Abstract
Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a cryptic species complex that includes some of the most significant pests of agriculture and horticulture worldwide. To understand the diversity and distribution of B. tabaci cryptic species in Yunnan, a famous biodiversity hotspot in China, a large-scale sampling was conducted from year 2010 to 2013 in 10 prefectures. Mitochondrial cytochrome oxidase I gene sequences were used to identify different cryptic species. Phylogenetic analyses were performed using Bayesian methods to assess the position of a new B. tabaci cryptic species in the context of the B. tabaci diversity in Asia. The survey indicates at least eight B. tabaci cryptic species are present in Yunnan, two invasive (MEAM1 and MED) and six indigenous (China 2, China3, China 4, Asia I, Asia II 1, and Asia II 6), MEAM1, MED, and Asia I being the three predominant cryptic species in Yunnan. Compared with MEAM1, MED has a wider distribution. Based on molecular data, a new cryptic species, here named China 4, was identified that appears to be related to China 1, China 2, and China 3. Future efforts should focus on the interactions between predominant B. tabaci cryptic species and begomoviruses and on the development of effective control strategies.
Keywords: Bemisia tabaci, mitochondrial cytochrome oxidase I, China 4 cryptic species
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a cryptic species complex widely distributed throughout tropical and subtropical regions and includes some of the most significant pests of agriculture and horticulture worldwide ( Dinsdale et al. 2010 , De Barro et al. 2011 ). Apart from direct damage to plants caused by feeding, B. tabaci acts as a vector for >120 plant viruses, particularly begomoviruses ( Jones 2003 , Hogenhout et al. 2008 ).
B. tabaci has long been thought to comprise a series of morphologically indistinguishable biotypes that differ in host range, fecundity, insecticide resistance, and the capability to transmit begomoviruses ( Costa and Brown 1991 , Bedford et al.1994 , Brown et al. 1995 , Jones 2003 , Pan et al. 2011 ). Recent studies, in turn, elucidated that B. tabaci is better regarded as a cryptic species complex rather than as one highly variable species. Such cryptic species have been initially identified using phylogenetic methods ( Dinsdale et al. 2010 , De Barro et al. 2011 ) and later confirmed by crossing experiments ( Liu et al. 2012 ). Initial phylogenetic analysis, conducted by Boykin et al. (2007) based on 366 mitochondrial cytochrome oxidase I (COI) DNA sequences of B. tabaci worldwide, led to the split of B. tabaci in 12 major genetic groups. Building over these results, Dinsdale et al. (2010) proposed a threshold of 3.5% mtCOI sequence divergence for species delimitation in the B. tabaci complex. This led to the identification of 24 distinct species: Mediterranean (Q, J, L, Sub-Saharan Africa Silverleaf); Middle East-Asia Minor 1 (B, B2); Middle East-Asia Minor 2; Indian Ocean (MS); Asia I (H, M, NA); Australia/Indonesia; Australia (AN); China 1 (ZHJ3); China 2; Asia II 1 (K, P, ZHJ2); Asia II 2; Asia II 3 (ZHJ1); Asia II 4; Asia II 5 (G); Asia II 6; Asia II 7 (Cv); Asia II 8; Italy (T); Sub-Saharan Africa 1; Sub-Saharan Africa 2 (S); Sub-Saharan Africa 3; Sub-Saharan Africa 4; New World (A, C, D, F, Jatropha, N, R, Sida); and Uganda (formerly associated biotype names are shown in parentheses, if any). At least 14 of these putative cryptic species have been subsequently confirmed based on crossing experiments, adding credibility to the procedure ( Elbaz et al. 2010 , Xu et al. 2010 , De Barro et al. 2011 , Sun et al. 2011 , Wang et al. 2011 , Liu et al. 2012 ). Using the same 3.5% boundary, Hu et al. (2011) identified four additional cryptic species in China (Asia II 9, Asia II 10, and Asia III and China 3), bringing the total number of identified B. tabaci species to 28, and more are being described worldwide ( Alemandri et al. 2012 , Chowda-Reddy et al. 2012 , Ahmed et al. 2013 , Firdaus et al. 2013 ). Although the close association observed today between B.tabaci and a number of different horticultural plants may suggest the opposite, it has been assessed that B.tabaci cryptic species, in fact, radiated well before the advent of agriculture, ∼60–30 millions of years ago ( Boykin et al. 2013 ).
Most B. tabaci cryptic species appear to have a localized geographic distribution and are regarded as indigenous ( Boykin et al. 2007 , Dinsdale et al. 2010 ). At variance, two well-known species, Middle East-Asia Minor 1 (formerly commonly known as biotype B, herein MEAM1) and Mediterranean (formerly biotype Q, herein MED), are highly invasive and have successfully colonized large areas worldwide. MEAM1 likely originated in the desert environments of northeastern Africa, the Middle East, and the Arabian peninsula ( Frohlich et al. 1999 , De Barro et al. 2000 , Elbaz et al. 2010 ), whereas MED seems to have originated in Saharan and sub-Saharan Africa and later spread throughout North Africa and the Mediterranean Basin ( Moya et al. 2001 , Boykin et al. 2007 , Elbaz et al. 2010 ). Noteworthy, a recent reappraisal of the original work by Gennadius based on DNA genotyping of one sample from his original 1889 collection confirmed that this latter cryptic species (MED) is to be regarded as the real B.tabaci in formal taxonomic terms ( Tay et al. 2012 ).
Invasive MEAM1 apparently entered China in the mid/late 1990s ( Luo et al. 2002 ), whereas MED was first reported from China in the province of Yunnan in year 2003 ( Chu et al. 2005 , 2006 ). An extensive field survey, conducted in China in years 2009–2010 by Hu et al. (2011) , indicates that MEAM1 is predominant in South and South-Eastern coastal areas, whereas MED is the dominant species in the Yangtze River Valley and Eastern coastal areas.
Yunnan is located in the Yunnan-Guizhou Plateau, in South-Western China, and is a worldwide renowned biodiversity hotspot due to its diverse climate and vegetation ( Myers et al. 2000 ). Moreover, Yunnan has the highest incidence and diversity of begomoviruses in China, with tomato yellow leaf curl China virus (TYLCCNV), tobacco curly shoot virus (TbCSV), tobacco leaf curl Yunnan virus (TbLCYNV), and tomato yellow leaf curl Thailand virus (TYLCTHV) being the most frequent on cultivated crops (tomato, pepper, and tobacco; Zhou et al. 2001 , Li et al. 2004 , Xie et al. 2006 ). Begomovirus belongs to the family Geminiviridae, a family of plant viruses with circular, single-stranded DNA genomes packaged within geminate particles. Begomoviruses are transmitted by B. tabaci to dicotyledonous plants, causing significant losses in many cultivated crops worldwide ( Varma and Malathi 2003 , Ndunguru et al. 2005 ). As of current knowledge, B. tabaci is the only insect vector capable of transmitting begomoviruses ( Brown 2007 , Hogenhout et al. 2008 ) and, noteworthy, different B. tabaci cryptic species differ with regards to their capability to transmit viruses ( Li et al. 2010 , Liu et al. 2010 ). As such, the identification of different B. tabaci cryptic species is crucial to our understanding of begomovirus epidemiology. The presence and distribution of different B. tabaci cryptic species in Yunnan is poorly known and limited to five localities sampled in a former campaign ( Hu et al. 2011 ). Thereby, this large-scale field survey was conducted in Yunnan to investigate the diversity of B. tabaci cryptic species and their distribution ranges. In delineating new cryptic species, we follow Dinsdale et al. (2010) in tentatively referring to lineages separated by >3.5% genetic divergence as “species,” although extensive crossing tests will be needed as a prerequisite for a formal description of these species.
