Abstract
Purpose
To investigate the relationship between intraocular pressure (IOP) and big bubble (BB) formation in an ex-vivo model of deep anterior lamellar keratoplasty (DALK).
Design
Ex-vivo
Methods
Corneo-scleral buttons from human donors were loaded onto an artificial anterior chamber connected to a column of balanced salt solution. A surgeon-in-training learned to perform DALK via the BB technique using swept-source microscope-integrated optical coherence tomography (SS-MIOCT) with heads-up-display (HUD). DALK procedures were performed at six different IOPs (5, 10, 15, 20, 30, or 40mmHg; n=6 per group) in a randomized fashion, with the surgeon-in-training masked to the pressure and guided by SS-MIOCT with HUD. For a subset of corneas within each pressure group, DALK was performed on matching donor tissue at a control IOP. BB diameter was recorded, and a diameter exceeding the trephine diameter was considered optimal.
Results
Wilcoxon rank sum test showed a difference in BB diameter amongst the different pressure groups (mean±standard deviation of 7.75±1.60mm, 8.33±1.99mm, 10.9±0.92mm, 9.08±1.07mm, 6.67±3.33mm, and 3.42±3.77mm in the 5, 10, 15, 20, 30, and 40mmHg groups, respectively; p=0.0014). Per Tukey test, this difference was attributable to comparisons between the 40mmHg group and the 5, 10, 15, or 20mmHg groups (p=0.04, 0.02, 0.0001, 0.004, respectively).
Conclusion
In this ex-vivo model of DALK, the BB technique guided by SS-MIOCT with HUD, yielded bubbles of optimal diameters only at physiologic pressures (10–20mmHg). Extremely high IOP (40mmHg) resulted in BBs of significantly smaller diameter than BBs obtained at physiologic and low (5mmHg) IOPs.
Keywords: deep anterior lamellar keratoplasty, intraocular pressure, optical coherence tomography
Introduction
Deep anterior lamellar keratoplasty (DALK) is an alternative corneal transplantation technique that is gaining popularity in treating pathologies limited to the upper layers of the cornea1. Advantages of DALK over conventional penetrating keratoplasty (PK) include a more rapid return of normal corneal biomechanics post-procedure2, faster surgical wound healing3, and better post-operative visual acuity4. DALK involves separating the epithelium, Bowman’s layer, and stroma from Descemet’s membrane and endothelium, then selectively removing the diseased anterior layers while leaving the healthy posterior layers intact5, 6. The graft can then be placed on top of the host Descemet’s membrane and endothelium. A popular method for separating the stroma from Descemet’s membrane involves an intrastromal air injection6, 7, termed the Big Bubble (BB) technique, which creates an air bubble between these layers. Aside from needle depth8 and residual stromal bed9, little is known about the factors that affect the success of BB formation, including intraocular pressure (IOP).
Given the prevalence of glaucoma, ocular hypertension, and associated pressure-lowering surgeries, studying the impact of IOP on corneal transplantation is important. DALK is well-suited for glaucoma patients as it eliminates the risk of endothelial rejection, reduces the number of medical or surgical interventions needed for post-operative elevated IOP, and shortens the course of post-operative steroids10–14. Much is known about the impact of glaucoma and ocular hypertension on the post-operative environment, including its ability to increase graft rejection rates by two to three-fold15–21; however, the impact of these conditions on successful completion of surgical procedures has not been studied.
Anecdotally, some corneal surgeons prefer lower IOPs for BB formation and have advocated for the use of pre-air injection paracenteses to actively lower IOP. To date, no study has investigated the effects of intraocular pressure on BB diameter during DALK. An awareness of the impact of IOP on BB formation could potentially impact a surgeon’s pre or peri-operative plan, if certain IOPs were more or less amenable to BB formation. This study sought to determine the relationship between IOP and BB formation in a controlled, ex-vivo model using swept-source microscope integrated optical coherence tomography (SS-MIOCT) with heads-up-display (HUD). Guidance of needle depth within the cornea was a novel use of SS-MIOCT with HUD technology and assisted in testing the null hypothesis that no difference in BB diameter exists in DALK performed at physiologic versus non-physiologic IOPs.
Materials and Methods
The work herein used tissue from de-identified human donors and accordingly received a research exemption by the Duke University Health System Institutional Review Board (Protocol number 00067174; September 29, 2015).
