Abstract
An emerging avenue for recalcitrant neurodegenerative disease treatment is neural progenitor cell (NPC) transplantation. In this study, we investigated the effectiveness of two different delivery routes of human-derived NPC inoculation: injection into the common carotid artery or unilateral stereotactic implantation into the degenerating cerebellum and hippocampus of spastic Han–Wistar (sHW) rats, a model of ataxia. At 30 days of age, sHW mutants were implanted with osmotic pumps preloaded with cyclosporine. Ten days after pump implantation, the animals were given either 3,000,000 live human-derived NPCs (hNPCs; n = 12) or 3,000,000 dead NPCs (dNPCs; n = 12) injected into the common carotid artery, or were given two unilateral implantations of 500,000 hNPCs into the cerebellum and 500,000 hNPCs into the hippocampus of each sHW rat (n = 12) or 500,000 dNPCs by unilateral implantation into the cerebellum and hippocampus (n = 12). We also compared treated sHW rats to untreated sHW rats: normal rats (n = 12) and sibling sHW rats (n = 12). Motor activity and animal weights were monitored every 5 days to ascertain effectiveness of the two types of delivery methods compared to the untreated mutant and normal animals. Mutant rats with hNPC implantations, but not dNPC or carotid artery injections, showed significant deceleration of motor deterioration (p < 0.05). These mutants with hNPC implantations also retained weight longer than dNPC mutants did (p < 0.05). At the end of the experiment, animals were sacrificed for histological evaluation. Using fluorescent markers (Qtracker) incorporated into the hNPC prior to implantation and human nuclear immunostaining, we observed few hNPCs in the brains of carotid artery-injected mutants. However, significant numbers of surviving hNPCs were seen using these techniques in mutant cerebellums and hippocampi implanted with hNPC. Our results show that direct implantation of hNPCs reduced ataxic symptoms in the sHW rat, demonstrating that stereotactic route of stem cell delivery correlates to improved clinical outcomes.
Keywords: Cerebellum, Hippocampus, Carotid artery infusion, Intracerebral transplantation, Stereotactic, Stem cells
Introduction
Stem cell transplantation is an emerging avenue of treatment for recalcitrant neurodegenerative diseases. Transplantation of allogeneic stem cells like neural progenitor cells (NPCs) provides the region of degeneration with a novel source of cells that has the potential to replace damaged neurons. This clinical approach is based on the capability of NPCs to adapt to novel environments, differentiate into relevant specialized neurons, and restore neuronal circuitry to defective host neuronal tissue1,2. Thus, neuronal stem cells like NPCs are hypothesized to function as a replacement therapy by supplementing or potentially restoring the degenerating neural tissue of patients suffering from neurodegenerative diseases.
Undifferentiated NPCs are significantly more plastic than adult neurons1. We hypothesize that transplanted NPCs possess the ability to reproduce in situ and differentiate into one or more cell types (e.g., different neurons and possibly glial cells) based on the biochemical signature of the defect and the severity of the degeneration. We further hypothesize that once infused into the patient, undifferentiated NPCs will detect the degeneration caused by disease progression and migrate toward the deficiency. Our study addresses two uncertainties regarding these hypotheses: (i) comparative efficacy of two delivery routes (direct stereotaxic injection vs. systemic intra-arterial injection) of progenitor cells into regions of neurodegeneration and (ii) ability of transplanted NPCs to alter disease progression.
Although there have been many different delivery sites and systems for successful NPC transplantation, these techniques can be divided into two delivery methods: indirect intravascular injection (normally using the carotid arterial system) and direct intracerebral implantation. Studies have shown that the indirect carotid delivery method is minimally invasive, and because of its systemic approach, it has the potential to cover a wider area of neurological damage. Guzman et al.3 utilized carotid injection of neuronal stem cells with a mouse model of stroke and concluded that their stem cells had migrated into affected brain regions. These mice also showed concomitant behavioral improvements (better rotarod performance) after stem cell treatment. In a similar study, Pendharkar et al.4 utilized another mouse stroke model and showed that carotid artery injection of neuronal stem cells produced a more wide-ranging distribution of stem cells in the brain than cells infused via the tail vein.
In contrast to the carotid route, intracerebral implantation of stem cells allows for direct delivery of potential replacement neurons into the region of neurodegeneration. However, this method is a technically difficult approach and involves invasive medical procedures. Yet the direct approach is more likely to deliver the cells precisely to where they are needed, which is reflected in reports of clinical improvements5. In a stereotactic study using an animal model of Parkinson's disease (PD), implanted neural stem cells (NSCs) were shown to integrate into the host brain, survive, express neurotrophic factors, and restore motor function that was previously lost6.
To study the effectiveness of transplantation as well as the ability of neuronal stem cells to migrate and survive in regions of neurodegeneration, we used a line of human-derived NPCs (hNPCs) developed by Celavie Biosciences, LLC (Oxnard, CA, USA) in this study. This cell line has been shown to express key stem cell markers such as octamer-binding transcription factor 4 (Oct-4) and sex-determining region Y-box 2 (SOX-2) and has been found to be nontumorigenic and hypoimmunogenic and to display a normal karyotype (unpublished data).
