Abstract
Mammal feces are the primary food and nesting resource for the majority of dung beetle species, and larval development depends on the quantity and quality of that resource. Physiological necessities, competitive interactions, and resource sharing are common and suggest that dung beetles may show preferences for feces of greater nutritional quality, which may in turn impact beetle assemblages and community structure. This study investigated whether attractiveness of dung beetles to different resource (feces) types varies depending on mammal trophic guild and associated nutritional content. This study was conducted in Atlantic Forest fragments in the Parque Estadual da Serra do Tabuleiro, Santa Catarina, Brazil. To evaluate attractiveness, the feces of the carnivore Puma concolor , the omnivores Cerdocyon thous and Sapajus nigritus, and the herbivore Tapirus terrestris were utilized as bait. Dung was collected from zoo animals fed a standard diet. Sampling was performed in triplicate in five areas in the summer of 2013. Four pitfall traps were established in each area, and each trap was baited with one type of mammal feces. Food preference of the species was analyzed by calculating Rodgers’ index for cafeteria-type experiments. In total, 426 individuals from 17 species were collected. Rodgers’ index showed that omnivorous mammal feces ( C. thous ) were most attractive to all dung beetle species , although it is known that dung beetles are commonly opportunistic with respect to search for and allocation of food resources. These results suggest that mammal loss could alter competitive interactions between dung beetles.
Keywords: food resource, sharing of resource, ecology, dung quality
Classical ecological theory predicts that community structure dynamics are principally influenced by competition, which in turn determines the competing species that can or cannot co-occur ( Hairston et al. 1960 , Connell 1980 , Tilman 1980 ). According to the principle of competitive exclusion, two populations cannot occupy the same ecological niche, and in the presence of limiting resources, one of them is either excluded or ecologically displaced. Various studies assume that competition and food limitation are the main factors in the structuring of biological communities (e.g., Richardson 1991 , Boggs and Ross 1993 ). Classical niche theory predicts that all dimensions of an ecological niche are involved in the probability of species persistence ( Hutchinson 1957 ). However, the dimensions of a niche do not remain unaltered; there can be displacement over evolutionary or spatial history, minimizing the competition for resources and having direct consequences on dispersion patterns and mechanisms structuring communities ( Paine et al. 1981 , Pulliam 2000 ).
Most dung beetle species compete for scarce and short-lived resources including feces or carcasses of vertebrates, both primarily of mammals. These resources are utilized for food and nesting for adults and larvae. Because the dung beetle life cycle involves complete metamorphosis, the development of the adult depends mainly on the quantity and quality of resource(s) consumed during the larval period ( Halffter and Edmonds 1982 ). The sharing of resources can, in many cases, decrease interspecific competition for a common resource. The biology and behavior of the species (e.g., functional guild, morphology, period of activity, behavior, search for and allocation of resource, and even chemical protection of the young) are important aspects that increase resource sharing among dung beetle species ( Halffter and Edmonds 1982 , Bellés and Favila 1983 , Hernández 2002 , Hernández et al. 2009 ). Functional differentiation and differences in activity period generally allow dung beetles to minimize competition for limited resources ( Hanski and Cambefort 1991 , Estrada et al. 1993 , Hernández 2002 ). Different food searching behavior, generally consisting of either flight close to the ground or perching on leaves in the subforest, may allow coexistence of competing species. Differences in resource allocation behavior may also facilitate coexistence (e.g., endocoprids: reside in the resource; paracoprids: store portions in tunnels just below the resource; and telecoprids: store portions in tunnels relatively far from the resource) ( Halffter and Matthews 1966 , Halffter and Edmonds 1982 ). Despite the existence of species with dietary specificity (i.e., stenophagous species) ( Halffter and Matthews 1966 ), in many areas (e.g., pastures), the most important food resource for dung beetles is herbivorous mammal feces ( Hanski and Cambefort 1991 ).
Studies in the Neotropical region have noted food preference of dung beetles for omnivorous mammal feces, which in general attracts a greater number of species compared with herbivore and carnivore feces ( Estrada et al. 1993 , Filgueiras et al. 2009 , Marsh et al. 2013 ). This preference is possibly due to the fact that omnivores have a greater variety of food items in their diet, in addition to substantial seasonal variation ( Uchoa and Moura-Britto 2004 , Rocha-Mendes et al. 2010 ). Depending on the trophic guild of the mammal, (carnivorous, herbivorous, or omnivorous), the composition and quality of food items will vary. In general, excrement is composed of plant and animal material, partially digested or not, which can include numerous components (e.g., feathers, bones, arthropod chitin, seeds and plant tissues, and pollen grains, in addition to mucus, secretions, cells, and abundance of bacteria) ( Bang and Dahlström 1975 , Bjune 2000 , Chame 2003 ). By feeding on feces, carcasses, and other decomposing materials, dung beetles play a role in nutrient cycling and secondary seed dispersal, and diminish parasitic diseases in mammals by controlling the increase in populations of flies and nematodes by killing larvae and eggs deposited in the feces ( Halffter and Matthews 1966 ).
It is possible that different dung beetle species have food preferences based on the relative nutritional quality of dung, which may differ among native mammals. To test this hypothesis, we evaluated the attractiveness of different types of mammal feces to different species of dung beetles. We hypothesize that beetle attractiveness to dung will vary according to mammal type and the trophic guild the mammal occupies.