We used an ex-vivo model to precisely alter IOP and to allow for testing of paired eyes at an experimental and control IOP. SS-MIOCT22 was used to provide the surgeon-in-training with live visualization23, 24 of the needle depth in the cornea, thereby controlling a known factor affecting the success of BB formation. The main outcome measure was BB diameter, as the optimal BB would allow for separation of stroma and Descemet’s membrane beyond the boundaries of the anterior cornea to be removed.
Materials
Tissue
Corneo-scleral buttons were obtained from the local eye bank (Miracles in Sight, Winston-Salem, NC). For all corneas, less than one week had elapsed since the donor’s death. All corneas were clear, non-edematous, without scars, and had no history of corneal trauma or surgery. The first of these corneas were used for training purposes, and the remainder were used to test BB formation rates at different IOPs. There were paired controls for each of the IOP groups.
4D Swept-Source Microscope-Integrated OCT with Heads-Up-Display
Technical details of the SS-MIOCT system with HUD have been previously described22, 25, 26. In brief, the SS-MIOCT system consisted of a swept-source based engine (Axsun Technologies, Billerica, MA, USA; λ0 = 1050nm) integrated with a Leica M844 ophthalmic surgical microscope (Leica Microsystems, Buffalo Grove, IL, USA). The SS-MIOCT system operated at the following parameters: A-scan rate = 100kHz, A-scans/B-scan = 500, B-scans/volume = 96, volume rate = 2Hz, resolution = 14 × 14 × 7.8μm [x,y,z], imaging range = 12 × 12 × 7.4mm [x,y,z], and sensitivity = 102dB. Custom graphics processing unit based software allowed for real-time visualization and recording of volumes, B-scans, and summed voxel projections (Figure 1a). A custom stereoscopic HUD24 (Figure 1b) projected the OCT images into the surgical oculars (Figure 1c), providing live OCT visualization for needle advancement during DALK. The system was also capable of recording the live stream of 3D SS-MIOCT data, which was used to review and analyze each procedure afterward.
Fig. 1. Experimental set-up with an artificial anterior chamber, balanced salt solution column, swept-source microscope-integrated optical coherence tomography (SS-MIOCT), and heads-up-display (HUD).
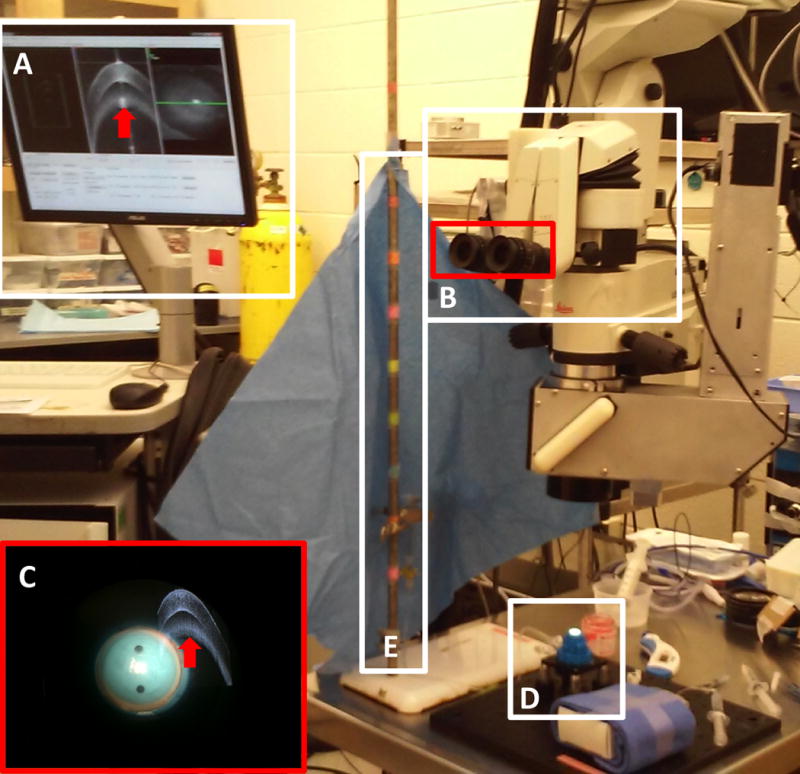
SS-MIOCT computer software controlled by the MIOCT technician (a) and HUD (b) with the surgical field and SS-MIOCT image viewed simultaneously (c). The red arrow in (a,c) denotes imaging artifact. A corneo-scleral button loaded onto an artificial anterior chamber (d) was attached to a column of balanced salt solution (e). Column height markings denote calibrated intraocular pressures.
