Abstract
Because the quality of mating partners varies, females of several taxa have evolved the ability to discriminate against low-quality mates. Although males in the Hymenoptera are usually haploid, diploid males may occur in species with complementary sex determination. Diploid males are almost always sterile in most of the species studied so far. They are thus of very low quality as mating partners, especially when females mate only once in life. We hypothesize that hymenopteran females might have evolved the ability to discriminate against infertile diploid males and avoid mating with them. To test this hypothesis, we studied diploid male fitness in the parasitoid wasp Bracon brevicornis Wesmael (Hymenoptera: Braconidae) by measuring survival rate and fertility and then estimated their chances of actually mating with a female. Flow cytometry was used to determine the ploidy level of wasps. The fitness costs of mating a diploid male are indeed high in this species: only 15% were able to sire daughters, of which 97% were triploid and hardly able to produce viable offspring. In contrast to the hypothesis of unsuitable mate discrimination though, no evidence was found for increased rejection of diploid males by females. Male discrimination against an unsuitable partner did also not occur: triploid females elicited the same intensity of courtship behavior in males than did diploid ones.
Keywords: inbreeding, sex ratio, diploid male vortex, mate choice, complementary sex determination
In the insect order Hymenoptera (ants, bees, wasps, and sawflies), females are produced from fertilized eggs and are diploid, whereas males hatch from unfertilized eggs and are haploid ( Cook and Crozier 1995 ). However, in many species it is not the ploidy level per se that determines an individual’s sex but the allelic composition at the sex-determining locus ( Whiting 1943 , Heimpel and de Boer 2008 ). These species have a so-called complementary sex determination (CSD) mechanism, which is thought to be ancestral in the Hymenoptera ( Asplen et al. 2009 , Schmieder et al. 2012 ). Under single-locus CSD diploid individuals develop into females only when they are heterozygous for the complementary sex determiner locus ( Beye et al. 2003 ) but become males when homozygous at this locus. Diploid males are usually inviable, sterile, or may occasionally produce infertile triploid daughters ( van Wilgenburg et al. 2006 , Heimpel and de Boer 2008 ). Only two species have been reported so far, out of >60 species having CSD ( van Wilgenburg et al. 2006 ), in which diploid males are able to produce fertile diploid daughters in relevant numbers ( Cowan and Stahlhut 2004 , Elias et al. 2009 ). Diploid male production depends on the level of inbreeding and the numbers of sex alleles and sex loci present in a population ( Cook and Crozier 1995 , de Boer et al. 2008 , Heimpel and de Boer 2008 ). The number of sex alleles present in a population usually appears to be high enough to prevent the production of large numbers of diploid males ( Adams et al. 1977 , Ross et al. 1993 , Owen and Packer 1994 , Heimpel et al. 1999 , Takahashi et al. 2001 , Antolin et al. 2003 , Fujiwara et al. 2004 , Francini et al. 2012 ). However, extensive diploid male production can easily happen in bottlenecked populations ( Ross et al. 1993 ) and drive them toward extinction ( Stouthamer et al. 1992 , Zayed and Packer 2005 , Zayed 2009 ). Several species of parasitic wasps possess traits that have been hypothesized to even further accelerate the extinction vortex ( Godfray 1994 ): first, they develop gregariously, increasing the probability of sib mating and thereby, diploid male production. Second, diploid males are viable in many species ( van Wilgenburg et al. 2006 ) and thus remain in the population to mate with females. Finally, females often mate only once in life, and if the chosen partner happens to be a sterile diploid male, the female will not be able to produce any daughters ( Harpur et al 2012 ). This is not necessarily detrimental for a female, as long as other females produce sufficient daughters her sons can mate with ( Godfray 1990 , West 2009 ). However, the more females in a population become constrained, i.e., can produce sons only, the more their sons will compete for the few females available, which reduces their mother’s fitness. In addition, the effective population size is reduced if fewer daughters occur, accelerating a “diploid male extinction vortex” ( Stouthamer et al. 1992 , Zayed and Packer 2005 , Zayed 2009 ).
On the other hand, female behavior needs to be considered before we can assess the true impact of diploid male production on an insect population ( Hein et al. 2009 ). It has been shown, e.g., that diploid male production can be greatly reduced through effective mechanisms of inbreeding avoidance ( Ode et al. 1995 , Metzger et al. 2010 ) or by the recognition of matching alleles in potential partners ( Thiel and Weeda 2013 ).
Nevertheless, diploid males have frequently been caught in wild populations of different parasitoid species, indicating a probability that diploid males may be encountered as potential mating partners ( Harpur et al 2012 ) and that the ability to discriminate against them could be beneficial to females. The threat diploid males may pose to population survival has so far only been considered in theoretical models, assuming panmictic populations ( Stouthamer et al. 1992 , Zayed and Packer 2005 , Hein et al. 2009 ). However, whether females indeed mate indiscriminately with sterile diploid males has not been analyzed to date (but see de Boer et al. 2007 for an isofemale line study), even though choice behavior might greatly impact population dynamics ( Hein et al. 2009 ).
To answer this question, the parasitic wasp Bracon ( Habrobracon ) brevicornis Wesmael (Hymenoptera: Braconidae) was used, which has a distribution reaching from central Europe to northern Africa and western India ( Temerak 1983 , Elzinga et al. 2007 , Venkatesan et al. 2009 ). This gregarious, larval ectoparasitoid uses different species of Lepidoptera as hosts ( Kares et al. 2009 ), and the occurrence of diploid males has been described in an earlier study ( Speicher and Speicher 1940 ). We thus analyzed diploid male survival probability, mating capacity, and fertility, to estimate the costs of diploid male production in this species. In addition, a female’s ability to reject a diploid male partner was determined in the laboratory. Because we found that diploid males can sire, triploid females’ fitness and the ability of haploid males to discriminate against them were analyzed as well.
Materials and Methods
Parasitoid Rearing Conditions
A laboratory colony of B. brevicornis was started from naturally parasitized corn borer larvae ( Ostrinia nubilalis (Hübner) [Lepidoptera: Crambidae]), collected in maize fields near Leipzig, Germany, in the summer of 2006. Parasitoids were since then bred on late instar larvae of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) at 25°C, 55% relative humidity (RH), and a photoperiod of 16:8 (L:D) h. All experiments were carried out under conditions similar to those of the rearing environment.
Diploid Male Production (Treatment of the Parental Generation P)
The aim of this procedure was to obtain a large number of haploid and diploid males, for which fitness parameters could be determined. The males obtained were also used in the mate choice experiments (see Diploid Male Mating Behavior).