To determine the best route for NPC delivery, we utilized the spastic Han–Wistar (sHW) rat as a prototype model of neurodegenerative disease. The sHW rat suffers from an undetermined autosomal, recessive disorder characterized by progressive neurodegeneration of Purkinje cells and CA3 pyramidal neurons in the cerebellum and hippocampus, respectively7. The developmental symptoms are characterized by hyperactivity and forelimb tremors that are visible at 25–30 days postnatally8. The mutant's condition is progressive and is manifested behaviorally by spastic paresis and hindlimb rigidity beginning at 40 days of age. The ataxic sHW mutants develop a clumsy, unsynchronized foot placement between 45 and 50 days, progressing to complete loss of motor function and death at 65–70 days of age8. The progressive Purkinje cell neurodegeneration in the cerebellum and the behavioral phenotype of this animal model closely resemble many forms of human ataxia. Easily measurable clinical manifestations combined with a quantifiable morphological deficiency make the sHW rat a good candidate for testing the ability of undifferentiated NPCs to repair known neurological deficits.
The main goal of our study was to compare the efficacy of carotid artery injection of stem cells to the stereotactic delivery of those same cells into ataxic rats. The secondary goal of this study was to initiate an investigation into the effectiveness of transplanted neuronal hNPCs, that is, their ability to improve weight, motor activity, and survival in sHW rats.
Materials and Methods
The protocol for this study was approved (1314-016B) by the Institutional Animal Care and Use Committee (IACUC) at California State University, Northridge.
Animals
A total of 60 male sHW and 12 male normal HW rats were obtained from the California State University, Northridge, breeding colony. At 30 days, mutant male siblings were randomly divided into four experimental mutant groups along with one mutant control group and one normal control group (Table 1): two common carotid artery injection mutant groups [hNPCs, n = 12; dead NPCs (dNPCs), n = 12], two mutant groups that received unilateral stereotactic implants into both the cerebellum and hippocampus (hNPCs, n = 12; dNPCs, n = 12), one untreated mutant group (n = 12), and one untreated normal HW rat group (n = 12). The untreated normal and mutant rats served as the baseline to compare the decrease in weight and motor activity in normal progression of the neurodegenerative disease versus treated mutants. Animal weights were taken every 5 days to assess overall health of the subjects. After treatment, all animals were housed in individual rat cages with access to LabDiet 5001 rodent chow and water ad libitum. The room was maintained at a temperature of 21 ± 1°C, with a 12-h light/12-h dark cycle.
Table 1.
Experimental Design Featuring Rat Genotype, Treatments, and Sample Size for Each Route of Injection
| Routes of Injection | Treatments | No. of Animals | |
|---|---|---|---|
| Mutant sHW rats | Common carotid artery | hNPCs | 12 |
| dNPCs | 12 | ||
| Unilateral stereotactic cerebellum and hippocampus | hNPCs | 12 | |
| dNPCs | 12 | ||
| Untreated | N/A | 12 | |
| Normal sHW rats | Untreated | N/A | 12 |
hNPCs, human-derived neural progenitor cells; dNPCs, dead neural progenitor cells; sHW, spastic Han–Wistar rats.
Derivation and Characterization of hNPCs
hNPCs were procured from the fetal brain tissue (6 weeks old) via routine sterile manual aspiration methods with informed consent from the donor, in accordance with NIH guidelines for use of neural progenitor tissue as well as federal and state laws. Neural tissue was separated from the meninges, minced with microscissors, and triturated with Pasteur pipettes until an NPC suspension was obtained. Cells were cultured in ultralow attachment culture flasks under feeder-free conditions in serum- and xeno-free minimum essential medium (MEM; SH30310; HyClone, Logan, UT, USA), supplemented with Gem21 (400-660; Gemini Bio-Products, Sacramento, CA, USA), epidermal growth factor (AF-100-15; PeproTech, Rocky Hill, NJ, USA), basic fibroblast growth factor (AF-100-18B; PeproTech), transforming growth factor-α (AF-100-16A; PeproTech), insulin-like growth factor 1 (AF-100-11; PeproTech), leukemia inhibitory factor (LIF1010; Millipore, Temecula, CA, USA), calcium chloride (1722; Thermo Fisher Scientific, Waltham, MA, USA), GlutaMAX (35050; Invitrogen, Carlsbad, CA, USA), nonessential amino acids (SH30238.01; HyClone), and an N2 supplement (17502; Invitrogen), all of which were added at proprietary concentrations (patent 7632681; Celavie Biosciences LLC).
NPCs were cultured in flasks incubated at 37°C under hypoxic conditions (5% O2/5% CO2) through four doublings. At the second doubling (D2), cell culture was tested for sterility (USP <71>), and at D4 culture was karyotyped and polymerase chain reaction (PCR) tested for presence of adventitious agents: HTLV-1 and HTLV-2; HIV-1 (A, B, D, F, H, N); hepatitis A, B, and C; Treponema pallidum (T. pallidum); CMV; HSV-1 and HSV-2; and HPV. Cells were then transferred to a closed bioreactor system (GE WAVE Bioreactor 2/10 System; GE Healthcare, Uppsala, Sweden) operating under the same physical and chemical conditions as the incubator expansion. The bioreactor was used to create the master cell bank (MCB), which was harvested, tested, characterized, and rate control cryopreserved after a total of seven doublings (D7). The hNPCs were stained with trypan blue (MP Biomedicals LLC, Solon OH, USA) and quantified on a hemocytometer to test viability and to estimate resuspension volume for injection prior to transplantation into live animals.
The dNPCs were used to control for the paracrine effects of cell inoculations/transplantations and were created by sequentially placing live NPC populations into a −20°C freezer for 30 min and a −80°C freezer for 30 min to freeze kill the cells. The dNPCs were then placed in a 2°C–8°C refrigerator for storage until use.
