Abstract
Introduction
Metastasis contributes to over 90% of cancer-related deaths. Numerous nanoparticle platforms have been developed to target and treat cancer, yet efficient delivery of these systems to the appropriate site remains challenging. Leukocytes, which share similarities to tumor cells in terms of their transport and migration through the body, are well suited to serve as carriers of drug delivery systems to target cancer sites.
Areas covered
This review focuses on the use and functionalization of leukocytes for therapeutic targeting of metastatic cancer. Tumor cell and leukocyte extravasation, margination in the bloodstream, and migration into soft tissue are discussed, along with the potential to exploit these functional similarities to effectively deliver drugs. Current nanoparticle-based drug formulations for the treatment of cancer are reviewed, along with methods to functionalize delivery vehicles to leukocytes, either on the surface and/or within the cell. Recent progress in this area, both in vitro and in vivo, is also discussed, with a particular emphasis on targeting cancer cells in the bloodstream as a means to interrupt the metastatic process.
Expert opinion
Leukocytes interact with cancer cells both in the bloodstream and at the site of solid tumors. These interactions can be utilized to effectively deliver drugs to targeted areas, which can reduce both the amount of drug required and various nonspecific cytotoxic effects within the body. If drug delivery vehicle functionalization does not interfere with leukocyte function, this approach may be utilized to neutralize tumor cells in the bloodstream to prevent the formation of new metastases, and also to deliver drugs to metastatic sites within tissues.
Keywords: cancer, leukocyte, metastasis, nanomedicine
1. Introduction
Cancer is one of the leading causes of death, with metastasis the cause of over 90% of cancer-related mortality [1]. Metastasis is initiated when cancer cells from a primary tumor invade the surrounding tissue, where they can then enter the bloodstream or lymphatic system to translocate to anatomically distant organs. Tumor cells may then exit the circulation, migrate into tissues, and proliferate to form secondary tumors. Surgical intervention, chemotherapy, and radiation are typically effective at treating primary tumors. However, metastases are difficult to detect, target, and treat therapeutically, and typically signal a poor patient prognosis, as only one in five patients diagnosed with metastatic cancer will survive > 5 years [1,2].
Nanoparticles have shown promise in the treatment of cancer. Perhaps one of the most well-known nanoparticle formulations currently in the clinic is liposomal doxorubicin (LP-Dox) (Doxil®), used to treat over 300,000 patients annually for Kaposi’s sarcoma and ovarian cancer [3]. The advent of nanoparticles has reduced systemic toxicity of traditionally administered chemotherapeutics, enabled the controlled release of multiple small-molecule drugs and proteins for continuous therapeutic delivery, and led to the development of targeting specific tumor cells within the body. Despite these advancements, it remains a challenge to deliver nanoparticle platforms in patients with advanced forms of cancer. Most nanoparticle formulations rely on the enhanced permeation and retention (EPR) effect for delivery to tumors, but the experimental patient data supporting this mechanism are limited [4], and elevated fluid pressures within the tumor can act to transport therapeutics back into the bloodstream. Additionally, when cancer cells enter the bloodstream as circulating tumor cells (CTCs), they are difficult to target before metastasis formation due to the fact that they are surrounded by billions of blood cells within vessels. Since nanoparticle platforms are typically administered systemically, disseminated tumor cells in tissues without a well-defined vascular structure can also be difficult to reach [2]. Additionally, hypoxic regions of tumors do not have a well-defined vasculature, making systemic of delivery of nanoparticles inefficient [5,6]. For advanced nanoparticle platforms to fulfill their potential, new strategies must be developed to locally guide nanoparticles to poorly vascularized tumor tissue and CTCs.
Leukocytes have recently received much attention in the treatment of cancer, particularly in the field of cancer immunotherapy, which utilizes the innate ability of leukocyte subpopulations to elicit antitumor immunity [7]. In a distinct avenue of therapy, leukocytes, which share similar migration patterns to tumor cells in blood and tissue, can also be utilized to carry current nanoparticle formulations to tumor sites that are difficult to reach via systemic administration of nanoparticles alone. Herein, we review recent advances in utilizing leukocytes as carriers of nanoparticles for targeted cancer drug delivery. We first discuss current nanoparticle platforms that are utilized in the treatment of cancer. Similarities in leukocyte and tumor cell migration in complex microenvironments such as blood and tumor tissue are then reviewed, with particular emphasis on cell margination in blood, adhesive interactions with the vessel wall, and migration along chemoattractant gradients to tumors and inflammatory sites. Methods to functionalize nanoparticles to leukocytes are also discussed, such as surface functionalization and internalization within cells. Finally, we review recent in vitro and in vivo strategies that have been developed to use leukocytes to deliver cancer drugs to tumors and CTCs.
2. Current nanoparticle platforms for delivery of cancer drugs
Various nanoparticle formulations have been developed for the delivery of cancer drugs, and have already been discussed in detail elsewhere [2,8,9]. Here, currently utilized nanoparticle platforms are overviewed within the following broad categories: polymeric nanoparticles, liposomes, metals, carbon and halloysite nanotubes, and molecular targeted nanoparticles (Table 1).
Table 1.
