Abstract
Background
Prevalence of heart failure (HF) increases significantly with age, coinciding with age-related changes in body composition that are common and consequential. Still, body composition is rarely factored in routine HF care.
Methods and Results
Health, Aging, and Body Composition (Health ABC) is a prospective cohort study of nondisabled adults. Using yearly dual energy X-ray absorptiometry (DXA), body composition was assessed in Health ABC over 6 years, comparing those who developed incident HF vs. those who did not. Among 2,815 Health ABC participants (48.5% men; 59.6% whites; mean age 73.6±2.9 years), 111 developed incident HF over the 6 year study period. At entry into Health ABC men and women who later developed HF had higher body mass compared to those vs. those who did not develop HF (Men: 80.9±10 kg vs. 78.6±12.9, p=0.05, women:72.7±15.0 vs. 68.2±14.2, p=0.01 respectively). However, after developing HF, loss of total lean body mass was disproportionate; men with HF lost 654.6 g/year vs. 391.4 in non-HF participants, p=0.02. Loss of appendicular lean was also greater with HF (−419.9 g/year vs. −318.2, p=0.02), even after accounting for total weight change. Among women with HF, loss of total and appendicular lean mass were also greater than in non-HF participants, but not to the extent seen among men.
Conclusions
Incident HF in older adults was associated with disproportionate loss of lean mass, particularly among men. Prognostic implications are significant, with key sex-specific inferences regarding physical function, frailty, disability, and pharmacodynamics that all merit further investigation.
Keywords: Aging, Sex-Specific, Body Composition, Sarcopenia, Frailty, Atrophy
The nomenclature of “heart failure” (HF) implies a cardio-centric disease which has reinforced the prioritization of HF research and therapies premised on ventricular morphologic and functional, arrhythmic, hemodynamic and other central cardiac pathophysiological mechanisms, with relatively less emphasis accorded to peripheral consequences of the disease.1 Nonetheless, several peripheral manifestations of HF have been identified, including diminished skeletal muscle microvascular architecture and perfusion2,3 as well as altered skeletal muscle histology and bioenergetics with intrinsic weakening.2,4 Prevalence of HF increases with age,5 and implications of peripheral manifestations of HF may be particularly important among older adults as aging is associated with loss of lean body mass6 and increased fat mass7 which in turn impact functional capacity, frailty risk, and general well-being.8,9,10
Focus on body composition in HF patients is further intensified by a separate consideration of the so-called “obesity paradox”. Multiple studies highlight risks associated with obesity both in causing and aggravating HF,11,12 while other studies suggest that once HF occurs, obesity may provide protective life-sustaining benefits.13,14
This study uses data from the Health, Aging, and Body Composition (Health ABC) study to analyze body composition trajectories in older adults who developed incident HF. Using yearly dual energy X-ray absorptiometry (DXA) scans, body composition was assessed serially over 6 years with comparisons of those who developed incident HF to those who did not. We hypothesized that HF would exacerbate the typical age-associated changes in body composition remodeling, i.e., that lean body mass would decrease at a greater rate in older adults with HF and fat mass would increase at a greater rate. We also delineated proportions of lean and fat mass in older adults with high BMI who developed HF to clarify relationships that might shed light on the obesity paradox.
Subjects and Methods
Health, Aging, and Body Composition (Health ABC) is a prospective cohort study of nondisabled adults with comprehensive assessments that included yearly DXAs for the first 6 years. Using DXA metrics, body composition was compared in participants who developed HF vs. those who did not. Changes in total body mass, lean mass, and fat mass were each evaluated. The institutional review boards of the University of Pittsburgh, the University of Tennessee, the University of California - San Francisco Coordinating Center and the National Institute on Aging approved the study, and all participants gave informed consent.
Health ABC
Health ABC enrolled 3,075 black (41.7%) and white men and women (51.5%) aged 70–79 years between March 1997 and April 1998, who resided in the Memphis, TN, and Pittsburgh, PA areas. Eligibility criteria included no self-reported difficulty walking a quarter mile, climbing 10 steps, or performing activities of daily living, no reported use of a walking aid and no active cancer treatment. Exclusion criteria included cognitive impairment and inability to communicate.
Dual energy X-ray absorptiometry scans were performed annually, in all participants, for the first 6 years of the study; thus we limited the surveillance period to this time frame (1997–98 to 2002–03). Of the 3075 Health ABC study participants, 2815 were eligible for this study; HF cases (n=111) and controls (n=2704). Figure 1 shows the flow of Health ABC participants to the analytic sample. Reasons for exclusion from the final analytic sample were: baseline prevalent HF (n=40), no follow-up DXA scans after incident HF (n=38) or after baseline study in the non-cases (n=182).
Figure 1. Distillation of the substudy population from the Health Aging and Body Composition total enrollment.
