HDL cholesterol (mmol/L) | 1 ± 0.1 | 1.1 ± 0.1 | 0.8 |
| Triglycerides (mmol/L) | 1 ± 0.1 | 1.8 ± 0.2 | 0.002 |
| Platelets (G/L) | 199 ± 11.4 | 181 ± 6.3 | 0.3 |
| Statins (%) | 20 (100) | 20 (100) | NS |
| Antiaggregant (%) | 20 (100) | 20 (100) | NS |
| Oral antidiabetics (%) | 0 | 18 (90) | <0.001 |
Results are expressed as means ± SEM. BMI, body mass index; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Stimulation of Platelet/CD34+ Conjugate Formation by RFYVVMWK
Circulating CD34+ and CD34− PBMCs were isolated from HD, T2D, and non-T2D blood by immunomagnetic separation and quantified. The purity of the isolated CD34+ cells was 92 ± 4%. No significant difference was found in total PBMCs (167.7 ± 50.2 × 106 PBMC/100 ml blood in T2D vs. 141.5 ± 32.5 × 106 in non-T2D and 136.4 ± 38.8 × 106 in HD; p = 0.2) and CD34− cells (77.2 ± 3.1 × 106 CD34−cells/100 ml blood in T2D vs. 62.0 ± 2.3 × 106 in non-T2D and 66.6 ± 2.5 × 106 in HD; p = 0.15). However, twice the amount of CD34+ PBMCs were retrieved from the blood of T2D participants compared to non-T2D and HD participants [218.9 ± 124.1 × 103 cells per 100 ml of blood vs. respectively, 101.6 ± 29.0 × 103 cells (p < 0.001) and 117.5 ± 49.8 × 103 (NS)]. The CD34+/total PBMC ratio was also significantly higher in cell fractions isolated from T2D participants [0.13 ± 0.06% in T2D vs. 0.075 ± 0.03% in non-T2D (p = 0.0011), and 0.1 ± 0.07% (p = 0.06) in HD]. Although a double enrichment process and extensive wash were used during the purification of CD34+ cells, platelets were still detectable in the final cell preparations (average of 23.5 × 103 platelets/103 CD34+ cells). Using flow cytometry, we measured the extent of plate-let/CD34+ cell conjugate formation (hereafter referred to as CD42b+/CD34+ conjugates) in samples and the effect of TSP-1 peptide preconditioning. RFYVVM preconditioning had no significant effect on conjugate formation, with 1.4% CD42b+/CD34+ conjugates in T2D and 2% in the non-T2D participants (NS). RFYVVMWK increased the percentage of CD42b+/CD34+ conjugates up to 11% in T2D (p < 0.0001 vs. RFYVVM) and 9% in non-T2D participants (p < 0.0001 vs. RFYVVM). Progenitor cell–platelet conjugate formation following RFYVVMWK treatment was confirmed by assessing the expression of CD42b and CD34 antigens by confocal microscopy (Fig. 1A).
Figure 1.
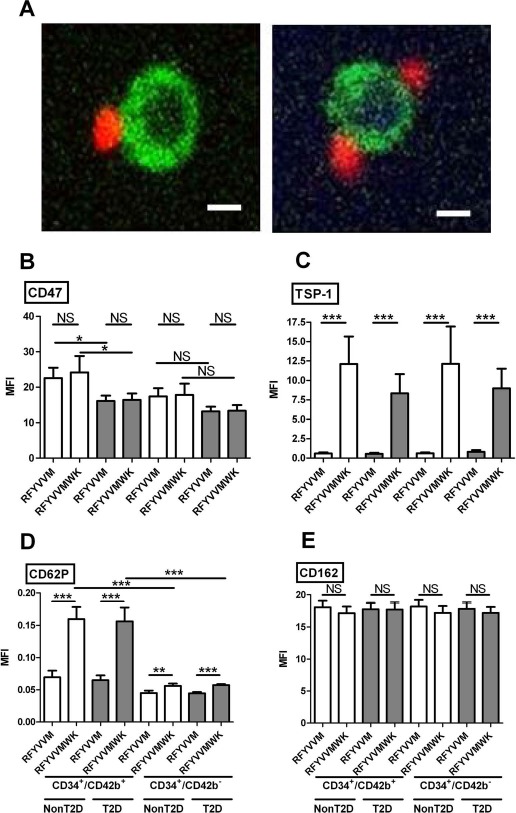
(A) Examples of CD42b+ (red)/CD34+ (green) conjugates formed after stimulation with RFYVVMWK observed by confocal microscopy. Scale bars: 5 μm. (B–E) Expression of CD47 (B), TSP-1 (C), CD62P (D), and CD162 (E) on CD34+/CD42b+ conjugates and CD34+/CD42b− cells after RFYVVM or RFYVVMWK preconditioning. TSP-1, thrombospondin-1; MFI, mean fluorescence intensity; T2D, type 2 diabetes (gray bars); non-T2D, nondiabetic (white bars); NS, not significant. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 [analysis of variance (ANOVA)].