In total, 28 virgin females produced haploid sons, to whom they were mated afterward. This ensured allelic matching between mating partners because haploid sons inherit one of their mother’s sex determining alleles. On average, 50% of all fertilized eggs produced from such a mother–son cross develop into diploid males instead of females, if developmental mortality of diploid males and females is the same ( Cook and Crozier 1995 ). Thus, an increased diploid male developmental mortality can be assumed if >50% of all diploid offspring are female. Although other mechanisms, e.g., selective fertilization of eggs or selective ovicide, could possibly also cause deviations from the 50% expectation ( van Wilgenburg et al. 2006 ), experimental evidence for these mechanisms to be effective is lacking in the Hymenoptera to date.
Females were kept at 10°C during the developmental time of their sons, to slow senescence. For those nine females that had refused several times to mate with a son, forced-contact mating ( Kitthawee 2008 , Thiel and Weeda 2013 ) was used, i.e., a female was immobilized by cooling and then presented to the male, which usually mated her immediately. During subsequent days, the mated females were provided with five hosts each to produce offspring. The eggs were counted within 24 h after oviposition. Furthermore, the number of pupae developing and the numbers of male and female offspring hatching were recorded.
Diploid Male Mating Behavior and Fertility Analysis (Treatment of the First Offspring Generation F1)
The aim of the described experiment was to analyze if haploid and diploid males differ in their courtship behavior, in their chance of mating a female, and in the probability of producing fertile offspring.
All male offspring (first offspring generation F1) produced by the mother–son crosses (see above) were individually provided with a virgin female to court and to mate with. The respective female had been randomly chosen from the offspring produced by one of the other experimental females. Because diploid and haploid males have similar phenotypes in B. brevicornis , male ploidy level was unknown at this point. Each male was placed in a closed Petri dish (ø = 3.5 cm) containing one virgin female (≥48 h postemergence). During each trial, we recorded courtship display (wing fanning), mating attempts (male on top of female), and copulation (genital regions of both partners for at least 5 s in contact, accompanied by characteristic antennal movement [ Weeda 2008 ]) using the observation software “The Observer” 2.0, Noldus, Wageningen. Observations lasted until copulation occurred but not longer than 10 min after male and female had made first contact. If no copulation was observed during this time, the mating trial was repeated within 48 h and up to two times, always using new females. Forced-contact mating ( Kitthawee 2008 , Thiel and Weeda 2013 ) was applied if a male had been rejected three times, for being able to assess the potential fitness of the rejected males. All females were subsequently provided with five hosts in total and the numbers of eggs laid, pupae produced, as well as number and sex ratio of adult offspring were recorded. After copulation, all males were frozen at −20°C for ploidy analysis (see below).
Triploid Female Mating Behavior and Fertility Analysis (Treatment of the Second Offspring Generation F2)
The aim of this experiment was to analyze the potential fitness of female offspring produced by diploid males. Because females can produce (male) offspring as virgins, we analyzed the reproductive potential of 85 females sired by 32 haploid males and 68 females sired by 15 diploid males before and after they had mated. Therefore, newly emerged virgin females (second offspring generation F2) were allowed to parasitize five hosts successively. Afterward, females were individually tested in a mating experiment similar to the one outlined for diploid males, using an unrelated male as mating partner. If no copulation occurred within 10 min, a new male was provided within 24 h. If, after encountering two males, still no copulation had occurred, the method of forced-contact mating was used ( Kitthawee 2008 , Thiel and Weeda 2013 ), for being able to assess the potential of producing biparental offspring for rejected or rejecting females. Afterward, females were allowed to parasitize another five hosts and were then frozen at −20°C. The ploidy of all females sired by diploid males was analyzed, whereas females sired by a normal haploid male were assumed to be diploid. The numbers of eggs laid, pupae developing, and male and female adults were recorded. All offspring of triploid females were frozen at −20°C for ploidy determination.
Ploidy Determination
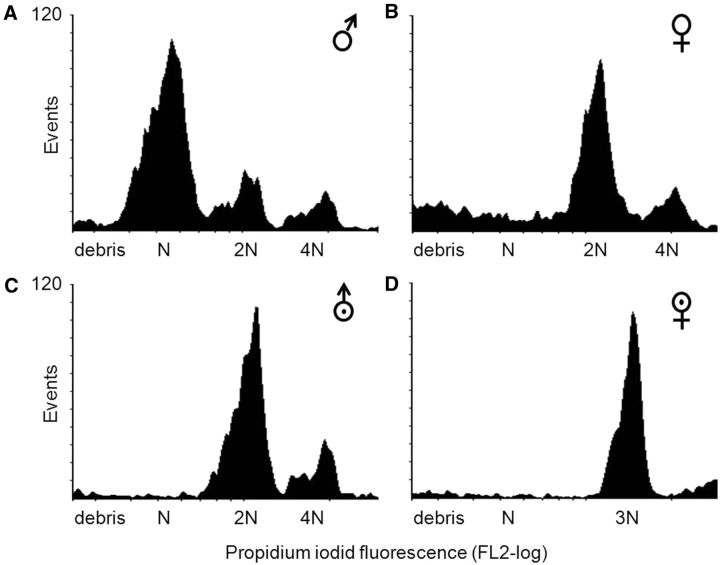
The ploidy level of all males produced from mother–son crosses, of all daughters sired by diploid males, and of all offspring of triploid females was determined using flow cytometry. For this, an individual’s head (without antenna) was pulverized in a Dounce tissue grinder containing 250 µl ice-cold Galbraith buffer (21 mM MgCl 2 ; 30 mM tri-sodium citrate dehydrate; 20 mM MOPS (3-[N-morpholino] propane sulfonic acid); 0.1% Triton X-100; 1 mg/l RNase A [ Galbraith et al. 1983 ]). The pestle was turned 20 times and the resulting cell suspension poured though a cell strainer cap on a 5-ml polystyrene tube (BD Falcon, www.bdbiosciences.com ). Nuclei were stained with 15 µl of propidium iodide (1.25 mg/ml) per sample. Finally, 250 µl of ultrapure water were added to guarantee sufficient volume. Propidium iodide fluorescence was detected with a flow cytometer (BD FACSCalibur, www.bdbiosciences.com ) using an excitation wave length of 488 nm and a band pass filter of 585 nm. Each sample was measured in an FSlog/FL2-log-gated region that contained haploid to tetraploid cells, using BD CellQuest Pro ( www.bdbiosciences.com ). A threshold on FL2-log was applied to exclude very small debris. We used flow cytometric DNA histograms of known haploid males (produced by virgin mothers) and diploid females as reference to determine the ploidy level of the unknown individuals ( Fig. 1 ).