Aims of this study are 1) to explore the diversity of B. tabaci in the region of Yunnan and to detect possible new cryptic species aiming at an improved knowledge of B. tabaci taxonomy and 2) to define the current local distribution of invasive MEAM1 and MED, as well as indigenous B. tabaci cryptic species, with the aim of providing the essential background information for improved pest management strategies and begomovirus disease control in Yunnan.
Materials and Methods
Whitefly Collection and DNA Extraction
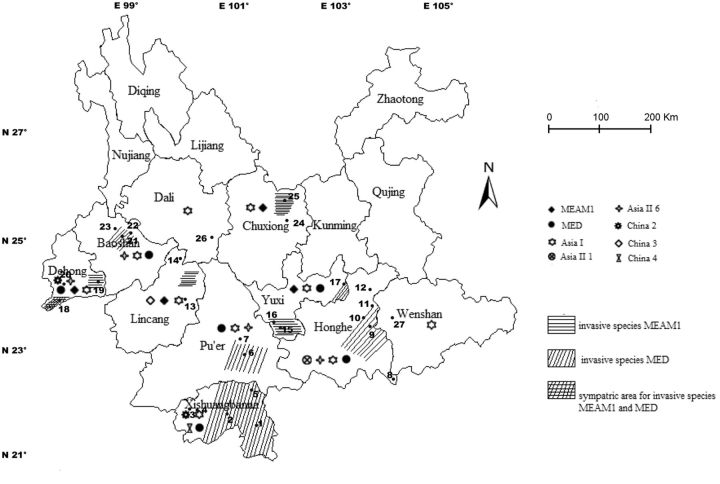
A large-scale sampling of B. tabaci was conducted from October 2010 to July 2013 covering 10 prefectures, ranging from 21°30′ N to 25°42′ N and 97°58′ E to 103°57′ E in the province of Yunnan ( Fig. 1 , Table 1 ). Yunnan lies in South-West China, covering an area of 0.39-million km 2 that accounts for 4.11% of the country. South-Eastern Yunnan is occupied by the Yunnan-Guizhou Plateau, while the North-West is characterized by the presence of the Hengduan Mountains. B. tabaci samples were collected from host plants that included cultivated vegetables, horticulture plants, and natural weeds from open fields. Samples from each of 1–4 different host plants were collected in each of 1–5 localities in each of the prefectures. In total, 47 samples from 27 localities were thus investigated (see Table 1 for extended sampling information).
Fig. 1.
Map of B. tabaci collection sites (see Table 1 for extended sampling information). Overlaid is the distribution of the two invasive cryptic species MEAM1 and MED.
Table 1.
B. tabaci sampling, extended information
| Location name | Latitude | Longitude | Elevation (m) | Host plant | Collection date | Individuals sequenced | Whitefly species | GenBank accession no. |
|---|---|---|---|---|---|---|---|---|
| Xishuangbanna | ||||||||
| Manlie, Mengla | 21° 30′ | 101° 33′ | 1,071 | C. annuum | Aug. 2012 | 3 | MED | KC113548 |
| Manda, Jinghong | 21° 56′ | 100° 43′ | 572 | G. max | Aug. 2012 | 2 | MED | KC113525 |
| Mengzhe, Menghai | 21° 59′ | 100° 16′ | 1,180 | S. lycopersicum | Aug. 2012 | 4 | MED | KC113551 |
| Manzhen, Menghai | 21° 59′ | 100° 25′ | 1,182 | G. max | Aug. 2012 | 2 | Asia I | KC113536 |
| D. stramonium | 2 | China 2 | KC113546 | |||||
| 3 | China 4* | KC113547 | ||||||
| Manhong, Jinghong | 22° 24′ | 101° 23′ | 1,310 | C. annuum | Aug. 2012 | 1 | Asia I | KC113535 |
| S. melongena | 5 | MED | KC113537 | |||||
| Pu'er | ||||||||
| Yongqing, Simao | 22° 47′ | 101° 07′ | 1,197 | S. lycopersicum | Aug. 2012 | 5 | MED | KC113565 |
| Xinping, Ning'er | 23° 02′ | 101° 02′ | 1,335 | I. batatas | Aug. 2012 | 3 | Asia II 6 | KC113561 |
| G. max | 1 | Asia I | KC113560 | |||||
| Honghe | ||||||||
| Shisandui, Hekou | 22° 30′ | 103° 57′ | 109 | S. melongena | Sep. 2011 | 1 | Asia I | KC113532 |
| I. batatas | 4 | Asia II 1 | KC113534 | |||||
| 3 | Asia II 6 | KC113577 | ||||||
| Caoba, Mengzi | 23° 29′ | 103° 24′ | 1,283 | Morus alba | Sep. 2011 | 5 | MED | KC113549 |
| Yusa, Kaiyuan | 23° 46′ | 103° 15′ | 1,063 | Cucumis sativus | May.2012 | 5 | MED | KC113543 |
| I. batatas | 3 | MED | KC113540 | |||||
| 2 | Asia I | KC113541 | ||||||
| Yiguo, Mile | 24° 06′ | 103° 23′ | 1,155 | C. annuum | May.2012 | 5 | MED | KC113558 |
| Mile county town | 24° 24′ | 103° 26′ | 1,438 | S. lycopersicum | May.2012 | 2 | Asia I | KC113554 |
| Lincang | ||||||||
| Mangpan | 23° 55′ | 100° 06′ | 1,460 | G. max | Aug. 2012 | 4 | China 3 | KC113544 |
| Tianxin, Yun county | 24° 55′ | 100° 08′ | 1,140 | Cucum. sativus | Aug. 2012 | 5 | MEAM1 | KC113574 |
| Tithonia diversifolia | Aug. 2012 | 2 | Asia I | KC113575 | ||||
| Yuxi | ||||||||
| Longtan, Yuanjiang | 23° 33′ | 102° 01′ | 467 | I. batatas | May.2012 | 1 | Asia I | KC113573 |
| Dong'e, Yuanjiang | 23° 41′ | 101° 49′ | 672 | Cucum. sativus | May.2012 | 5 | MEAM1 | KC113578 |
| I. batatas | 3 | MEAM1 | KC113566 | |||||
| Dendrocnide urentissima | 4 | MEAM1 | KC113569 | |||||