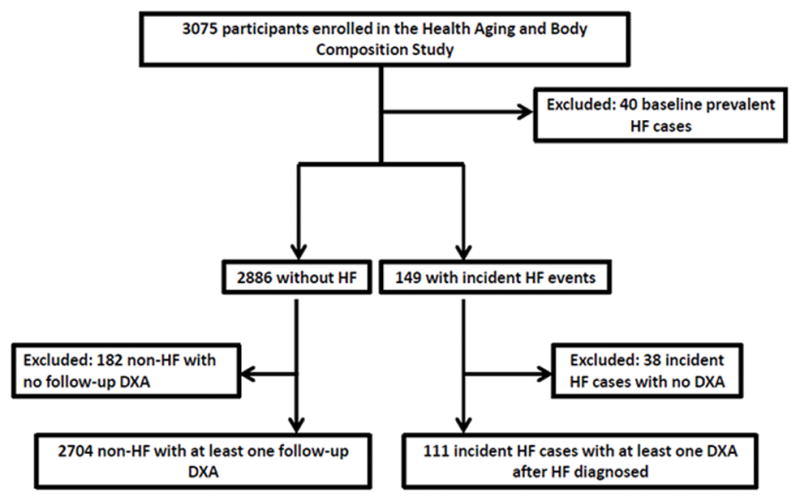
Health ABC is a prospective cohort study of 3,075 nondisabled black (41.7%) and white men and women (51.5%) aged 70–79 years, enrolled from March 1997 to April 1998. Of the 3,075 enrolled, 2815 participants had both a baseline DXA scan and either a follow-up DXA scan (controls, n=2704) or a DXA scan after incident HF (cases. n=111). DXA–Dual X-Ray Absorptiometry. HF–Heart Failure
Heart Failure
The primary criterion for incident HF was a hospitalization for a HF event, which was adjudicated at each field center using medical records. Criteria used to diagnose HF included: (1) symptoms (e.g., shortness of breath, fatigue, orthopnea, or paroxysmal nocturnal dyspnea); (2) physical signs (e.g., edema, rales, tachycardia, a gallop rhythm, or a displaced PMI); (3) medical therapy (a diuretic and digitalis or a beta blocker or vasodilator). Although not required, additional supporting evidence included a chest x-ray showing cardiomegaly and pulmonary edema or cardiac imaging (echocardiography or contrast ventriculography) showing evidence of a dilated ventricle and global or segmental wall motion abnormalities with decreased systolic function.
Baseline prevalent HF was defined as a self-reported history of HF confirmed with the usage of diuretic and either vasodilator or cardiac glycoside medications. These Health ABC participants were excluded from this analysis. Among those with incident HF, heart failure with reduced ejection fraction (HFrEF) was distinguished from heart failure with preserved ejection fraction (HFpEF) on the basis of left ventricular ejection fraction. This information was obtained from medical records and not directly from assessments performed in the Health ABC study. Those with left ventricular ejection fractions 45% and below were considered to have HFrEF. Left ventricular ejection fraction was obtained through review of imaging studies performed during hospitalization, principally echocardiography, but invasive contrast ventriculography or radionuclide ventriculography was used if echocardiography was not available. If there was a range of ejection fraction assessments, the lowest value was selected.
Dual energy X-ray absorptiometry
Lean mass of the upper and lower extremities as well as the total body were assessed using DXA (Hologic QDR 4500, software version 8.21; Waltham, MA). Bone mineral content was subtracted from the total and regional lean mass to define total non-bone lean mass, which represents primarily skeletal muscle in the extremities.15 Fat mass was estimated for the whole body as well. Both the percent fat and total fat were examined in these analyses. Total body non-bone mass was also calculated. DXA scans were conducted yearly from baseline to the year 6 visit (i.e., 5-year follow-up)..
Body weight and height were measured by calibrated balance beam scale and stadiometer with all participants restricted to light clothing and no shoes. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height in meters. Three weight change groups were also determined: stable (<3% change), gainers (≥3% increase), and losers (≥3% decrease).6
Potential Confounders
Potential confounders known to be associated with weight loss, muscle loss or HF included baseline age, self-reported race, sex, baseline prevalent chronic conditions, and baseline physical activity (kilocalories per week, as assessed by walking/exercise/recreational activities/chores determined by questionnaire16). Chronic obstructive pulmonary disease (COPD) was defined as self-reported physician diagnosis of chronic bronchitis, emphysema or asthma. Hypertension was defined as self-report of physician diagnosis, confirmed by use of an antihypertensive medication and presenting with a systolic blood pressure of ≥140mmHg at the baseline clinic visit. Diabetes was defined as self-report of physician diagnosis confirmed by use of diabetic medication, or fasting blood glucose ≥126mg/dL or plasma glucose levels ≥200mg/dl after an oral glucose tolerance test. Stroke and coronary heart disease (including myocardial infarction, percutaneous or surgical revascularization, and/or angina) were adjudicated using medical records. Chronic kidney disease (CKD) was defined as having an estimated Glomerular Filtration Rate (eGFR) <60 ml/min/1.73 m2, which was estimated using serum creatinine and the 4-variable Modification of Diet in Renal Disease Equation.17 Depression was defined as a score ≥10 on the modified Center for Epidemiologic Studies-Depression scale.18 Cognition was assessed using the Modified Mini-Mental State Examination (3MSE) score and mild cognitive impairment was defined as a score <80.19 Multimorbidy was defined as the number of prevalent chronic conditions (COPD, hypertension, diabetes, stroke, CHD, CKD, depression, cognitive impairment).
Statistical Analyses
Baseline means and standard deviations or frequencies and percentages were calculated stratified by sex and incident HF status. Baseline levels were compared between men and women and HF cases and controls within sex-strata using t-tests and chi-squared tests as appropriate. A p value <.05 was considered significant. When referring to baseline in this manuscript we are referring to the initial Health ABC visit.
