Abstract
Being a potential candidate for stroke treatment, bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) have been demonstrated to be able to enhance angiogenesis and proliferation of reactive astrocytes, which subsequently leads to the amelioration of neurological injury. Increasing evidence further indicates that combining BM-MSCs with certain agents, such as simvastatin, may improve therapeutic effects. Sodium ferulate (SF) and n-butylidenephthalide (BP), two main components of Radix Angelica Sinensis, are proven to be important regulators of stem cells in cell migration, differentiation, and pluripotency maintenance. This study aimed to investigate whether combining BM-MSCs with SF and BP had better therapeutic effect in the treatment of stroke, and the underlying molecular basis for the therapeutic effects was also investigated. The results showed that combination treatment notably reduced neurological injury after stroke and increased the expression of astrocyte-derived vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and von Willebrand factor-positive vascular density in the ischemic boundary zone as evaluated by immuno fluorescence staining. After treatment with BM-MSCs plus SF and BP, astrocytes showed increased expression of VEGF and BDNF by upregulating protein kinase B/mammalian target of rapamycin (AKT/mTOR) expression in an oxygen- and glucose-deprived (OGD) environment. Human umbilical vein endothelial cells (HUVECs) incubated with the conditioned medium (CM) derived from OGD astrocytes treated with BM-MSCs plus SF and BP showed significantly increased migration and tube formation compared with those incubated with the CM derived from OGD astrocytes treated with BM-MSCs alone. These results demonstrate that combination treatment enhances the expression of astrocyte-derived VEGF and BDNF, which contribute to angiogenesis after cerebral ischemia, and the underlying mechanism is associated with activation of the astrocytic AKT/mTOR signaling pathway. Our study provides a potential therapeutic approach for ischemic stroke.
Keywords: Angiogenesis, Astrocytes, Bone marrow-derived stromal cells (BM-MSCs), n-Butylidenephthalide (BP), Sodium ferulate (SF)
Introduction
Globally, stroke is the second leading cause of death and the major cause of disability, especially in developing countries1. Reports indicated that angiogenesis and neurogenesis occur in response to ischemic stroke. New formation of collateral circulation in the penumbra could contribute to enhancing neuronal survival and reducing mortality, so angiogenesis seemed to be more important2,3. In this sense, enhancement of angiogenesis is an emerging therapeutic strategy in the treatment of stroke.
It is believed that bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) are able to promote neovascularization and tissue regeneration after stroke. Indeed, recent reports have demonstrated the therapeutic potential of BM-MSCs in the treatment of stroke, including stimulating synaptogenesis and neurogenesis, reducing neuronal apoptosis, and promoting angiogenesis in the ischemic zone after stroke4–7. In addition, BM-MSCs could activate the bone morphogenetic protein (BMP) 2/4 signaling pathway in oxygen- and glucose-deprived (OGD) astrocytes, enhance the astrocytic phenotype in subventricular progenitor cells in vitro, and improve glial cell line-derived neurotrophic factor (GDNF) secretion in reactive astrocytes after middle cerebral artery occlusion (MCAo)8,9, as well as facilitate the release of neurovascular trophic factors from activated astrocytes, for example, brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) through activating astrocytic phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK) pathways10. In addition to VEGF, another key molecule in poststroke angiogenesis, BDNF, also directly modulates vascular density and neurological functional recovery after stroke11. Together, BM-MSC-mediated activation of astrocytes and secretion of VEGF and BDNF contribute to angiogenesis and neuroprotective effects.
Recent evidence suggests that the therapeutic effect of combined BM-MSCs and a therapeutic agent was superior to any single-treatment method12. Combination treatment of BM-MSCs and simvastatin facilitated the expression of VEGF, BDNF, stromal cell-derived factor-1α (SDF-1α), and GDNF in astrocytes during the treatment of stroke. Also, simvastatin was shown to enhance the differentiation into endothelial cells (ECs) and migration of BM-MSCs13–15. Our previous study also demonstrated that sodium ferulate (SF; an active component derived from Radix Angelica Sinensis) promoted the migration of BM-MSCs toward the ischemic boundary zone (IBZ) via upregulation of SDF-1α/chemokine (CXC motif) receptor-4 axis and the differentiation into astrocytes via activating the BMP 2/4 signaling pathway on day 3 poststroke16,17. n-Butylidenephthalide (BP), an active component of Radix Angelica Sinensis, was found to be capable of maintaining stem cell pluripotency and increasing induced pluripotent stem cell (iPSC) generation efficacy18. Despite the therapeutic potential of combination treatments, there is still lack of evidence as to whether combined treatment with BM-MSCs + SF + BP brings a more effective therapeutic outcome in stroke. The aim of this study was to evaluate the effects of combined treatment of BM-MSCs + SF + BP on stroke, including the expression of astrocyte-derived neurovascular trophic factors, angiogenesis, and neurological functional amelioration, as well as the molecular mechanisms after focal cerebral ischemia.
Materials and Methods
Primary Cell Culture and Identification of BM-MSCs
The present study conforms to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996 for scientific purposes. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Macau University of Science and Technology. BM-MSCs were obtained from young male rats according to our previously reported method16. In brief, male Sprague–Dawley (SD) rats (Guangdong Medical Laboratory Animal Center, Foshan, P.R. China) weighing 50–60 g were sacrificed by cervical dislocation, and both sides of the tibias and femurs were disinfected by 75% alcohol for 3 min. Afterward, the tibias and femurs were washed by phosphate-buffered saline (PBS) three times, and the bone marrow was extracted by syringe needles. The freshly isolated cells were placed in a 95% air/5% CO2 incubator at 37°C and cultured with Dulbecco's modified Eagle's medium (DMEM)/F12 cell culture medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin–streptomycin (Thermo Fisher Scientific). Forty-eight hours later, the culture medium was replaced.
We sought to characterize cultured BM-MSCs because the cells isolated from the bone marrow might contain non-BM-MSCs (e.g., hematopoietic cells). BM-MSCs from passage 3 were harvested and respectively incubated with fluorescence-conjugated primary antibodies including CD44-PE, CD90-PE, CD45-FITC, CD34-PerCP, and PBS (negative control) in a black chamber at 4°C for 30 min. After washing with PBS, the cells were fixed with 4% paraformaldehyde (PFA) and analyzed by a flow cytometer equipped with the CellQuest system (Becton Dickinson, Franklin Lakes, NJ, USA).