Fig. 1.
Flow cytometric histograms of the DNA contents in cells of a representative haploid male (A), a diploid female (B), a diploid male (C), and a triploid female (D) of B. brevicornis .
Males for which we could not determine ploidy level, e.g., because either no definite haploid peak appeared or because the absence of a haploid peak could not be verified, were scored as “unknown.”
Statistical Analysis
For statistical analysis, generalized linear models (GLMs; Nelder and Wedderburn [1972] ) were fitted to the data. The use of GLMs was the most powerful approach for our data, which did often not follow a normal distribution. The tests were performed using the statistical program “R 2.15.1” ( R Development Core Team 2012 ), with package “car” ( Fox and Weisberg 2011 ). Count data were analyzed with Poisson distribution or quasipoisson, if dispersion parameters were >2. Proportions were analyzed using binomial or quasibinomial distribution, the latter was used for dispersion parameters >2. Tests of proportions were always done including the “cbind” command, to account for the different numbers of offspring produced by individual females. The proportions of offspring types produced in the F2 and F3 generations needed special treatment because the individuals all related back to those 20 females in the parental generation P that had been successfully crossed with their sons. We can view these 20 successful matings as different family lines. Because family lines did not contribute with equal numbers of individuals to the analyses, generalized linear mixed models (GLMM) were used from package “lme4” ( Bates et al. 2014 ), with “family” as random factor.
Results
Diploid Male Production (by Parental Generation P)
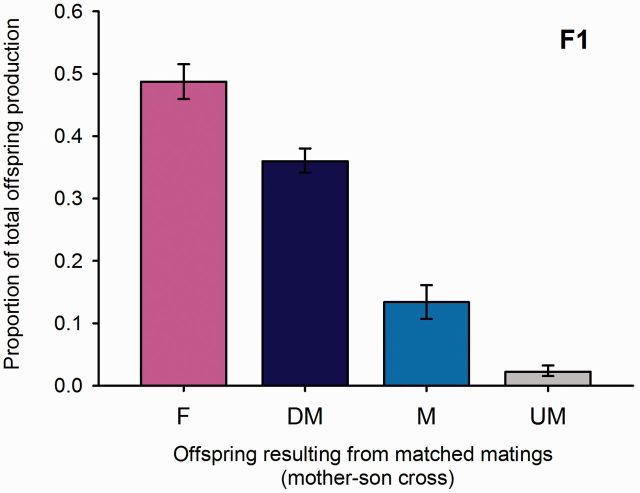
To ensure a high production of diploid males in our experimental wasps, mother–son crosses were used in P. Twenty mother–son crosses were successful, as they resulted in diploid, and thus biparental, offspring. In total, 335 adult offspring resulted from 747 eggs, indicating a high background mortality. The offspring consisted of 162 males and 174 females. Among males, there were 42 haploid and 113 diploid individuals, the ploidy level of 7 males could not be determined. The mean proportions of offspring types produced were determined using a GLM with binomial error distribution (ED) and are shown in Fig. 2 .
Fig. 2.
Proportions of offspring types produced in the F1 generation (mother–son crosses). F, females (presumably diploid); DM, diploid males; M, haploid males; UM, males for which the ploidy level could not be determined. Bars show the estimated values from a GLM-analysis (ED: binomial) with associated SE bars.
Whether a mating occurred freely or via forced contact did neither influence the total number of eggs laid by a female ( = 0.64, P = 0.42, ED = quasipoisson) nor the proportion of diploids that developed as males instead of females ( = 0.05, P = 0.82, ED = quasibinomial; see Table 1 for the primary data).
Table 1.
Primary data of the F 1 generation (mother–son crosses)
| Female | Mating | N eggs | N pupae | N HM | N DM | N UM | N F |
|---|---|---|---|---|---|---|---|
| 1 | Forced | 41 | 27 | 5 | 11 | 0 | 11 |
| 2 | Accepted | 26 | 18 | 4 | 4 | 1 | 6 |
| 3 | Forced | 33 | 18 | 1 | 11 | 0 | 4 |
| 5 | Accepted | 42 | 19 | 2 | 7 | 0 | 10 |
| 6 | Accepted | 27 | 19 | 1 | 7 | 0 | 11 |
| 7 | Forced | 30 | 22 | 0 | 10 | 2 | 10 |
| 8 | Forced | 43 | 21 | 0 | 0 | 0 | 21 |
| 9 | Accepted | 53 | 15 | 4 | 5 | 0 | 5 |
| 13 | Accepted | 25 | 9 | 0 | 2 | 0 | 1 |
| 14 | Accepted | 47 | 14 | 1 | 3 | 0 | 7 |
| 15 | Accepted | 30 | 13 | 0 | 1 | 0 | 12 |
| 16 | Forced | 49 | 27 | 6 | 11 | 0 | 8 |
| 17 | Accepted | 27 | 16 | 0 | 2 | 0 | 3 |
| 18 | Forced | 36 | 13 | 3 | 3 | 1 | 6 |
| 19 | Forced | 46 | 27 | 1 | 8 | 1 | 14 |
| 20 | Forced | 38 | 16 | 2 | 2 | 0 | 12 |
| 21 | Accepted | 43 | 30 | 1 | 14 | 1 | 11 |
| 24 | Forced | 38 | 20 | 6 | 4 | 0 | 10 |
| 25 | Accepted | 20 | 9 | 4 | 1 | 0 | 4 |
| 28 | Accepted | 53 | 23 | 1 | 7 | 1 | 8 |
Mating type and numbers of eggs, pupae, haploid males (HM), diploid males (DM), males for which the ploidy level could not be determined (UM), and females (F, presumably diploid).
Because a female has two different sex determining alleles, of which a son will inherit only one, half of her eggs fertilized by her son are expected to be homozygous at the sex determining locus, and thus develop into diploid males ( Cook and Crozier 1995 ). The other half should be heterozygous and develop into females. In our study, however, fewer diploid males occurred than diploid females ( Fig. 2 ). In total, there developed 61 diploid males less than females (174). If we assume differential diploid male mortality as a reason, this translates into a diploid-male specific mortality rate of ∼35%.
Diploid Male Mating Behavior and Fertility Analysis (F1 Generation)
Courtship and mating behavior of 42 haploid and 110 diploid males produced by 20 mother–son crosses (P generation) were analyzed. Of those, 19 haploid and 60 diploid males mated at the first encounter with a female (without any enforcement). The probability for being accepted by a female upon first encounter was not different between haploid and diploid males ( = 1.33, P = 0.25, ED = binomial).