The primary exposure was incident HF with body composition changes in each compartment from DXA as dependent variables. Separate mixed effects random slopes and intercepts models for each body composition measure were used to examine the rates of body composition change after incident HF compared to controls and to the period before HF. The time from incident HF to follow-up DXA scans was based on the hospital admission date. For ease of interpretation, days to HF and days between clinic visits were converted to years by dividing by 365.25. The primary predictor in each model was the estimated beta coefficient corresponding to the interaction term of time by incident HF, where the time was between the date of the first HF hospitalization and the follow-up DXA scan. These estimated betas represent the yearly rate of change in body composition (grams/year) after incident HF compared to non-HF and the period before HF. The interaction term accounts for the fact that an incident HF event could have occurred at any time between yearly DXA scans by factoring in the exact amount of time between the incident HF event and each subsequent follow-up DXA scan. In other words, in the models, starting DXA values in controls were based on the initial Health ABC study scans and starting DXA values for cases were based on the most recent yearly scan before the incident HF event. Time 0 for controls was study entry and time 0 for cases was the date of first HF hospitalization, which accounts for differences in the time between the HF event and subsequent DXAs.
No adjustments were made for multiple testing, as these were hypothesis driven analyses addressing a priori research questions. Nonetheless, type I error may be inflated as a result.”
To determine if the amount of lean or fat mass change was excessive following HF for a given amount of total body mass change, data were also adjusted for the annualized rate of total body mass change (from DXA), which was calculated from linear regression using all available time points (baseline – year 6). To illustrate absolute body composition changes between HF and non-HF, absolute yearly changes were estimated by solving the mixed effects model. Furthermore, to clarify if body composition changes were excessive for a given change in total mass, absolute body composition changes were also indexed to the average yearly total mass change of a HF case (−802.9 g/yr for men and −841.0 g/yr for women).
Due to significant interactions between overall weight change directions, models pertaining to weight losers, gainers and those who were weight stable were all assessed separately. To evaluate possible benefits of overweight or obese status in those who developed HF, interactions between baseline BMI category (normal: BMI < 25.0kg/m2, overweight: BMI 25.0–29.9kg/m2, and obese: BMI ≥ 30kg/m2) and lean body mass were analyzed. Finally, subgroups (HFpEF vs. HFrEF) were each compared. In all analyses, men and women were examined separately as factors associated with body composition change differ by sex.6 Simulations using artificially generated datasets where the rate of lean, fat and total mass before and after HF was known were used to confirm the accuracy of all models and all analyses were conducted using SAS v9.3 (Cary, NC, United States).
Results
Mean age of the Health ABC study population was 73.6 ± 2.9 years and included 48.5% men and 59.6% Whites. Baseline characteristics of the Health ABC study population are listed in Supplementary Table 1. Relatively more women than men had hypertension and depression, and relatively more men than women had coronary heart disease, diabetes, and cognitive impairment. Incident HF occurred in only 3.9% of the overall population, with similar rates in men and women. Table 1 shows baseline (entry into Health ABC) characteristics of the Health ABC study population, focusing on comparisons between those who developed incident HF vs. those who did not, and also subdivided by sex. Men and women with HF had relatively greater comorbidity at baseline than those without HF, but comorbidity was also relatively low in both groups. Among men with HF there were 2.0 (interquartile range [IQR]: 1–3) other comorbid health conditions vs. 1.4 (IQR: 0–2) in men without HF. Among women with HF, there were 1.9 (IQR: 1–3) total comorbid health conditions vs. 1.3 (IQR: 0–2) in women without HF. Coronary heart disease at baseline was more common in men and women with HF compared to the non-HF population. Diabetes was more frequent in men with HF compared to those without HF. Among women, stroke and chronic obstructive pulmonary disease were more common among women with HF compared to those without HF.
Table 1.