Materials and Methods
Study Area
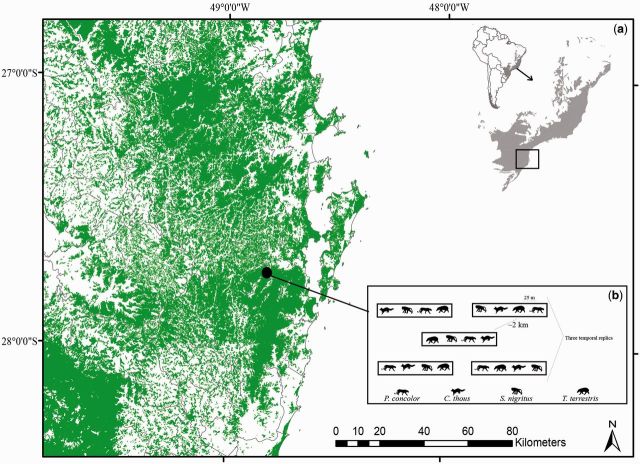
The study was conducted in Dense Ombrophilous Forest areas (Atlantic Forest Biome) in the Parque Estadual da Serra do Tabuleiro (PEST), located in the municipality of Santo Amaro da Imperatriz, Santa Catarina, Brazil (27° 44′ S, 48° 48′ W; 480 meters above sea level) ( Fig. 1 ). The study region falls within the humid subtropics (Cfa according to the Köppen–Geiger classification), with defined seasons and well-distributed rainfall throughout the year, with an annual rainfall average of 1,700 mm. Temperatures vary greatly throughout the year, ranging between 0 and 40°C with an annual average of 19°C ( Pell et al. 2007 ).
Fig. 1.
( a ) Location map of the sampling area in forest remnants (green) located in Parque Estadual da Serra do Tabuleiro (Santa Catarina, Brazil) into Atlantic Forest Biome (original area showed in gray) and ( b ) sampling design to evaluate the attractiveness of dung beetles species by native mammals feces of different trophic guilds.
Baits and Nutritional Quality
The attractiveness of Scarabaeinae species was evaluated using fresh—newly defecated—feces of native mammals from four different trophic guilds as bait. The mammals occur in the southern region of the Atlantic Forest and consisted of the following species and trophic guilds: the carnivore Puma concolor (L., 1771) (puma); two omnivores with different diets: Cerdocyon thous (L., 1766) (graxaim/crab-eating fox): with omnivorous diet but more prone to eat the protein contents of animal origin and Sapajus nigritus (Goldfuss, 1809) (macaco-prego/black-horned capuchin): with more generalist diet prone to eating a greater quantity of plant matter; and the herbivore Tapirus terrestris (L., 1758) (anta/lowland tapir). Animal feces were supplied by the Fundação Herman Weege (Pomerode Zoo) in Pomerode, Santa Catarina, Brazil.
The nutritional quality of the mammalian feces was determined using a nutritional description based on the detailed diet provided by zookeepers. The dietary information consisted of the quantity (kg per animal) of each food item, including feed, supplements, and mineral salts. Although nutritional quality of dung may be affected by sex and age of the animals ( Touma and Palme 2005 ), we opted to employ standardized methods for nutritional measurements. Using the known quantities and types of food items provided to captive animals, the following was calculated using pre-established equations: 1) quantity of nitrogen in the feces (g N/d) in relation to mean species weight (see Parera 2002 , Oliveira and Cassaro 2005 ) via subtraction of nitrogen intake and considering protein synthesis, demand, and exogenous excretion, based on the equations for intake ( y = 1,600 W0.65 ) ( Evans and Miller 1968 ), synthesis ( y = 367 W0.65 ) ( Munro 1969 ), demand ( y = 250 W0.65 ) ( Miller and Payne 1964 ), and exogenous excretion ( y = 146 W0.65 ) ( Brody 1945 ); 2) quantity of available nitrogen in the feces (g N/d) based on the efficiency of assimilation in relation to the items consumed ( Ricklefs 1974 ); 3) calculation of the percentage of protein, fats, and carbohydrates of items consumed ( Ricklefs 1974 ); and 4) quantity of fecal mass produced (g/d) based on the mean weight of the animal according to the equation y = 0.85 W−0.37 ( Blueweiss et al. 1978 ). For all these equations, W is the average body weight.
Captive animals were kept in the Zoológico de Pomerode and received a balanced diet similar to the natural species diet (e.g., Uchoa and Moura-Britto 2004 , Rocha-Mendes et al. 2010 ), however, with the addition of supplements, mineral salts, and feed consisting of mostly protein content (especially soy and animal protein). P . concolor had a strict protein-based diet, consuming on average 1.125 kg of meat daily and fasting 2 d/wk. The diets of C. thous and S. nigritus were more variable, based on fruits and protein (meats, eggs, and feed). T. terrestris consumed a large amount of food, which was composed essentially of vegetables and fruits. In the analysis of the proportionality of each dietary item, particularly protein, fats, and carbohydrates, C. thous in particular consumed a relatively high quantity of dietary protein without significant reduction in the other components. P. concolor consumed mostly protein, without carbohydrates; S. nigritus , as well as C. thous , showed little difference in the percentages of the three components observed. T . terrestris had a low percentage of all three items, especially protein ( Table 1 ).
Table 1.
Type, composition, and qualitative parameters of the diets of mammals: P. concolor, C. thous , S. nigritus, and T. terrestris and their feces on the basis of received feed in captivity and literature
| Species | Trophic guild | Diet in captivity (kg/wk) |
|---|---|---|
| P. concolor | C | Chicken (2.8), bovine (3.3), swine (1.7), and supplement (0.085) |
| C. thous | O | Fruits (1.0), bovine (1.4), egg (0.175), and animal feed (0.5) |
| S. nigritus | O | Fruits (0.55), greens (0.35), flesh (0.13), egg (0.2), and animal feed (0.35) |
| T. terrestris | H | Greens (49.7), fruits (8.4), animal feed (7.0), and mineral salt (0.35) |
|
Average nutritional quality
| ||||||
|---|---|---|---|---|---|---|
| Species | Nf (g N/d −1 ) | Nfd (g N/d −1 ) | P (%) | F (%) | C (%) | D (g/d) |
| P. concolor | 9.1 | 6.8 | 22.9 | 5.7 | 0 | 665.5 |
| C. thous | 2.8 | 2.0 | 15.9 | 4.0 | 4.0 | 214.5 |
| S. nigritus | 2.0 | 1.3 | 10.3 | 2.6 | 4.3 | 153.0 |
| T. terrestris | 30.3 | 18.2 | 2.8 | 0.7 | 1.6 | 2,138.6 |
C, carnivore; O, omnivore; H, herbivore; Nf, nitrogen in faeces (g N/d −1 ); Nfd, nitrogen of stools available for assimilation (gN/d −1 ); P, proteins; F, fats; C, carbohydrates; D, amount of feces produced (g/d).