Surgical Procedure
A corneo-scleral button was loaded onto a Barron artificial anterior chamber (Figure 1d) (Katena, Denville, NJ, USA) and connected via tubing to a 10ml syringe of balanced salt solution at a column height of 20cm above the corneal surface for training or at 6, 13, 27, 40, or 54cm above the corneal surface to generate specific cm H2O pressures within the artificial anterior chamber (Figure 1e). The column heights of 6, 13, 20, 27, 40, and 54cm corresponded to artificial anterior chamber pressures of 5, 10, 15, 20, 30, and 40mmHg, respectively. Three measurements of the pressure in mmHg, using a Tono-Pen AVIA (Reichert, Depew, NY, USA), were recorded and averaged for comparison against pressure measures determined from column height calculations. Both column height and Tono-Pen measurements were masked from the surgeon-in-training. The surgeon-in-training trephined to a depth of approximately 25% using a Barron 8mm radial vacuum trephine. This trephination depth was preferred by the surgeon-in-training to allow for a greater range of maneuverability in depth once the needle entered the cornea. An SS-MIOCT image was recorded post trephination to verify trephination depth (Figure 2a). A 27-gauge needle, bent at approximately 60 degrees and attached to a 5ml syringe of air, was advanced from a trephined edge towards the apex of the cornea until a needle depth of 80% or greater was visually approximated by the surgeon-in-training under SS-MIOCT with HUD guidance. While maintaining a needle depth of greater than 80%, the surgeon-in-training promptly injected 5ml of air in an effort to form a BB. SS-MIOCT images of the needle advancement and air injection were recorded (Figures 2b–d).
Fig. 2. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) images post trephination, during needle advancement, and during air injection.
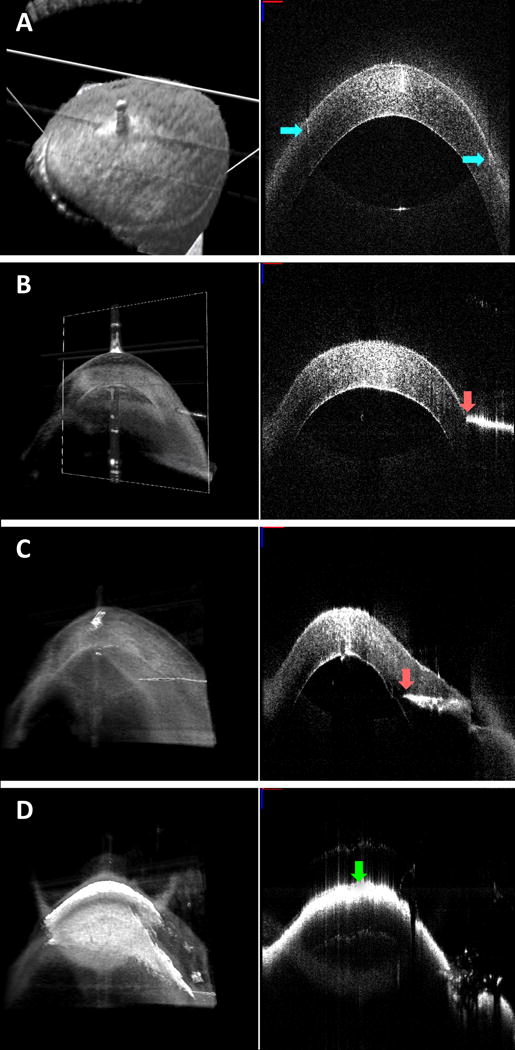
Volume (left) and B-scan (right) views generated by SS-MIOCT. Trephination lines (blue arrows) extending into the cornea are visible in the B-scan (a). Needle tip (pink arrow) advances from superficial cornea (b) to deep cornea (c) prior to air injection and opacification from intrastromal air emphysema (green arrow) (d). In all SS-MIOCT images, the red scale bar in the 2D B-scan measures 1mm laterally, and the blue scale bar measures 1mm axially.