Baseline characteristics of Health ABC participants subdivided by sex and incident HF
| Characteristics | Men (n=1366) | Women (n=1449) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean ± SD or n (%) | HF (n=52) | No HF (n=1314) | P-value | HF (n=59) | No HF (n=1390) | P-value |
|
| ||||||
| Age, y | 74.0 ± 3.0 | 73.7 ± 2.8 | 0.53 | 73.5 ± 2.9 | 73.5 ± 2.9 | 0.99 |
|
| ||||||
| Black | 19 (37) | 473 (36) | 0.94 | 36 (61) | 609 (44) | 0.009 |
|
| ||||||
| Pittsburgh | 35 (67) | 640 (49) | 0.009 | 40 (68) | 669 (48) | 0.003 |
|
| ||||||
| Smoking | ||||||
| Never | 10 (19) | 402 (31) | 0.06 | 32 (54) | 801 (58) | 0.03 |
|
| ||||||
| Former | 39 (75) | 771 (59) | 18 (31) | 461 (33) | ||
|
| ||||||
| Current | 3 (6) | 141 (11) | 9 (15) | 128 (9) | ||
|
| ||||||
| Alcohol, | ||||||
| more than 1 drink/day, yes | 5 (10) | 148 (11) | 0.71 | 3 (5) | 48 (4) | 0.50 |
|
| ||||||
| Education, | ||||||
| less than high school | 14 (27) | 348 (27) | 0.99 | 14 (24) | 308 (22) | 0.76 |
|
| ||||||
| high school | 13 (25) | 336 (25) | 20 (34) | 535 (39) | ||
|
| ||||||
| post-secondary | 25 (48) | 630 (48) | 25 (42) | 547 (39) | ||
|
| ||||||
| Hypertension | 26 (50) | 495 (38) | 0.07 | 34 (58) | 660 (48) | 0.13 |
|
| ||||||
| Coronary Heart Disease | 25 (48) | 280 (21) | <0.0001 | 16 (27) | 159 (11) | 0.0003 |
|
| ||||||
| Stroke | 3 (6) | 84 (6) | 0.99 | 8 (14) | 101 (7) | 0.08 |
|
| ||||||
| COPD | 4 (8) | 148 (11) | 0.42 | 12 (20) | 151 (11) | 0.02 |
|
| ||||||
| CKD | 20 (39) | 381 (29) | 0.14 | 20 (34) | 386 (28) | 0.30 |
|
| ||||||
| Diabetes | 18 (35) | 268 (20) | 0.01 | 16 (27) | 195 (14) | 0.005 |
|
| ||||||
| Depression | 1 (2) | 58 (4) | 0.72 | 3 (5) | 94 (7) | 0.79 |
|
| ||||||
| MMSE<80 | 7 (14) | 155 (12) | 0.72 | 4 (7) | 111 (8) | 0.99 |
|
| ||||||
| No comorbid disease | 10 (19) | 335 (26) | 0.31 | 10 (17) | 378 (27) | 0.08 |
|
| ||||||
| Multimorbidity, | ||||||
| number of conditions | 2.00 ± 1.40 | 1.42 ± 1.19 | 0.001 | 1.92 ± 1.49 | 1.34 ± 1.15 | 0.002 |
|
| ||||||
| Physical Activity (kcal/week) | 4195 ± 2892 | 4311 ± 3926 | 0.68 | 3461 ± 2190 | 3923 ± 2849 | 0.33 |
MMSE–mini mental status exam
These data reflect body composition at entry in the Health ABC study (year 1) for both cases and controls.
Table 2 shows baseline DXA measures of body composition, subdivided by HF and sex. Despite similar mean BMI in men and women, men had higher total mass, total lean body mass and appendicular lean mass than women. In men, BMI and lean body mass were higher in those who developed incident HF. In women, BMI, total mass, lean body mass and appendicular lean mass were all higher in those who developed HF.
Table 2.
Baseline DXA measures of body composition, subdivided by incident HF and sex
| Total | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics, mean ± SD | No HF (n=2704) | HF (n=111) | HF (n=52) | No HF (n=1314) | P-value | HF (n=59) | No HF (n=1390) | P-value |
| Body mass index, kg/m2 | 27.3 ± 4.7 | 28.8 ± 4.9 | 28.2 ± 3.3 | 27.0 ± 3.9 | <0.01 | 29.4 ± 6.0 | 27.6 ± 5.4 | 0.02 |
| Total mass, kg | 75.4 ± 14.9 | 78.5 ± 13.6 | 80.9 ± 10.0 | 78.6 ± 12.9 | 0.05 | 72.7 ± 15.0 | 68.2 ± 14.2 | 0.01 |
| Total lean mass, kg | 46.6 ± 10.0 | 48.0 ± 9.1 | 55.2 ± 5.8 | 54.4 ± 7.1 | 0.24 | 41.6 ± 6.3 | 39.4 ± 6.0 | 0.001 |
| Appendicular lean mass, kg | 20.1 ± 5.0 | 20.7 ± 4.5 | 24.2 ± 3.0 | 23.9 ± 3.6 | 0.38 | 17.7 ± 3.3 | 16.6 ± 3.2 | 0.002 |
| Total fat mass, kg | 26.6 ± 8.7 | 28.6 ± 9.0 | 25.7 ± 6.3 | 24.2 ± 7.2 | 0.08 | 31.1 ± 10.2 | 29.1 ± 9.3 | 0.11 |
| Percent Body Fat, % | 34.9 ± 7.8 | 35.7 ± 7.8 | 30.2 ± 4.9 | 29.2 ± 5.0 | 0.24 | 40.6 ± 6.5 | 40.4 ± 5.8 | 0.63 |
HF–heart failure
These data reflect body composition at entry in the Health ABC study (year 1) for both cases and controls.
Table 3 and Figure 2a/b show the main results from models used to assess body composition changes in HF cases vs. controls. Model 1 adjusts for confounders to examine whether participants who developed HF experienced absolute differences regarding body composition change. Model 2 adjusts additionally for annualized weight loss to assess whether those with HF experienced accelerated loss of lean or fat mass for a given weight change. As shown in Table 3, total mass, lean mass, and appendicular lean mass all decreased more in those who developed incident HF compared to those who did not, among both men and women (model 1). After adjusting for annualized weight loss, appendicular mass decline remained significant only in men. Among women, the loss of lean mass was largely explained by the annualized weight changes.
Table 3.