Expression of CD47 and its Ligand TSP-1
CD47 expression was not modified by the RFYVVMWK preconditioning compared with the nonactive RFYVVM control peptide (Fig. 1B). We observed a 30% lower expression on T2D CD34+ cells compared to non-T2D cells (p < 0.01). CD47 expression was higher on CD42b+/CD34+ conjugates compared to CD42b−/CD34+ cells, probably due to the presence of CD47 on platelets (p < 0.05). TSP-1 was barely expressed on CD42b+/CD34+ conjugates and CD42b−/CD34+ preincubated with RFYVVM (Fig. 1C). RFYVVMWK induced a high expression of TSP-1 on both T2D and non-T2D CD34+ cells, in the presence or absence of platelets (all p < 0.001).
Expression of CD62P and CD162
RFYVVMWK induced a significant increase in CD62P on T2D and non-T2D CD42b+/CD34+ conjugates [+146% (p < 0.001) and +129% (p < 0.001) vs. RFYVVM, respectively], and a low increase in CD42b−/CD34+ cells [+26% (p < 0.01) vs. +25% (p < 0.001)] (Fig. 1D). By contrast, CD162 expression remained unchanged (Fig. 1E).
Expression of Adhesion Receptors CD29 and CD51/CD61
RFYVVMWK preconditioning of CD34+ cells significantly increased the expression of CD29 in both T2D and non-T2D participants [74% (p < 0.01) and 42% (p < 0.05) in CD42b+/CD34+ conjugates, respectively] (Fig. 2A). A strong increase in CD51/CD61 expression was also measured in T2D and non-T2D CD34+ cells [+2,715% (p < 0.001) and +3,260% (p < 0.001) in CD42b+/CD34+ conjugates, respectively] (Fig. 2B). Similar results were observed with CD42b−/CD34+ cells. Integrin polarization and clustering, which are indicators of integrin activation state, were also detected in RFYVVMWK stimulated cells by confocal microscopy (Fig. 2C).
Figure 2.
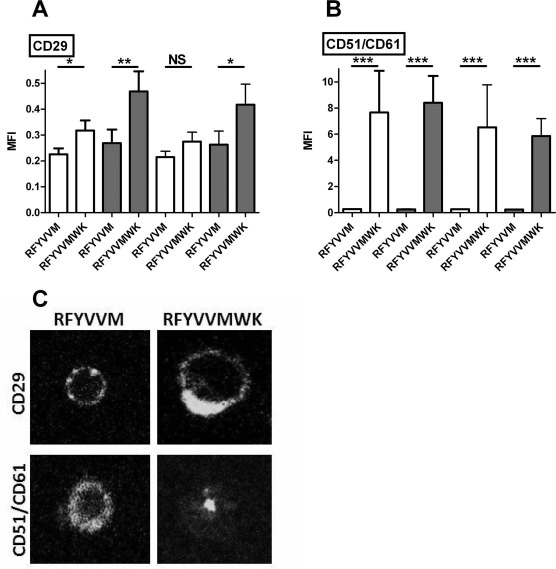
Expression of CD29 (A) and CD51/CD61 (B) on CD34+/CD42b+ conjugates and CD34+/CD42b− cells after RFYVVM or RFYVVMWK preconditioning. (C) Examples of CD29 (top) and CD51/CD61 (bottom) distribution on RFYVVM (left)- and RFYVVMWK (right)-stimulated cells observed by confocal microscopy are shown. MFI, mean fluorescence intensity; T2D, type 2 diabetes (gray bars); non-T2D, nondiabetic (white bars); NS, not significant. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (ANOVA).