Training at Physiologic IOP
The surgical procedure described above was first performed on 12 corneas by a surgeon-in-training, for learning purposes, at an IOP of 15mmHg. This IOP was selected because it rests in the middle of the physiologic IOP range of 10 to 21mmHg. The initial six corneas were used to familiarize the surgeon-in-training with the proper steps of the procedure and instrument handling under the guidance of a corneal surgeon. The subsequent six corneas were used to test the surgeon-in-training’s ability to successfully form BBs under SS-MIOCT with HUD guidance. The surgeon-in-training was deemed ready to proceed after six consecutive BB formations.
DALK at Varying IOPs
After successful completion of the training phase of the experiment, the surgeon-in-training performed the BB technique on 30 corneas at one of five (5, 10, 20, 30, or 40mmHg) different IOPs. IOPs of 10 and 20mmHg were tested due to these values being the upper and lower boundaries of physiologic IOP. IOPs of 5, 30, and 40mmHg were tested to determine the effects of low, high, and extremely high pressures on BB diameter. An IOP of 5mmHg was included in to determine if there could be a lower bound to successful BB formation post paracentesis, which has been advocated by surgeons at ophthalmology conferences. An IOP of 30mmHg was included to see if such an elevated pressure could still be amenable to successful BB formation. If so, it could suggest paracentesis may not be necessary, reducing the number of invasive steps in surgery. An IOP of 40mmHg was included to test the upper bounds of pressure for successful BB formation. These groupings also allowed for a balanced number of physiologic (10, 15, and 20mmHg) and non-physiologic (5, 30, and 40mmHg) pressure points. The IOPs were randomized by the MIOCT technician and masked from the surgeon-in-training. The surgeon-in-training then performed the surgical procedure as described above.
For three out of the six corneas tested at any particular pressure, the cornea from the same donor’s other eye was tested at an IOP of 15mmHg, as a control. These paired control procedures were performed to exclude the possibility that small diameter BBs were formed due to inherent differences in tissue rather than pressure alone.
Measurement of BB
Injection of air into the cornea frequently caused opacification of the stroma, preventing visualization of the BB through the cornea as loaded on the artificial anterior chamber. In order to assess BB diameter, the cornea was first unloaded from the artificial anterior chamber and flipped endothelial side up. A BB, whether it be type 1 or type 2, was considered to be formed if Descemet’s membrane was separated from the stroma. A surgical caliper was then used to measure BB diameter to the nearest 0.5mm (Figure 3a). An SS-MIOCT image of each BB was captured (Figures 3b–c).
Fig. 3. Assessment of big bubble (BB) formation.
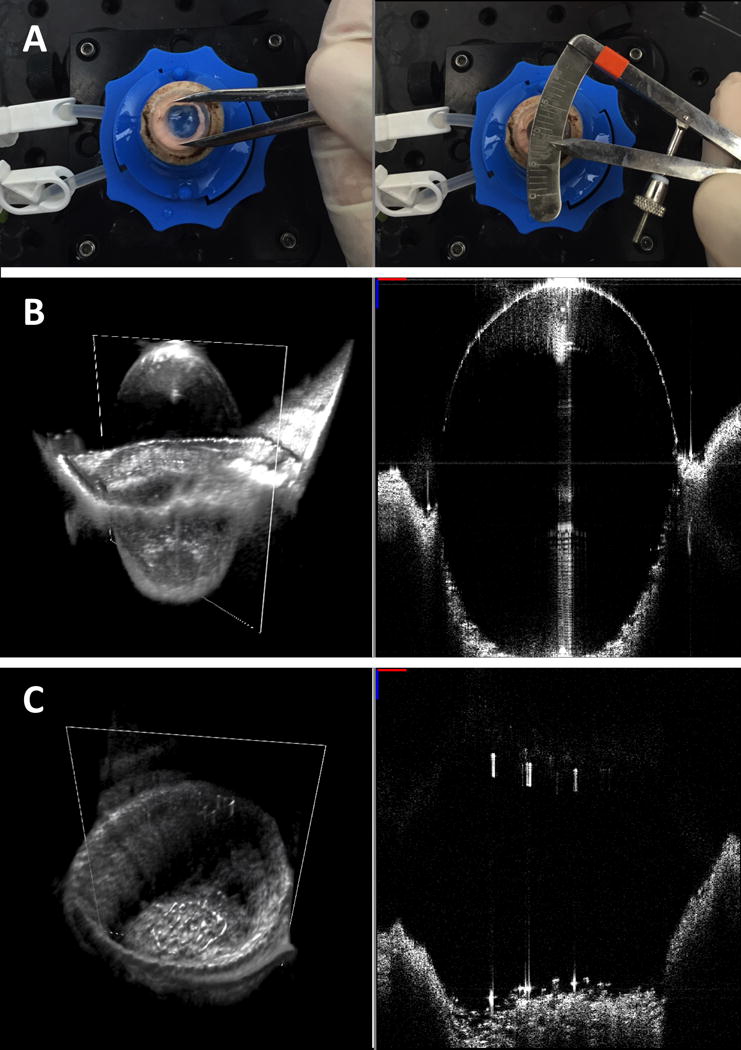
Surgical calipers were used to measure BB diameter with the cornea positioned endothelial side up (a). Swept-source microscope-integrated optical coherence tomography generated volumes (left) and B-scans (right) showing successful (b) and unsuccessful (c) BB formation.