Rat Permanent Middle Cerebral Artery Occlusion (pMCAo) Model and Experimental Groups
According to our previous report16, 50 male SD rats weighting 230–250 g were anesthetized with 10% (w/v) chloral hydrate [3.0 ml/kg; intraperitoneal (IP) injection; Sigma-Aldrich Shanghai Trading Co. Ltd., Shanghai, P.R. China]; subsequently, 4-0 surgical nylon suture (length of 20–22 mm determined by body weight) coated with polylysine was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the middle cerebral artery (MCA). In order to control the body temperatures of the rats under satisfactory conditions during the operation, we put the rats on animal heating pads to maintain their rectal temperature at 37°C; moreover, the room temperature and the humidity in the surgery room were kept at 23°C and 69%, respectively, using an air conditioner. Finally, the neurological function was evaluated using a 5-point scale neurological deficit score as reported by Yilmaz and colleagues19. Only animals with the score of “2” were selected for the subsequent studies (0 = no deficit, 1 = failure to extend right paw, 2 = circling to the right, 3 = falling to the right, and 4 = unable to walk spontaneously). During operation, nine rats died, and five rats did not comply with the standard and were excluded in the present study. Sham-operated animals underwent the same surgical procedure, but the nylon suture was not inserted. After pMCAo model establishment, all rats were given free access to food and water, and living room temperature and humidity were maintained as described for surgery; a 12:12-h light–dark cycle was implemented.
Thirty-six MCAo rats were randomly divided into three groups (12 rats per group): MCAo group, BM-MSC group, and BM-MSC + SF + BP group. One milliliter of PBS or BM-MSC suspension solution (2 × 106 cells/ml) was respectively injected into rats in the MCAo and BM-MSC or BM-MSC + SF + BP groups via the caudal vein at 3 h after model establishment; then 1 ml of PBS or SF (60 mg/kg) was intraperitoneally injected into rats in the MCAo, BM-MSC, or BM-MSC + SF + BP group once a day for 7 days; in addition, BP (10 mg/kg) was subcutaneously injected into the BM-MSC + SF + BP group once a day for 3 days.
Neurological Functional Evaluations
Garcia and colleagues' neurological score evaluation20 was performed at 6 h, and 1, 3, 5, and 7 days after operation in each group. In brief, the test included the evaluation of animals' spontaneous activity: symmetry of four limb movements when rats were held suspended by the tail and symmetric forepaws, climbing wall of wire cage, body proprioception, reaction to touch on either side of the trunk, and response to vibrissae touch. The scores were awarded by an investigator who was blinded to the experimental groups.
Immunofluorescence Staining
Rats were sacrificed on the seventh day. Their brains were fixed in fresh 4% PFA for histological studies. Fresh frozen coronal sections of 40-μm thickness were cut by a cryostat microtome (Shandon Cryotome FSE; Thermo Fisher Scientific). Every fifth slice for a total of six sections was used for immunofluorescence staining. To identify whether VEGF and BDNF were expressed in astrocytes after stroke, an antibody against glial fibrillary acidic protein (GFAP; 1:1,000; Abcam, Cambridge, UK) was couple stained with VEGF (1:200; Abcam) and BDNF (1:1,000; Abcam) according to the manufacturer's protocol. Furthermore, brain sections harvested from each group were stained with von Willebrand factor (vWF) primary antibody (a marker of ECs; 1:1,000; Abcam) in order to observe new capillary network formation in IBZ. Samples were then washed and incubated with the Alexa Fluor® 488 goat anti-rabbit immunoglobulin (IgG; H + L) (1:200; Life Technologies) or goat anti-rabbit IgG H&L (Cy3®) preadsorbed (1:100; Abcam) secondary antibodies for 1 h at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Shanghai Trading Co. Ltd.). Fluorescent labeling was examined with an LSM710 laser scanning confocal microscope (Carl Zeiss, Göttingen, Germany). Five nonoverlapping fields of one slice in the penumbral cortex and striatum were evaluated for each rat under a magnification of 10×40 in confocal images. Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA) was applied to analyze the proteins' positive signal. In control experiments, primary antibodies were replaced with PBS.
Oxygen–Glucose-Deprived Astrocytes and Experimental Groups In Vitro
In order to ideally mimic focal cerebral ischemia in vitro, we used OGD astrocytes. Astrocytes were procured from the American Type Culture Collection (ATCC® CRL2006TM; Manassas, VA, USA) and derived from the cortex of normal Rattus norvegicus. Specifically, cultured cells were washed three times with CMF-HBSS (Ca2+, Mg2+-free Hank's balanced salt solution; Thermo Fisher Scientific) and then replaced with glucose- and serum-free DMEM. Afterward, the astrocytes were placed in an anaerobic chamber (HP015; HITECH, Guangzhou, P.R. China) with an atmosphere of 95% N2 and 5% CO2 for 1 h at 37°C. The OGD process was terminated by removing the astrocytes from the anaerobic chamber, rinsing with PBS, and feeding with DMEM/F12. Control cells were maintained in complete DMEM/F12 medium under normal culture conditions.
According to our previously determined dosages of SF and BP, normal and OGD astrocytes were divided into five experimental groups, and OGD astrocytes (5 × 105 cells/ml) were incubated with different culture mediums. The experimental groups included the OGD group, BM-MSC group, BM-MSC + SF + BP group, BM-MSC + SF + BP + AT7867 group, and normal cultured astrocytes group (as control). In the OGD group, OGD astrocytes were incubated with DMEM/F12 culture medium alone; in the BM-MSC group, CM of cultured BM-MSCs (5 × 104 cells/ml) after 12 h was added into OGD astrocytes; in the BM-MSC + SF + BP group, CM of cul tured BM-MSCs treated with SF (5 μg/ml) and BP (0.75 μg/ml) for 12 h was added into OGD astrocytes; in the BM-MSC + SF + BP + AT7867 group, AT7867 (1 μM; Akt inhibitor; Selleckchem, Houston, TX, USA) was added into the medium of cultured astrocytes for 1 h in order to inhibit Akt activation, and the cells were simultaneously induced by the OGD procedure for 1 h. Finally, these astrocytes (5 × 105 cells/ml) were incubated with CM of the BM-MSC + SF + BP group. After incubation with different culture media for 15 min, astrocytic protein kinase B/mechanistic target of rapamycin (AKT/mTOR) pathway in five groups was detected, and then astrocyte-derived VEGF and BDNF protein expressions were analyzed after 36 h of incubation by Western blot.