Qtracker Cell Labeling and Quantification
The Qtracker 625 Cell Labeling Kit (Invitrogen), with excitation at 405–585 nm and emission at 625 nm, was used to label live hNPCs and to track migration within the rat brain and other tissues. Briefly, 10 nM Qtracker labeling solution was prepared by combining 1 μl of component A (2 μM in 50 mM borate buffer, pH 8.3) with 1 μl of component B [phosphate-buffered saline (PBS), pH 7.2] for 5 min at room temperature. Finally, the working solution was diluted with 2 ml of growth medium. For cell preparation, 10 million live hNPCs were centrifuged at 201 × g for 5 min, resuspended in 1 ml of growth media, and incubated with 10 nM Qtracker labeling solution at 37°C for 1 h. The labeled hNPCs were washed two times with growth media and resuspended in 15 ml of cell growth media prior to animal inoculation.
Stem Cell Transplantation
Prior to stem cell treatment, cyclosporine (Med-Vet International, Mettawa, IL, USA) was used to suppress the sHW rats' immune response to the presence of allogeneic hNPCs. At 30 days, mutants underwent subcutaneous implantation (scapular) of an osmotic pump (Alzet Model 2004; Durect Corp., Cupertino, CA, USA), which continuously delivered the immunosuppressant cyclosporine (15 mg/kg/day) for the duration of the experiment (18 days). At 40 days of age, the animals were randomly assigned to either common carotid artery injection (n = 12 mutant pairs) or stereotactic intracerebral implantation (n = 12 pairs). The sHW rats in the common carotid artery experiment were anesthetized with chloral hydrate [intraperitoneal (IP), 350 mg/kg] and placed on a surgical board under a dissecting microscope. The common carotid-injected animals received either 3,000,000 hNPCs in 0.5 ml of growth culture medium or the identical number of dNPCs in 0.5 ml of culture medium solution. For the stereotactic implanted stem cells, the animals were anesthetized with chloral hydrate (IP, 350 mg/kg), placed into the David Kopf Model 900 Small Animal Stereotaxic Instrument (David Kopf Instruments, Tujunga, CA, USA), and received either 500,000 hNPCs in 5 ml of culture medium or an equivalent number of dNPCs in 5 ml of culture medium, which were implanted directly into the cerebellum (AP, −11.0; ML, −2.0; DV, 5.5) and hippocampus (AP, −3.0; ML, −2.5; DV, 3.5). These brain coordinates were chosen because they represented areas of significant neurodegeneration in the sHW rats7,8. All animals were placed on an electric heating pad until they displayed a righting reflex and then were returned to individual cages in the colony.
Motor Activity Testing
In order to monitor their locomotor activity, mutant rats were tested in an open-field arena every 5 days for the duration of the experiment, starting at 30 days and just prior to pump implantation8. Each rat was placed individually in the center of the arena (ABS black plastic box measuring 100 cm × 100 cm × 35.5 cm) and was allowed to move freely. The test consisted of three, non-consecutive trials of 120-s duration with a 150-s rest period between trials. A Logitech Pro 9000 web camera (Logitech, Romanel-sur-Morges, Switzerland) using the Virtual Dub software was utilized to record movements, and an open-field analyzer determined the total distance traveled (cm) within the activity arena. The total distance traveled for each rat was then averaged among the three open-field trials. A single motor activity score (averaged distance traveled) was determined for each rat at each time point tested. Finally, all individual motor activity scores for all rats in each treatment were then averaged (with standard error) to obtain a quantitative measure of their motor abilities for each age tested (every fifth day).
Tissue Processing (Qtracker Analysis)
To examine the presence of hNPCs in various tissues, 58-day-old mutant littermates were anesthetized deeply with chloral hydrate (IP, 400 mg/kg). The rats were transcardially perfused with saline solution (0.9% saline solution containing 1% sodium sulfate and 1,000 U/ml heparin) followed by fixation using 4% paraformaldehyde (PFA) (Thermo Fisher Scientific, Portsmouth, NH, USA) in 0.1 M PBS. Tissue (brains and lungs) was removed, postfixed in 4% PFA for at least 48 h, and transferred to 20% sucrose in PBS for 24 h prior to sectioning. A Thermo Fisher HM550 cryostat was used to slice the whole cerebellum in the sagittal plane, the hippocampus in the coronal plane, and the lung tissue in the longitudinal plane into 25 mm. For the intracarotid injections, 280 sections (starting from 1 mm before the bregma) of the perfused brain and 320 sections of the cerebellum were examined for the presence of any hNPCs. In the stereotactically injected animals, 40 sections anterior and 40 sections posterior of the needle track were examined adjacent to the hippocampus site; 40 sections lateral and 40 sections medial of the needle track were examined in the cerebellum. For Qtracker labeling observation, all tissue slices were counterstained with Hoechst 33342 (1:10,000 dilution; Invitrogen) for 10 min and rinsed with PBS for 5 min. The tissue sections from all animals were mounted on glass slides and examined for the presence of NPCs labeled with Qtracker (Invitrogen) using an Olympus BX60 fluorescent microscope (100×–400×; Olympus, Shinjuku, Tokyo, Japan) with U-MNB excitation cube (BP470-490) and ToupView 7.3 software (Touptek, Zhejiang, P.R. China).