Experimental Design and Sampling
Dung beetles sampling was done at five points (spatial repetitions [e]) with a mean distance of 2 km between them. Four pitfall traps were placed at each point, consisting of plastic containers (15-cm depth by 18-cm diameter), placed 25 m apart and buried with the tops of the containers flush with the ground. Traps were half filled with water along with a small amount of neutral detergent 2%. Traps were sampled three times in the summer of 2013 (temporal repetitions [t]). Each of the four traps was baited with 10 g of one type of feces (i.e., from one species only per trap). Placed in the morning, baits remained in traps for 48 h prior to sampling for each repetition, with configuration of the spatial and temporal placements was determined randomly. Thus, a total of 15 traps (5e by 3t) were placed per bait type, generating a sampling effort of 60 traps in total ( Fig. 1 ). Baits were suspended above traps, so that the insects fell into the containers before reaching the bait. As a preventive measure, feces were frozen prior to use to minimize the risk of pathogen or parasite infection of wild species present in the areas. Controlled tests showed that freezing did not reduce the bacterial populations to the point of compromising recolonization after thawing ( Haines 1938 , Morley et al. 1983 ). Potential vector access was reduced by means of a physical barrier around the feces, diminishing direct contact by the dung beetles as well as other potentially attracted insects. After trapping, the feces were recovered from sampling areas and properly discarded.
Data Analysis
The data were initially analyzed in a descriptive manner, exploring the number and percentage of species, and number of individuals attracted by each type of feces. To determine the use of the resource in terms of food preference, Rodgers’ index for “cafeteria” type experiments was calculated ( Rodgers and Lewis 1985 ), excluding from the analysis those species considered rare ( n < 7) and yielding as minimal units the species and the respective total number of individuals per species. In “cafeteria” type experiments, a matrix of food types is presented to an animal in equal abundance, such that availability does not enter directly in the measurement of the preference ( Krebs 1999 ). Rodgers and Lewis (1985) suggested that the most appropriate measurement of preference is the area under each of the cumulative curves of standardized consumption, with a maximum value of 1.0. Accordingly, the use of the resource is calculated under the curve of use, partitioning it into a series of triangles, trapeziums, and/or rectangles and summing the areas. Next, the scoring of preference is normalized for the range between 0.0 and 1.0, such that the most preferred resource has the maximum score (1.0) and the least preferred resource has the lowest score. The scores are obtained using the following formula:
where = Rodgers’ index for food preference for “cafeteria” experiments for i species; = area under the curve of cumulative proportion consumed by i species based on the partition of the curve (triangles, trapeziums, and/or rectangles); and = highest value of .
The same index was calculated for the species separately, taking as minimal units the spatial and temporal repetitions, including all individuals of each species. The analyses were carried out using R 3.0.1 ( R Core Team 2013 ).
Results
In total, 426 dung beetles were collected belonging to 17. The bait with the highest attractiveness was that of omnivorous mammals C. thous (59% of the beetles collected) and S. nigritus (23% of the beetles collected). Traps with C. thous and S. nigritus feces captured 15 and 12 species of dung beetles, respectively, whereas traps with resources from carnivore and herbivore species each captured eight beetle species ( Table 2 ).
Table 2.
Capture number of individuals (and percentage) per species of dung beetles (Coleoptera: Scarabaeinae) in relation to the types of resources (feces of mammals) in the Atlantic forest in Santa Catarina, Brazil
| Species |
Resource (feces)
|
|||
|---|---|---|---|---|
| Pc | Ct | Sn | Tt | |
| Canthi. aff. trinodosum (Boheman, 1858) | 7 (14%) | 29 (59%) | 7 (14%) | 6 (12%) |
| Canthidium dispar Harold, 1867 | 1 (14%) | 2 (29%) | 2 (29%) | 2 (29%) |
| Canthon luctuosus (Harold, 1868) | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) |
| Cantho. rutilans cyanescens (Harold, 1868) | 15 (10%) | 98 (63%) | 31 (20%) | 11 (7%) |
| Coprophanaeus cerberus (Harold, 1869) | 0 (0%) | 1 (33%) | 2 (67%) | 0 (0%) |
| Co. saphirinus (Sturm, 1826) | 3 (12%) | 13 (52%) | 6 (24%) | 3 (12%) |
| Deltochilum brasiliense (Laporte, 1840) | 0 (0%) | 4 (80%) | 1 (20%) | 0 (0%) |
| D. furcatum (Laporte, 1840) | 0 (0%) | 8 (44%) | 9 (50%) | 1 (6%) |
| D. morbillosum Burmeister, 1848 | 4 (29%) | 8 (57%) | 2 (14%) | 0 (0%) |
| Deltochilum multicolor Balthasar, 1939 | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) |
| Dichotomius fissus (Harold, 1867) | 0 (0%) | 6 (46%) | 6 (46%) | 1 (8%) |
| Dichotomius mormon (Ljungh, 1799) | 0 (0%) | 1 (50%) | 1 (50%) | 0 (0%) |
| Dichotomius sericeus (Harold, 1867) | 12 (12%) | 57 (56%) | 21 (21%) | 11 (11%) |
| Eurysternus cyanescens Balthasar, 1939 | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| E. inflexus (Germar, 1824) | 0 (0%) | 15 (63%) | 9 (38%) | 0 (0%) |
| Phanaeus splendidulus (F., 1781) | 0 (0%) | 4 (100%) | 0 (0%) | 0 (0%) |
| Uroxys sp1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) |
| Individuals | 44 (10%) | 249 (59%) | 97 (23%) | 36 (8%) |
| Species | 8 (47%) | 15 (88%) | 12 (70%) | 8 (47%) |
Pc, P. concolor ; Ct, C. thous ; Sn, S. nigritus ; Tt, T. terrestris .