Western Blot Analysis
Protein samples were extracted from cultured astrocytes of over five groups at different time points, and protein concentration was determined by enhanced bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Shanghai, P.R. China) according to the method suggested by the manufacturer. Protein samples were electrophoresed on gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel (Bio-Rad, Hercules, CA, USA) and subsequently electrotransferred to polyvinylidene difluoride (PVDF) mem branes (Bio-Rad) in Tris-glycine transfer buffer. Membranes were blocked in 5% (w/v) nonfat dry milk for 1 h at room temperature, followed by protein incubation with primary antibodies including anti-AKT (1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-p-AKT (1:2,000; Cell Signaling Technology), anti-mTOR (1:200; Cell Signaling Technology), anti-p-mTOR (1:200; Cell Signaling Technology), anti-VEGF (1:1,000; Abcam), and anti-BDNF (1:100; Abcam) at 4°C overnight. Then the membranes were gently washed with TBST [50 mM Tris-HCl (pH 7.4; Acros Organics BVBA, Geel, Belgium), 150 mM NaCl, 0.05% Tween 20 (Acros Organics BVBA)] three times and incubated with goat anti-rabbit IgG (H + L) secondary antibodies (1:5,000; LI-COR Biosciences, Lincoln, NE, USA) at room temperature for 1 h. Finally, the membranes were scanned, and the signals of reactive bands were quantified using the Odyssey Infrared Imager (LI-COR). β-Actin (1:1,000) was detected as internal control, VEGF and BDNF expressions in five groups were normalized against that of β-actin, and phosphorylation levels of the targeted proteins were analyzed by total levels of corresponding proteins. Western blots were duplicated three independent times.
Wound Healing and Tube Formation Assay
Cultured human umbilical vein endothelial cells (HUVECs; ATCC® CRL-1730; ATCC) were used to evaluate cell migration by wound healing assay. During the HUVEC logarithmic growth period, the confluent layers of cells in six-well plates were scratched with a pipette tip to create an artificial straight line wound that was used to monitor cell migration and repopulation in the scratched area, and then HUVECs were rinsed with PBS three times. Subsequently, CM after 36 h of incubation in the normal, OGD, BM-MSC, BM-MSC + SF + BP, and BM-MSC + SF + BP + AT7867 groups was respectively added into the well plates. Images of wound closure were taken using a phase-contrast microscope (Leica MC170 HD; Leica Microsystems GmbH, Wetzlar, Germany), and the width of each wound from each group was measured at 0, 12, and 24 h. The percentage of the remaining cell-free area of the OGD, BM-MSC, BM-MSC + SF + BP, and BM-MSC + SF + BP + AT7867 groups compared to normal group was used to quantify the characteristics of HUVEC migration. Simultaneously, HUVECs were grown on 96-well plates coated with Matrigel followed by incubation with CM of the five groups for 24 h. Cells were photographed using a phase-contrast microscope, and the number of branch points was counted to verify the capacity of angiogenesis in each group.
Statistical Analysis
All results were expressed as means ± standard deviation. Two-way repeated measures analysis of variance (ANOVA) was performed to determine the interaction effect between group and time period on neurological function, and one-way ANOVA was used to analyze other data followed by least significant difference test for multiple comparisons. Differences were considered to be statistically significant at p < 0.05. All data were analyzed using SPSS 19.0 (IBM, Armonk, NY, USA).
Results
Characterization of Rat BM-MSCs
To characterize the cultured BM-MSCs, expression of CD44 and CD90 (BM-MSC antigens), and CD45 and CD34 (hematopoietic cell surface markers) was determined in cells at passage 3 using flow cytometry. The results indicated 7.09% and 2.17% of CD45 and CD34 expression in cultured cells, respectively, whereas 92.41% and 96.92% of cells expressed CD44 and CD90, respectively (Fig. 1). This result indicated a relatively high purity of BM-MSCs, which were used in the following experiments.
Figure 1.

Bone marrow-derived mesenchymal stem/stromal cell (BM-MSC) phenotype identification by flow cytometry. BM-MSCs were harvested from bone marrow of Sprague–Dawley (SD) rats, and adherent stromal cells were isolated. BM-MSCs were identified by fluorescence and incubated with fluorescence-conjugated antibodies including CD44-PE, CD90-PE, CD45-FITC, CD34-PerCP, and phosphate-buffered saline (PBS). The positive expressions of CD44 (A) and CD90 (B) were 92.41% and 96.92%, and those of CD45 (C) and CD34 (D) were 7.09% and 2.17%.
pMCAo Model Establishment and Combination Treatment Improved Neurological Function
In the present study, we successfully established the pMCAo model. Our dropout rate was 9% because of exclusion of unstandardized experimental animals, and the mortality rate as reported by Shanbhag and colleagues was about 12.5% using a new filament21, while our animal survival rate was about 82%. Additionally, in order to more clearly illustrate animal experimental design, a scheme of animal experimental timeline is presented in Figure 2A.
Figure 2.
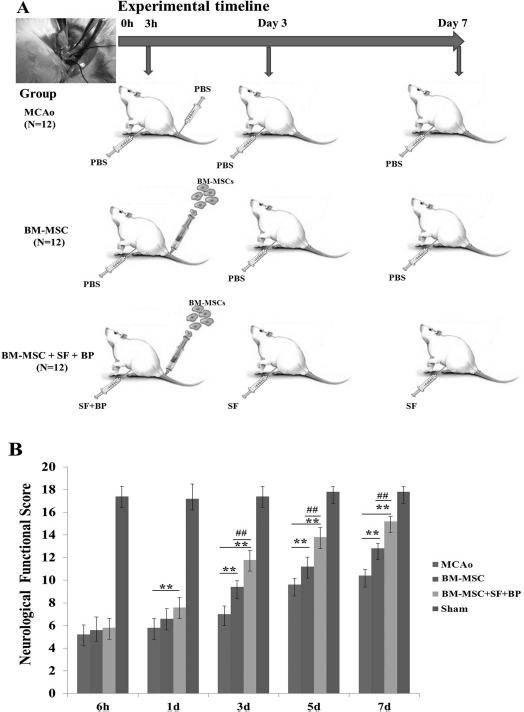
Scheme of animal experimental timeline and neurological functional outcomes in different times after surgery. (A) Focal cerebral ischemia was induced by MCAo. One milliliter of PBS or BM-MSC suspension solution (2 × 106 cells/ml) was respectively injected into rats in MCAo and BM-MSC or BM-MSC + SF + BP groups via caudal vein at 3 h after operation; then 1 ml of PBS or SF (60 mg/kg) was intraperitoneally injected into rats in the MCAo, BM-MSC, or BM-MSC + SF + BP group once a day for a continuous 7 days, while BP (10 mg/kg) was subcutaneously injected into the BM-MSC + SF + BP group once a day for a continuous 3 days. (B) Garcia et al.'s20 neurological score evaluation was performed from 6 h to 7 days after operation in each group. Combination treatment could significantly promote neurological functional recovery compared with the MCAo and BM-MSC groups. Data are presented as means ± SD. ∗∗p < 0.01, compared with MCAo; ##p < 0.01, compared with BM-MSC. SF, sodium ferulate; BP, n-butylidenephthalide; PBS, phosphate-buffered saline; MCAo, middle cerebral artery occlusion; BM-MSC, bone marrow-derived mesenchymal stem/stromal cell; SD, standard deviation.