Calculation of Central Corneal Thickness, and Needle Depth
Post-procedure, MATLAB (MathWorks, Natick, MA, USA) was used to calculate the central corneal thickness (CCT), needle depth, and residual stromal bed in the recorded stream of SS-MIOCT volumes that were captured during the procedure. A calculated corneal group refractive index of 1.384 was used for refraction correction based on prior data27, 28. Calculating CCT involved loading a recorded SS-MIOCT volume from before the needle insertion event into MATLAB, identifying the B-scan at the corneal apex, and marking the epithelium and endothelium along the central plane within this B-scan. Needle depth prior to air injection and residual stromal bed beneath the needle tip was calculated by similarly loading recorded SS-MIOCT volumes from immediately prior to air injection into MATLAB. The needle tip was marked, and its position between the epithelial and endothelial boundaries, expressed both as a percentage and absolute distance, was recorded.
Statistical Analyses
Statistical analyses were performed using JMP statistical software (SAS Institute, Cary, NC, USA). A Wilcoxon rank sum test was used to compare BB diameter at physiologic versus non-physiologic IOPs, and a Wilcoxon signed rank test was used to compare BB diameter at a given IOP versus the control IOP of 15mmHg. BB diameter comparisons between the five different IOP groups were made via a Kruskal Wallis test and were followed by Tukey tests for comparisons between each pair of IOPs. BB diameter was also compared across endothelial cell density (ECD), age, sex, race, CCT, and needle depth prior to air injection for physiologic versus non-physiologic IOPs, using linear regression for continuous variables and t-tests for categorical variables. These factors were also compared for physiologic versus non-physiologic IOPs, using Wilcoxon rank sum tests for continuous variables and chi-square tests for categorical variables. Tests were two-sided with an α value of < 0.05.
Results
Of the 64 corneas used for this study, seven were excluded from the analysis due to the following reasons: needle breakage at the point of angulation due to bending of the needle during insertion into the cornea (n=2), excess reflectivity of the needle causing difficulties in imaging (n=2), abortion of procedure due to full-thickness trephination (n=1), and needle displacement due to the surgeon’s hand slipping upon air injection and resulting in shallower than intended air injection (n=2). The remaining 57 corneas were used as follows: 12 training corneas, 30 experimental corneas, and 15 paired controls.
BB diameters (mean±standard deviation) were significantly larger for procedures performed at physiologic (9.53±1.75mm) versus non-physiologic (5.94±3.43mm) IOPs (p=0.0003) (Figure 4a). There was a significant difference in BB diameter amongst the pressure groups (7.75±1.60mm, 8.33±1.99mm, 10.90±0.92mm, 9.08±1.07mm, 6.67±3.33mm, and 3.42±3.77mm in the 5, 10, 15, 20, 30, and 40mmHg groups, respectively; p=0.001). The difference corresponded to physiological pressures of 5, 10, 15, or 20mmHg compared to the 40mmHg (p=0.04, 0.02, 0.0001, and 0.004, respectively) (Figure 4b).
Fig. 4. Big bubble (BB) diameters at different intraocular pressures (IOPs).