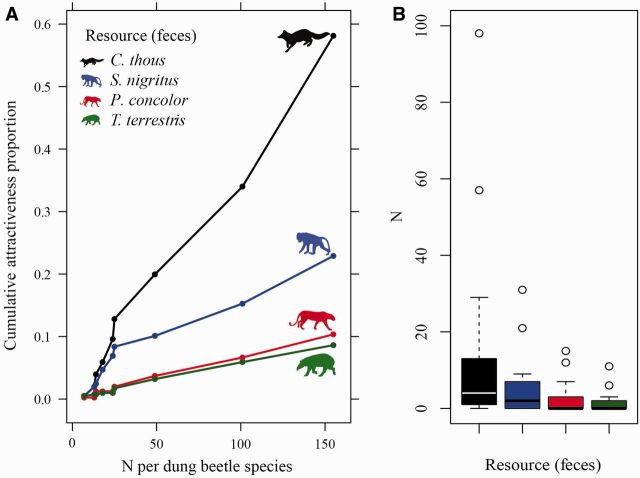
Rodgers’ index for all individuals captured showed through curve partitioning scores ( Fig. 2 A) that the most attractive resource was C. thous feces ( Act = 43.72; Rct = 1.000), followed by feces of S. nigritus ( Asn = 19.80; Rsn = 0.452). Feces from animals with the most restricted diets were the least attractive, including that of P. concolor ( Apc = 8.13; Rpc = 0.186) and T. terrestris ( Att = 7.06; Rtt = 0.161). The cumulative attractiveness curve for food resources demonstrated a higher number of beetles per species captured by omnivore species, in comparison to that of P. concolor and T. terrestris. In addition, there was low variation in abundance among baits ( Fig. 2 B).
Fig. 2.
( A ) Curve of cumulative (accumulated by individuals and species) proportion of bait attractiveness (feces of mammals) and ( B ) space–time variation per bait (by individuals of dung beetles) collected in five Atlantic Forest points located in the Parque Estadual da Serra do Tabuleiro, Santa Catarina, Brazil.
Among those analyzed, Eurysternus inflexus was the only species captured exclusively by omnivorous mammal feces. Deltochilum furcatum and Deltochilum fissus were not found in P. concolor feces traps, and Deltochilum morbillosum did not occur in traps using T. terrestris feces. For Canthon rutilans cyanescens, Coprophanaeus saphirinus , Deltochilumsericeus, and Canthidium aff. trinodosum , the highest proportion of individuals were captured in traps with C. thous feces ( Fig. 3 ). Based on the analysis of preference by means of Rodgers’ index, all species showed greater preference for feces of C. thous . The second most preferred bait type for all beetle species was S. nigritus feces, with the exception of Canthi . aff. trinodosum ; this order of attractiveness was maintained regardless of relocation guild ( Table 3 ).
Fig. 3.
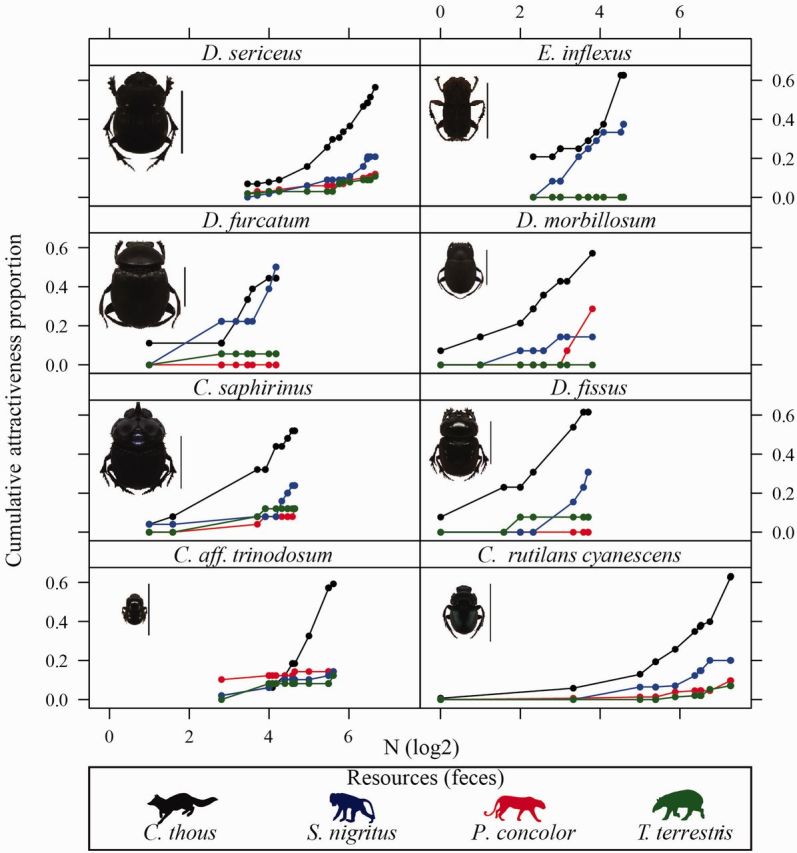
Curve of cumulative proportion of resource usage (feces of mammals) by species of dung beetles (Coleoptera: Scarabaeinae) collected in five Atlantic Forest points located in the Parque Estadual da Serra do Tabuleiro, Santa Catarina, Brazil. Lateral bars = 1 cm.