To determine whether combination treatment improves neurological function, Garcia et al.'s neurological score evaluation20 was performed on rats at 6 h and at 1, 3, 5, and 7 days after stroke. A two-way repeated-measures ANOVA showed significant effects in both group and time period regarding neurological function of all four groups (group: F = 179.746, p < 0.01; time period: F = 758.45, p < 0.01), as well as obvious effect in the factor of group and time period (F = 21.28, p < 0.01). MCAo rats treated with BM-MSCs showed significantly improved neurological function compared to rats in the MCAo group from day 3 to day 7 (p < 0.01) (Fig. 2B). Furthermore, MCAo rats receiving combined treatment of BM-MSCs + SF + BP exhibited significant improvement of neurological function as early as 1 day after MCAo operation, which was obviously superior to rats in the MCAo or BM-MSC group. Similar results were also observed on days 3–5 after stroke (p < 0.01, vs. MCAo group and BM-MSC group) (Fig. 2B). In addition, in our other published article, 2,3,5-triphenyltetrazolium chloride (TTC) staining was applied to evaluate the volume of infarction zone in the rat brain on the seventh day after treatment, and it suggested that SF and BP combined with BM-MSCs significantly decreased infarct volume after stroke22.
Combination Treatment Facilitated Astrocyte-Derived VEGF and BDNF Expression and Enhanced vWF+ Vascular Density In Vivo
The expression of GFAP is widely considered as a marker of reactive astrogliosis. BM-MSCs reportedly amplified proliferation of reactive astrocytes after ischemia and increased astrocytic VEGF and BDNF gene expression when they were cocultured with astrocytes under hypoxic conditions8,23. As shown in Figure 3A and B, expression of VEGF and BDNF (green) primarily colocalized with GFAP+ (red) astrocytes in the IBZ of the brain on day 7 after stroke. Quantitative analysis showed that astrocyte-derived VEGF and BDNF expression in the BM-MSC group increased markedly compared with those in the MCAo group (p < 0.01) (Fig. 3A and B). Furthermore, combination treatment dramatically enhanced the expression of astrocytic VEGF and BDNF, compared to the BM-MSC group (p < 0.01) (Fig. 3A and B).
Figure 3.
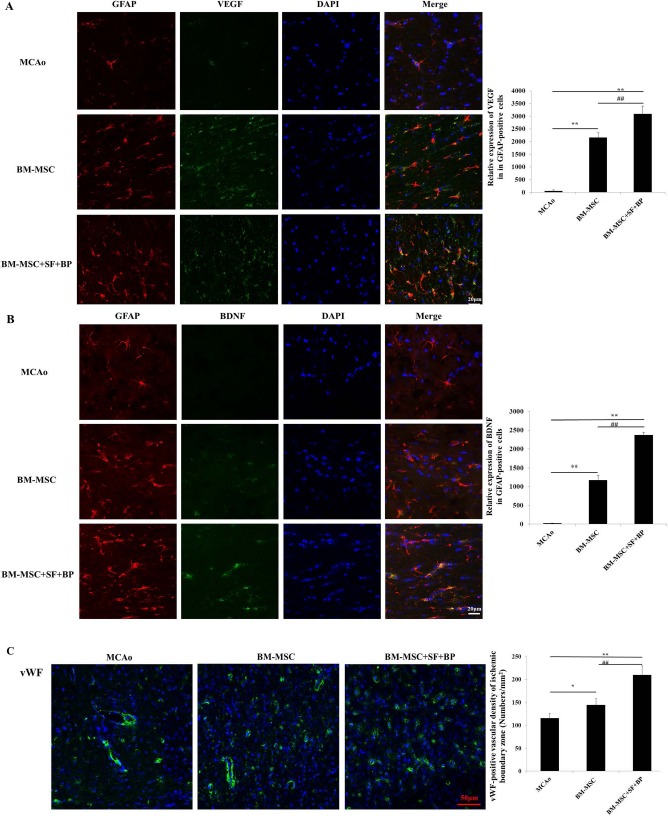
Astrocyte-derived VEGF and BDNF expression and vWF+ vascular density in the ischemic boundary zone on the seventh day after stroke. The fresh-frozen coronal sections in each group were collected, and triple fluorescence immunostaining was performed. (A, B) Immunofluorescence stainings of GFAP (red), VEGF (green), and BDNF (green) were presented, and relative expressions of VEGF and BDNF in the GFAP+ area were analyzed. It indicated that combination of BM-MSCs with SF and BP notably improved astrocytic VEGF and BDNF expression. (C) vWF immunofluorescence staining (green) was presented, and positive signal was analyzed. Data are expressed as mean±SD. ∗p < 0.05, ∗∗p < 0.01, compared with MCAo; ##p < 0.01, compared with BM-MSC. The experiment was repeated three times, and representative pictures are shown. Scale bars: 20 μm (A, B), 50 μm (C). vWF, von Willebrand factor primary antibody; VEGF, vascular endothelial growth factor; BDNF, brain-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; BM-MSCs, bone marrow-derived mesenchymal stem/stromal cells; MCAo, middle cerebral artery occlusion; SF, sodium ferulate; BP, n-butylidenephthalide; DAPI, 4′,6-diamidino-2-phenylindole.
Angiogenesis is defined as sprouting of new vessels from preexisting vessels, resulting in new capillary networks24. In Figure 3C, treatment of BM-MSCs alone increased the density of vWF+ endothelium cells in IBZ compared with the MCAo group (p < 0.05). Moreover, the vWF+ cell density was significantly higher in rats treated with BM-MSCs + BP + SF than in those treated with BM-MSCs alone (p < 0.01).