DXA changes over time in participants with incident HF, subdivided by sex
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 1* | Model 2† | |||||
| Beta (SE)* | P-value | Beta (SE) | P-value | Beta (SE) | P-value | Beta (SE) | P-value | |
| Dual X-Ray Absorptiometry‡ | ||||||||
| • Total Mass Change, g | −444.3 (225.6) | 0.05 | - | - | −313.3 (231.8) | 0.18 | - | - |
| • Lean Mass Change, g | −263.2 (110.4) | 0.02 | −99.8 (72.1) | 0.17 | −181.0 (90.8) | 0.04 | −16.7 (62.5) | 0.79 |
| • Appendicular Lean Mass Change, g | −176.3 (59.9) | <0.01 | −101.7 (44.7) | 0.02 | −105.0 (48.2) | 0.03 | 7.7 (36.6) | 0.83 |
| • Fat Mass Change, g | −184.3 (155.7) | 0.24 | 103.7 (83.9) | 0.22 | −197.6 (176.1) | 0.26 | 46.3 (90.4) | 0.61 |
| • Percent Fat Mass Change, % | −0.10 (0.13) | 0.36 | 0.10 (0.09) | 0.28 | −0.15 (0.14) | 0.28 | 0.03 (0.10) | 0.79 |
DXA measurements were assessed at baseline and repeated annually for 5 years. Starting DXA values for controls was study entry and the DXA immediately prior to HF for cases. Each subsequent DXA was used to calculate rate of change. Time 0 for controls was study entry and time 0 in cases was the date of first HF hospitalization, which accounts for differences in the time between the HF event and subsequent DXAs. Regression coefficients are for the interaction of HF * time and represent differences in yearly changes between cases and controls
DXA–Dual X-Ray Absorptiometry; HF–Heart Failure
Model 1 – adjusted for age, race, site, baseline: smoking, alcohol consumption, education, BMI, hypertension, coronary heart disease, stroke, chronic obstructive pulmonary disease, diabetes, chronic kidney dysfunction, depression, low Teng mini mental score and physical activity main effects and time interactions in mixed models
Model 2 – Model 1 additionally adjusted for annualized rate of change in total body mass
Random Slopes and Intercepts Mixed Model with total, lean, or fat mass or percent fat by time interaction as outcome
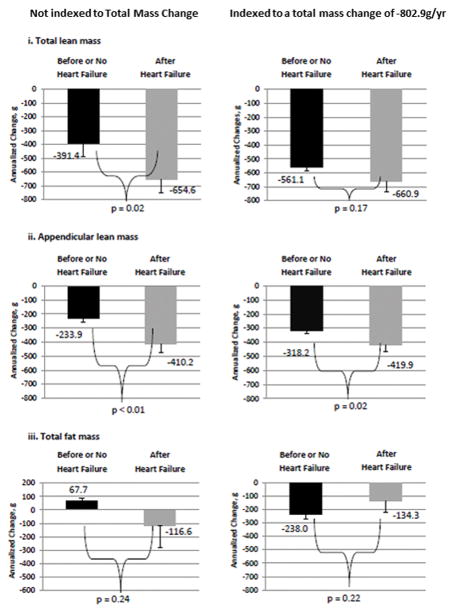
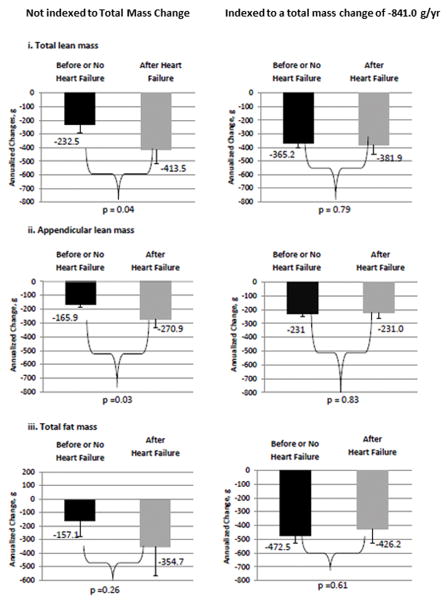
Figure 2. Differences in total lean, appendicular lean, and total fat mass between the HF vs. non-HF groups*.
A: Male participants with HF group developed significantly greater losses in total lean mass than and appendicular lean than non-HF over time. When data are indexed to annual changes of total mass per year, the loss of appendicular lean associated with HF vs. non-HF remained significant. Total fat decreased HF and non-HF before and after annualized weight changes were considered with no significant differences between the groups. B: Female participants with HF also developed greater losses of total lean mass and appendicular lean compared to non-HF. However, once annualized weight changes were considered, the loss of total lean mass and appendicular lean were no longer greater among those with HF. Total fat decreased HF and non-HF before and after annualized weight changes were considered with no significant differences between the groups. *Starting DXA values in controls were based on the initial Health ABC study scans and starting DXA values for cases were based on the most recent yearly scan before the incident HF event. Time 0 for controls was study entry and time 0 in cases was the date of first HF hospitalization, which accounts for differences in the time between the HF event and subsequent DXAs. DXA–Dual X-Ray Absorptiometry. HF–Heart Failure
Figure 2 depicts body composition changes calculated from the models described in Table 3. In Figure 2a-i and 2a-ii loss of lean mass and annualized lean mass were significantly greater in those who developed HF vs. those who did not among men, and loss of appendicular lean mass remained greater in HF vs. those who did not develop HF even after adjusting for annualized changes.
While Figure 2b-i and 2b-ii suggest similar relationships between lean mass and appendicular lean mass in women with HF, the loss of lean mass and appendicular lean were no longer significant compared to the women who did not develop HF after accounting for annualized weight changes. Similarly, among women with stable or gains in weight, relatively proportions of lean (total or appendicular) or fat mass did not change significantly.