Table 3.
Rodgers index and their scores for experiment type “cafeteria” for the feeding preference of species of dung beetles (Coleoptera: Scarabaeinae) by resource type (feces) of different species of native mammals of different trophic guilds
| Species | G |
Resource (feces)
|
Att. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
P. concolor
|
C. thous
|
S. nigritus
|
T. terrestris
|
|||||||
| A | R | A | R | A | R | A | R | |||
| D. furcatum | Te | 0.00 | 0.00 | 4.47 | 1.00 | 3.78 | 0.84 | 0.75 | 0.17 | C S T |
| E. inflexus | En | 0.00 | 0.00 | 7.42 | 1.00 | 4.58 | 0.62 | 0.00 | 0.00 | C S |
| Dichotomius sericeus | Pa | 6.79 | 0.24 | 28.57 | 1.00 | 9.61 | 0.33 | 5.52 | 0.19 | C S P T |
| Dichotomius fissus | Pa | 0.00 | 0.00 | 3.04 | 1.00 | 2.73 | 0.90 | 0.73 | 0.24 | C S T |
| Co. saphirinus | Pa | 1.14 | 0.16 | 7.20 | 1.00 | 2.36 | 0.33 | 1.80 | 0.25 | C S T P |
| D. morbillosum | Te | 0.93 | 0.19 | 4.78 | 1.00 | 1.29 | 0.27 | 0.00 | 0.00 | C S P |
| Canthi. aff. trinodosum | Pa | 5.91 | 0.52 | 11.47 | 1.00 | 3.97 | 0.35 | 3.14 | 0.27 | C P S T |
| Cantho. rutilans cyanescens | Te | 6.30 | 0.13 | 48.90 | 1.00 | 18.11 | 0.37 | 4.17 | 0.08 | C S T P |
The last column is the resource type ordered by preference. G, relocation guilds; Te, telecoprids; En, endocoprids; Pa, paracoprids; A, summed area partitioned curve; R, Rodgers index scores; P, P. concolor ; C, C. thous ; S, S. nigritus ; T, T. terrestris ; Att., attractiveness order.
Discussion
The principal result of this study revealed that beetles are most attracted to feces of omnivorous mammals, although the range and composition of the diet of dung beetles can be diverse ( Halffter and Matthews 1966 , Hanski and Cambefort 1991 ). C. thous feces were the resource that attracted the highest number of beetle species and individuals, indicating a strong preference. Other studies evaluating the food preference of dung beetles among a greater variety of feces types have also shown that the greatest number of individuals was attracted to feces of omnivorous mammals (e.g., Estrada et al. 1993 , Filgueiras et al. 2009 , Noriega 2012 ).
The diet of C. thous consists of a large variety of food items and with considerable seasonal variation; the diet of the specimens in captivity utilized in this study showed fewer food types without negatively impacting nutritional content (e.g., Uchoa and Moura-Britto 2004 , Rocha-Mendes et al. 2010 ). This range of food includes different sources of protein and other nutrients that are not fully assimilated by the mammal, therefore a substantial portion is eliminated in feces ( Vulla et al. 2009 ) is in general high in calcium resulting from ingestion of bones and as such shows a more rigid texture ( Bang and Dahlström 1975 ). The age, extent of desiccation, and texture of feces generally predict its nutritional status, especially the levels of nitrogen and carbohydrates, and also influence preferences of dung beetles ( Anderson and Coe 1974 , Sowig and Wassmer 1994 ).
Dung beetle preference for feces of C. thous corroborates the notion that the feces of omnivores are the principal resource for food and nesting ( Halffter and Matthews 1966 , Halffter and Edmonds 1982 , Hanski and Cambefort 1991 ). Although there are stenophagous species ( Halffter and Matthews 1966 ), for the majority of dung beetles species, food preferences have been documented only at relatively large scales (e.g., generalist, coprophagous, and necrophagous) without evaluation of the quality of these resources ( Halffter and Matthews 1966 , Halffter and Edmonds 1982 , Hanski and Cambefort 1991 ).
As discussed, dung beetles and other detritivores do not only ingest material in the feces chemically derived from the producers but also the associated microflora ( Odum and Barret 2008 ). Quaternary bacterial diversity is higher in omnivores than in carnivorous mammals and herbivores, and their feces also contain a large quantity of associated microorganisms ( Ley et al. 2008 ). It is not clear which of the many components of the feces supply nutrients for developing beetle larvae, but some studies show that larvae, unlike adults, do not require symbiotic microorganisms in the diet ( Byrne et al. 2013 ). Coprophagous adults appear to be dependent on symbionts for digestion. Species that feed on feces containing large amounts of plant material utilize bacteria for help with digestion of cellulose, whereas the species that feed on other types of feces (e.g., carnivore or omnivore feces) using the microorganisms present in the feces, which are ingested along with the excrement, facilitating digestion ( Halffter and Matthews 1971 , Cambefort 1982 ). Thus, it is possible to see that species that feed on feces of herbivores are necessarily more highly specialized than are species that feed on other types of feces, thereby the feces of omnivores are more disputed among dung beetle assemblages.
Dung beetles are generally opportunistic with respect to exploitation of feces and thus utilize a wide variety of types ( Hanski and Cambefort 1991 ). Thus, the partitioning of dung beetles into assemblages based on trophic habits also depends on the capacity of the insect to detect and select the different types of resources when available ( Dormont et al. 2004 ). Feces texture and water content may also have an influence on resource choice, because these factors affect desiccation rate and extent of odor emission, the latter being an important aspect of resource localization by beetles ( Halffter and Edmonds 1982 ).