Combination Treatment Activated Astrocytic AKT/mTOR Pathway and Elevated Astrocyte-Derived VEGF and BDNF Expressions In Vitro
A previous report demonstrated that MSC-mediated activation of AKT/mTOR signaling in astrocytes was the major pathway to enhance the expression of astrocytederived VEGF and BDNF8. In the current study, treatment of BM-MSCs + SF + BP on rats after stroke increased the expression of astrocyte-derived VEGF and BDNF in IBZ more significantly than treatment of BM-MSCs alone. We further examined the activation status of the AKT/mTOR pathway in astrocytes after incubation with the CM derived from BM-MSCs + SF + BP or BM-MSCs alone. As shown in Figure 4A, quantitative analysis indicated that the expression of phosphorylated AKT and mTOR in the BM-MSC + SF + BP group was significantly higher than those of normal, OGD, and BM-MSC groups (p < 0.01). Treatment of BM-MSCs + SF + BP also markedly enhanced the expression of astrocyte-derived VEGF and BDNF compared with the other four groups after 36 h of incubation (Fig. 4B). The elevated VEGF and BDNF expression in the BM-MSC + SF + BP-treated group was effectively inhibited by AT7867 (AKT inhibitor). Together, these results suggest that combination treatment of BM-MSCs + SF + BP enhances the expression of astrocyte-derived VEGF and BDNF via activation of AKT/mTOR pathway more significantly than treatment of BM-MSCs alone.
Figure 4.
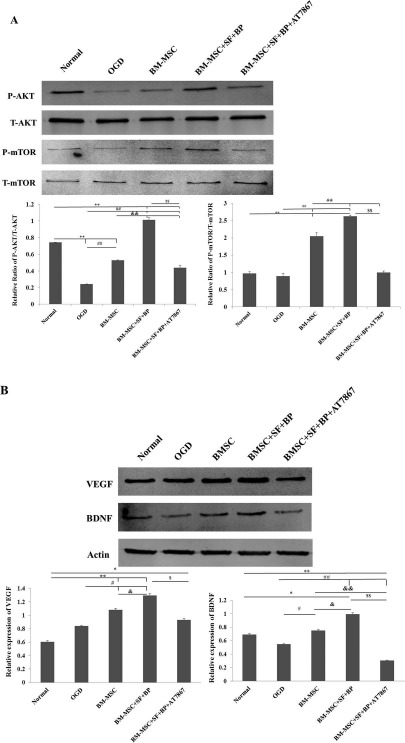
AKT/mTOR signaling pathway regulated VEGF and BDNF expression in OGD-induced astrocytes. Western blot detected astrocytic AKT/mTOR, VEGF, and BDNF expressions in each group. (A) Representative Western blot results for p-Akt, Akt, p-mTOR, mTOR, and quantitative analysis at 15 min after treatment. (B) Representative Western blot results for VEGF and BDNF, as well as quantitative analysis after 36 h of incubation. It showed combined BM-MSCs with SF and BP could notably activate astrocytic AKT/mTOR signaling pathway to improve VEGF and BDNF protein synthesis, but the effects were inhibited by the AKT inhibitor (AT7867). Data are expressed as means ± SD. ∗p < 0.05, ∗∗p < 0.01, versus Normal; #p < 0.05, ##p < 0.01, versus OGD; & p < 0.05, & &p < 0.01, versus BM-MSC; $p < 0.05, $$p < 0.01, versus BM-MSC + SF + BP. AKT/mTOR, protein kinase B/mechanistic target of rapamycin; OGD, oxygen and glucose deprived; VEGF, vascular endothelial growth factor; BDNF, brain-derived neurotrophic factor; SD, standard deviation; SF, sodium ferulate; BP, n-butylidenephthalide; BM-MSC, bone marrow-derived mesenchymal stem/stromal cell.
Combination Treatment Promoted Migration and Capillary Formation of HUVECs
To further clarify whether treatment of BM-MSCs + SF + BP increases angiogenesis, we performed wound healing and tube formation assay by incubating HUVECs with different CMs. As shown in Figure 5, the result of wound healing assay showed that HUVECs incubated in the CM of the BM-MSC + SF + BP group exhibited significantly higher cell migration than cells incubated with the CM of OGD and BM-MSCs. Similarly, HUVECs incubated with CM of the BM-MSC + SF + BP group showed significantly higher endothelial tube formation and angiogenic activity than those incubated with CM of OGD and BM-MSCs (p < 0.05, vs. BM-MSC; p < 0.01, vs. OGD). Cell migration and tube formation of HUVECs in CM of the BM-MSC + SF + BP group were effectively inhibited in the presence of AT7867 and significantly lower than that of cells in CM of the BM-MSC alone and BM-MSC + SF + BP groups. This may be attributed to the counteracting effect of AT7867 in the activation of AKT signaling pathway and expression of astrocyte-derived VEGF and BDNF. Together, these results demonstrated that combination treatment was able to improve angiogenesis in vitro.
Figure 5.
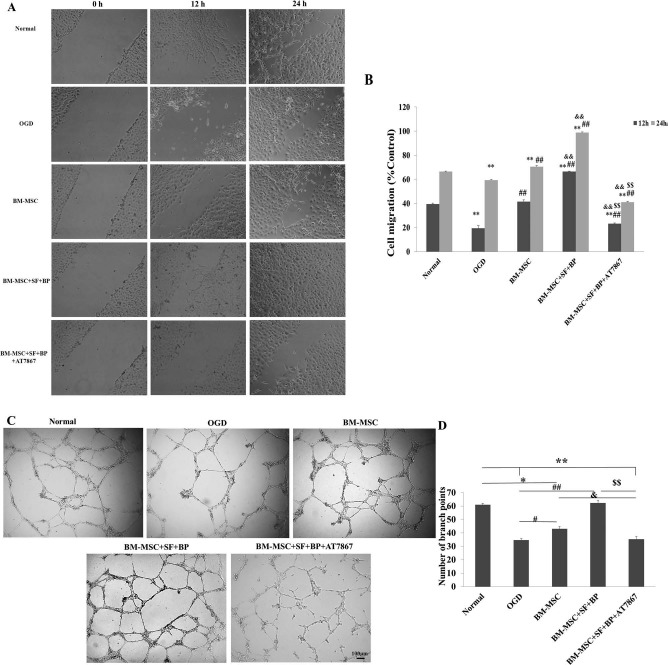
Wound healing and tube formation assay of HUVECs. (A, B) Cultured HUVECs were wounded using multichannel straight scratch with a pipette tip and treated with different conditional media for 24 h. The images of wound closure were taken, and a percentage of mean wound width of each treatment group was compared to control. It showed that the BM-MSC + SF + BP group significantly promoted HUVEC migration into denuded areas. (C, D) Cultured HUVECs on Matrigel-coated 96-well plates incubated with different conditional media for 24 h. Representative images of HUVEC tube formation were presented, and branch points in each group were counted to quantify endothelial tube formation. It indicated that BM-MSC + SF + BP group obviously enhanced the number of branch points compared to other groups. Data are expressed as means ± SD. ∗p < 0.05, ∗∗p < 0.01, versus Normal; #p < 0.05, ##p < 0.01, versus OGD; &p < 0.05, & & p < 0.01, versus BM-MSC; $$p < 0.01, versus BM-MSC + SF + BP. HUVECs, human umbilical vein endothelial cells; OGD, oxygen and glucose deprived; SD, standard deviation; SF, sodium ferulate; BP, n-butylidenephthalide; BM-MSC, bone marrow-derived mesenchymal stem/stromal cell.