| 2 | Asia I | KC113568 | ||||||
| Panxi, Huaning | 24° 13′ | 103° 06′ | 1,153 | I. batatas | May.2012 | 3 | MED | KC113529 |
| G. max | 2 | Asia I | KC113528 | |||||
| S. lycopersicum | 5 | MED | KC113526 | |||||
| Dehong | ||||||||
| Jiegang, Ruili | 24° 01′ | 97° 51′ | 776 | I. batatas | Oct. 2010 | 3 | Asia II 6 | HQ916813 |
| Laggera pterodonta | 3 | Asia I | KC123182 | |||||
| S. melongena | 4 | MEAM1 | KC123185 | |||||
| 3 | MED | KC123186 | ||||||
| Mangli, Mangshi | 24° 21′ | 98° 34′ | 900 | S. lycopersicum | Oct. 2010 | 5 | MEAM1 | HQ916816 |
| L. pterodonta | 4 | Asia I | HQ916818 | |||||
| Maliba, Longchuan | 24° 23′ | 97° 58′ | 1,026 | Lantana camara | Oct. 2010 | 1 | Asia I | KC123184 |
| Lan. camara | Oct. 2010 | 4 | China 2 | HQ916820 | ||||
| Baoshang | ||||||||
| Mangdan, longyang | 24° 10′ | 98° 48′ | 1,313 | I. batatas | Oct. 2010 | 3 | MED | HQ916814 |
| S. melongena | 1 | Asia I | HQ916817 | |||||
| Shitouzhai, longyang | 25° 01′ | 99° 01′ | 1,389 | Nicotiana tabacum | Oct. 2010 | 4 | MED | HQ916815 |
| Mangbang, Tengchong | 25° 03′ | 98° 50′ | 798 | I. batatas | Oct. 2010 | 2 | Asia I | HQ916819 |
| 3 | Asia II 6 | |||||||
| Chuxiong | ||||||||
| Dapingdi, Lufeng | 24° 58′ | 101° 52′ | 1,291 | L. pterodonta | Oct. 2010 | 1 | Asia I | HQ916821 |
| Guanneng, Yuanmou | 25° 42′ | 101° 53′ | 1,129 | S. lycopersicum | Aug. 2012 | 5 | MEAM1 | KC113571 |
| Dali | ||||||||
| Yingdi, Nanjian | 25° 02′ | 100° 31′ | 1,370 | Phaseolus vulgaris | Aug. 2012 | 3 | Asia I | KC113562 |
| Wenshan | ||||||||
| De’an, Yanshan | 23° 44′ | 103° 47′ | 1,461 | G. max | Jul. 2013 | 5 | Asia I | KF534718 |
Location numbers refer to Fig. 1 .
Samples were preserved in 95% ethanol prior to molecular analysis. Total DNA was extracted from individual adult specimens using DNeasy Blood&Tissue Kit (QIAGEN, Hilden, Germany). Voucher specimens were deposited in the Biotechnology and Germplasm Resources Institute of the Yunnan Academy of Agricultural Sciences.
COI Gene Amplification and Sequencing
Approximately half of the COI coding region (758 bp) was polymerase chain reaction (PCR) amplified in all samples using universal primers C1-J-2195 (5′-TTGATTTTTTGGTCATCCAGAAGT-3′) and TL2-N-3014 (5′-TCCAATGCACTAATCTGCCATATTA-3′) ( Simon et al. 1994 ). Reaction conditions consisted in an initial denaturation of 94°C for 5', followed by 35 cycles of 94°C for 45", 50°C for 1' and 72°C for 1'30", and a final extension at 72°C for 10'. PCR products were gel purified using the AxyprepTM DNA Gel Extraction Kit (AXYGEN, Union City, CA) and directly sequenced in an ABI 3730 XL DNA analyzer. In total, 77 new sequences were deposited in GenBank under accession numbers KC123182–KC123194, KC113525–KC113578, HQ916813–HQ916821, and KF534718 ( Table 1 ).
Cryptic Species Identification and Designation of a New Species
Different B. tabaci cryptic species were identified using the 3.5% boundary proposed by Dinsdale et al. (2010) . Genetic distances among 18 B. tabaci species (17 reported in Asia and one new species discovered in this study) were calculated as mean Kimura two-parameter sequence divergences as implemented in MEGA 5.05 ( Tamura et al. 2011 ) and tabled as a distance matrix ( Table 2 ). The new species (see Results) was nominated referring to Hu et al. (2011) and De Barro et al. (2011) . To confirm that the new species had actually not been sampled before and overlooked in our reference dataset, we further compared the novel sequence with the latest Global Bemisia dataset (release version 31 December 2012, compiled by L. Boykin and P. De Barro, available at http://dx.doi.org/10.4225/08/50EB54B6F1042 ). A combined dataset that includes the 558 sequences present in the aforementioned dataset as well as the new sequences reported here was assembled, and K2P and ML (GTR+I+Γ) distances were calculated using PAUP* ( Swofford 2003 ) and revised to identify the closest relatives to the new sequence.
Table 2.