Competition—influenced by resource sharing, and promoting an expansion and/or reduction of the species niches—for spatial and temporal resources plays a large role in population dynamics of dung beetles ( Hanski and Cambefort 1991 ). Dung beetles undergo complete metamorphosis, and the resource(s) utilized by larvae directly influence morphology, size, and biomass of adults. These characteristics in turn exert an influence on sexual selection and other life history processes ( Kotiaho 2002 , Cotter et al. 2008 , Chamorro-Florescano and Favila 2009 , Hernández et al. 2009 , Simmons and Ridsdill-Smith 2011 ). Trophic generalists show a decreased tendency to compete for short-lived and/or scarce food resources, preventing specialists from occupation of new habitats where there are no specific foods available ( Silva 2011 ).
The most abundant dung beetles species collected in this study have previously been classified into different trophic guilds. D . furcatum and D. morbillosum are generally considered as necrophagous ( Falqueto et al. 2005 , Silva and Di Mare 2012 ), E. inflexus, D. sericeus, and D. fissus as coprophagous ( Huertas and Arias 2007 , Campos and Hernández 2013 ), and Co. saphirinus and Cantho. rutilans cyanescens as generalists (identified as Canthon latipes Blanchard, 1845 in the following studies: Silva and Di Mare 2012 , Campos and Hernández 2013 ). This suggests that D. furcatum and D. morbillosum were collected in feces possibly because they are opportunists and/or due to the fact the resources they typically feed on were scarce. However, with this greater preference for feces from omnivorous mammals, the dung beetles community is potentially structuring by competition for these resource (feces of C. thous and others omnivorous), unless there is sharing of resources or, in the long term, contraction or expansion of their niches to minimize competition.
Optimal foraging theory describes the calculations for ideal amount of time spent, resource quality, and energy expenditure in the search for food resources ( MacArthur and Pianka 1966 ), including adjustment strategies that improve foraging efficiency and reduce search time necessary to meet dietary needs. This calculation considers the quantity of biomass or calories, and the ease of manipulation and capture compared with the amount of time spent foraging ( Pianka 2008 ). Competitive dynamics will certainly have an influence on the structuring of dung beetle assemblages, where food preference is likely defined by the diet of the different beetle species based on space–time availability of the resource, and on possible changes in availability resulting in the use of alternative resources in ephemeral conditions.
Coprophagy is predominant and well studied in both evolutionary and ecological contexts, and dung beetle species are known to compete for nesting and feeding resources. Regardless, there is a preference for resources coming from generalist sources (omnivores), which have a greater number of food items and are more nutritive compared with other types studied. Feces are predicted to be a limiting resource and may serve as structuring resources for dung beetle assemblages, particularly in scenarios of reduction in mammal fauna. Local loss of specific mammal groups may have direct effects on dung beetle fauna, which in turn may alter nutrient cycling processes and secondary dispersion of seeds ( Andresen 2002 ). In addition, the biological effects of reduction in fauna may impact different environmental scales (e.g., local, regional, ecosystemic, and global) and processes (e.g., behavioral/physiological, ecological, and evolutionary) ( Galetti and Dirzo 2013 ).
In conclusion, despite opportunism remaining common, dung beetles seem to be more attracted to omnivorous mammalian feces than to feces types from mammals of other trophic guilds. This perspective suggests that loss of mammals (i.e., and their feces as a food resource) may alter competitive interactions between dung beetles species and may even cause local extinction of highly specialized species.
Acknowledgments
We thank CAPES (Education Ministry of Brazil) for the scholarship to J.A.B. and CNPq (Science and Technology Ministry of Brazil) for funding the project (Process 553880/2010) and for the Research Productivity Grant to M.I.M.H. (Proc. 303800/2010-0). We also thank Fernando Zagury Vaz-de-Mello (Federal University of Mato Grosso) for confirming the identification of species, Fernando Brüggemann (Hotel Plaza Caldas da Imperatriz) for allowing access to field area, Cláudio Maas (Zoo de Pomerode) for providing the animal feces, Pedro Giovâni da Silva and Renata Calixto Campos for the valuable support during fieldwork, and Maurício Eduardo Graipel, Luis Maurício Bini, Paulo César de Azevedo Simões-Lopes, and Luciana Iannuzzi for their contribution to the paper.