Tissue Processing (hNPC Immunostaining)
Immunohistochemical staining was conducted in order to confirm the identity of injected or implanted NPCs in the sHW rat brain. After tissue sectioning on the cryostat as described above, brain slices were rinsed in washing buffer consisting of 1× PBS (Gibco, Grand Island, NY, USA), 0.5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA), and 0.1% Triton-X (Acros Organics, Waltham, MA, USA) three times for 5 min each. Slices were permeabilized with washing buffer containing 0.1% Tween 20 (Thermo Fisher Scientific) for 30 min and then rinsed once again in washing buffer solution for 5 min. Tissue was blocked in 5% goat serum (Vector Laboratories, Burlingame, CA, USA) in washing buffer for 20 min at room temperature. The sections were incubated with anti-human nuclei MAB1281 (1:1,000; Millipore, Billerica, MA, USA) for 24 h at 4°C. Tissue was rinsed three times for 5 min each in washing buffer solution, and then incubated with the fluorescent secondary antibody Alexa Fluor 488® (1:2,000; Invitrogen) for 1 h at room temperature. Upon completion of incubation, the tissue was incubated with the background stain Hoechst 33342 (1:10,000) for 5 min. The sections were then sequentially dehydrated in 95% and 100% ethanol and coverslipped with mounting medium containing 1× PBS, glycerol, and n-propyl gallate (MP Biomedicals, Santa Ana, CA, USA). Tissue sections from all animals were mounted on glass slides and examined for labeled hNPCs using an Olympus BX60 fluorescent microscope with ToupView 7.3 software.
Statistical Analysis
Repeated-measures analysis of variance (ANOVA) was performed using the SYSTAT analysis program (Cranes Software, Bangalore, India) in order to detect differences in weights and motor activity scores among and within hNPC, dNPC, and untreated rats over the 28-day course of the experiment. Statistical analysis for animal weights and activity testing at each time point were performed using t-tests. All values shown are means ± standard error of the mean (SEM), and significance levels were set at p < 0.05 for all tests.
Results
Effects of Stem Cell Route of Delivery on Weight Gain
Animal weights (Fig. 1) were used as an assessment of overall health and were documented every 5 days starting at 30 days of age (when cyclosporine pumps were implanted into the treatment groups). As expected, the untreated normal and mutant sHW rats showed considerable differences in weight over the course of the experiment with distinct differences beginning at 40 days. Both mutant groups that received carotid injections showed significant increases in weight, as the animals grew significantly over the first 20 days of the experiment (F = 112.65, p < 0.001) (Fig. 1). However, there were no significant differences in weight gain between the hNPC and dNPC carotid injection groups (F = 2.16, p > 0.05) (Fig. 1) throughout the posttreatment period (18 days). Thus, there were no significant advantages to those sHW rats that received hNPCs via carotid injection compared to rats that received dNPCs via the carotid artery. Similar to the carotid artery groups, the stereotactic implantation study showed consistent and significant weight gain as the mutants matured from 30 to 55 days of age (F = 18.12, p < 0.01) (Fig. 1). However, by the end of the experiment (58 days of age), mutant rats stereotactically implanted with hNPCs displayed significantly greater weights (t = 2.63, p < 0.05) (Fig. 1) compared to their paired stereotactically injected dNPC mutants, whose mean weight plateaued by day 50 and then dropped significantly after day 55.
Figure 1.
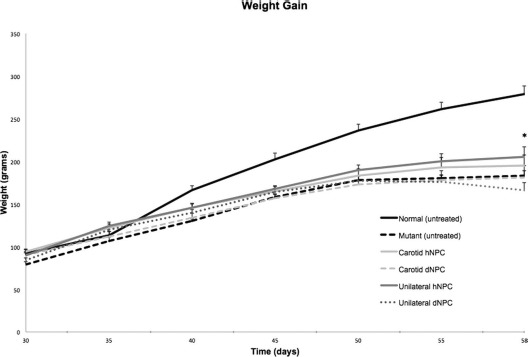
Mean weight gain was utilized to assess overall health. Treatments included mutant sHW rats injected with hNPCs (n = 12) or dNPCs (n = 12) into the common carotid artery, mutant rats unilaterally implanted with hNPCs (n = 12) or dNPCs (n = 12) into the hippocampus and the cerebellum, and untreated mutant (n = 12) and normal rats (n = 12). The carotid injections of hNPCs elicited similar weight gain/loss compared to the dNPC group. Mutant sHW rats unilaterally implanted with hNPCs elicited significant weight gain compared to the dNPC group (p < 0.05). As expected, untreated normal rats showed the highest weight gain, while untreated mutant rats showed a decrease in weight due to the natural progression of their ataxia. All values are means ± SEM; asterisk indicates significant difference in weight between the live hNPC group and other mutants (p < 0.05). hNPCs, human-derived neural progenitor cells; dNPCs, dead neural progenitor cells; sHW, spastic Han–Wistar rats; SEM, standard error of the mean.
Effects of Stem Cell Route of Delivery on Motor Activity
An open-field test was used to evaluate if stem cell treatment had any positive effects on the progressive decline in motor activity normally seen in the sHW mutants. Figure 2 illustrates that the untreated normal and mutant rats had considerable differences in their motor abilities over the course of the experiment, with distinct differences seen beginning at 40 days. There were no significant differences in motor activity between the carotid artery-injected hNPC and dNPC animals from 30 to 58 days (F = 0.342, p > 0.05). All mutants from 30 to 58 days of age that received carotid-infused hNPCs or dNPCs developed symptoms of neurodegeneration with a corresponding drop in motor activity. With one exception: at 58 days of age, the carotid-infused hNPC group showed significantly higher activity scores compared to the carotid-infused dNPC group (t = 2.35, p < 0.05). In contrast, the stereotactic implanted hNPC and dNPC groups showed significantly different activity scores for most of the experiment (F = 4.88, p < 0.05) (Fig. 2). Starting at 45 days of age, the NPC group demonstrated sustained gains in motor activity scores, while the dNPC group continued the downward trend in their motor activity scores as seen in the untreated mutants. The stereotactic implantation data indicated that the direct graft of hNPCs significantly improved motor ability as animals aged compared to the dNPC group at 55 and 58 days of age (t = 3.69, p < 0.001).