Mean Kimura two-parameter genetic distances among B. tabaci cryptic species found in Asia
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Asia I | 0 | |||||||||||||||||
| 2 Asia II 1 | 0.161 | 0 | ||||||||||||||||
| 3 Asia II 2 | 0.146 | 0.067 | 0 | |||||||||||||||
| 4 Asia II 3 | 0.177 | 0.132 | 0.077 | 0 | ||||||||||||||
| 5 Asia II 4 | 0.172 | 0.128 | 0.055 | 0.048 | 0 | |||||||||||||
| 6 Asia II 5 | 0.181 | 0.099 | 0.108 | 0.134 | 0.132 | 0 | ||||||||||||
| 7 Asia II 6 | 0.176 | 0.123 | 0.136 | 0.152 | 0.159 | 0.069 | 0 | |||||||||||
| 8 Asia II 7 | 0.156 | 0.103 | 0.123 | 0.132 | 0.143 | 0.114 | 0.112 | 0 | ||||||||||
| 9 Asia II 8 | 0.158 | 0.139 | 0.124 | 0.123 | 0.147 | 0.132 | 0.143 | 0.132 | 0 | |||||||||
| 10 Asia II 9 | 0.172 | 0.124 | 0.097 | 0.055 | 0.085 | 0.118 | 0.142 | 0.118 | 0.127 | 0 | ||||||||
| 11 Asia II 10 | 0.137 | 0.133 | 0.111 | 0.113 | 0.123 | 0.138 | 0.132 | 0.122 | 0.129 | 0.105 | 0 | |||||||
| 12 Asia III | 0.082 | 0.154 | 0.141 | 0.161 | 0.154 | 0.168 | 0.161 | 0.145 | 0.163 | 0.156 | 0.136 | 0 | ||||||
| 13 China 1 | 0.134 | 0.157 | 0.142 | 0.161 | 0.162 | 0.15 | 0.153 | 0.163 | 0.155 | 0.154 | 0.136 | 0.12 | 0 | |||||
| 14 China 2 | 0.13 | 0.153 | 0.152 | 0.161 | 0.17 | 0.153 | 0.157 | 0.145 | 0.155 | 0.147 | 0.133 | 0.121 | 0.039 | 0 | ||||
| 15 China 3 | 0.142 | 0.156 | 0.137 | 0.159 | 0.17 | 0.163 | 0.165 | 0.154 | 0.134 | 0.144 | 0.138 | 0.131 | 0.118 | 0.107 | 0 | |||
| 16 China 4 | 0.137 | 0.144 | 0.129 | 0.139 | 0.154 | 0.153 | 0.165 | 0.149 | 0.141 | 0.14 | 0.143 | 0.133 | 0.113 | 0.1 | 0.103 | 0 | ||
| 17 MEAM1 | 0.164 | 0.17 | 0.13 | 0.179 | 0.164 | 0.179 | 0.187 | 0.189 | 0.162 | 0.173 | 0.175 | 0.173 | 0.169 | 0.161 | 0.153 | 0.159 | 0 | |
| 18 MED | 0.168 | 0.176 | 0.149 | 0.179 | 0.186 | 0.189 | 0.176 | 0.182 | 0.162 | 0.163 | 0.179 | 0.183 | 0.171 | 0.169 | 0.169 | 0.165 | 0.055 | 0 |
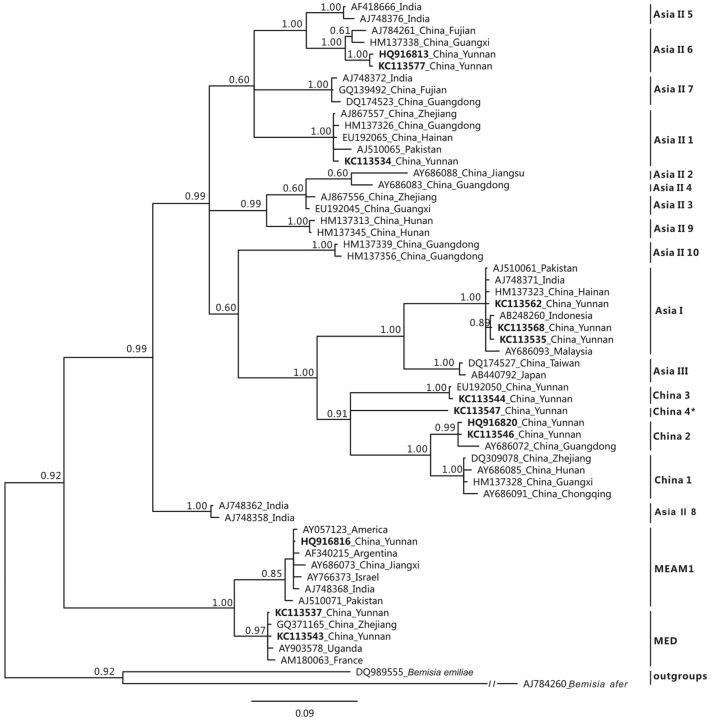
The position of the new B. tabaci cryptic species in the context of B. tabaci diversity in Asia was evaluated based on a phylogenetic analysis. The COI sequences obtained during this study, as well as others retrieved from GenBank to represent all known Asian and invasive cryptic species ( Table 3 ), were aligned using ClustalX (ver. 1.81; Thompson et al. 1997 ). Bemisia emiliae (GenBank accession DQ989555) and Bemisia afer (GenBank accession AJ784260) were also included as outgroups. The final dataset consisted of a total of 58 COI sequences for a total length of 666 bp. The phylogenetic analysis was performed applying Bayesian methods as implemented in MrBayes (ver 3.1; Huelsenbeck et al. 2001 ) that employs a Markov chain Monte-Carlo sampling to approximate the posterior probabilities of phylogenies ( Green 1995 , Boykin et al. 2007 ). The most appropriate model of sequence evolution (GTR+I+Γ) was selected using MrModeltest (ver. 2.2; Nylander 2004 ). Two parallel sets of four chains were run for 50 million generations, with trees and parameters sampled every 1,000th generation. Based on likelihood and parameter traces visualized in Tracer (available at: http://tree.bio.ed.ac.uk/software/tracer/ ), the 10% initial states were discarded as burn in of the analysis. The best topology, as well as posterior probabilities at each node, was obtained by summarizing the remaining tree states.
Table 3.