References Cited
- Anderson J. M., Coe M. J. . 1974. . Decomposition of elephant dung in an arid, tropical environment . Oecologia 14 : 111 – 125 . [DOI] [PubMed] [Google Scholar]
- Andresen E . 2002. . Dung beetles in a Central Amazonian rainforest and their ecological role as secondary seed dispersers . Ecol. Entomol. 27 : 257 – 270 . [Google Scholar]
- Bang P., Dahlström P. . 1975. . Huellas y Señales de los Animales de Europa , 264 p. Omega; , Barcelona: . [Google Scholar]
- Bellés X., Favila M. E. . 1983. . Protection chimique du nid chez Canthon cyanellus cyanellus LeConte [Col. Scarabaeidae] . Bulletin de la Société Entomologique de France 88 : 602 – 607 . [Google Scholar]
- Bjune A. E . 2000. . Pollen analysis of faeces as a method of demonstrating seasonal variations in the diet of Svalbard reindeer ( Rangifer tarandus platyrhynchus ) . Polar Res. 19 : 183 – 192 . [Google Scholar]
- Blueweiss L., Fox H., Kudzma D., Nakashima D., Peters R., Sams S. . 1978. . Relationships between body size and some life history parameters . Oecologia 37 : 257 – 272 . [DOI] [PubMed] [Google Scholar]
- Boggs C. L., Ross C. L. . 1993. . The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae) . Ecology 74 : 433 – 441 . [Google Scholar]
- Brody S. 1945. . Bioenergetics and growth , 1023 p. Reinhold Publishing Corporation; , New York, NY: . [Google Scholar]
- Byrne M. J., Watkins B., Bouwer G. . 2013. . Do dung beetles larvae need microbial symbionts from their parents to feed on dung? Ecol . Entomol. 38 : 250 – 257 . [Google Scholar]
- Cambefort Y. 1982. . Nidification behaviour of Old World Onticellini (Coleoptera: Scarabaeidae) . InHalffter G., Edmonds W. (eds.), The nesting behaviour of dung beetles (Scarabaeinae): an ecological and evolutive approach . Instituto de Ecologia; , Mexico: , pp. 139 – 143 . [Google Scholar]
- Campos R. C., Hernández M.I.M. . 2013. . Dung beetle assemblages (Coleoptera: Scarabaeinae) in Atlantic Forest fragments in southern Brazil . Revista Brasileira de Entomologia 57 : 47 – 54 . [Google Scholar]
- Chame M . 2003. . Terrestrial mammal feces: a morphometric summary and description . Memórias do Instituto Oswaldo Cruz 98 : 71 – 94 . [DOI] [PubMed] [Google Scholar]
- Chamorro-Florescano I. A., Favila M. E. . 2009. . The reproductive status of both sexes affects the frequency of mating and the reproductive success of males in the ball roller beetle: Canthon cyanellus cyanellus (Coleoptera: Scarabaeinae) . Behaviour 146 : 1449 – 1512 . [Google Scholar]
- Connell J. H . 1980. . Diversity and the coevolution of competitors or the ghost of competition past . Oikos 35 : 131 – 138 . [Google Scholar]
- Cotter S. C., Beveridge M., Simmons L. W. . 2008. . Male morph predicts investment in larval immune function in the dung beetle Onthophagus taurus . Behav. Ecol. 19 : 331 – 337 . [Google Scholar]
- Dormont L., Epinat G., Lumaret J. P. . 2004. . Trophic preferences mediated by olfactory cues in dung beetles colonizing cattle and horse dung . Environ. Entomol. 33 : 370 – 377 . [Google Scholar]
- Estrada A., Halffter G., Coates-Estrada R., Meritt D. A., Jr . 1993. . Dung beetles attracted to mammalian herbivore ( Alouatta palliata ) and omnivore ( Nasua narica ) dung in the tropical rainforest of Los Tuxtlas Mexico . J. Trop. Ecol. 9 : 45 – 54 . [Google Scholar]
- Evans E., Miller D. S. . 1968. . Comparative nutrition: growth and longevity . Proc. Nutr. Soc. 27 : 121 – 129 . [DOI] [PubMed] [Google Scholar]
- Falqueto S. A., Vaz-de-Mello F. Z., Schoereder J. H. . 2005. . Are fungivorous Scarabaeidae less specialist? Ecología Austral 15 : 17 – 22 . [Google Scholar]
- Filgueiras B.K.C., Liberal C. N., Aguiar C.D.M., Hernández M.I.M., Iannuzzi L. . 2009. . Attractivity of omnivore carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera Scarabaeidae) in a tropical Atlantic Forest remnant . Revista Brasileira de Entomologia 53 : 422 – 427 . [Google Scholar]
- Galetti M., Dirzo R. . 2013. . Ecological and evolutionary consequences of living in a defaunated world . Biol. Conserv. 163 : 1 – 6 . [Google Scholar]
- Haines R. B . 1938. . The effect of freezing on bacteria . Proc. R. Soc. B 124 : 451 – 463 . [Google Scholar]
- Hairston N., Smith F. E., Slobodkin L. B. . 1960. . Community structure population control and competition . Am. Nat. 94 : 421 – 425 . [Google Scholar]
- Halffter G., Edmonds W. D. . 1982. . The nesting behavior of dung beetles (Scarabaeinae): an ecologic and evolutive approach , 176 p. Man and Biosphere Program UNESCO; , Mexico City: . [Google Scholar]
- Halffter G., Matthews E. G. . 1966. . The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera Scarabaeidae) . Folia Entomológica Mexicana 12 : 1 – 312 . [Google Scholar]
- Halffter G., Matthews E. G. . 1971. . The natural history of dung beetles. A supplement on associated biota . La Revista Latinoamericana de Microbiología 13 : 147 – 163 . [PubMed] [Google Scholar]
- Hanski I., Cambefort Y. . 1991. . Resource partitioning , pp. 330 – 349 . InHanski I., Cambefort Y. (eds.), Dung beetle ecology . Princeton University Press; , Princeton, NJ: . [Google Scholar]
- Hernández M.I.M . 2002. . The night and day of dung beetles (Coleoptera: Scarabaeinae) in the Serra do Japi Brazil: elytra colour related to daily activity . Revista Brasileira de Entomologia 46 : 597 – 600 . [Google Scholar]
- Hernández M.I.M., Monteiro L. R., Favila M. E. . 2009. . The role of body size and shape in understanding competitive interactions within a community of Neotropical dung beetles . J. Insect Sci. 11 : 1 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas B. C., Arias J. J. . 2007. . Estudio preliminar de la entomofauna de la Serranía de los Churumbelos: mariposas diurnas y escarabajos coprófagos . Conservación Colombiana 3 : 67 – 76 . [Google Scholar]