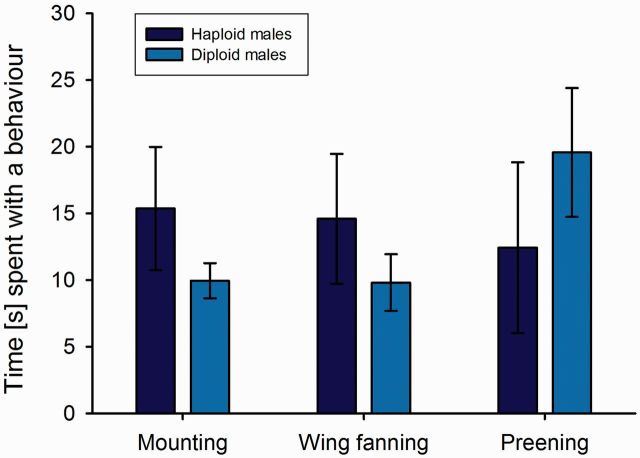
For those males that were accepted upon first encounter, the latency until a mating occurred was not different for haploid and diploid males ( = 2.33, P = 0.13, ED = gamma with log link function; Fig. 3 A). A behavioral comparison ( Fig. 4 ) showed that haploid and diploid males did not differ significantly in their behavior during the time until a copulation occurred: they spent similar times with cleaning behavior ( = 0.58, P = 0.45, ED = Gaussian), courtship (wing fanning; = 1.05, P = 0.30, ED = Gaussian), and mounting attempts ( = 2.42, P = 0.12, ED = Gaussian). Copulations also took about the same time, irrespective of male ploidy ( = 0.001, P = 0.98, ED = Gaussian; Fig. 3 B).
Fig. 3.
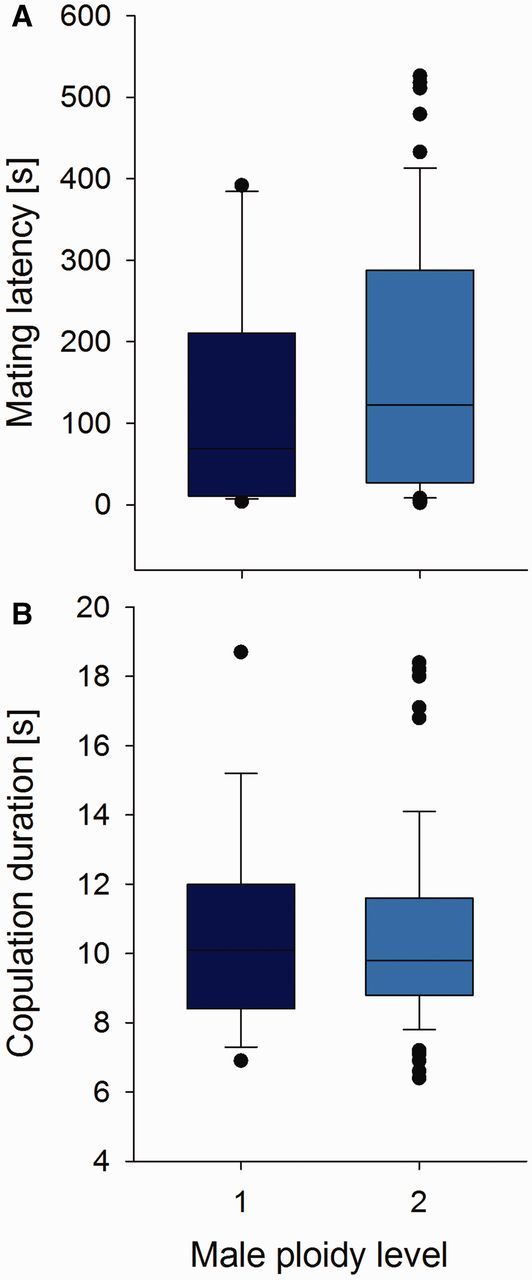
Mating behavior of haploid and diploid males. Box plots showing mating latency (A) and copulation duration (B) for those 19 fully functional haploid (dark blue bars) and 60 (almost) sterile diploid males (light blue bars) that copulated in our experiment.
Fig. 4.
Analysis F 1 male courtship behavior. Bars showing the average (±SE) amount of time spent with wing fanning, mounting attempts, or cleaning behavior in the mate choice experiments for 19 haploid (dark blue bars) and 60 diploid (light blue bars) males.
Females in the F 1 laid on average 37.0 (standard error [SE] 0.9) eggs; this number did not depend upon the ploidy of the male they had mated with (father DM: 38.0 [SE 1.0], N = 107; father DM: 37.9 [SE 1.7], N = 40; = 0.003, P = 0.95, ED = quasipoisson). The average number of daughters sired by each haploid male (8.8) was about 10 times higher than that of diploid males (0.7) ( = 61.6, P < 0.01, ED = quasipoisson, three sets from diploid males excluded, in which all offspring had died). Diploid males had a lower probability for mating successfully (15% producing daughters) than had haploid males (79% producing daughters) ( = 51.1, P < 0.01, ED = binomial), data set including “successful males” (females had produced at least one daughter) and “unsuccessful males” (no daughter but at least six sons had been produced); nine daughterless females with fewer than six sons were not used in this subset, since they might have produced daughters if they had had more offspring in total).
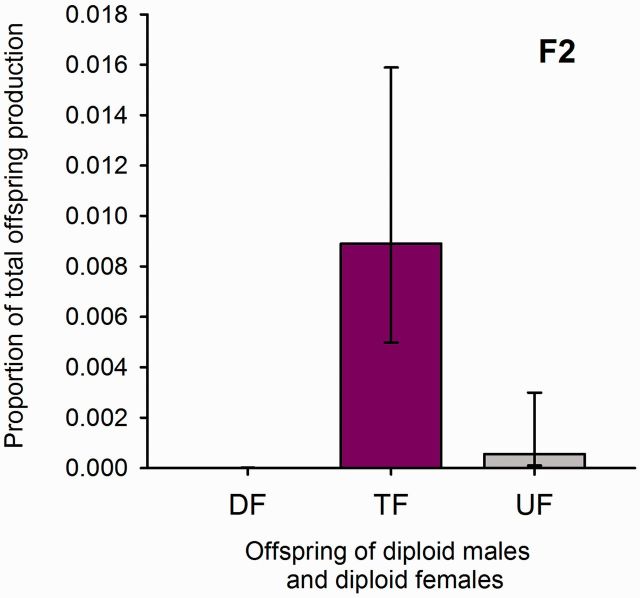
Those 15 diploid males that had sired daughters often sired more than one (5.3 on average, SE 1.4). The daughters produced were mainly triploid (68), but two diploid females also occurred. The ploidy level of six females could not be determined unambiguously. The proportions of offspring types sired on average were determined using GLMM (see Materials and Methods) and are shown for diploid males in Fig. 5 and for haploid males in Fig. 6 .