B. tabaci COI sequences obtained from GenBank
| Location | Accession no. | Cryptic species | Host plant | References |
|---|---|---|---|---|
| Pakistan | AJ510061 | Asia I | S. melongena | — |
| India | AJ748371 | Asia I | Parthenium hysterophorus | Rekha et al. (2005) |
| Indonesia | AB248260 | Asia I | G. max | — |
| Malaysia | AY686093 | Asia I | Hibiscus syriacus | — |
| Hainan, China | HM137323 | Asia I | Manihot esculenta | Hu et al. (2011) |
| Hainan, China | EU192065 | Asia II 1 | — | — |
| Zhejiang, China | AJ867557 | Asia II 1 | I. batatas | — |
| Guangdong, China | HM137326 | Asia II 1 | G. max | Hu et al. (2011) |
| Pakistan | AJ510065 | Asia II 1 | Gossypium hirsutum | — |
| Jiangsu, China | AY686088 | Asia II 2 | Go. hirsutum | — |
| Zhejiang, China | AJ867556 | Asia II 3 | Go. hirsutum | — |
| Guangxi, China | EU192045 | Asia II 3 | — | — |
| Guangdong, China | AY686083 | Asia II 4 | Cucurbita moschata | Brown and Idris (2005) |
| India | AF418666 | Asia II 5 | M. esculenta | Maruthi et al. (2004) |
| India | AJ748376 | Asia II 5 | Ageratum conyzoides | Rekha et al. (2005) |
| Fujian, China | AJ784261 | Asia II 6 | Go. hirsutum | — |
| Guangxi, China | HM137338 | Asia II 6 | Luffa aegyptiaca | Hu et al. (2011) |
| Guangdong, China | DQ174523 | Asia II 7 | Codiaeum variegatum | Hsieh et al. (2006) |
| India | AJ748372 | Asia II 7 | P. hysterophorus | Rekha et al. (2005) |
| Fujian, China | GQ139492 | Asia II 7 | — | — |
| India | AJ748362 | Asia II 8 | — | Rekha et al. (2005) |
| India | AJ748358 | Asia II 8 | — | Rekha et al. (2005) |
| Hunan, China | HM137313 | Asia II 9 | I. batatas | Hu et al. (2011) |
| Hunan, China | HM137345 | Asia II 9 | I. batatas | Hu et al. (2011) |
| Guangdong, China | HM137339 | Asia II 10 | Cucur. moschata | Hu et al. (2011) |
| Guangdong, China | HM137356 | Asia II 10 | Cucur. moschata | Hu et al. (2011) |
| Taiwan, China | DQ174527 | Asia III | Mesona chinensis | Hsieh et al. (2006) |
| Japan | AB440792 | Asia III | Wedelia biflora | Ueda et al. (2009) |
| Chongqing, China | AY686091 | China 1 | E. pulcherrima | — |
| Jiangxi, China | HM137328 | China 1 | I. batatas | Hu et al. (2011) |
| Zhejiang, China | DQ309078 | China 1 | I. batatas | — |
| Hunan, China | AY686085 | China 1 | S. melongena | — |
| Guangdong, China | AY686072 | China 2 | Lycopersicon esculentum | — |
| Yunnan, China | EU192050 | China3 | G. max | — |
| Pakistan | AJ510071 | MEAM1 | Go. hirsutum | — |
| India | AJ748368 | MEAM1 | — | Rekha et al. (2005) |
| Israel | AY766373 | MEAM1 | — | — |
| America | AY057123 | MEAM1 | E. pulcherrima | Brown and Idris (2005) |
| Argentina | AF340215 | MEAM1 | — | Viscarret et al. (2003) |
| Jiangxi, China | AY686073 | MEAM1 | S. melongena | — |
| Zhejiang, China | GQ371165 | MED | C. annuum | — |
| Uganda | AY903578 | MED | Nicotiana tabacum | Sseruwagi et al. (2005) |
| France | AM180063 | MED | — | Tahiri et al. (2006) |
— denotes unknown host plant or unpublished sequences from NCBI.
Results
In total, 27 locations were surveyed in the study, with 47 samples collected from host plants belonging to 14 species in 8 families ( Table 1 ). Cryptic species MEAM1 was found in the prefectures of Chuxiong, Yuxi, Lincang, and Dehong. MED was found in the prefectures of Yuxi, Honghe, Pu’er, Xishuangbanna, Baoshan, and Dehong ( Table 1 ; Fig. 1 ). In the prefecture of Dehong, species MEAM1 and MED were observed to coexist on individual plants of Solanum melongena ( Table 1 ). Taken together, our records indicate that the MED has a wider distribution than MEAM1 in Yunnan.
Six indigenous B. tabaci cryptic species were identified ( Fig. 1 ; Table 1 ). Asia I is characterized by the widest distribution among all indigenous species, being present in all the 10 prefectures surveyed. No other indigenous species was found in Chuxiong and Dali except Asia I. Asia II 6 was found in Honghe, Pu’er, Baoshan, and Dehong and appears to be the second predominant indigenous species in Yunnan. Asia II 1 was found only in Honghe and China 3 only in Lincang. China 2 was found in two noncontiguous areas: Dehong and Xishuangbanna. Indigenous B.tabaci were observed on a number of different plant species, nevertheless Ipomoea batatas and Glycine max appeared the most frequent hosts.
According to the 3.5% boundary proposed by Dinsdale et al. (2010) to separate candidate species, a new lineage was identified here for the first time and was tentatively given specific rank. Based on its phylogenetic affinities, it was named China 4 (see below). The comparison conducted with the 558 worldwide sequences present in the Global Bemisia dataset confirmed that this species had not been sampled before, as the most similar previously reported sequence (EU19205) is as distant as 9.6% (K2P distances) or 12.4 (ML), well above the generally accepted 3.5% limit for species delimitation.
The new indigenous B. tabaci cryptic species China 4 clusters with China1, 2, and 3 with a high posterior probability (0.91). These are further associated with Asia I and Asia III in a larger clade supported by a posterior probability of 1 ( Fig. 2 ). Genetic distances between China 4 and the other 17 B. tabaci cryptic species reported in Asia range from 0.1 to 0.165 ( Table 2 ).
Fig. 2.
Bayesian tree inferred from mtCOI data. Posterior probabilities are indicated at nodes. The proposed new species is indicated with *. New sequences obtained in this study are indicated in bold.
The new cryptic species China 4 was collected in the mountainous region of Menghai (Xishuangbanna) close to the Myanmar border, at an elevation of 1,182 m. The only three individuals found were sampled on Datura stramonium , a solanaceous flowering plant with an annual cycle that is not considered of economic importance, although some parts of the plant are sometime used in herbal medicine due to their high content in alkaloids. In the same area, two additional B.tabaci cryptic species were collected: Asia I on G . max and China 2 similarly on D . stramonium , although China 2 and China 4 were never found on the very same plant.
Discussion
The global diversity of the whitefly B. tabaci in Yunnan, as revealed by the current survey, totals to eight cryptic species, including two invasive species (MEAM1 and MED) and six indigenous species (Asia I, Asia II 1, Asia II 6, China 2, China 3, and China 4). In general, invasive MEAM1 and MED are characterized by a wider distribution than indigenous species in Yunnan ( Fig. 1 ). Furthermore, MEAM1 and MED are mostly found in association with valuable cultivated crops such as Solanum lycopersicum, Capsicum annuum, and S . melongena . Both MEAM1 and MED are found in a number of geographically noncontiguous areas, possibly due to the diverse landforms that characterize the region of Yunnan, that include high mountains, rivers, canyons, and deep valleys that may act as geographical barriers capable of impairing the dispersal of these species.