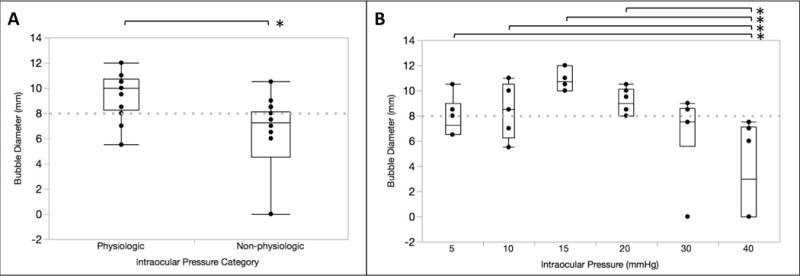
Box and whisker plots of BB diameter in the physiologic versus non-physiologic IOP groups (p=0.0003, n=18 in each group) (a) and six different IOP groups (n=6 in each group) (b). In the latter, significant differences in BB diameter were seen between the 40mmHg IOP group and 5, 10, 15, and 20mmHg groups (p=0.04, 0.02, 0.0001, 0.004, respectively). Dashed line corresponds to trephine diameter of 8mm.
All DALK procedures performed on matched donor pairs at an IOP of 15mmHg yielded BBs with diameters greater than 8mm (p=0.007).
BBs were formed in 41/45 (93%) procedures performed at various IOPs. 27/32 (84%) of BBs with diameters that cleared the trephine diameter were found in the physiologic IOP groups.
BB diameter did not change as the surgeon-in-training’s experience increased (R2=0.003, p=0.77).
There were no differences in BB diameter across ECD (p=0.41), age (p=0.49), sex (p=0.94), race (p=0.51), CCT (p=0.17), needle depth (p=0.51), or residual stromal bed (p=0.20). No differences in these factors were seen for physiologic versus non-physiologic IOPs (Table 1).
Table 1.
Baseline characteristics of research tissue.
| Baseline Characteristics | |||
|---|---|---|---|
| Measures | Physiologic IOP | Non-physiologic IOP | P Value |
| Endothelial Cell Density (cells/mm2) | 2194±737 | 2026±715 | 0.57 |
| Age (years) | 55±15 | 54±12 | 0.57 |
| Males (n) | 14 | 16 | 0.37 |
| Females (n) | 4 | 2 | |
| Caucasians (n) | 10 | 13 | 0.30 |
| Non-Caucasians (n) | 8 | 5 | |
| Central Corneal Thickness (μm) | 537±27 | 512±48 | 0.07 |
| Needle Depth (%) | 88±4 | 89±6 | 0.41 |
| Residual Stromal Bed (μm) | 80±50 | 62±31 | 0.57 |
Comments recorded by the MIOCT technician while the surgeon-in-training was masked to IOP and performed the BB technique revealed mechanical challenges at IOPs of 5mmHg, 10mmHg, and 40mmHg. In the 5mmHg and 10mmHg groups, controlled needle advancement was noted to be challenging, as the cornea tended to deform and move with the needle tip. In the 40mmHg group, the surgeon-in-training met resistance when attempting to inject air requiring increased effort to advance the syringe plunger. Two BBs in this group collapsed during the air injection. In these two cases, significant plunger acceleration was needed to overcome the chamber pressure to initiate and form the BB. This led to air leakage rather than a single sustained bubble.
Discussion
Our study showed that BBs may be formed at any IOP ranging from 5mmHg to 40mmHg; however, physiologic IOPs yielded BBs of optimal diameters for dissection during DALK. Different mechanical explanations may exist for smaller BB diameters seen at low versus high pressures. Low IOPs may not provide sufficient counter-traction for good control of the needle while the surgeon attempts to advance it to an appropriate depth in the cornea. The surgeon-in-training in our study commented on the difficulty of needle advancement in the 5mmHg and 10mmHg groups, stating that the tissue had a tendency to deform and move with the needle, a challenge that had to be overcome. High IOPs may prevent or undo infiltration of air into the tissue upon injection. Resistance encountered during air injection may lead to more forceful or rapid injection, which may not allow sufficient time for the continued separation of the stroma and Descemet’s membrane.