Fig. 5.
Proportions of offspring types (daughters) sired on average by a diploid male in the F2 generation (including males that did not sire any offspring). DF, diploid females; TF, triploid females; UF, females for which the ploidy level could not be determined. Bars show the estimated values from a GLMM analysis (see Materials and Methods) with associated. The GLMM took male family into account.
Fig. 6.
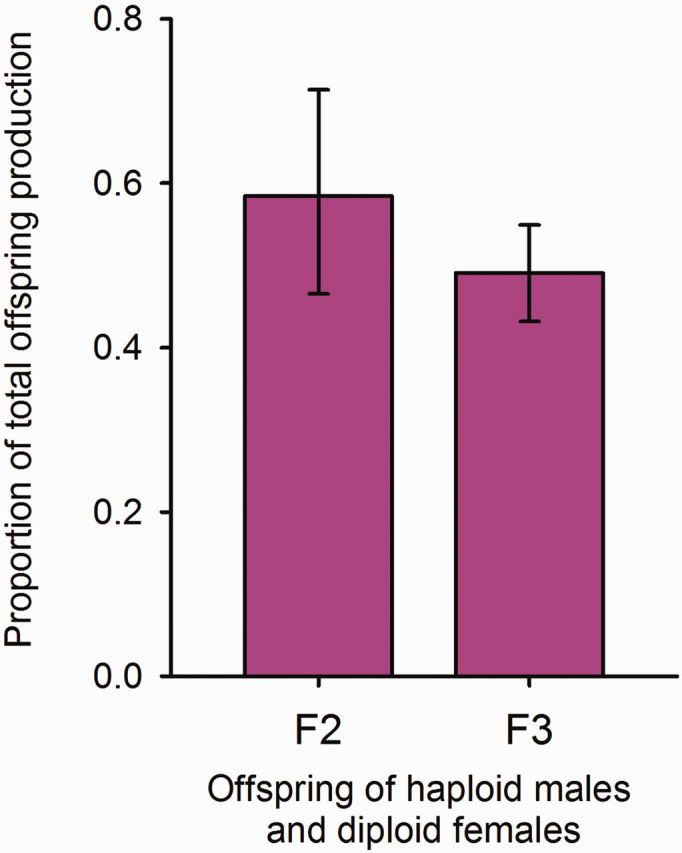
Proportions of daughters sired on average by a presumably haploid male that mated presumably diploid females in the F2 and the F3 generation. Bars show the estimated values from a GLMM-analysis (see Materials and Methods), with associated SE. The GLMM took male family (F2) and female family (F3) into account.
Most females in our study that mated a diploid male produced no daughters (85%). Sterility of diploid males can be caused by a reduction of reproductive organs ( Duchateau and Marien 1995 , Tavares et al. 2003 ), failure of sperm production or transfer ( Holloway et al. 1999 ), and failure of sperm to fertilize eggs ( MacBride 1946 , Holloway et al. 1999 ). Diploid males in B. brevicornis are sometimes able to produce diploid sperm, because most daughters were triploid, but haploid sperm must have occurred in at least one male, which sired two diploid daughters. This male also had several triploid daughters.
Triploid Female Mating Success and Fertility Analysis (F2 Generation)
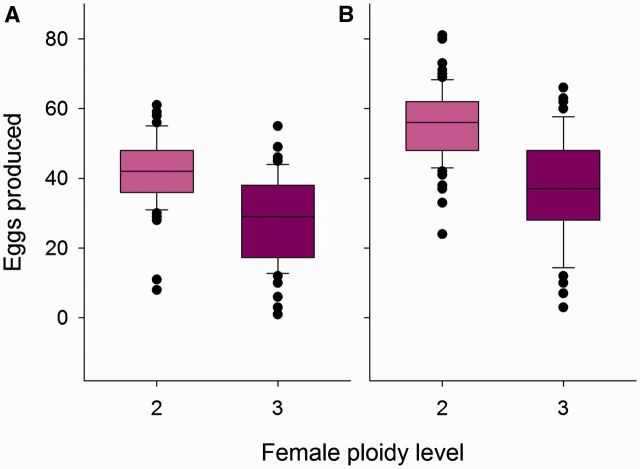
Mating success and fertility of 76 diploid females (with haploid fathers) and 43 triploid females (with diploid fathers) were analyzed when placed with an unrelated, haploid male. Of these, 48 diploid and 31 triploid females were observed to mate at first encounter. The probability for mating at first encounter was not different between diploid and triploid females ( = 1.00, P = 0.32, ED = binomial). For those that did mate at first encounter, the latency until a mating occurred was the same for diploid and triploid females ( = 1.59, P = 0.20, ED = gamma; Fig. 7 A). Diploid and triploid females received similar numbers of wing fanning bouts from males ( = 2.32, P = 0.12, ED = quasipoisson; Fig. 7 B). Copulation took about the same time, irrespective of female ploidy ( = 0.001, P = 0.97, ED = Gaussian; Fig. 7 C).
Fig. 7.
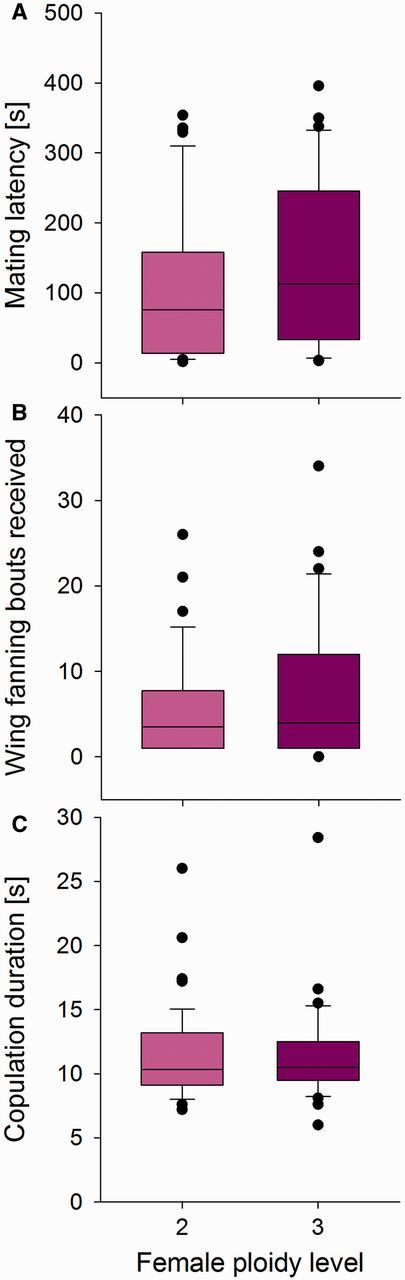
Mating behavior of diploid and triploid females. Box plots showing mating latency (A), number of wing fanning bouts received (B), and copulation duration (C) for diploid (light, pink bars) and triploid (dark, violet bars) females.