Based on their overall distributions, MED seem to have colonized a larger area than MEAM1 in Yunnan, in line with what has been observed in Shandong ( Chu et al. 2010 ), Hubei ( Rao et al. 2011 ) and the Yangtze River Valley in China ( Hu et al. 2011 , Guo et al. 2012 ). However, a predictive model for the distribution of MED and MEAM1 in Yunnan is difficult to develop at present, underlining the importance of frequent field surveys on the population dynamics of MED and MEAM1 as a support to efficient pest protection strategies. In fact, MED was shown to be able to displace MEAM1 under intensive insecticide pressure due to its higher resistance to insecticides ( Horowitz et al. 2005 , Prabhaker et al. 2005 , Wilson et al. 2007 , Crowder et al. 2010a , b , Luo et al. 2010 ), whereas in the absence of intense insecticide pressure, MEAM1 is more competitive than MED ( Pascual and Callejas 2004 ) and was shown to displace other B. tabaci cryptic species through the mechanism of asymmetric mating interactions ( Liu et al. 2007 ). Although of primary importance for control purposes, these dynamics are difficult to study in the region of Yunnan due to the largely disjunct distribution of MEAM1 and MED and the presence of geographical barriers that may affect their spread. Ruili, on the Western border of the area under scrutiny, is the only sampled region where invasive MEAM1 and MED are found in sympatry. Although no collection data are available about the order in which the two species colonized this area, apart from an early survey of Ahmed et al. (2009) that reported the presence of MEAM1 on Ipomea batatas in this area, Ruili appears the best location to investigate the interactions and possible displacement among the two species. Ahmed et al. (2009) and Hu et al. (2011) collected MED adult samples in the prefecture of Kunming from ornamental plant, Euphorbia pulcherrima ; however, no open field populations of MEAM1 or MED are present in Kunming, at variance with the neighboring region of Yuanmou (Chuxiong) where MEAM1 can indeed survive. A possible explanation can be found in the prevailing outdoor temperature. In fact, Yuanmou is a hot and dry valley, with annual mean temperature of 21.9°C and a winter daily mean minimum temperature in winter of 9°C, whereas the annual mean temperature in Kuming is 15°C and the winter daily mean minimum temperature is 3°C. As such, invasive MEAM1 and MED cannot be able to complete their life history in an open environment in this latter region.
Beside invasive MEAM1 and MED, the dominant indigenous B. tabaci cryptic species in Yunnan is Asia I. It was collected from all prefectures investigated ( Fig. 1 ), although typical Asia I population sizes were far less than those observed for MEAM1 or MED. With the exception of Asia I, other indigenous cryptic species Asia II 1, Asia II 6, China 2, China 3, and China 4 were observed to feed almost exclusively on a very limited number of host plants that include I . batatas , G . max, and natural weeds in the least altered environments ( Table 1 ). On the other hand, invasive B. tabaci MEAM1 and MED are mostly associated with disturbed environments characterized by the presence of intense anthropic activities. Based on this observation, it is possible to predict that a total replacement of invasive over indigenous species is unlikely to take place, as indigenous species seem to be able to persist in the least altered habitats ( Delatte et al. 2009 ).
Following the initial evidence that B. tabaci is in fact a species complex composed of numerous cryptic species, studies on species delimitation based on molecular genetic data have accumulated at a steady pace ( Dinsdale et al. 2010 , Hu et al. 2011 , Esterhuizen et al. 2013 , Marubayashi et al. 2013 ), and improved analytical procedures of species delimitation based on molecular data have also been applied to this taxon ( Boykin et al. 2012 ). In the phylogenetic analysis, the new cryptic species China 4 clustered with China 2, China 3, and China 1, suggesting a close genetic relationship among three indigenous B. tabaci cryptic species. Moreover, China 4, China 3, China 2, and China 1 clustered with Asia I and Asia III to form a larger clade. Considering that two indigenous Asian species, Asia II 5 and Asia II 8, are seemingly not present in China, our data, as well as reports in Hu et al. (2011) , indicate the presence of 16 B. tabaci cryptic species in China, 2 invasive and 14 indigenous. Nevertheless, not all species appear to attack economically important crops. In fact, the new China 4 species described here was sampled only on D . stramonium and at very low frequencies overall. As such, even in the light of a certain interest for our understanding of B.tabaci diversity, its biological significance appears to be rather limited.
Beside the importance of this taxon in terms of the damages, it can induce to horticultural crops by direct phloem feeding, a better knowledge of B. tabaci cryptic species diversity is crucial for our understanding of the occurrence and epidemiology of begomoviruses. Li et al. (2010) reported that MEAM1 and MED are capable of transmitting the tomato yellow leaf curl virus much more efficiently than Asia II 1. Furthermore, evidences for indirect mutualism were observed between begomoviruses and given B. tabaci cryptic species. The fecundity and longevity of MEAM1, in fact, was fostered by feeding on plants infected by the TbCSV or the TYLCCNV, whereas no such effect was observed for Asia II 3 ( Jiu et al. 2007 ). The same phenomenon was observed in MED feeding on TYLCCNV-infected plants ( Liu et al. 2010 ). Such evidences are of marked interest for the development of efficient plant protection strategies in Yunnan, the region characterized by the highest incidence of begomoviruses in China ( Zhou et al. 2001 , Xie and Zhou 2003 , Li et al. 2004 , Xie et al. 2006 ), as an improved definition of B. tabaci diversity, and the relative transmission capabilities of different cryptic species can led to a better understanding of the epidemics of begomovirus diseases and the possibility to develop more efficient control strategies.
The data and analyses presented here, apart from providing an extensive picture of B. tabaci diversity in Yunnan at present, may be used in the future as background information to help monitor the spread of exotic invasive B. tabaci MEAM1 and MED and their possible replacement of indigenous species in some areas. Future studies should also focus on the interactions between predominant B. tabaci cryptic species and begomoviruses in Yunnan and on the development of effective management strategies to control the epidemics of begomovirus diseases in this area.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (U1136606, 31360429), the Applied Basic Research of Yunnan Province (2011FB122), and the Reserve Talents of Young and Middle-Aged Academic and Technical Leaders of the Yunnan Province (2012HB038).