Effect of RFYVVMWK on CD34+ Cell Viability
RFYVVMWK induced increased phosphatidylserine exposure (PI−/annexin V+ cells) in both non-T2D CD34+ (7.2% vs. 0.15% with RFYVVM, p < 0.001) and T2D CD34+ cells (12.4% vs. 0.04% with RFYVVM, p < 0.001) (Fig. 3A). The percentage of PI+/annexin V+ cells in response to RFYVVMWK was also significantly higher compared to RFYVVM but remained negligible (0.25% in non-T2D and 0.17% in T2D, p < 0.001 vs. RFYVVM) (Fig. 3B).
Figure 3.
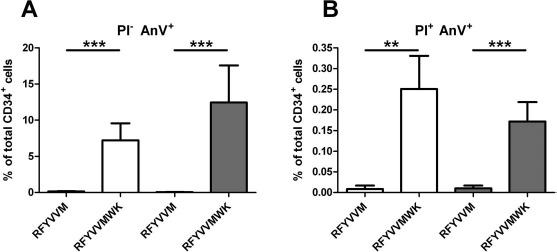
Percentage of CD34+ annexin V+/PI− and annexin V+/PI+ cells after preconditioning with RFYVVM or RFYVVMWK. T2D, type 2 diabetes (gray bars); non-T2D: nondiabetic (white bars); AnV, annexin V; PI, propidium iodide. ∗∗p < 0.01; ∗∗∗p < 0.001 (ANOVA).
Cell Adhesion to Vitronectin–Collagen Matrix
CD34+ cells preincubated with vehicle (saline) adhered with values reaching 59.9 ± 8 cells/20 × 103 seeded cells, compared to 18.8 ± 2.9 CD34- cells (p < 0.0001) (Fig. 4A). RFYVVMWK strongly increased the adhesion of CD34+ and CD34-, respectively, by +368% and +468%. RFYVVM peptide had no significant effect, giving results comparable to vehicle. T2D CD34+ cells showed 67% less basal adherence (30 ± 4 cells) compared to non-T2D cells (90 ± 14 cells, p < 0.0001) (Fig. 4B). RFYVVMWK strongly increased the adhesion of T2D and non-T2D CD34+ cells by +786% (266 ± 54 cells) and +232% (296 ± 31 cells), respectively (p < 0.0001 compared to vehicle-treated cells).
Figure 4.
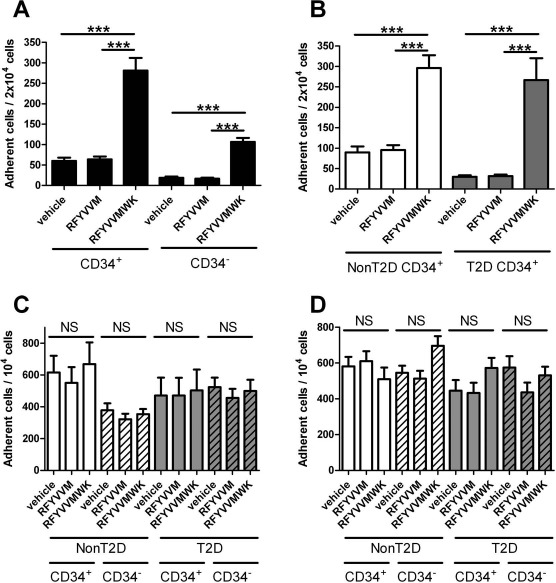
Effect of peptide preconditioning on the adhesion of CD34- and CD34+ cells onto vitronectin–collagen matrix. (A) All patients (diabetic plus nondiabetic). (B) Diabetic (gray bars) versus nondiabetic patients (white bars). Results are expressed as number of adherent cells per 2 × 103 seeded cells. ∗∗∗p < 0.001 (Kruskal–Wallis test). Effect of preconditioning with TSP-1 peptides on the adhesion of CD34+ (nonhashed bars) and CD34- cells (hashed bars) in diabetic (gray bars) versus nondiabetic patients (white bars) on HUVEC monolayers prestimulated by TNF-α (C) or IL-1b (D). Results are expressed as number of adherent cells per 103 seeded cells (p > 0.05, Kruskal–Wallis test). HUVEC, human umbilical vein endothelial cell; TNF, tumor necrosis factor; TSP, thrombospondin; T2D, type 2 diabetes; non-T2D, nondiabetic; NS, not significant. ∗∗∗p < 0.001 (ANOVA).