Discussion
Angiogenesis and recovery of vasculature have been the emerging therapeutic strategies for patients suffering from stroke. BM-MSCs are reportedly able to enhance angiogenesis, which contributes to the recovery of neurological function. When cocultured with astrocytes, BM-MSCs have been shown to increase the expression of astrocyte-derived VEGF and BDNF, a crucial mechanism of BM-MSC-induced angiogenesis and neuronal protection8,11,25,26. In the present study, compared to treatment of BM-MSCs alone, treatment of BM-MSCs + SF + BP showed significantly better improvement of angio genesis, higher expression of astrocyte-derived VEGF and BDNF in the IBZ, as well as decreased neurological injury.
BM-MSC transplantation has been regarded as a candidate for ameliorating neurological injury in stroke patients, as it is especially beneficial to neuroplasticity and neurological outcome through the enhancement of neurogenesis, angiogenesis, and oligodendrogenesis after stroke27,28. However, the mechanism of how intravenously transplanted BM-MSCs repair neurological injury from ischemic stroke remains unclear. Most of the infused BM-MSCs did not migrate to the stroke lesion and interact with cerebral parenchymal cells. Therefore, replacement of injured neurons by neuronal cells newly differentiated from BM-MSCs was not the main effect of BM-MSCs in ameliorating neurological function. Inflammation and interaction between the immune and central nervous system (CNS) are the crucial processes in the pathophysiology of stroke29. It has been reported that splenic monocytes in peripheral blood contributed to attenuation of cerebral ischemic injury, and BM-MSCs infused via the tail vein could reprogram pulmonary macrophages to secret interleukin-10 (IL-10) in an animal sepsis model30,31. These results suggested that a convincing mechanism on attenuating cerebral damage was through the immunomodulation by infused BM-MSCs. The two active components derived from Radix Angelica Sinensis, SF and BP, were demonstrated to be capable of reducing inflammation and inhibiting activated microglia from releasing various proinflammatory molecules32,33. We hypothesized that treatment of SF and BP would exert a synergistic effect on immunomodulation when combined with BM-MSCs. In the current study, we demonstrated that combined BM-MSC administration with SF and BP could notably facilitate OGD astrocytes to release VEGF and BDNF. It showed that the paracrine effect of BM-MSCs by which immunomodulatory and/or neuro/vasculartrophic factors are secreted is considered as the key action on the improvement of neurological function after stroke.
When acute cerebral ischemia occurs, reactive astrocytes are believed to possess a protective function through releasing neurotrophic factors, decreasing inflammation and lesion volume, enhancing interplay of neurogenic and vascular elements, and regulating the CNS homeostasis after stroke34–36. In addition, when cocultured with astrocytes, BM-MSCs substantially suppress the expression of genes related to inhibition of axonal regeneration and therefore contribute to axonal remodeling. Such positive effects from BM-MSCs counteracted the side effect of reactive astrocytes: excessive thickness of glial scar formation, inhibition of axonal and synaptic growth, and regeneration in the later stage of cerebral ischemia37,38. However, a recent report indicated that astrocyte-mediated scar formation was beneficial to axonal regeneration in severe spinal cord injury39. The advantages and disadvantages of reactive astrocytes in ischemic brain injury suggest the existence of different subtypes of astrocytes. Wagner et al. demonstrated that both S100β and Musashi1 staining colocalized with GFAP+ astrocytes at the infarct border, while there was high glutamine synthetase expression in GFAP+ astrocytes in the remote zone instead of the IBZ, suggesting at least two subtypes of astrocytes characterized by different migratory abilities40. Thus, different effects of astrocyte subclasses after stroke should be further investigated in future experiments.
Evidence indicated that grafted BM-MSCs facilitated the expression of VEGF and BDNF in astrocytes when BM-MSCs and astrocytes were cocultured, as well as increased gliogenesis in the subventricular zone and proliferation of reactive astrocytes in the MCAo rats treated with BM-MSCs8,23,41. These results may suggest a therapeutic effect from BM-MSCs, which modulate the astrocytes to be protective and restorative in the acute stroke period or later recovery phase. In the present the study, we demonstrated that BM-MSCs combined with SF and BP significantly enhanced the expression of astrocyte-derived VEGF and BDNF compared with BM-MSC treatment alone at day 7 after ischemia, indicating that with the aid of BP and SF, treatment of BM-MSCs induced higher secretion of neurovascular trophic factor of astrocytes.
Among various angiogenic factors, VEGF is important for the migration and proliferation of ECs as well as their role in vascular sprouting and tube formation. Also, VEGF regulates the expression of angiopoietin-1 (Ang1)/Tie2, which leads to the stabilization of new blood vessel networks in the IBZ42. BDNF is another factor participating in angiogenesis after stroke. Fouda et al. found that with knockdown expression of BDNF, rats after MCAo showed notably decreased neurobehavioral recovery and vascular density, even with candesartan treatment11. In MCAo rats, we observed that BM-MSCs combined with SF and BP not only increased the expression of astrocyte-derived VEGF and BDNF but also significantly elevated the vWF+ vascular density in the IBZ, compared with the BM-MSC and MCAo treatment groups. Simultaneously in the in vitro study, OGD astrocytes cultured with CM of the BM-MSC + SF + BP group notably expressed VEGF and BDNF. Also, CM derived from OGD astrocytes in the BM-MSC + SF + BP group distinctly increased the ability of migration and tube formation of HUVECs. The consistency of results from in vitro and in vivo studies indicated that combination treatment was able to induce therapeutic angiogenesis through upregulating astrocyte-derived VEGF and BDNF after stroke.