When given hosts as virgins, 3 out of 82 diploid females did not lay eggs at all; the remaining 79 produced on average 41.4, SE 1.1 eggs. In triploid females, 12 out of 68 did not lay a single egg, while the remaining 56 produced 28.1, SE 1.7 on average, the difference is highly significant ( = 44.8, P < 0. 01, ED = quasipoisson; Fig. 8 ). About 73.1% of all diploid females’ eggs developed into pupae, while there was almost no survival of eggs of triploid females (only 0.2% survived). In the end, 79 diploid females had on average 19 sons each. The number of offspring produced by 68 virgin triploid female was remarkably smaller: a single haploid son.
Fig. 8.
Analysis of triploid female fitness. Box plots showing the number of eggs produced on five host larvae for virgin (A) and mated (B) diploid (light, pink bars) and triploid (dark, violet bars) females.
After mating, 1 out of 77 diploid females did not lay eggs at all; the remaining 76 produced on average 55.4, SE 1.2 eggs. In triploid females, 2 out of 49 mated females did not lay a single egg, while the remaining 47 produced 37.0, SE 2.2 on average. The difference is highly significant ( = 50.9, P < 0. 01, ED = quasipoisson; Fig. 8 ). About 55.1% of all diploid females’ eggs developed into pupae, while there was almost no survival of eggs of triploid females (only 0.7% survived).
On average, 14 adult offspring were produced by each diploid female ( N = 76), at an almost 1:1 sex ratio ( Fig. 6 ). The 47 triploid females had again a much lower number of offspring: in total, they produced one diploid son, three diploid daughters, and one triploid daughter. Triploid females probably produced haploid eggs at a very low rate, as one haploid male developed. They likely produce diploid eggs also, as they had triploid female offspring sired by a haploid male. Thus, diploid offspring (male or female) produced by triploid females could be of either uniparental or biparental origin.
Discussion
Diploid males seem to be an unavoidable by-product of the CSD mode ( Cook and Crozier 1995 , van Wilgenburg et al. 2006 , Heimpel and de Boer 2008 ). Beside the cost sterile diploid males impose on their parents, they can also be detrimental for the fitness of their mating partners ( Harpur et al 2012 ). Many parasitic wasps mate only once ( Godfray 1994 ) and accepting a sterile diploid male as partner results in a loss of the ability to produce female offspring ( Heimpel and de Boer 2008 ). Our experiments show that diploid males occur in the parasitic wasp B. brevicornis under inbreeding conditions. However, fewer diploid individuals had been male than expected, which might have been caused by different mortality rates in diploid male and female offspring. From the numbers of developing females and diploid males ( Fig. 2 ), we calculated a 35% mortality rate for diploid male offspring. In the closely related Bracon ( Habrobracon ) hebetor , diploid male mortality rates of 90% or more have been recorded, varying with population or study ( Whiting 1943 , Petters and Mettus 1979 , Ode et al. 1997 ). Nevertheless, diploid males have been detected in naturally occuring B. hebetor populations ( Heimpel et al. 1999 , Antolin et al. 2003 ). Diploid males have also been detected in a number of other Hymenopteran species in the field ( Adams et al. 1977 , Ross et al. 1993 , Owen and Packer 1994 , Takahashi et al. 2001 , Fujiwara et al. 2004 , Francini et al. 2012 ). This makes the presence of diploid males likely in natural populations of B. brevicornis , too, even though direct evidence from field data is missing, yet. Diploid male offspring, triploid females, are also found rather frequently in some species ( Krieger et al. 1999 , Liebert et al. 2004 ). However, despite the likelihood of diploid male encounters under natural conditions and despite the high fitness costs mating with them pose to females, we have not been able to detect discrimination against them. Of course, a lack of discrimination ability would not be surprising in species with fertile diploid males ( Elias et al. 2009 ). However, there is also no evidence of discrimination against sterile diploid males in the few other species that have been surveyed so far. In a study by de Boer et al. (2007) , e.g., Cotesia vestalis females easily accepted diploid males. However, not even half of the males tested had actually approached the female, which may be explained by the fact that all experiments were done with full siblings and inbreeding avoidance might have occurred. An overall low mating tendency may well have masked differences in haploid or diploid male behavior or in their acceptance by C. vestalis females ( de Boer et al. 2007 ). However, the absence of discrimination against diploid males in B. brevicornis must be due to another factor than inbreeding avoidance, because unrelated partners were used and an overall high mating tendency was observed, despite the severe fitness costs.
Diploid male recognition has been described to occur in honey bee ( Apis mellifera ). Workers are able to recognize diploid male larvae, most likely by their emitted odor ( Santomauro et al. 2004 ), and eliminate them from the brood to save the rearing costs ( Woyke 1963 ). For performing this task, bees only need to recognize males (drones) developing in female (worker) brood cells and they do presumably not detect ploidy levels directly. Their ability is therefore not comparable to the situation of mate choice. Although odor components may be sex specific, indicate relatedness between individuals (kin recognition, e.g., Simmons 1990 , Herzner et al. 2006 , Metzger et al. 2010 ), or the presence of (in)compatible alleles ( Penn and Potts 1999 , Reusch et al. 2001 ), they can probably not reliably reveal the ploidy level of an individual.
In summary, the fitness gained on average by mating a diploid male is comparable to not mating at all in B. brevicornis . Even though the proportion of constrained females is unlikely to be high in most populations most of the time, there might be sufficient fitness benefit for successfully mated females to select for the ability to recognize a diploid male and avoid mating with him ( Godfray 1990 ), because only this would allow for mating a more suitable partner later and producing fertile female offspring. The same can be said for a male courting a triploid female, only that males can remate in this species, which lessens the selection pressure to avoid triploid females. However, there is no evidence that diploid males or triploid females were recognized or avoided in our experiments. One might argue that the females (or males) in our experiment did not have a choice, because only one male (or female) was presented at a time. On the other hand, B. brevicornis females do reject haploid males with a matching sex allele in no-choice tests of the same design ( Thiel and Weeda 2013 ). The discrimination ability of an individual might even be better in no-choice compared with choice tests because the odors produced by two males can mix and confuse the female ( Metzger et al. 2010 ). Thus, we conclude that B. brevicornis individuals would most likely have rejected their diploid male or triploid female mating partner if they had been able to recognize their partner’s ploidy level. Our results strongly imply that it cannot be counted on females’ ability to avoid mating with diploid males when it comes to estimating the costs of diploid male occurrence in insect populations. Therefore, high diploid male survival rates are indeed likely to accelerate the diploid male extinction vortex.