Table 3 as well as Figure 2a-iii and 2b-iii focus on body fat, which decreased similarly in participants with and without HF. Among the men who developed HF, 11 were normal weight, 23 were overweight, and 18 were obese.
We conducted several subgroup analyses, testing for whether baseline BMI, the direction of weight change (gain vs loss) or the type of heart failure (HFrEF vs HFpEF) influenced the degree of lean loss. No significant interactions were evident between baseline BMI category and loss of lean mass after development of HF in men (p=0.98) or women (p=0.36), suggesting loss of lean is similar across BMI categories. Within weight change categories, only men who lost weight manifested differences between HF vs. those who did not develop HF, i.e., participants who developed HF lost a higher proportion of lean mass. Relative losses of lean (total or appendicular) or fat mass among women with HF were not similarly significant (Table 4). Finally, associations between HF and lean mass tend to be stronger in HFrEF than HFpEF (Supplementary Table 2). However, in the women who developed HFpEF increases in fat mass and percent fat mass change persisted even after accounting for annualized changes in weight. While such of sex-related changes in fat in HFpEF are intriguing, the small number of HFpEF limits decisive conclusions.
Table 4.
Annual DXA changes associated with incident HF in men and women, subdivided by the direction of weight change and sex
| Weight Stable | Weight Gainer | Weight Loser | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 1* | Model 2† | Model 1* | Model 2** | |||||||
| Beta (SE)* | P-value | Beta (SE) | P-value | Beta (SE) | P-value | Beta (SE) | P-value | Beta (SE) | P-value | Beta (SE) | P-value | |
| DXA Indices‡ | ||||||||||||
| MEN | ||||||||||||
| • Total Mass Change, g | 0.1 (154.8) | 0.99 | - | - | −161.8 (527.5) | 0.76 | - | - | −285.5 (275.9) | 0.30 | - | - |
| • Lean Mass Change, g | 33.9 (106.7) | 0.75 | 53.2 (99.7) | 0.59 | 236.6 (309.5) | 0.44 | 266.2 (293.6) | 0.36 | −329.3 (153.1) | 0.03 | −236.5 (117.3) | 0.04 |
| • Appendicular Lean mass Change, g | −9.1 (62.1) | 0.88 | −1.8 (60.0) | 0.98 | 86.5 (182.5) | 0.64 | 101.7 (175.8) | 0.56 | −232.3 (89.3) | 0.01 | −179.8 (71.7) | 0.01 |
| • Fat Mass Change, g | −26.4 (123.3) | 0.83 | 2.4 (108.7) | 0.98 | −441.5 (453.5) | 0.33 | −382.0 (3.5.5) | 0.26 | 46.3 (197.3) | 0.81 | 199.2 (138.6) | 0.15 |
| • Percent Fat Mass Change, % | −0.06 (0.13) | 0.65 | −0.04 (0.12) | 0.73 | −0.49 (0.40) | 0.22 | −0.48 (0.35) | 0.17 | 0.16 (0.18) | 0.36 | 0.27 (0.14) | 0.05 |
| WOMEN | ||||||||||||
| • Total Mass Change, g | 131.3 (297.5) | 0.66 | - | - | 234.6 (382.5) | 0.54 | - | - | −156.0 (242.5) | 0.52 | - | - |
| • Lean Mass Change, g | −162.7 (169.7) | 0.34 | −218.8 (165.5) | 0.19 | 175.8 (195.5) | 0.37 | 86.9 (173.8) | 0.62 | −114.7 (101.5) | 0.26 | 7.9 (78.6) | 0.92 |
| • Appendicular Lean Mass Change, g | −91.7 (97.0) | 0.34 | −119.1 (95.7) | 0.21 | 135.2 (105.5) | 0.20 | 91.0 (96.5) | 0.35 | −46.5 (55.0) | 0.40 | 27.0 (46.1) | 0.56 |
| • Fat Mass Change, g | 288.8 (235.1) | 0.22 | 156.5 (223.4) | 0.48 | 135.0 (321.1) | 0.67 | −12.7 (231.2) | 0.96 | −77.3 (188.2) | 0.68 | 59.5 (120.6) | 0.62 |
| • Percent Fat Mass Change, % | 0.34 (0.24) | 0.15 | 0.29 (0.22) | 0.20 | 0.02 (0.27) | 0.94 | −0.10 (0.23) | 0.67 | −0.07 (0.16) | 0.66 | 0.02 (0.12) | 0.87 |
DXA measurements were assessed at baseline and repeated annually for 5 years. Starting DXA values for controls was study entry and the DXA immediately prior to HF for cases. Each subsequent DXA was used to calculate rate of change. Time 0 for controls was study entry and time 0 in cases was the date of first HF hospitalization, which accounts for differences in the time between the HF event and subsequent DXAs. Time is from the first HF hospitalization in the incident HF group. Weight change groups were defined by the following criteria: stable (<3% change), gainers (≥3% increase), and losers (≥3% decrease). Regression coefficients are for the interaction of HF * time and represent differences in yearly changes between cases and controls
Model 1 – adjusted for age, race, site, baseline: smoking, alcohol consumption, education, BMI, hypertension, coronary heart disease, stroke, chronic obstructive pulmonary disease, diabetes, chronic kidney dysfunction, depression, low Teng mini mental score and physical activity main effects and time interactions in mixed models
Model 2 – Model 1 additionally adjusted for annualized rate of change in total body mass
Random Slopes and Intercepts Mixed Model with total, lean, or fat mass or percent fat by time interaction as outcome
DXA–Dual X-Ray Absorptiometry; HF–Heart Failure
Discussion
In this study, we studied participants in Health ABC, and showed greater losses of lean body mass in older men and women who developed HF and lost weight compared to those without HF. Notably, loss of appendicular lean tissue remained significant in men even after accounting for annualized weight change, which implies that HF accelerates appendicular lean tissue atrophy beyond that associated with typical aging. Though lean change after HF in women tracked well with total weight loss, it still notable that women with HF lost more lean and total weight than controls. These changes were most pronounced in men and women with heart failure who were losing overall body weight in this time period.