A previous report showed that BM-MSCs stimulated the secretion of trophic factors from astrocytes, such as VEGF and BDNF, which were involved in the activation of the PI3K/AKT signaling pathway8. In order to clarify the mechanism that the combination treatment induced (the expression of astrocyte-derived VEGF and BDNF) more effectively, we investigated the astrocytic AKT/mTOR cascade. Akt is mainly phosphorylated by class I PI3Ks and plays an important role in neuronal survival and angiogenesis. Phosphorylated Akt activates mTOR and upregulates the expression of hypoxia-inducible factor (HIF)-1α by inhibiting prolyl-hydroxylases 2 (PHD2; an intracellular oxygen sensor) activity and subsequently turns on the transcription of erythropoietin and VEGF genes43. Chai et al. found that Scutellarin and caffeic acid ester fraction improved the expression of BDNF, nerve growth factor (NGF), and GDNF in astrocytes under the hypoxia/reoxygenation condition, and the mechanism was associated with cAMP response element-binding protein (CREB) and Akt signaling44. Recently, it was reported that blocking the mTOR pathway led to the inhibition of astrocyte migration, proliferation, and from producing inflammation mediators45. In the present study, the degrees of AKT phosphorylation in each group were parallel to those of mTOR. Moreover, the combination treatment group activated the AKT/mTOR pathway more significantly compared with the group treated with BM-MSCs alone. Subsequently, quantitative analysis showed that the expression of VEGF and BDNF in the combination treatment group was the highest and was inhibited by AT7867 (AKT inhibitor). Interestingly, slightly decreased phosphorylation of mTOR was noted in the OGD group in comparison with the normal group, while AKT phosphorylation in the OGD group was markedly lower than that in the normal group. We postulated that certain signaling pathways other than the PI3K/AKT pathway may be involved in the regulation of mTOR (e.g., activation of amino acid signal has been considered as an upstream modulator of mTORC1 independent of the PI3K/AKT pathway)46. To conclude, enhanced expression of astrocyte-derived VEGF and BDNF induced by the BM-MSC + SF + BP group closely correlates with activation of the AKT/mTOR pathway. Inhibition of HUVEC migration and tube formation by AT7867 further demonstrated the activation of AKT/mTOR signaling by combination treatment as an important factor in angiogenesis.
Although much data have demonstrated that BM-MSC transplantation may be an alternative approach for the treatment of stroke, the safety of grafted BM-MSCs should be considered. A previous study indicated that intravenous injection of a high dosage of BM-MSCs (3 × 106 cells per animal) resulted in 80% mortality of mice due to pulmonary microembolis47. However, when the transplanted cell number was reduced to 2 × 106, the mortality decreased to 10% due to less pulmonary embolism47. Further, He et al. demonstrated that transplantation of 2 times; 106 BM-MSCs significantly reduced the volume of the infarct area, enlarged the diameters of pial vessels and the basilar artery, and increased the capillary area in the peri-infarct zone of the cerebral cortex48. In addition to cell dosage, the velocity of cell infusions was also critical for the safety criteria in MSC therapy49. In the present study, few deaths of rats were found after transplantation of 2 times; 106 /ml allogeneic BM-MSCs via the caudal vein, suggesting that a cell dose of 2 × 106 /ml also met the criteria of safety of MSC transplantation in our experiment. Additionally, immunological rejection should be a concern due to different origins of BM-MSCs. Yet, it was reported that allogeneic (rat BM-MSCs) or xenogeneic (human BM-MSCs) transplantation could produce significant recovery of motor behavior after rat cerebral ischemia23, which suggested that allograft rejection was minimal. Therefore, allogeneic transplantation in the current study is appropriate.
From results of previous studies, not all combined treatments of BM-MSCs with pharmacological agents were beneficial to stroke therapy. Pösel et al. found that during combined treatment of bone marrow-derived mononuclear cells (BM-MNCs) and granulocyte-colony-stimulating factor (G-CSF), transplantation of cells at 48 h after stroke obviously abolished the improvement of neurological function induced by G-CSF treatment alone in a rat model of spontaneously hypertensive stroke50. A similar result was observed in another study that combined treatment of G-CSF, and predifferentiated BM-MSCs did not show more improvement of functional neurological recovery and neurogenesis, as well as decrease of infarct volume compared with G-CSF monotherapy in aged MCAo rats, although combined treatment enhanced angiogenesis significantly51. Moreover, because of excessive angiogenic factor expression, it was reported that BM-MSC transplantation had adverse effects on stroke in diabetic rats52. The detrimental effects of BM-MSC therapy were perhaps attributed to multiple factors, which at least included the influence of various pathological models and aged brain. In hypertensive and diabetic models, endothelial cellular dysfunction and/or inflammatory reaction might have disadvantageous impact on grafted BM-MSC at a certain pathological period, and aged brain might result in a detrimental microenvironment to the survival and regenerative capacity of BM-MSCs. Nevertheless, our previous report22 and current study suggested a beneficial and synergistic effect of combined treatment. Of course, in order to clearly illustrate if side effects exist, long-term observation needs to be completed in the next experiment.
In summary, the present study demonstrated that combination treatment is able to enhance the expression of astrocyte-derived VEGF and BDNF, leading to angiogenesis in the ischemic penumbra. The underlying molecular mechanism is at least partly associated with the activation of the astrocytic AKT/mTOR pathway. The benefits should help to ameliorate neurological injury after cerebral ischemia. It suggests the synergic therapeutic potential of combined BM-MSCs with BP and SF in the treatment of ischemic stroke.
Acknowledgments
This work was supported by the Science and Technology Development Fund of Macau (FDCT: No. 089/2012/A3). The authors thank Dr. Dawei Wang and Dr. Shigui Deng in Guangdong Provincial Hospital of Traditional Chinese Medicine who provided the laser confocal microscope for our research. The authors declare no conflicts of interest.