Acknowledgments
This study was supported by the German Federal Ministry of Economy and Technology (BMWi) with grant KF 2152901MD8 to T.S. Hoffmeister. We thank H. Schnee (Saxon regional office for agriculture) for collecting the parasitoids, B. Wührer and O. Zimmermann (AMW Nützlinge GmbH, Pfungstadt) for support, D. Kosior, N.B. Linek, and E. Lorenz (University of Bremen) for insect rearing, A.L. Roberts (University of Bremen) for assistance in the mate choice experiments, B. Fuchs (Max-Planck-Institute for Marine Microbiology, Bremen) for help with the flow cytometry, and J.G. de Boer (Wageningen University, NL) for discussion and valuable comments.
References Cited
- Adams J., Rothman E. D., Kerr W. E., Paulino Z. L. . 1977. . Estimation of the number of sex alleles and queen matings from diploid male frequencies in a population of Apis mellifera . Genetics 86 : 583 – 596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolin M. F., Ode P. J., Heimpel G. E., O'Hara R. B., Strand M. R. . 2003. . Population structure, mating system, and sex-determining allele diversity of the parasitoid wasp Habrobracon hebetor . Heredity 91 : 373 – 381 . [DOI] [PubMed] [Google Scholar]
- Asplen M. K., Whitfield J. B., de Boer J. G., Heimpel G. E. . 2009. . Ancestral state reconstruction analysis of hymenopteran sex determination mechanisms . J. Evol. Biol. 22 : 1762 – 1769 . [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. . 2014. . Fitting linear mixed-effects models using lme4. Journal of Statistical Software, arXiv:1406.5823v1 . [Google Scholar]
- Beye M., Hasselmann M., Fondrk M. K., Page R. E., Omholt S. W. . 2003. . The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein . Cell 114 : 419 – 429 . [DOI] [PubMed] [Google Scholar]
- Cook J. M., Crozier R. H. . 1995. . Sex determination and population biology in the Hymenoptera . Trends Ecol. Evol. 10 : 281 – 286 . [DOI] [PubMed] [Google Scholar]
- Cowan D. P., Stahlhut J. K. . 2004. . Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination . Proc. Natl. Acad. Sci. USA. 101 : 10374 – 10379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. G., Ode P. J., Vet L.E.M., Whitfield J. B., Heimpel G. E. . 2007. . Diploid males sire triploid daughters and sons in the parasitoid wasp Cotesia vestalis . Heredity 99 : 288 – 294 . [DOI] [PubMed] [Google Scholar]
- de Boer J. G., Ode P. J., Rendahl A. K., Vet L.E.M., Whitfield J. B., Heimpel G. E. . 2008. . Experimental support for multiple-locus complementary sex determination in the parasitoid Cotesia vestalis . Genetics 180 : 1525 – 1535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau M. J., Marien J. . 1995. . Sexual biology of haploid and diploid males in the bumblebee Bombus terrestris . Insectes Sociaux 42 : 255 – 266 . [Google Scholar]
- Elias J., Mazzi D., Dorn S. . 2009. . No need to discriminate? reproductive diploid males in a parasitoid with complementary sex determination . PLoS One 4 : e6024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga J. A., Zwakhals K., Harvey J. A., Biere A. . 2007. . The parasitoid complex associated with the herbivore Hadena bicruris (Lepidoptera: Noctuidae) on Silene latifolia (Caryophyllaceae) in the Netherlands . J. Nat. Hist. 41 : 101 – 123 . [Google Scholar]
- Fox J., Weisberg S. . 2011. . An (R) companion to applied regression . Sage; , Thousand Oaks, CA: . [Google Scholar]
- Francini I. B., Nunes-Silva C. G., Carvalho-Zilse G. A. . 2012. . Diploid male production of two Amazonian Melipona bees (Hymenoptera: Apidae) . Psyche . doi:10.1155/2012/484618 . [Google Scholar]
- Fujiwara Y., Akita K., Okumura W., Kodaka T., Tomioka K., Naito T. . 2004. . Estimation of allele numbers at the sex-determining locus in a field population of the turnip sawfly ( Athalia rosae ) . J. Hered. 95 : 81 – 84 . [DOI] [PubMed] [Google Scholar]
- Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. . 1983. . Rapid flow cytometric analysis of the cell-cycle in intact plant tissues . Science 220 : 1049 – 1051 . [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J. 1990. . The causes and consequences of constrained sex allocation in haplodiploid animals . J. Evol. Biol. 3 : 3 – 17 . [Google Scholar]
- Godfray H.C.J. 1994. . Parasitoids: behavioral and evolutionary ecology . Princeton University Press; , Princeton, NY: . [Google Scholar]
- Harpur B. A., Sobhani M., Zayed A. . 2012. . A review of the consequences of complementary sex determination and diploid male production on mating failures in the Hymenoptera . Entomologia Experimentali et Applicata 146 : 156 – 164 . [Google Scholar]
- Heimpel G. E., de Boer J. G. . 2008. . Sex determination in the Hymenoptera . Annu. Rev. Entomol. 53 : 209 – 230 . [DOI] [PubMed] [Google Scholar]
- Heimpel G. E., Antolin M. F., Strand M. R. . 1999. . Diversity of sex-determining alleles in Bracon hebetor . Heredity 82 : 282 – 291 . [DOI] [PubMed] [Google Scholar]
- Hein S., Poethke H. J., Dorn S. . 2009. . What stops the ‘diploid male vortex’? a simulation study for species with single locus complementary sex determination . Ecol. Model. 220 : 1663 – 1669 . [Google Scholar]
- Herzner G., Schmitt T., Heckel F., Schreier P., Strohm E. . 2006. . Brothers smell similar: variation in the sex pheromone of male European Beewolves Philanthus triangulum F. (Hymenoptera: Crabronidae) and its implications for inbreeding avoidance . Biol. J. Linnean Soc. 89 : 433 – 442 . [Google Scholar]
- Holloway A. K., Heimpel G. E., Strand M. R., Antolin M. F. . 1999. . Survival of diploid males in Bracon sp. near hebetor (Hymenoptera: Braconidae) . Ann. Entomol. Soc. Am. 92 : 110 – 116 . [Google Scholar]