References Cited
- Ahmed M. Z., Shen Y., Jin G. H., Ren S. X. . 2009. . Population and host plant differentiation of the sweetpotato whitefly, Bemisia tabaci (Homoptera:Aleyrodidae), in East, South and Southwest China . Acta Entomol. Sin. 52 : 1132 – 1138 . [Google Scholar]
- Ahmed M. Z., De Barro P. J., Ren S. X., Greeff J. M., Qiu B. L. . 2013. . Evidence for horizontal transmission of secondary endosymbionts in the Bemisia tabaci cryptic species complex . PLoS One 8 : e53084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemandri V., De Barro P., Bejerman N., Argüello Caro E. B., Dumón A. D., Mattio M. F., Rodriguez S. M., Truoli G. . 2012. . Species within the Bemisia tabaci (Hemiptera: Aleyrodidae) complex in soybean and bean crops in Argentina . J. Econ. Entomol. 105 : 48 – 53 . [DOI] [PubMed] [Google Scholar]
- Bedford I. D., Briddon R. W., Brown J. K., Rosell R. C., Markham P. G. . 1994. . Geminivirus transmission and biological characterisation of Bemisia tabaci (Gennadius) biotypes from different geographic regions . Ann. Appl. Biol. 125 : 311 – 325 . [Google Scholar]
- Boykin L. M., Shatters R. G., Rosell R. C., McKenzie C. L., Bagnall R. A., De Barro P., Frohlich D. R. . 2007. . Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial CO1 DNA sequences . Mol. Phylogenet. Evol. 44 : 1306 – 1319 . [DOI] [PubMed] [Google Scholar]
- Boykin L. M., Armstrong K. F., Kubatko L., De Barro P. . 2012. . Species delimitation and global biosecurity . Evol. Bioinform. 8 : 1 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykin L. M., Bell D. C., Evans G., Small L., De Barro P. J. . 2013. . Is agriculture driving the diversification of the Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodidae)? Dating, diversification and biogeographic evidence revealed . BMC Evol. Biol. 13 : 228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. K. 2007. . The Bemisia tabaci complex: genetic and phenotypic variability drives begomovirus spread and virus diversification . APSnet Features . http://www.apsnet.org/online/feature/btabaci/ . [Google Scholar]
- Brown J. K., Frohlich D. R., Rosell R. C. . 1995. . The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 40 : 511 – 534 . [Google Scholar]
- Chowda-Reddy R. V., Kirankumar M., Seal S. E., Muniyappa V., Vland G. B., Govindappa M. R., Colvin J. . 2012. . Bemisia tabaci phylogenetic groups in India and the relative transmission efficacy of Tomato leaf curl Bangalore virus by an indigenous and an exotic population . J. Integr. Agric. 11 : 235 – 248 . [Google Scholar]
- Chu D., Zhang Y. J., Wang F. H. . 2010. . Cryptic invasion of the exotic Bemisia tabaci biotype Q occurred widespread in Shandong Province of China Fla. Entomol. 93 : 203 – 207 . [Google Scholar]
- Chu D., Zhang Y. J., Cong B., Xu B. Y., Wu Q. J., Zhu G. R. . 2005. . Sequences analysis of mtDNA COI gene and molecular phylogeny of different geographical populations of Bemisia tabaci (Gennadius) . Sci. Agric. Sin. 38 : 76 – 85 . [Google Scholar]
- Chu D., Zhang Y. J., Brown J. K., Cong B., Xu B. Y., Wu Q. J., Zhu G. R. . 2006. . The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops . Fla. Entomol. 89 : 168 – 174 . [Google Scholar]
- Costa H. S., Brown J. K. . 1991. . Variation in biological characteristics and in esterase patterns among populations of Bemisia tabaci (Genn.) and the association of one population with silverleaf symptom development . Entomol. Exp. Appl. 61 : 211 – 219 . [Google Scholar]
- Crowder D. W., Horowitz A. R., De Barro P. J., Liu S. S., Showalter A. M., Kontsedalov S., Khasdan V., Shargal A., Liu J., Carrière Y. . 2010a. . Mating behaviour, life history and adaptation to insecticides determine species exclusion between whiteflies . J. Anim. Ecol. 79 : 563 – 570 . [DOI] [PubMed] [Google Scholar]
- Crowder D. W., Sitvarin M. I., Carriere Y. . 2010b. . Plasticity in mating behaviour drives asymmetric reproductive interference in whiteflies . Anim. Behav. 79 : 579 – 587 . [Google Scholar]
- De Barro P. J., Driver F., Trueman J. W., Curran J. . 2000. . Phylogenetic relationships of world populations of Bemisia tabaci (Gennadius) using ribosomal ITS1 . Mol. Phylogenet. Evol. 16 : 29 – 36 . [DOI] [PubMed] [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M., Dinsdale A. B. . 2011. . Bemisia tabaci : a statement of species status . Annu. Rev. Entomol. 56 : 1 – 19 . [DOI] [PubMed] [Google Scholar]
- Delatte H., Duyck P. F., Triboire A., David P., Becker N., Bonato O., Reynaud B. . 2009. . Differential invasion success among biotypes: case of Bemisia tabaci . Biol. Invas. 11 : 1059 – 1070 . [Google Scholar]
- Dinsdale A., Cook L., Riginos C., Buckley Y. M., De Barro P. . 2010. . Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoiea: Aleyrodidae) mitochondrial cytochrome oxidase I to identify species level genetic boundaries . Ann. Entomol. Soc. Am. 103 : 196 – 208 . [Google Scholar]
- Elbaz M., Lahav N., Morin S. . 2010. . Evidence for pre-zygotic reproductive barrier between the B and Q biotypes of Bemisia tabaci . Bull. Entomol. Res. 100 : 581 – 590 . [DOI] [PubMed] [Google Scholar]
- Esterhuizen L. L., Mabasa K. G., van Heerden S. W., Czosnek H., Brown J. K., van Heerde H., Rey M. E. C. . 2013. . Genetic identification of members of the Bemisia tabaci cryptic species complex from South Africa reveals native and introduced haplotypes . J. Appl. Entomol. 137 : 122 – 135 . [Google Scholar]
- Firdaus S., Vosman B., Hidayati N., Supena E. D. J., Visser R. G. F., van Heusden A. W. . 2013. . The Bemisia tabaci species complex: additions from different parts of the world . Insect Sci. 20 : 723 – 733 . [DOI] [PubMed] [Google Scholar]
- Frohlich D. R., Torres-Jerez I., Bedford D., Markham P. G., Brown J. K. . 1999. . A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers . Mol. Ecol. 8 : 1683 – 1691 . [DOI] [PubMed] [Google Scholar]
- Green P. J. 1995. . Reversible jump Markov chain Monte Carlo computation and Bayesian model determination . Biometrika 82 : 711 – 732 . [Google Scholar]
- Guo X. J., Rao Q., Zhang F., Luo C., Zhang H. Y., Gao X. W. . 2012. . Diversity and genetic differentiation of the whitefly Bemisia tabaci species complex in China based on mtCOI and cDNA-AFLP analysis . J. Integr. Agric. 11 : 206 – 214 . [Google Scholar]
- Hogenhout S. A., Ammar E. D., Whitfield A. E., Redinbaugh M. G. . 2008. . Insect vector interactions with persistently transmitted viruses . Annu. Rev. Phytopathol. 46 : 327 – 359 . [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Kontsedalov S., Khasdan V., Ishaaya I. . 2005. . Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance . Arch. Insect Biochem. Physiol. 58 : 216 – 225 . [DOI] [PubMed] [Google Scholar]