Our results suggest that IOP should be a consideration in the pre and peri-operative environments. A patient’s surgical candidacy for DALK via the BB technique may be improved by having an IOP within the physiologic range. For those with elevated pressures, medications to lower pressure to the physiological range before surgery may be advantageous. Pre-operative pressure lowering medications may also help reduce the number of pre-air injection paracenteses. Additionally, surgeons may avoid maneuvers that may increase IOP during the procedure, such as inadvertently pressing down on the globe or adnexa or insufficient normalization of IOP after a retrobulbar block. Our results also highlight the challenges of attaining BBs of sufficient diameter in low IOPs and may provide information about the likelihood of having to convert a DALK into a PK.
IOP may explain the occurrence of partial bubbles during BB formation. While we recognize that others may choose to define success differently, we considered an optimal bubble diameter to be one that is greater than the diameter of the trephine used during surgery. This is because the trephination line serves as the boundary for the anterior cornea to be removed post air injection. In order to minimize disruption of Descemet’s membrane during anterior corneal removal, there must be some degree of separation between Descemet’s membrane and the stroma at the trephination line. We used an 8mm trephine, but acknowledge that surgeons may elect to use other trephine sizes, which may relax or further constrict the parameters for an “optimal diameter.” We also acknowledge that in contrast to this experimental setting, which used a fixed volume of 5 mL for air injection in order to limit confounding variables, in clinical practice, surgeons can continue to inject more air in an attempt to increase the size of the bubble once the initial bubble is created.
This study required controlled manipulation of IOP and paired donor tissue, making it well suited for an ex-vivo study. Paired DALK in a single patient would be infrequent, and at best, would be separated by time; hence an ex-vivo setting, as used in this study, would be optimal for creating a paired experimental/control scenario. Precise control of IOP in the in-vivo setting would also have been impractical, given native individual fluctuations in IOP and variable pressure changes from active attempts to change IOP (e.g. a pre-air injection paracentesis may over or undershoot a targeted IOP). In our ex-vivo model, we mimicked in-vivo parameters as best as possible by using human corneo-scleral buttons from donors who had deceased within less than one week. This was to minimize corneal edema and maintain CCT values at baseline. However, previous work9 has shown no relationship between CCT and BB formation. As DALK becomes more widespread clinically, future work to investigate the in-vivo correspondence of this relationship between IOP and BB formation can be performed. Such work may also allow investigators to explore alternative techniques for performing and troubleshooting DALK procedures.
In summary, physiologic, but not non-physiologic, IOPs yielded BBs of optimal size for dissection during DALK. While BBs also formed at lower and higher IOPs, needle-tissue interactions and resistance led to lower BB formation rates, and BBs were of sub-optimal size. To the best of our knowledge, this work represents the first demonstration of the effect of IOP on BB formation. The use of an ex-vivo model and SS-MIOCT with HUD allowed us to control for other factors that may impact BB formation. Our results have implications for surgical practice and may impact a surgeon’s pre and peri-operative plans.
Acknowledgments
Funding/Support: NIH Biomedical Research Partnership Grant R01-EY023039, NIH R01-EY024312, and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Duke University School of Medicine.
Other Acknowledgements: We would like to thank Miracles in Sight for graciously providing us with all the human corneo-scleral buttons used in this study.
Footnotes
Financial Disclosures: At the time this study was conducted, J.A.I. was Chairman and Chief Scientific Advisor for Bioptigen, Inc. (since acquired by Leica Microsystems) and had corporate, equity, and intellectual property interests (including royalties) in this company. C.A.T. and J.A.I. are inventors on pending and disused patents pertaining to the intraoperative visualization technologies described in this paper. C.A.T. has surgical technologies patents licensed by Duke University to Alcon and receives financial support from Genentech. A.N.K. has an image correction algorithm patent licensed by Duke University to Bioptigen. The remaining authors do not have any disclosures to declare.