Figure 2.
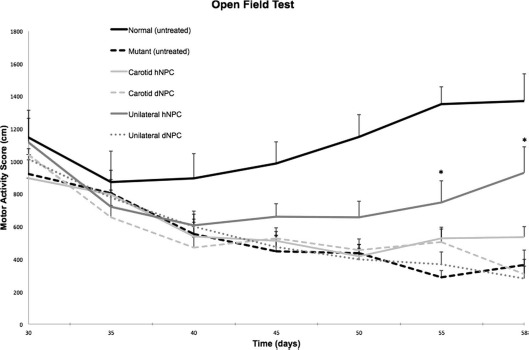
Open-field test was used to assess the effect of NPCs on motor activity in five groups of sHW rats: hNPCs (n = 12) or dNPCs (n = 12) injected into the common carotid artery, hNPCs (n = 12) or dNPCs (n = 12) implanted directly into the hippocampus and cerebellum, and untreated mutants (n = 12) and normal Han–Wistar rats (n = 12). All mutant rats exhibited decreased motor activity regardless of treatment. The data show that the carotid injection of hNPCs did not improve motor coordination as the ataxia progressed except at 58 days of age (p < 0.05). After the initial drop in activity (prior to stem cell inoculation), the unilaterally implanted hNPC group showed increased motor activity scores compared to the dNPC group (p < 0.001). As expected, untreated normal rats showed the highest rate of motor activity, while untreated mutants had a slow, steady decrease in activity due to the progression of neurodegenerative disease. All values are means ± SEM; asterisks indicate significant difference in motor activity score between the live hNPC group and other mutants (p < 0.05). NPC, neural progenitor cell; hNPCs, human-derived neural progenitor cells; dNPCs, dead neural progenitor cells; sHW, spastic Han–Wistar rats; SEM, standard error of the mean.
Histological Comparison of Stem Cell Distribution
Histological examination of the lungs, cerebellum, and hippocampus was performed on all mutants in the carotid artery and stereotactic implantation groups. All mutants were sacrificed and perfused at the end of the experiment (58 days of age), cryosectioned, and examined for the presence of surviving human stem cells using two different methods. Histological examination revealed that a large percentage of carotid artery-injected hNPCs labeled with Qtracker were deposited in the lungs (Fig. 3A). The presence of human cells inside the rat lungs was confirmed using immunohistochemical detection with an anti-human nuclear antibody (Fig. 3B). This experiment, as well as other studies looking at arterial injections9 and intravenous infusions9–11, indicates that the majority of stem cells never reach the organ of interest because they are trapped in the lungs.
Figure 3.
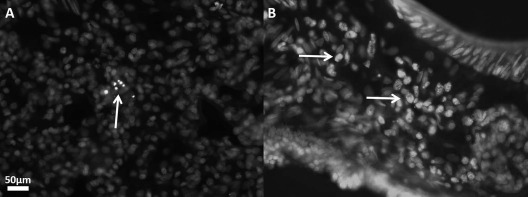
Photomicrographs of lung tissue of two 58-day-old sHW rats (18 days posttransplantation) showing incorporation of hNPCs after carotid artery injection. (A) A small mass of hNPCs in the rat lungs illuminated with the fluorescent label Qtracker. (B) hNPCs labeled with human nuclear immunofluorescent stain in the lung after carotid artery injection. Magnifications for both images were taken at 400×. sHW, spastic Han–Wistar rats; hNPCs, human-derived neural progenitor cells.
After careful examination of the brains of carotid artery-injected NPC mutants, we observed very few, isolated human cells in the brain. For example, a single NPC in the cortex was detected by human nuclear immunofluorescence (Fig. 4A), while Qtracker labeling (Fig. 4B) detected two NPCs in the lower cerebrum near the circle of Willis.
Figure 4.
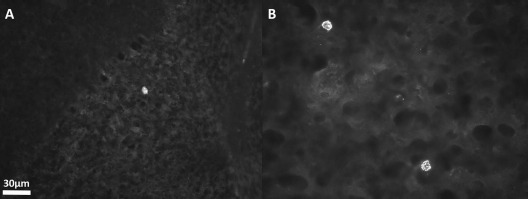
Photomicrographs of the brain of a 58-day-old sHW rat 18 days after carotid injection of hNPCs. (A) A single hNPC detected in the cerebellum by immunofluorescent staining with anti-human nuclei antibodies. (B) Two hNPCs in the temporal lobe of the brain. Magnifications for both images were taken at 400×. sHW, spastic Han–Wistar rats; hNPCs, human-derived neural progenitor cells.
The inability of hNPCs to migrate into the mutant brain when injected through the carotid artery was in direct contrast with stereotactic placement of stem cells into the cerebellum and hippocampus. After direct unilateral implantation of hNPCs, a significant number of surviving human cells were found in the brains, while after carotid injection only a few cells were detected as visualized by the Qtracker detection method. Figure 5 demonstrates distribution of Q-dot-labeled hNPCs through the cerebellar tissue (Fig. 5A) and hippocampus (Fig. 5B).
Figure 5.