Cell Adhesion to HUVEC Monolayers Stimulated with TNF-α or IL-1β
We next measured the adhesion of CD34+ cells on HUVEC monolayers prestimulated with TNF-α or IL-1β. T2D and non-T2D CD34+ cells equally adhered to prestimulated HUVEC monolayers. Neither RFYVVMWK nor RFYVVM had a significant effect on cell adhesiveness (Fig. 4C and D).
Discussion
Proangiogenic cell therapy offers many potential applications in regenerative medicine for the treatment of patients with ischemic diseases, particularly in cardiology. Promising preclinical studies have prompted the initiation of numerous clinical trials based on administration of progenitor cells. Several cellular functions are involved in the process of neoangiogenesis such as homing and recruitment of cells, proliferation, endothelial differentiation, and survival. However, cardiovascular risk factors such as T2D were associated with dysfunctional progenitor cells, including impaired adhesiveness5,6, which undermines their therapeutic value in autologous cell therapies20. CD34+ PBMC recruitment to damaged vessels is a crucial step to initiate the process of vascular repair and neovascularization9. In ex vivo settings, we observed a lower basal adhesion of T2D CD34+ cells to vitronectin–collagen matrix compared to the non-T2D CD34+ cells. We thus sought to investigate whether stimulating the adhesiveness of CD34+ PBMCs was feasible in an attempt to improve cell therapy efficiency in T2D.
The transmembrane protein CD47, TSP-1 receptor, associates with CD51/CD61, CD41a/CD61, CD49d/CD29, and CD49b/CD29 integrins to mediate cell adhesion and motility17. Herein we provide evidence that prestimulating CD34+ PBMCs with RFYVVMWK, a TSP-1-related peptide that activates CD47, restores and amplifies their adhesiveness to vitronectin–collagen matrix beyond the basal adhesion values obtained in non-T2D patients. In addition, we showed a strong increase in surface expression of CD29 and CD51/CD61 integrins following RFYVVMWK stimulation, thereby providing a possible mechanism to the increased adhesion of CD34+ cells to the subendothelial matrix components. Confocal microscopy strengthened this hypothesis by revealing polarization of integrin at the cell surface, consistent with the clustering process occurring during integrin activation.
The endothelial expression of CD51/CD61 (αvβ3 integrin) and its interaction with extracellular matrix components are crucial during angiogenesis21. This interaction triggers vascular endothelial growth factor (VEGF)-A-mediated full activation of VEGF receptor 2 (VEGFR-2), but also yields a strong antiapoptotic effect through the suppression of the p53 and the p53- inducible cell cycle inhibitor p21WAF1/CIP1 activities and the increase in the Bcl-2/Bax ratio22,23. Consistent with the later functions of CD51/CD61, RFYVVMWK priming did not compromise CD34+ cell survival, as assessed by annexin V/PI labeling. CD29 (β1) integrin subsets diversely contribute to angiogenesis. Li and collaborators have associated CD29 expression levels with the rate of implantation and colonization of ischemic limbs with bone marrow-derived endothelial precursors, which is of critical importance for inducing therapeutic angiogenesis by cell implantation24.
Albeit standardized CD34+ PBMC isolation and purification techniques were used in this study, there were still platelet remnants in the positive fraction, as recurrently reported in the literature addressing CD34+ cell isolation and enrichment25. We observed a significant increase in platelet–CD34+ conjugate formation upon RFYVVMWK stimulation, along with an increased expression of CD62P restricted to platelet–CD34+ conjugates. These results are consistent with the previously reported activating effect of RFYVVMWK on platelets26. This activation, concomitant to CD34+ cell stimulation, induces platelet secretion and surface CD62P expression, thereby enabling platelets to interact with CD162 (PSGL-1) on CD34+ cells. As previously described by others, platelets are instrumental in neovascularization by targeting CD34+ cell recruitment to injured vessels and promoting their homing and maturation8,10,11.