References
- 1.Terry MA. The evolution of lamellar grafting techniques over twenty-five years. Cornea. 2000;19(5):611–6. doi: 10.1097/00003226-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Abdelkader A. Influence of different keratoplasty techniques on the biomechanical properties of the cornea. Acta Ophthalmol. 2013;91(7):e567–72. doi: 10.1111/aos.12136. [DOI] [PubMed] [Google Scholar]
- 3.Bahar I, Kaiserman I, Srinivasan S, et al. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146(6):905–12.e1. doi: 10.1016/j.ajo.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Tan DT, Anshu A, Parthasarathy A, et al. Visual acuity outcomes after deep anterior lamellar keratoplasty: a case-control study. Br J Ophthalmol. 2010;94(10):1295–9. doi: 10.1136/bjo.2009.167528. [DOI] [PubMed] [Google Scholar]
- 5.Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002;21(4):374–83. doi: 10.1097/00003226-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ang M, Mohamed-Noriega K, Mehta JS, et al. Deep anterior lamellar keratoplasty: surgical techniques, challenges, and management of intraoperative complications. Int Ophthalmol Clin. 2013;53(2):47–58. doi: 10.1097/IIO.0b013e31827eb746. [DOI] [PubMed] [Google Scholar]
- 7.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 8.Pasricha ND, Shieh C, Carrasco-Zevallos OM, et al. Needle Depth and Big Bubble Success in Deep Anterior Lamellar Keratoplasty: An Ex Vivo Microscope-Integrated OCT Study. Cornea. doi: 10.1097/ICO.0000000000000948. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorcia V, Busin M, Lucisano A, et al. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology. 2013;120(3):471–6. doi: 10.1016/j.ophtha.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Borderie VM, Sandali O, Bullet J, et al. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119(2):249–55. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YY, Visser N, Schouten JS, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology. 2011;118(2):302–9. doi: 10.1016/j.ophtha.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117–124. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Han DC, Mehta JS, Por YM, et al. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009;148(5):744–751.e1. doi: 10.1016/j.ajo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–50. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sit M, Weisbrod DJ, Naor J, et al. Corneal graft outcome study. Cornea. 2001;20(2):129–33. doi: 10.1097/00003226-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116(6):1023–8. doi: 10.1016/j.ophtha.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anshu A, Lim LS, Htoon HM, et al. Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol. 2011;151(3):442–8.e1. doi: 10.1016/j.ajo.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Stewart RM, Jones MN, Batterbury M, et al. Effect of glaucoma on corneal graft survival according to indication for penetrating keratoplasty. Am J Ophthalmol. 2011;151(2):257–62.e1. doi: 10.1016/j.ajo.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Wagoner MD, Ba-Abbad R, Al-Mohaimeed M, et al. Postoperative complications after primary adult optical penetrating keratoplasty: prevalence and impact on graft survival. Cornea. 2009;28(4):385–94. doi: 10.1097/ICO.0b013e31818d3aef. [DOI] [PubMed] [Google Scholar]
- 20.Rahman I, Carley F, Hillarby C, et al. Penetrating keratoplasty: indications, outcomes, and complications. Eye (Lond) 2009;23(6):1288–94. doi: 10.1038/eye.2008.305. [DOI] [PubMed] [Google Scholar]
- 21.Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855–65. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 22.Viehland C, Keller B, Carrasco-Zevallos OM, et al. Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT. Biomed Opt Express. 2016;7(5):1815–29. doi: 10.1364/BOE.7.001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steven P, Le Blanc C, Lankenau E, et al. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT) Br J Ophthalmol. 2014;98(7):900–4. doi: 10.1136/bjophthalmol-2013-304585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L, Carrasco-Zevallos O, Keller B, et al. Novel microscope-integrated stereoscopic heads-up display for intrasurgical optical coherence tomography. Biomed Opt Express. 2016;7(5):1711–26. doi: 10.1364/BOE.7.001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep. 2016;6:31689. doi: 10.1038/srep31689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrasco-Zevallos OM, Keller B, Viehland C, et al. Optical Coherence Tomography for Retinal Surgery: Perioperative Analysis to Real-Time Four-Dimensional Image-Guided Surgery. Invest Ophthalmol Vis Sci. 2016 Oct;57(9):37–50. doi: 10.1167/iovs.16-19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin RC, Shure MA, Rollins AM, et al. Group index of the human cornea at 1.3-microm wavelength obtained in vitro by optical coherence domain reflectometry. Opt Lett. 2004;29(1):83–5. doi: 10.1364/ol.29.000083. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Kuo AN, Izatt JA. 3D refraction correction and extraction of clinical parameters from spectral domain optical coherence tomography of the cornea. Opt Express. 2010;18(9):8923–36. doi: 10.1364/OE.18.008923. [DOI] [PubMed] [Google Scholar]