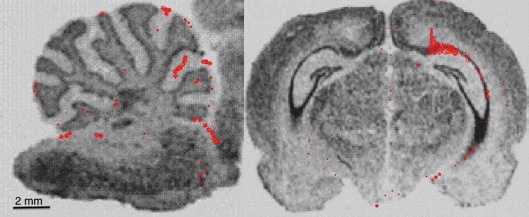
Representation of locations of surviving hNPCs labeled with Qtracker where red dots show locations of individual cells after unilateral implantation in the cerebellum (A). (B) The hippocampus of a 58-day-old sHW rat. Images clearly show that the hNPCs not only survived after 18 days posttransplantation but also appeared to have migrated from their initial injection sites. Magnifications for both images were taken at 12.5×. sHW, spastic Han–Wistar rats; hNPCs, human-derived neural progenitor cells.
The photomicrographs in Figure 6 show the location and survival of hNPCs in the cerebellum and hippocampus 18 days after stereotactic implantation. Figure 6A shows the location of hNPCs labeled with Qtracker at the cerebellar injection site. Figure 6B shows the cerebellum transplantation site containing hNPCs immunostained with mouse, anti-human nuclei antibodies, confirming the presence and survival of hNPCs 18 days posttransplantation. In addition, these hNPCs are observed in the adjacent granule cell region, suggesting that the hNPCs are migrating from the inoculation site toward the region of Purkinje cell neurodegeneration in the sHW mutant. Figure 6C and D shows implantation of hNPCs in the hippocampus via Qtracker fluorescent detection and mouse anti-human nuclei immunostaining, respectively. Untreated mutant cerebellum controls (negative controls) for Qtracker fluorescence (Fig. 6E) and immunohistochemistry (Fig. 6F) reveal the lack of background staining. Figure 6G shows the human prefrontal cortex tissue stained with anti-human nuclear antibodies (positive control), confirming the specificity of the antibody.
Figure 6.
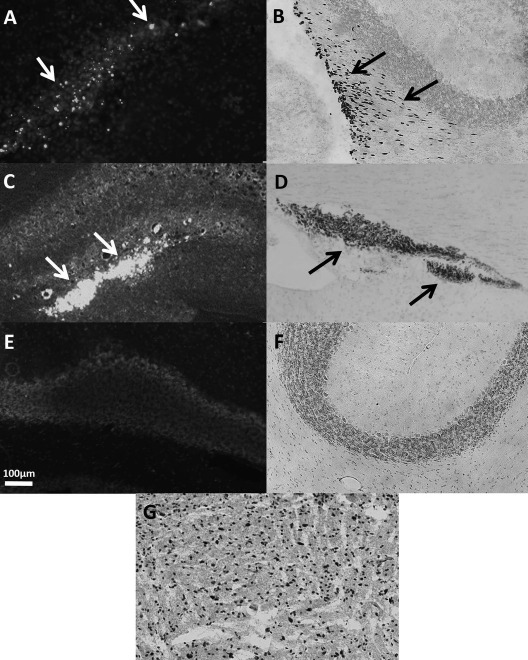
Photomicrographs of transplanted hNPCs in the cerebellum (A, B) and hippocampus (C, D) of sHW rats 18 days after implantation. (A) Qtracker-labeled cells in the cerebellum, with arrows indicating the locations of surviving hNPCs. (B) Immunohistochemical staining using anti-human nuclei antibodies in the cerebellum, with arrows indicating hNPCs migrating away from the needle tract into the cerebellar tissue. (C) Qtracker-labeled hNPCs in the hippocampus, with arrows showing locations of surviving cells. (D) Immunohistochemical staining with anti-human nuclei antibodies in the hippocampus, with arrows that indicate hNPCs in the CA2–CA3 regions. (E) Negative controls for Qtracker (no Qtracker added) in the mutant cerebellum. (F) Negative control (no primary antibody) for human nuclei immunohistochemistry in the mutant cerebellum. (G) Positive control of immunohistochemical staining using human prefrontal cortex. Magnifications for all images were taken at 100×. Calibration bar in (E) refers to all images. sHW, spastic Han–Wistar rats; hNPCs, human-derived neural progenitor cells.
Discussion
As clinical applications of regenerative medicine advance, questions regarding the safest and most efficacious way to transplant regenerative cells like NPCs become crucial. Answers to these uncertainties should integrate the nature of the disorder, such as local (stroke or contusion), diffuse (Alzheimer's disease, multiple sclerosis, or concussion), or a combination of both (PD) with considerations like the degree of delivery method invasiveness and the overall safety of stem cell transplantation. In our current study, two common delivery routes were investigated. The results of this study suggest that direct implantation of NPCs into brain tissue serves as a more effective, albeit invasive, route of administration for treatment of neurodegeneration in this animal model of ataxia. The significant dissimilarities in efficacies of these two cell delivery systems are clear, as demonstrated by the observed improvements in weight gain, motor activity, and stem cell survival.
We used different doses of the hNPCs for direct stereotaxic and intracarotid injections based on past research and practicality. Based on past experience, it is impossible to inject more than 5–8 ml into the brain parenchyma. In our research, we have discovered that when any transplantation uses larger volume, the cells and media are pushed back up through the needle track, thus making it impossible to concentrate more than 500,000 hNPCs without making the cell suspension too viscous, possibly damaging injected hNPCs. In the case of intracarotid injections, the injection of 3,000,000 hNPCs (sixfold more than hNPCs transplanted into the brain) was an attempt to increase the number of cells that crossed the blood–brain barrier (BBB) and entered the brain. An additional concern was that we were injecting into the common carotid artery (the diameter of the internal carotid artery is too small that it made this route technically very difficult). As the diameter of the external carotid artery is significantly bigger than that of the internal, we compensated for this difference by expecting that the majority of the hNPCs would go into the external carotid artery.