Clinical implications of lean tissue loss are substantial; diminished lean mass is associated with increased mortality,20,21 functional decline, weakening, frailty, disability, diminished quality of life, altered pharmacokinetics and dynamics, increased risks of falls, and many other harmful sequelae.8,9 In particular, loss of appendicular lean mass corresponds to poor clinical prognosis.22
The implications of these data are intensified by the high prevalence of HF among older adults. HF is endemic among today’s expanding population of senior adults5, and prognosis (mortality, morbidity, and quality of life) worsens with age.23,24 Whereas contemporary treatment standards remain oriented primarily to central pathophysiology, the accelerated lean body tissue loss has substantial bearing on outcome, but is rarely addressed as part of routine care.
Just as with men, loss of lean tissue was greater in women with HF than those without, but was largely explained by greater annualized weight loss. These patterns are consistent with studies, including Health ABC, demonstrating greater lean body mass in men than women during youth, but relatively greater atrophy of lean mass in men than women with aging.6,25 Women have relatively less lean mass, but it is better conserved with age. Bazzonni et al.26 describe greater stability of lean mass in healthy women versus men, suggesting there is a natural protective capacity in women with normal aging, which also seems to moderate the added atrophying effects of disease in old age. Ongoing work aims to uncover biological mechanisms underlying sex differences.
High BMI at baseline (entry into Health ABC) was associated with subsequent incident HF. However, in contrast to many studies which rely on BMI or other anatomical measurements to assess body composition, using DXA data revealed loss of lean tissue over time and with relatively preserved fat mass, and perhaps even increased fat mass in women with HFpEF. High BMI seems to mask the loss of lean tissue, and the shift towards sarcopenic obesity.27 It therefore seems consistent that we did not detect an interaction between BMI category and lean body mass change in those who developed HF; refuting the premise of a beneficial obesity paradox. Nonetheless, the small number of HF cases limits any definitive conclusions.
Physical challenges associated with fat mass escalate as underlying skeletal muscle mass decreases. The relatively higher proportion of fat mass is also conducive to greater inflammation28 (i.e., accelerating atrophy) and lower muscle quality29 (i.e., increasing infiltrative fat) and seem generally likely to exacerbate risks of mortality and disability.
Frailty is commonly described as a phenotype that includes diminished physical activity, weakness, slowing, and exhaustion.30 It is highly prevalent in older adults with HF,31,32 and correlates strongly with increased risks of disability and dependency.33 The loss of lean mass with aging and HF also seems related to frailty 34 and reinforces the rationale to target body composition in HF management. Ongoing studies are exploring the relationship with between frailty and HF, and the potential benefits of diet, exercise, and other interventions.35, 36,37,38 Strength training may have particular utility.39,40 Future analyses in Health ABC will aim to determine the impact of HF on physical performance and whether or not body composition changes mediate any effect.
The associations of HF with respect to body composition were more apparent in HFrEF than HFpEF. In many respects this seems counterintuitive as HFpEF includes degenerative effects on skeletal muscle that parallel those in HFrEF.2,41 Recent literature highlights the constitutive changes in body composition with related cellular and subcellular changes that occur with HFpEF.42 Other studies highlight the dominant role inflammation plays in HFpEF pathophysiology,43 which may compound risk of lean tissue loss. The differences in body composition HFpEF vs. HFrEF in this analysis may merely reflect insufficient statistical power to assess HFpEF reliably, but they may also indicate that changes in lean mass associated with HFpEF may occur much earlier (i.e., long before onset of prototypical heart failure symptoms and signs) and may be largely complete before the time period of Health ABC’s assessments. Additional studies are needed with larger HFpEF populations to better clarify prognostic implications of body composition changes.
Strengths of this study relate to the distinctions of Health ABC. Heart failure was assessed with careful, adjudicated assessments with clear delineation of disease onset and body composition was serially ascertained using DXA. Further, we had exact time to event data and the models were able to take into account the number of days between the HF event and subsequent DXA scans. The analysis also stands out for assessing changes in lean mass associated with disease relative to annualized weight loss, which allowed us to determine if body composition changes were disproportionate for a given weight change.
Weaknesses of these analyses include its relatively small sample of HF cases, and particularly few with HFpEF. Moreover, HF was based only on hospital diagnoses. While this can be construed as a strength as it ensures standardized accuracy, it is possible that adults diagnosed with HF as out-patients were not included. This may be particularly true among those with HFpEF. Health ABC also enrolled relatively healthy older adults at baseline, who developed HF amidst little comorbidity, limiting generalizability to older HF populations in which multimorbidity is endemic.