Consistent with this rationale, RFYVVMWK stimulation had no significant effect on CD34+ PBMC adhesion on HUVEC monolayers, stimulated with either TNF-α or IL1-β. These results echo our observations in a TSP-1 knockout mouse model of FeCl3-induced intravascular thrombosis, in which we observed that TSP-1 was essential for bone marrow cell (BMC) recruitment to vascular injury sites18. We also reported that CD47 preactivation with RFYVVMWK strongly stimulated BMC adhesion and specific recruitment to sites of thrombosis in vivo19. The present findings suggest that stimulation by RFYVVMWK confers to CD34+ PBMCs an increased adhesiveness restricted to most damaged and de-endothelialized vascular areas exposing the matrix components, with limited stickiness to healthier areas.
It has previously been suggested that increased expression of adhesion molecules [including CD11a/CD18 (LFA-1), CD49d/CD29 (VLA-4), CD54 (ICAM-1), CD51/CD61, and CD162] on CD34+ or endothelial progenitor cells and/or increased adhesiveness in vitro could translate into enhanced endothelial repair or neovascularization capacity in vivo27–29. In coherence with these observations, we have previously reported that priming BM-MNCs with RFYVVMWK results in increased proangiogenic activity in a mouse model of hindlimb ischemia and cell therapy19. However, additional studies are required to demonstrate whether the priming of CD34+ cells isolated from PB improves vascularization in vivo.
In a recent study, Albiero et al. suggested that increased adhesiveness of stem cells may hamper their ability of being mobilized from the bone marrow30. Our results are in line with the prospect of using the peptide ex vivo as a pretreatment strategy prior to administration of an autologous cell-based therapy product, rather than using RFYVVMWK in vivo. Thus, we anticipate that the endogenous mobilization of stem cells would not be affected. In addition, since the majority of current cell-based therapy strategies are using local injection in ischemic areas, it is unlikely that RFYVVMWK preconditioning of cells can favor homing of injected CD34+ cells into the bone marrow.
RFYVVMWK induced surface expression of TSP-1. This neo-expression was observed even in CD42b−/CD34+ cells. The timeline of our experimental conditions suggest that TSP-1 originated from exocytosis or platelet secretion rather than from neosynthesis per se. The consequences of TSP-1 expression on CD34+ cells are difficult to anticipate as TSP-1 induces both positive and negative modulation of endothelial cell adhesion, motility, and growth through its interaction with a plethora of cell adhesion receptors, including CD47, CD36, CD51/CD61, and CD29 integrins, and syndecan12.
CD47 expression was not modulated upon RFYVV MWK stimulation. CD47 interaction with signal regulatory protein α (SIRP-α), expressed on macrophages and dendritic cells, negatively regulates phagocytosis of hematopoietic cells31. Interestingly, we observed that T2D CD42b+/CD34+ conjugates express significantly less CD47 on their surface compared to non-T2D cells. This lower expression of CD47 may contribute to a higher susceptibility of T2D CD34+ to phagocytosis in vivo. Yet, we could not demonstrate lower amounts of CD34+ in T2D PB as previously observed by others32. Surprisingly, using a single-blinded counting approach, we measured significantly higher levels of CD34+ cells recovered from T2D patients (n = 20) compared to non-T2D (n = 20). This could be due to the fact that counting of CD34+ cells was performed after enrichment of cells with an immunomagnetic CD34 antibody column rather than on the PB of patients, which may have introduced an unexpected bias in the quantification. In addition, several studies have suggested that glycemic control could impact circulating progenitor cell levels in diabetic patients. Indeed, oral antidiabetics were shown to attenuate the quantitative deficit and improve angiogenic function of progenitor cells in diabetics33,34. The underlying mechanisms probably involve reduction in inflammation, oxidative stress, and insulin resistance. Furthermore, a recent study reported a positive correlation between circulating CD34+ cell count and serum triglycerides in nonhypertensive elderly Japanese men, suggesting that triglycerides may stimulate an increase in circulating CD34+ by inducing vascular disturbance35. In our patient cohort, triglycerides were significantly higher in T2D patients despite statin treatment. In agreement with the study by Shimizu and collaborators35, CD34+ cell count significantly correlated with triglyceride levels in nonhypertensive patients (n = 11; Spearman test; r = 0.81; p < 0.004), but also to a lesser extent in the hypertensive group (n = 27; Spearman test; r = 0.43; p < 0.03).