- Hutchinson G. E . 1957. . Concluding remarks . Cold Spring Harb. Symp. Quant. Biol. 22 : 415 – 427 . [Google Scholar]
- Kotiaho J. S . 2002. . Sexual selection and condition dependence of courtship display in three species of horned dung beetles . Behav. Ecol. 13 : 791 – 799 . [Google Scholar]
- Krebs C. J. 1999. . Ecological methodology , 2nd ed. , 620 p. Addison-Wesley Longman; , New York: . [Google Scholar]
- Ley R. E., Hamady M., Lozupone C., Turnbaugh P. J., Ramey R. R., Bircher S., Schlengel M. L., Tucker T. A., Schrenzel M. D., Knight R., Gordon J. I. . 2008. . Evolution of mammals and their gut microbes . Science 320 : 1647 – 1651 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R. H., Pianka E. R. . 1966. . On optimal use of a patchy environment . Am. Nat. 100 : 603 – 609 . [Google Scholar]
- Marsh C. J., Louzada J., Beiroz W., Ewers R. M. . 2013. . Optimising bait for pitfall trapping of Amazonian dung beetles (Coleoptera: Scarabaeinae) . PLoS One 8 : 1 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. S., Payne P. R. . 1964. . Dietary factors influencing nitrogen balance . Proc. Nutr. Soc. 23 : 11 – 19 . [DOI] [PubMed] [Google Scholar]
- Morley C. R., Trofymow J. A., Coleman D. C., Cambardella C. . 1983. . Effects of freeze-thaw stress on bacterial populations in soil microcosms . Microb. Ecol. 9 : 329 – 340 . [DOI] [PubMed] [Google Scholar]
- Munro H. N. 1969. . Mammalian protein metabolism , vol. 3 , 590 p. Academic Press; , New York, NY: . [Google Scholar]
- Noriega J. A. 2012. . Dung beetles (Coleoptera: Scarabaeinae) attracted to Lagothrix lagotricha (Humboldt) and Alouatta seniculus (Linnaeus) (Primates: Atelidae) dung in a Colombian Amazon Forest . Psyche 2012: Article ID: 437589. (doi: ) 10.1155/2012/437589 . [DOI] [Google Scholar]
- Odum E., Barret G. W. . 2008. . Fundamentos de ecologia , 5th ed. , 612 p. Cengage Learning; , São Paulo, Brazil: . [Google Scholar]
- Oliveira T. G., Cassaro K. . 2005. . Guia de Campo dos Felinos do Brasil , 80 p. Instituto Pró-Carnívoros; , São Paulo, Brazil: . [Google Scholar]
- Paine T. D., Birch M. C., Svihra P. . 1981. . Niche breath and resource partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae) . Oecologia 48 : 1 – 6 . [DOI] [PubMed] [Google Scholar]
- Parera A. 2002. . Los Mamíferos de la Argentina y la Región Austral de Sudamérica , 453 p. El Ateneo; , Buenos Aires, Argentina: . [Google Scholar]
- Pell M. C., Finlayson B. L., McMahon T. A. . 2007. . Updated world map of the Köppen-Geiger climate classification . Hydrol. Earth Syst. Sci. 11 : 1633 – 1644 . [Google Scholar]
- Pianka E. R. 2008. . Optimal foraging . InJorgensen S. E. (ed.), Encyclopedia of ecology , pp. 2559 – 2561 . Elsevier; , Amsterdam, The Netherlands: . [Google Scholar]
- Pulliam H. R . 2000. . On the relationship between niche and distribution . Ecol. Lett. 3 : 349 – 361 . [Google Scholar]
- R Core Team . 2013. . R: a language and environment for statistical computing . R Foundation for Statistical Computing; , Vienna, Austria: . ( http://wwwR-projectorg/ ) . [Google Scholar]
- Richardson J. S . 1991. . Seasonal food limitation of detritivores in a montane stream: an experimental test . Ecology 72 : 873 – 887 . [Google Scholar]
- Ricklefs R. E. 1974. . Energetics of reproduction in birds . InPaynter R. A., Jr. , (ed), Avian energetics , pp. 152 – 297 . Nuttall Ornithological Club; , Cambridge: . [Google Scholar]
- Rocha-Mendes F., Mikich S. B., Quadros J., Pedro W. A. . 2010. . Feeding ecology of carnivores (Mammalia Carnivora) in Atlantic Forest remnants Southern Brazil . Biota Neotropica 10 : 2 – 10 . [Google Scholar]
- Rodgers A. R., Lewis M. C. . 1985. . Diet selection in Arctic lemmings ( Lemmus sibericus and Dicrostonyx groenlandicus ): food preferences . Can. J. Zool. 63 : 1161 – 1173 . [Google Scholar]
- Silva P. G . 2011. . Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) of two non-native habitats in Bagé Rio Grande do Sul Brazil . Zool. Stud. 50 : 546 – 559 . [Google Scholar]
- Silva P. G., Di Mare R. A. . 2012. . Escarabeíneos copro-necrófagos (Coleoptera Scarabaeidae Scarabaeinae) de fragmentos de Mata Atlântica em Silveira Martins Rio Grande do Sul Brasil . Iheringia Série Zoológica 102 : 197 – 205 . [Google Scholar]
- Simmons L. W., Ridsdill-Smith T. J. (eds.). 2011. . Ecology and evolution of dung beetles , 347 p. Blackwell Publishing; , Oxford: . [Google Scholar]
- Sowig P., Wassmer T. . 1994. . Resource partitioning in coprophagous beetles from sheep dung: Phenology and microhabitat preferences . Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere 121 : 171 – 192 . [Google Scholar]
- Tilman D . 1980. . Resources: a graphical-mechanistic approach to competition and predation . Am. Nat. 116 : 362 – 393 . [Google Scholar]
- Touma C., Palme R. . 2005. . Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation . Ann. N Y Acad. Sci. 1046 : 54 – 74 . [DOI] [PubMed] [Google Scholar]
- Uchoa T., Moura-Britto M. . 2004. . Hábito alimentar e uso do hábitat por canídeos no Parque Estadual do Cerrado: avaliação da situação atual da família Canidae no limite sul do bioma Cerrado no Brasil . Cadernos de Biodiversidade 4 : 59 – 65 . [Google Scholar]
- Vulla E., Hobson K. A., Korsten M., Leht M., Martin A. J., Lind A., Männil P., Valdmann H., Saarma U. . 2009. . Carnivory is positively correlated with latitude among omnivorous mammals: evidence from brown bears badgers and pine martens . Annales Zoologici Fennici 46 : 395 – 415 . [Google Scholar]