Summary
Overall, this study highlights the impact of HF on changes in body composition. Specifically, we showed greater loss of lean mass after HF compared to non-HF controls. In men, we show an accelerated loss of lean mass after HF for a given weight change, whereas lean loss tracked more closely with overall weight change in women who developed HF. Lean mass is a known determinant of health, and loss of lean mass is particularly dangerous in HF as it has bearing on functional degradation as well as frailty. This study provides strong rationale for future studies that delineate the mechanisms underlying reduced lean mass, with relative differences in respect to sex. Inflammation, adipokines and other key factors may determine these relationships. Moreover, opportunities for therapeutic enhancement also seem likely, with diet, novel medications, and exercise all likely candidates for improved care and outcomes.
Supplementary Material
What is new?
While heart failure (HF) is usually described as a pumping pathophysiology, this analysis of healthy older adults over 6 years shows that in those who newly develop HF, a striking loss of lean body mass occurs, particularly among men.
What are the clinical implications?
Clinical implications are substantial as loss of lean mass in older HF patients likely increases their risks of physical functional decline, falls, and frailty.
This intuitively implies that HF management should broaden to include greater emphasis on nutrition and other approaches to preserve lean mass.
It also reinforces rationale for more research regarding body composition, sex differences, and novel therapeutic approaches.
Acknowledgments
Sources of Funding: This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. This work was also supported by P30 AG024827 and the intramural research program at the NIA
Footnotes
Disclosures: Dr. Forman is supported in part by NIA grant 1R56AG051637-01A1, PCORI grant IH-1304678, and VA Office of Rehabilitation Research and Development grant F0834-R. Dr. Newman is supported in part by NIA AG023629 and P30 AG024827. Dr. Santanasto is supported by NIA T32AG000181. Dr. Kizer: stock ownership, Pfizer, Inc; Gilead Sciences, Inc; both significant
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2014;307:H1512–20. doi: 10.1152/ajpheart.00527.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennel PJ, Mancini DM, Schulze PC. Skeletal Muscle Changes in Chronic Cardiac Disease and Failure. Compr Physiol. 2015;5:1947–69. doi: 10.1002/cphy.c110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–8. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard DR, Beliaeff S, Dionne IJ, Brochu M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: nutrition as a determinant of successful aging (NuAge) - the Quebec Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2007;62:1382–8. doi: 10.1093/gerona/62.12.1382. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 9.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–21. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 10.Jahangir E, De Schutter A, Lavie CJ. Low weight and overweightness in older adults: risk and clinical management. Prog Cardiovasc Dis. 2014;57:127–33. doi: 10.1016/j.pcad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc. 2016;5:e003921. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail. 2016;9:e002883. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oga EA, Eseyin OR. The Obesity Paradox and Heart Failure: A Systematic Review of a Decade of Evidence. J Obes. 2016:9040248. doi: 10.1155/2016/9040248. Epub 2016 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV, Ventura HO. Update on Obesity and Obesity Paradox in Heart Failure. Prog Cardiovasc Dis. 2016;58:393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J Appl Physiol. 1999;87:1513–20. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 16.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–9. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 18.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 20.Santanasto AJ, Goodpaster BH, Kritchevsky SB, Miljkovic I, Satterfield S, Schwartz AV, Cummings SR, Boudreau RM, Harris TB, Newman AB. Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2017;72:513–9. doi: 10.1093/gerona/glw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, Dam TT, Lapidus JA, Cawthon PM, Ensrud KE, Orwoll ES Osteoporotic Fractures in Men Study Group. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–40. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen I, Heymsfield SB, Ross R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J Am Geriatric Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 23.Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Jr, Krumholz HM, Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–10. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forman DE, Ahmed A, Fleg JL. Heart failure in very old adults. Curr Heart Fail Rep. 2013;10:387–400. doi: 10.1007/s11897-013-0163-7. [DOI] [PubMed] [Google Scholar]
- 25.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH Health, Aging, and Body. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazzocchi A, Diano D, Ponti F, Andreone A, Sassi C, Albisinni U, Marchesini G, Battista G. Health and ageing: a cross-sectional study of body composition. Clin Nutr. 2013;32:569–78. doi: 10.1016/j.clnu.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–75. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 28.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017 May;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB Health ABC study. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–95. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 31.Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–75. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- 32.Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, Bauer DC, Kritchevsky SB, Butler J. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166:887–94. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 34.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:1–4. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman DE, Sanderson BK, Josephson RA, Raikhelkar J, Bittner V American College of Cardiology’s Prevention of Cardiovascular Disease Section. Heart Failure as a Newly Approved Diagnosis for Cardiac Rehabilitation: Challenges and Opportunities. J Am Coll Cardiol. 2015;65:2652–9. doi: 10.1016/j.jacc.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part II. Ongoing and unpublished randomized trials. Prog Cardiovasc Dis. 2014;57:144–51. doi: 10.1016/j.pcad.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–43. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, Lewis EF, Myers J, Reeves GR, Shen WK American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing Functional Capacity as a Principal End Point for Therapies Oriented to Older Adults With Cardiovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artero EG, Lee DC, Lavie CJ, España-Romero V, Sui X, Church TS, Blair SN. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32:351–8. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, Forman DE, Arena R. Bulking Up Skeletal Muscle to Improve Heart Failure Prognosis. JACC Heart Fail. 2016;4:274–6. doi: 10.1016/j.jchf.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119:739–44. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.