- Hu J., De Barro P., Zhao H., Wang J., Nardi F., Liu S. S. . 2011. . An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China . PLoS One 6 : e16061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J. P., Ronquist F., Nielsen R., Bollback J. P. . 2001. . Bayesian inference of phylogeny and its impact on evolutionary biology . Science 294 : 2310 – 2314 . [DOI] [PubMed] [Google Scholar]
- Jiu M., Zhou X. P., Tong L., Xu J., Yang X., Wan F. H., Liu S. S. . 2007. . Vector-virus mutualism accelerates population increase of an invasive whitefly . PLoS One 2 : e182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. R. 2003. . Plant viruses transmitted by whiteflies . Eur. J. Plant Pathol. 109 : 195 – 219 . [Google Scholar]
- Li Z. H., Zhou X. P., Zhang X., Xie Y. . 2004. . Molecular characterization of tomato-infecting begomoviruses in Yunnan, China . Arch. Virol. 149 : 1721 – 1732 . [DOI] [PubMed] [Google Scholar]
- Li M., Hu J., Xu F. C., Liu S. S. . 2010. . Transmission of tomato yellow leaf curl virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci . Int. J. Pest Manage. 56 : 275 – 280 . [Google Scholar]
- Liu S. S., De Barro P. J., Xu J., Luan J. B., Zang L. S., Ruan Y. M., Wan F. H. . 2007. . Asymmetric mating interactions drive widespread invasion and displacement in a whitefly . Science 318 : 1769 – 1772 . [DOI] [PubMed] [Google Scholar]
- Liu J., Li M., Li J. M., Huang C. J., Zhou X. P., Xu F. C., Liu S. S. . 2010. . Viral infection of tobacco plants improves performance of Bemisia tabaci but more so for an invasive than for an indigenous biotype of the whitefly . J. Zheijang Univ. Sci. B 11 : 30 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. S., Colvin J., De Barro P. J. . 2012. . Species concepts as applied to the whitefly Bemisia tabaci systematics: how many species are there? J . Integr. Agric. 11 : 176 – 186 . [Google Scholar]
- Luo C., Yao Y., Wang R. J., Yan F. M., Hu D. X., Zang Z. L. . 2002. . The use of mitochondrial cytochrome oxidase I (mtCO1) gene sequences for the identification of biotype of Bemisia tabaci (Gennadius) in China . Acta Entomol. Sin. 45 : 759 – 763 . [Google Scholar]
- Luo C., Jones C. M., Devine G., Zhang F., Denholm I., Gorman K. . 2010. . Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China . Crop Prot. 29 : 429 – 434 . [Google Scholar]
- Marubayashi J. M., Yuki V. A., Rocha K. C. G., Mituti T., Pelegrinotti F. M., Ferreira F. Z., Moura M. F., Navas-Castillo J., Moriones E., Pavan M. A., et al. . 2013. . At least two indigenous species of the Bemisia tabaci complex are present in Brazil . J. Appl. Entomol. 137 : 113 – 121 . [Google Scholar]
- Moya A., Guirao P., Cifuentes D., Beitia F., Cenis J. L. . 2001. . Genetic diversity of Iberian populations of Bemisia tabaci (Hemiptera: Aleyrodidae) based on random amplified polymorphic DNA polymerase chain reaction . Mol. Ecol. 10 : 891 – 897 . [DOI] [PubMed] [Google Scholar]
- Myers N., Mittermeier R. A., Mittermeier C. G., da Fonceca G. A. B., Kent J. . 2000. . Biodiversity hotspots for conservation priorities . Nature 403 : 853 – 858 . [DOI] [PubMed] [Google Scholar]
- Ndunguru J., Legg J. P., Aveling T. A. S., Thompson G., Fauquet C. M. . 2005. . Molecular biodiversity of cassava begomoviruses in Tanzania: evolution of cassava geminiviruses in Africa and evidence for east Africa being a center of diversity of cassava geminiviruses . Virol. J. 2 : 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander J. A. A. 2004. . MrModeltest v2 program distributed by the author . Evolutionary Biology Centre, Uppsala University; , Uppsala, Sweden: . [Google Scholar]
- Pan H. P., Li X. C., Ge D. Q., Wang S. L., Wu Q. J., Xie W., Jiao X. G., Chu D., Liu B. M., Xu B. Y., Zhang Y. J. . 2011. . Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci . PLoS One 7 : e30760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual S., Callejas C. . 2004. . Intra- and interspecific competition between biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) from Spain . Bull. Entomol. Res. 94 : 369 – 375 . [DOI] [PubMed] [Google Scholar]
- Prabhaker N., Castle S., Henneberry T. J., Toscano N. C. . 2005. . Assessment of cross-resistance potential to neonicotinoid insecticides in Bemisia tabaci (Hemiptera: Aleyrodidae) . Bull. Entomol. Res. 95 : 535 – 543 . [DOI] [PubMed] [Google Scholar]
- Rao Q., Luo C., Zhang H., Guo X., Devine G. J. . 2011. . Distribution and dynamics of Bemisia tabaci invasive biotypes in central China . Bull. Entomol. Res. 101 : 81 – 88 . [DOI] [PubMed] [Google Scholar]
- Simon C., Frati F., Beckenback A., Crespi B., Hong B. L., Flook P. . 1994. . Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers . Ann. Entomol. Soc. Am. 87 : 651 – 701 . [Google Scholar]
- Sun D. B., Xu J., Liu S. S. . 2011. . Reproductive incompatibility between the B and Q biotypes of the whitefly Bemisia tabaci in China: genetic and behavioural evidence . Bull. Entomol. Res. 101 : 211 – 220 . [DOI] [PubMed] [Google Scholar]
- Swofford D. L. 2003. . PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods), version 4 . Sinauer Associates; , Sunderland, MA: . [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. . 2011. . MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods . Mol. Biol. Evol. 28 : 2731 – 2739 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay T. W., Evans G. A., Boykin M. L., De Barro P. J. . 2012. . Will the real Bemisia tabaci please stand up? PLoS One 7 : e50550 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. . 1997. . The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acids Res. 22 : 4673 – 4680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A., Malathi V. G. . 2003. . Emerging geminivirus problems: a serious threat to crop production . Ann. Appl. Biol. 142 : 145 – 164 . [Google Scholar]
- Wang P., Sun D. B., Qiu B. L., Liu S. S. . 2011. . The presence of six putative species of the whitefly Bemisia tabaci complex in China as revealed by crossing experiments . Insect Sci. 18 : 67 – 77 . [Google Scholar]
- Wilson M., Moshitzky P., Laor E., Ghanim M., Horowitz A. R., Morin S. . 2007. . Reversal of resistance to pyriproxyfen in the Q biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) . Pest Manage. Sci. 63 : 761 – 768 . [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhou X. P. . 2003. . Molecular characterization of squash leaf curl Yunnan virus , a new begomovirus and evidence for recombination . Arch. Virol. 148 : 2407 – 2054 . [DOI] [PubMed] [Google Scholar]
- Xie Y., Jiang T., Zhou X. P. . 2006. . Agroinoculation shows tobacco leaf curl Yunnan virus is a monopartite begomovirus . Eur. J. Plant Pathol. 115 : 369 – 375 . [Google Scholar]
- Xu J., De Barro P. J., Liu S. S. . 2010. . Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex . Bull. Entomol. Res. 100 : 359 – 366 . [DOI] [PubMed] [Google Scholar]
- Zhou X. P., Xie Y., Zhang Z. K. . 2001. . Molecular characterization of a distinct begomovirus infecting tobacco in Yunnan, China . Arch. Virol. 146 : 1599 – 1606 . [DOI] [PubMed] [Google Scholar]