In conclusion, priming CD34+ PBMCs from T2D patients with the TSP-1 carboxy-terminal peptide RFYVVMWK restores and amplifies their adhesion properties without compromising their viability. These findings may be instrumental to improve proangiogenic autologous cell therapy in several disease settings such as T2D.
Acknowledgments
This work was supported in part by the Agence Nationale de la Recherche (Grant No. ANR-07-PHYSIO-025-02). The authors declare no conflicts of interest.
References
- 1.Scheubel RJ, Holtz J, Friedrich I, Borgermann J, Kahrstedt S, Navarrete Santos A, Silber RE, Simm A,. Paracrine effects of CD34 progenitor cells on angiogenic endothelial sprouting. Int J Cardiol. 2010; 139: 134–41. [DOI] [PubMed] [Google Scholar]
- 2.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003; 9: 702–12. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, Bankson JA, Shpall E, Willerson JT, Gelovani JG, Yeh ET. Human CD34+ cells in experimental myocardial infarction: Long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010; 106: 1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, Zemljic G, Haddad F, Wu JC, Vrtovec B. Effects of transendocardial CD34+ cell transplantation in patients with ischemic cardiomyopathy. Circ Cardiovasc Interv. 2014; 7: 552–9. [DOI] [PubMed] [Google Scholar]
- 5.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781–6. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A,. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 2013; 36: 943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadini GP, Ciciliot S, Albiero M. Concise review: Perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells 2017; 35(1): 106–16. [DOI] [PubMed] [Google Scholar]
- 8.Broquères-You D, Leré-Déan C, Merkulova-Rainon T, Mantsounga CS, Allanic D, Hainaud P, Contrères JO, Wang Y, Vilar J, Virally M, Mourad JJ, Guillaussear PJ, Silvestre JS, Lévy BI. Ephrin-B2-activated peripheral blood mononuclear cells from diabetic patients restore diabetes-induced impairment of postischemic neovascularization. Diabetes 2012; 61: 2621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malecki M, Sabo C, Putzer E, Stampe C, Foorohar A, Quach C, Beauchaine M, Tombokan X, Anderson M. Recruitment and retention of human autologous CD34+ CD117+ CD133+ bone marrow stem cells to infarcted myocardium followed by directed vasculogenesis: Novel strategy for cardiac regeneration. Mol Cell Ther. 2013; 1(pii): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M,. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 2008; 117: 206–15. [DOI] [PubMed] [Google Scholar]
- 11.Lev EI, Estrov Z, Aboulfatova K, Harris D, Granada JF, Alviar C, Kleiman NS, Dong JF. Potential role of activated platelets in homing of human endothelial progenitor cells to subendothelial matrix. Thromb Haemost. 2006; 96: 498–504. [PubMed] [Google Scholar]
- 12.Bonnefoy A, Moura R, Hoylaerts MF. The evolving role of thrombospondin-1 in hemostasis and vascular biology. Cell Mol Life Sci. 2008; 65: 713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D. Human thrombospondin's (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: A molecular modeling study. Arch Biochem Biophys. 2008; 478: 103–9. [DOI] [PubMed] [Google Scholar]
- 14.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol. 1996; 135: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung J, Gao AG, Frazier WA. Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J Biol Chem. 1997; 272: 14740–6. [DOI] [PubMed] [Google Scholar]
- 16.Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J Cell Biol. 1999; 147: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Regulation of integrin function by CD47 ligands. Differential effects on alpha vbeta 3 and alpha 4beta1 integrin-mediated adhesion. J Biol Chem. 2002; 277: 42859–66. [DOI] [PubMed] [Google Scholar]