The common carotid artery injection failed to generate a significant effect on our subjects' motor activity scores. Animals receiving either live or dead hNPCs via carotid injection displayed a progressive decrease in motor activity that resembled that of untreated mutants, indicating no significant benefit of treatment. While there was a slight improvement in motor activity at the end of the experiment with carotid artery-injected hNPC mutants, the motor activity of these animals did not come close to the increased activity scores of mutants transplanted with hNPCs. The mean weight gain data also displayed no significant differences among live hNPCs, dead hNPCs, and untreated sHW rat groups with carotid artery injections. This lack of effectiveness by carotid artery inoculation was confirmed by histological analysis of the mutant brain where only isolated hNPCs were found scattered throughout the brain.
The carotid artery infusion is a minimally invasive method of delivery of hNPCs but, as shown by our experiments, certainly is not an effective way for delivery of our hNPCs to the brain. Carotid artery injection relies on the ability of hNPCs to migrate and translocate directly through the BBB into the damaged area in the brain. In our experiment, stem cells delivered via common carotid infusion failed to demonstrate an ability to cross the BBB in significant numbers and were trapped in the lung tissue. In addition, the finding of a large percentage of stem cells becoming entrapped in the lungs has been supported by other studies11–14 whose researchers also discovered that injected human stem cells were found in lesser amounts in other filtering organs, including liver, spleen, kidney, and bone marrow. Since our hNPCs are found in abundance in lung tissue, the BBB is probably the main reason why the intra-arterial injection did not result in significant improvement in both health and motor coordination. Because of the paucity of hNPCs in the brains of carotid artery-perfused mutants, we have confirmed other researchers' findings15–17 that the BBB is potentially a major obstacle to stem cell therapy.
Direct stereotactic implantation delivered a bolus of hNPCs to both disease-affected areas of the sHW rat— the cerebellum and hippocampus. It is an invasive surgery with its own set of possible complications, including increased risk of infection, intracranial bleeding, and neurological damage to unaffected tissues at the injection sites. However, this study suggests that direct implantation is a relatively more effective procedure where the biomedical risks are outweighed by significant improvements observed after hNPC injections. Improved motor activity of the hNPC group correlated with improvement in weight gain and resembled that of normal rats. The animals treated with hNPCs showed significantly better behavioral scores than those receiving dNPCs, suggesting that the surviving stem cells contributed to the improvements. Brain histology indicated an abundant number of hNPCs not only survived the initial transplantation but also showed signs of incorporation into both the cerebellum and hippocampus. In a similar study where four different methods of human stem cell transplantation were attempted, including stereotactic, carotid, venous, and ventricular injection, a significant number of surviving human stem cells in the brain tissue were detected only after stereotactic implantation18.
Improvements in behavioral effectiveness due to stem cell incorporation have been reported by other research groups. In a study of motor neurodegeneration19, a single intracerebroventricular injection of human cord blood mononuclear cells was administered into SOD1-G93A and wobbler mice, two models of amyotrophic lateral sclerosis. The results showed significant improvements in symptom progression in both animal groups and increased longevity in the SOD1-G93A mice. In addition, implanted NSCs into the brains of an animal model of PD were shown to survive in the host brain and express neurotrophic factors representative of the neurons they replaced6.
Since our experiment was strictly a short-term study of delivery, we did not examine the mechanisms supporting the physical and behavioral improvements in our ataxia model. Other researchers have investigated the possible mechanisms responsible for the beneficial outcome of transplanting stem cells into neurodegenerative tissue. In vitro studies20 have shown that stem cells protect other cells against glutamate excitotoxicity-induced cell death by secreting neurotrophic factors, including brain-derived neurotrophic factors (BDNFs), vascular endothelial growth factors (VEGFs), and hepatocyte growth factor (HGF). Blurton-Jones et al.21 showed increased synaptic density and improved cognition in a mouse model of Alzheimer's disease after implantation of NSCs expressing elevated BDNF. NSCs have also been known to replace and repair neurons in lesioned areas of the mammalian brain22 and protect against glutamate-induced excitotoxicity by secreting glial cell line-derived factor and nerve growth factor23.
Last, we must consider the effect the immunosuppressive agent cyclosporine had on the results of the study. The use of cyclosporine is crucial to prevent rejection of human cell xenografts in rodent models of neurological diseases24. Cyclosporine affects T-cell production by interfering with signaling pathways that decrease proliferation, differentiation, and cytokine production of mammalian T cells25. In previous studies, there was a high variability in cyclosporine dosage and ways of administration that includes oral, subcutaneous, IP, and intramuscular. The most common dosages ranged from 5 to 20 mg/kg/day. These short-term studies found that daily IP injections26 or IP injections combined with cyclosporine in drinking water showed positive results in preventing human stem cell rejection23. In our current study, we chose Alzet osmotic pumps to deliver a cyclosporine dose of 15 mg/kg/day for 28 days for all treated mutant groups. This method of chronic delivery prevents painful daily injection and subsequent behavioral changes in treated animals. We did not detect any negative effects of cyclosporine, and no behavioral alterations were observed in treated mutants other than natural disease progression.