- Kares E. A., Ebaid G. H., El-Sappagh I. A. . 2009. . Biological studies on the larval parasitoid species Bracon brevicornis Wesm. (Hymenoptera: Braconidae), reared on different insect hosts . Egypt. J. Biol. Pest Control 19 : 165 – 168 . [Google Scholar]
- Kitthawee S. 2008. . Forced-contact mating: a technique for crossing experiments with the fruit fly parasitoid, Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) . Biol. Control 44 : 73 – 78 . [Google Scholar]
- Krieger M.J.B., Ross K. G., Chang C.W.Y., Keller L. . 1999. . Frequency and origin of triploidy in the fire ant Solenopsis invicta . Heredity 82 : 142 – 150 . [Google Scholar]
- Liebert A. E., Johnson R. N., Switz G. T., Starks P. T. . 2004. . Triploid females and diploid males: underreported phenomena in Polistes wasps? Insectes Sociaux 51 : 205 – 211 . [Google Scholar]
- MacBride D. H. 1946. . Failure of sperm of Habrobracon diploid males to penetrate eggs . Genetics 31 : 224 . [PubMed] [Google Scholar]
- Metzger M., Bernstein C., Hoffmeister T. S., Desouhant E. . 2010. . Does kin recognition and sib-mating avoidance limit the risk of genetic incompatibility in a parasitic wasp? Plos One 5 : e13505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder J. A., Wedderburn R.W.M. . 1972. . Generalized linear models . J. R. Stat. Soc. Ser. A 135 : 370 – 384 . [Google Scholar]
- Ode P. J., Antolin M. F., Strand M. R. . 1995. . Brood-mate avoidance in the parasitic wasp Bracon hebetor Say . Anim. Behav. 49 : 1239 – 1248 . [Google Scholar]
- Ode P. J., Antolin M. F., Strand M. R. . 1997. . Constrained oviposition and female-biased sex allocation in a parasitic wasp . Oecologia 109 : 547 – 555 . [DOI] [PubMed] [Google Scholar]
- Owen R. E., Packer L. . 1994. . Estimation of the proportion of diploid males in populations of Hymenoptera . Heredity 72 : 219 – 227 . [Google Scholar]
- Penn D. J., Potts W. K. . 1999. . The evolution of mating preferences and major histocompatibility complex genes . Am. Nat. 153 : 145 – 164 . [DOI] [PubMed] [Google Scholar]
- Petters R. M., Mettus R. V. . 1979. . Diploid male viability in Habrobracon juglandis . Genetics 91 : S95 – S95 . [Google Scholar]
- R Development Core Team . 2012. . R: a language and environment for statistical computing, version 2.15.1. R Foundation for Statistical Computing. http://www.r-project.org . [Google Scholar]
- Reusch T.B.H., Haberli M. A., Aeschlimann P. B., Milinski M. . 2001. . Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism . Nature 414 : 300 – 302 . [DOI] [PubMed] [Google Scholar]
- Ross K. G., Vargo E. L., Keller L., Trager J. C. . 1993. . Effect of a founder event on variation in the genetic sex-determining system of the fire ant Solenopsis invicta . Genetics 135 : 843 – 854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomauro G., Oldham N. J., Boland W., Engels W. . 2004. . Cannibalism of diploid drone larvae in the honey bee ( Apis mellifera ) is released by odd pattern of cuticular substances . J. Apic. Res. 43 : 69 – 74 . [Google Scholar]
- Schmieder S., Colinet D., Poirie M. . 2012. . Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants . Nat. Commun. 3 : 895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W. 1990. . Pheromonal cues for the recognition of kin by female field crickets, Gryllus bimaculatus . Anim. Behav. 40 : 192 – 195 . [Google Scholar]
- Speicher B. R., Speicher K. G. . 1940. . The occurence of diploid males in Habrobracon brevicornis . Am. Nat. 74 : 379 – 382 . [Google Scholar]
- Stouthamer R., Luck R. F., Werren J. H. . 1992. . Genetics of sex determination and the improvement of biological control using parasitoids . Environ. Entomol. 21 : 427 – 435 . [Google Scholar]
- Takahashi N. C., Peruquetti R. C., Del Lama M. A., Campos L.A.D. . 2001. . A reanalysis of diploid male frequencies in euglossine bees (Hymenoptera: Apidae) . Evolution 55 : 1897 – 1899 . [DOI] [PubMed] [Google Scholar]
- Tavares M. G., Irsigler A.S.T., Campos L.A.D. . 2003. . Testis length distinguishes haploid from diploid drones in Melipona quadrifasciata (Hymenoptera: Meliponinae) . Apidologie 34 : 449 – 455 . [Google Scholar]
- Temerak S. A. 1983. . Longevity of Bracon brevicornis [Hym, Braconidae] adults as influenced by nourishment on artificial and natural foods . Entomophaga 28 : 145 – 150 . [Google Scholar]
- Thiel A., Weeda A. C. . 2013. . Genetic incompatibility drives mate choice in a parasitic wasp . Front. Zool. 10 : 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg E., Driessen G., Beukeboom L. W. . 2006. . Single locus complementary sex determination in Hymenoptera: an “unintelligent” design? Front . Zool. 3 : 1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan T., Jalali S. K., Srinivasamurthy K. . 2009. . Competitive interactions between Goniozus nephantidis and Bracon brevicornis , parasitoids of the coconut pest Opisina arenosella . Int. J. Pest Manag. 55 : 257 – 263 . [Google Scholar]
- Weeda A. C. 2008. . Paarungsverhalten und Auswirkungen von Inzucht bei der Brackwespe Habrobracon brevicornis . Diploma Thesis, Institute of Ecology, University of Bremen, Germany . [Google Scholar]
- West S. A. 2009. . Sex allocation . Princeton University Press; , Princeton, NJ: . [Google Scholar]
- Whiting P. W. 1943. . Multiple alleles in complementary sex determination in Habrobracon . Genetics 28 : 365 – 382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyke J. 1963. . What happens to diploid drone larvaein a honeybee colony . J. Apic. Res. 2 : 73 – 75 . [Google Scholar]
- Zayed A. 2009. . Bee genetics and conservation . Apidologie 40 : 237 – 262 . [Google Scholar]
- Zayed A., Packer L. . 2005. . Complementary sex determination substantially increases extinction proneness of haplodiploid populations . Proc. Natl. Acad. Sci. USA. 102 : 10742 – 10746 . [DOI] [PMC free article] [PubMed] [Google Scholar]