- 18.Eren P, Bonnefoy A, Lebret M, Foubert P, Merval R, Legrand C, Rosa J-P, Tedgui A, Blanc-Brude O. Abstract 1299: Thrombospondin-1 is essential for bone marrow mononuclear cell recruitment to sites of vascular injury. Circulation 2007;116: II_265 Available from http://circ.ahajournals.org/content/116/Suppl_16/II_265.4 [Google Scholar]
- 19.Blanc-Brude OP, Eren P, Bonnefoy A, Matrone G, Foubert P, Duriez M, Legrand C, Silvestre J-S, Tedgui A,. Abstract 895: CD47 activation by thrombospondin peptides enhances bone marrow mononuclear cell adhesion, recruitment during thrombosis, endothelial differentiation and stimulates pro-angiogenic cell therapy. Circulation 2007; 116;II_175 Available from http://circ.ahajournals.org/content/116/Suppl_16/II_175.3.abstract [Google Scholar]
- 20.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB,. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007; 56: 960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994; 264: 569–71. [DOI] [PubMed] [Google Scholar]
- 22.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999; 18: 882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strömblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA,. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996; 98: 426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li TS, Ito H, Hayashi M, Furutani A, Matsuzaki M, Hamano K. Cellular expression of integrin-beta 1 is of critical importance for inducing therapeutic angiogenesis by cell implantation. Cardiovasc Res. 2005; 65: 64–72. [DOI] [PubMed] [Google Scholar]
- 25.Richel DJ, Johnsen HE, Canon J, Guillaume T, Schaafsma MR, Schenkeveld C, Hansen SW, McNiece I, Gringeri AJ, Briddell R, Ewen C, Davies R, Freeman J, Miltenyi S, Symann M. Highly purified CD34+ cells isolated using magnetically activated cell selection provide rapid engraftment following high-dose chemotherapy in breast cancer patients. Bone Marrow Transplant. 2000; 25: 243–9. [DOI] [PubMed] [Google Scholar]
- 26.Dorahy DJ, Thorne RF, Fecondo JV, Burns GF. Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J Biol Chem. 1997; 272: 1323–30. [DOI] [PubMed] [Google Scholar]
- 27.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Lere-Dean C, Contreres JO, Sulpice E, Levy BI, Plouet J, Tobelem G, Le Ricousse-Roussanne S. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007; 117: 1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Koga H, Iwamoto H, Tsutsumi V, Imamura Y, Naitou M, Masuda A, Ikenzono Y, Abe M, Wada F, Sakaue T, Ueno T, Ii M, Alev C, Kawamoto A, Asahara T, Torimura T. Ex vivo expansion of circulating CD34(+) cells enhances the regenerative effect on rat liver cirrhosis. Mol Ther Methods Clin Dev. 2016; 3: 16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brehm M, Ebner P, Picard F, Urbien R, Turan G, Strauer BE. Enhanced mobilization of CD34(+) progenitor cells expressing cell adhesion molecules in patients with STEMI. Clin Res Cardiol. 2009; 98: 477–86. [DOI] [PubMed] [Google Scholar]
- 30.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, Vigili de Kreutzenberg S, Moura R, Giorgio M, Pelicci P, Avogaro A, Fadini GP,. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes 2014; 63: 1353–65. [DOI] [PubMed] [Google Scholar]
- 31.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: A perspective. J Leukoc Biol. 2006; 79: 896–903. [DOI] [PubMed] [Google Scholar]
- 32.Jialal I, Devaraj S, Singh U, Huet BA. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: Implications for increased cardiovascular risk. Atherosclerosis 2010; 11: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.YU JW, Deng YP, Han X, Ren GF, Cai J, Jiang GJ. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc Diabetol. 2016; 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complications 2013; 27: 519–25. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y, Sato S, Koyamatsu J, Yamanashi H, Nagayoshi M, Kadota K, Maeda T. Circulating CD34-positive cells, glomerular filtration rate and triglycerides in relation to hypertension. Atherosclerosis 2015; 243: 71–6. [DOI] [PubMed] [Google Scholar]