Conclusions
In conclusion, our study has demonstrated that stereotactically implanted, undifferentiated NPCs are capable of engrafting into damaged areas of host brain. Stereotactic injection of undifferentiated hNPCs resulted in an improved clinical picture for sHW rats. Specifically, animals that received injection of live progenitor cells into both the hippocampus and cerebellum demonstrated weight gain and improved motor scores compared to their dNPC counterparts. Intracarotid injection of these same cells failed to produce any significant clinical improvements. Histological examination showed that a large number of NPCs injected into the blood were retained by the lungs and failed to arrive into the site of disease (brain) in significant numbers. In conclusion, this study utilizing two common delivery systems reveals that a direct and focused delivery method is essential to stem cell treatment success and also suggests that undifferentiated NPCs have a strong potential for becoming a viable treatment of neurodegenerative diseases.
Acknowledgments
The authors thank Lindsay Peltz and Tatianna Adelstein for stem cell culturing and immunohistochemistry, and William Van Trigt and Prachi Javale for editing and proofreading the manuscript. We appreciate Fritz Libby and the vivarium staff for animal care. This study was partially funded by a Joint Venture CSUPERB Grant. Dr. Oleg Kopyov is a cofounder and co-owner of Celavie Biosciences LLC, and Alex Kopyov and Jessica Ochoa are employees of the company.
References
- 1.Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006; 24(6): 1433–40. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003; 23(7): 2557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, Schaar B, Steinberg G,. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke 2008; 39(4): 1300–6. [DOI] [PubMed] [Google Scholar]
- 4.Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, De A, Choi R, Chen S, Rutt BK, Gambhir SS, Guzman R. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke 2010; 41(9): 2064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias-Carrión O, Yuan TF,. Autologous neural stem cell transplantation: A new treatment option for Parkinson's disease? Med Hypotheses 2009; 73(5): 757–9. [DOI] [PubMed] [Google Scholar]
- 6.Blandini F, Cova L, Armentero M-T, Zennaro E, Levandis G, Bossolasco P, Calzarossa C, Mellone M, Giuseppe B, Deliliers GL, Polli E, Nappi G, Silani V,. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 2010; 19(2): 203–17. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RW, Cepeda C, Miyashiro-Turman J, Levine M,. Development of morphological and physiological alterations in the hippocampus of the spastic Han Wistar rat. Dev Brain Dysfunct. 1997; 10(1): 1–14. [Google Scholar]
- 8.Brunson KL, Khanna A, Cromwell HC, Cohen RW. Effect of the noncompetitive NMDA antagonists MK-801 and ketamine on the spastic Han-Wistar mutant: A rat model of excitotoxicity. Dev Neurosci. 2001; 23(1): 31–40. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001; 169(1): 12–20. [DOI] [PubMed] [Google Scholar]
- 10.Daldrup-Link HE, Rudelius M, Metz S, Piontek G, Pichler B, Settles M, Heinzmann U, Schlegel J, Oostendorp RAJ, Rummeny EJ. Cell tracking with gadophrin-2: A bifunctional contrast agent for MR imaging, optical imaging, and fluorescence microscopy. Eur J Nucl Med Mol Imaging 2004; 31(9): 1312–21. [DOI] [PubMed] [Google Scholar]
- 11.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS,. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009; 18(5): 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 2008; 131(Pt 3): 616–29. [DOI] [PubMed] [Google Scholar]
- 13.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: The lung barrier. Transplant Proc. 2007; 39(2): 573–6. [DOI] [PubMed] [Google Scholar]
- 14.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleynik A, Gernavage KM, Mourad YS, Sherman LS, Liu K, Gubenko YA, Rameshwar P. Stem cell delivery of therapies for brain disorders. Clin Transl Med. 2014; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K,. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 2011; 6(11): e27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Eckert MA, Riazifar H, Kang D-K, Agalliu D, Zhao W,. From blood to the brain: Can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013; 2013: 435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, Lang J, Sun X, Zhang B, Liu Y, An R. Monitoring bone marrow stem cells with a reporter gene system in experimental middle cerebral artery occlusion rat models. J Nucl Med. 2013; 54(6): 984–9. [DOI] [PubMed] [Google Scholar]
- 19.Bigini P, Veglianese P, Andriolo G, Cova L, Grignaschi G, Caron I, Daleno C, Barbera S, Ottolina A, Calzarossa C, Lazzari L, Mennini T, Bendotti C, Silani V. Intracerebroventricular administration of human umbilical cord blood cells delays disease progression in two murine models of motor neuron degeneration. Rejuvenation Res. 2011; 14(6): 623–39. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Lu C, Han Q, Li J, Du Z, Liao L, Zhao RC. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology 2011; 279(1–3): 189–95. [DOI] [PubMed] [Google Scholar]
- 21.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM,. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 2009; 106(32): 13594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, Lachyankar MB, Redmond DE, Snyder EY. Global gene and cell replacement strategies via stem cells. Gene Ther. 2002; 9(10): 613–24. [DOI] [PubMed] [Google Scholar]
- 23.Lladó J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD,. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004; 27(3): 322–31. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MB, Krishnaney-Davison R, Cohen LK, Zhang SC. Injected versus oral cyclosporine for human neural progenitor grafting in rats. J Stem Cell Res Ther. 2012; Suppl 10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner J, Drobits K, Pickl WF, Majdic O, Zlabinger G, Steinberger P. The effects of cyclosporine A and azathioprine on human T cells activated by different costimulatory signals. Immunol Lett. 2011; 140(1–2): 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998; 149(2): 310–21. [DOI] [PubMed] [Google Scholar]