References
- 1.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011; 10: 471–80. [DOI] [PubMed] [Google Scholar]
- 2.Slevin M, Krupinski J. Cyclin-dependent kinase-5 targeting for ischaemic stroke. Curr Opin Pharmacol. 2009; 9: 119–24. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Zhu L, Fan M. Oxygen, a key factor regulating cell behavior during neurogenesis and cerebral diseases. Front Mol Neurosci. 2011; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003; 73: 778–86. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004; 1005: 21–8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience 2006; 141: 687–95. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A, Li ZH, Feng ZP, Li RK. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 2008; 16: 993–1005. [PubMed] [Google Scholar]
- 8.Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce bmp2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006; 83: 1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen LH, Li Y, Chopp M. Astrocytic endogenous glial cell derived neurotrophic factor production is enhanced by bone marrow stromal cell transplantation in the ischemic boundary zone after stroke in adult rats. Glia 2010; 58: 1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience 2005; 136: 123–34. [DOI] [PubMed] [Google Scholar]
- 11.Fouda AY, Alhusban A, Ishrat T, Pillai B, Eldahshan W, Waller JL, Ergul A, Fagan SC. Brain-derived neurotrophic factor knockdown blocks the angiogenic and protective effects of angiotensin modulation after experimental stroke. Mol Neurobiol. 2017; 54(1): 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Yu F, Lei T, Gao H, Li P, Sun Y, Huang H, Mu Q. Bone marrow mesenchymal stem cell therapy in ischemic stroke: Mechanisms of action and treatment optimization strategies. Neural Regen Res. 2016; 11: 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Liu X, Chen J, Zacharek A, Cui X, Savant-Bhonsale S, Liu Z, Chopp M,. Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via notch signaling pathway. Am J Physiol Cell Physiol. 2009; 296: C535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Chopp M, Zacharek A, Roberts C, Lu M, Savant-Bhonsale S, Chen J,. Chemokine, vascular and therapeutic effects of combination Simvastatin and BMSC treatment of stroke. Neurobiol Dis. 2009; 36: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhang Q, Chen Z, Liu N, Ke C, Xu Y, Wu W. Simvastatin combined with bone marrow stromal cells treatment activates astrocytes to ameliorate neurological function after ischemic stroke in rats. Turk J Biol. 2016; 40: 519–28. [Google Scholar]
- 16.Zhao Y. Guan Y, Xu Y, Li Y, Wu W. Sodium ferulate combined with bone marrow stromal cell treatment ameliorating rat brain ischemic injury after stroke. Brain Res. 2012; 1450: 157–65. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Lai W, Xu Y, Li L, Chen Z, Wu W. Exogenous and endogenous therapeutic effects of combination sodium ferulate and bone marrow stromal cells (BMSCs) treatment enhance neurogenesis after rat focal cerebral ischemia. Metab Brain Dis. 2013; 28: 655–66. [DOI] [PubMed] [Google Scholar]
- 18.Liu SP, Harn HJ, Chien YJ, Chang CH, Hsu CY, Fu RH, Huang YC, Chen SY, Shyu WC, Lin SZ. n-Butylidenephthalide (BP) maintains stem cell pluripotency by activating Jak2/Stat3 pathway and increases the efficiency of iPS cells generation. PLoS One 2012; 7: e44024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006; 113: 2105–12. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26: 627–34. [DOI] [PubMed] [Google Scholar]
- 21.Shanbhag NC, Henning RH, Schilling L. Long-term survival in permanent middle cerebral artery occlusion: A model of malignant stroke in rats. Sci Rep. 2016; 6: 28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Zhao Y, Xu Y, Chen Z, Liu N, Ke C, Liu B, Wu W. Sodium ferulate and n-butylidenephthalate combined with bone marrow stromal cells (BMSCs) improve the therapeutic effects of angiogenesis and neurogenesis after rat focal cerebral ischemia. J Transl Med. 2016; 14: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010; 19: 1073–84. [DOI] [PubMed] [Google Scholar]
- 24.Folkman J. Tumor angiogenesis: Therapeutic implications. N Eng J Med. 1971; 285: 1182–86. [DOI] [PubMed] [Google Scholar]
- 25.Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007; 27: 1684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng YB, Ye WB, Hu ZZ, Yan Y, Wang Y, Takon BF, Zhou GQ, Zhou YF. Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol Res. 2010; 32: 148–56. [DOI] [PubMed] [Google Scholar]
- 27.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005; 57: 874–82. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–11. [DOI] [PubMed] [Google Scholar]
- 29.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D,. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011; 10(5): 471–80. [DOI] [PubMed] [Google Scholar]
- 30.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006; 176: 6523–31. [DOI] [PubMed] [Google Scholar]
- 31.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E,. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009; 15: 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008; 36: 1105–19. [DOI] [PubMed] [Google Scholar]
- 33.Nam KN, Kim KP, Cho KH, Jung WS, Park JM, Cho SY, Park SK, Park TH, Kim YS, Lee EH. Prevention of inflammation-mediated neurotoxicity by butylidenephthalide and its role in microglial activation. Cell Biochem Funct. 2013; 31: 707–12. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M,. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008; 28: 468–81. [DOI] [PubMed] [Google Scholar]
- 35.Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010; 119: 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014; 565: 30–8. [DOI] [PubMed] [Google Scholar]
- 37.Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M,. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia 2008; 56: 1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol Rev. 2014; 94: 1077–98. [DOI] [PubMed] [Google Scholar]
- 39.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV,. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016; 532: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner DC, Scheibe J, Glocke I, Weise G, Deten A, Boltze J, Kranz A. Object-based analysis of astroglial reaction and astrocyte subtype morphology after ischemic brain injury. Acta Neurobiol Exp. (Wars) 2013; 73: 79–87. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005; 49: 407–17. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014; 8: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun YY, Lin SH, Lin HC, Hung CC, Wang CY, Lin YC, Hung KS, Lien CC, Kuan CY, Lee YH. Cell type-specific dependency on the PI3K/Akt signaling pathway for the endogenous epo and VEGF induction by baicalein in neurons versus astrocytes. PLoS One 2013; 8: e69019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chai L, Guo H, Li H, Wang S, Wang YL, Shi F, Hu LM, Liu Y, Adah D. Scutellarin and caffeic acid ester fraction, active components of dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. J Ethnopharmacol. 2013; 150: 100–7. [DOI] [PubMed] [Google Scholar]
- 45.Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem Int. 2015; 83–84: 9–18. [DOI] [PubMed] [Google Scholar]
- 46.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013; 126: 1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 2009; 113: 816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He B, Yao Q, Liang Z, Lin J, Xie Y, Li S, Wu G, Yang Z, Xu P. The dose of intravenously transplanted bone marrow stromal cells determines the therapeutic effect on vascular remodeling in a rat model of ischemic stroke. Cell Transplant. 2016; 25(12): 2173–85. [DOI] [PubMed] [Google Scholar]
- 49.Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, Janowski M, Walczak P, Boltze J, Lukomska B, Jolkkonen J,. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pösel C, Scheibe J, Kranz A, Bothe V, Quente E, Fröhlich W, Lange F, Schäbitz WR, Minnerup J, Boltze J, Wagner DC,. Bone marrow cell transplantation time-dependently abolishes efficacy of granulocyte colony-stimulating factor after stroke in hypertensive rats. Stroke 2014; 45: 2431–7. [DOI] [PubMed] [Google Scholar]
- 51.Balseanu AT, Buga AM, Catalin B, Wagner DC, Boltze J, Zagrean AM, Reymann K, Schaebitz W, Popa-Wagner A. Multimodal approaches for regenerative stroke therapies: Combination of granulocyte colony-stimulating factor with bone marrow mesenchymal stem cells is not superior to G-CSF alone. Front Aging Neurosci. 2014; 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke 2011; 42: 3551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