Advantages and challenges for delivery of current nanoparticle systems.
| Type of nanoparticle | Advantages | Challenges to delivery |
|---|---|---|
| Polymeric nanoparticles | Biodegradable, biocompatible, tunable release profile, well characterized | Structural heterogeneity as reflected by high polydispersity index |
| Liposomes | Effective delivery of both water-soluble and insoluble drugs, easily tailored size and carrying capacity | Instability, burst drug release, clearance to reticuloendothelial system |
| Metal nanoparticles | Effective thermoablative vehicles, imaging agents for cancer detection and diagnosis | Toxic effects on body |
| Carbon nanotubes | Extensive surface area, effective thermoablative agents, can be loaded efficiently with drugs | Inflammatory side effects, potentially toxic to some cell types |
| Molecularly targeted nanoparticles | Targeting of specific cell types, partitioning of more nanoparticles to targeted tissues, variety of targeting ligands available | Selection of proper ligands expressed on targeted cells and not undesired cell populations, reliance on EPR effect |
2.1 Polymers
Polymeric materials comprise perhaps the largest category of nanoparticles for the delivery of cancer drugs, with many formulations currently in preclinical or clinical trials [9,10]. Composed mainly of biocompatible and biodegradable polymers, most polymeric nanoparticles are synthesized using a self-assembly process using block copolymers consisting of multiple polymers of varying hydrophobicity. In an aqueous environment the copolymers will form core-shell particles, with hydrophobic polymers forming the core to minimize aqueous exposure, and hydrophilic polymers forming the shell to stabilize the core (Table 1) [11–13]. The shell of the nanoparticle provides steric protection, while the core allows for a high loading capacity of hydrophobic small-molecule drugs, in addition to macromolecules such as nucleic acids and proteins [14]. Poly (lactic-co-glycolic acid) (PLGA)-based biodegradable nanoparticles are perhaps the most notable, as these materials are approved by the US Food and Drug Administration and can be utilized to deliver a variety of cancer drugs [15,16].
Polymers such as poly(lactic acid) (PLA) and chitosan have also been utilized to develop polymeric nanoparticles [17,18]. Like PLGA, PLA nanoparticles possess desirable biodegradable properties with low toxicity [19], in addition to slightly negative surface charge. PLA nanoparticles have been used to create nanoparticle–aptamer bioconjugates with RNA aptamers to specifically target drugs to prostate-specific membrane antigen, a marker overexpressed in prostate cancer epithelial cells [11]. Chitosan, a polysaccharide with structural characteristics similar to glycosaminoglycans [20,21], has been widely utilized in the pharmaceutical and biomedical fields due to its biodegradable and biocompatible properties, in addition to its ability to serve as a carrier of hydrophilic drugs [22,23]. Encapsulation of doxorubicin–dextran conjugates within chitosan nanoparticles has been shown to reduce the toxic side effects of doxorubicin while enhancing the therapeutic efficacy in the treatment of solid tumors in vivo [22].
One concern with utilizing polymeric nanoparticles is that most possess a heterogeneous nanoparticle structure, as evidenced by a high polydispersity index (Table 1). However, certain methods can now produce polymeric nanoparticles with near-homogeneous size distributions [24]. A key advantage of polymeric nanoparticle systems is their numerous controlled-release properties. The release of drugs from polymeric nanoparticles can be controlled by a variety of mechanisms, including surface or bulk erosion, diffusion through the polymer matrix, swelling or shrinking followed by diffusion, and by local changes in the environment, such as pH and temperature changes [2].
2.2 Liposomes
Lipids are amphiphilic small molecules that can self-assemble into nanoparticles referred to as liposomes, spherical nanoscale vesicles that possess an aqueous core. Perhaps one of the first nanoparticle platforms to be utilized in medicine [25], liposomes contain either a single or multiple bilayers consisting of a variety of lipid types, both natural and synthetic [26]. Liposomes can be easily tailored in terms of size and carrying capacity, and can range from tens to hundreds of nanometers in diameter (Table 1). Their versatility as a therapeutic carrier for both water-soluble and insoluble drugs is a key advantage, as the aqueous core of liposomes allows for the encapsulation of hydrophilic agents, while the lamellae can be utilized for the encapsulation of hydrophobic agents.
Liposomal nanoparticles do pose delivery challenges, however, due to their instability and burst drug release. Additionally, liposomes rapidly clear to the reticuloendothelial system upon injection into the bloodstream, and can also be uptaken by phagocytic leukocytes. To combat this effect, liposomes have been coated with PEG to enhance circulation time and improve stability [26]. Clinically, over a dozen liposomal formulations are approved for use, including Myocet™ and Doxil, with many others currently undergoing preclinical and clinical trials [27,28].
2.3 Metals
Metal nanoparticles, typically composed of biocompatible, inert metals such as gold and titanium, have been extensively studied as a therapeutic platform for thermoablation of tumor cells [29]. Under exposure to near-infrared (NIR) light, gold nanoparticles such as nanoshells and nanorods can release energy, which heat tumor tissue and result in tumor cell death (Table 1) [30]. This can also cause coagulation within the tumor vasculature, which can enhance the effects of other targeted therapeutics [31]. Conversely, metal nanoparticles have also been utilized for the controlled release of chemotherapeutics [32], and can be functionalized with therapeutic ligands [33]. Various forms of metal nanoparticles have proven to be effective imaging agents for diagnosis and early detection of cancer [34,35]. The long-term effects of metal nanoparticle delivery of cancer therapeutics are still being investigated, as a fraction of the particles are retained within the body after administration, and can induce unwanted toxicity after repeated doses [36].
2.4 Carbon nanotubes
Carbon nanotubes (CNTs) are well-ordered allotropes of carbon with a cylindrical nanostructure. CNTs have a uniquely high aspect ratio, with lengths ranging from hundreds of nanometers to several micrometers, and diameters ranging from 0.4 to 2.0 nm for single-walled CNTs (SWNTs) to 2–100 nm for multi-walled CNTs [37]. Similar to metal nanoparticles, SWNTs can emit heat when they absorb energy from NIR light. The extensive surface area of SWNTs has been utilized for functionalization of antibodies specific to tumor cells for highly specific thermal ablation (Table 1) [38]. The surface area of SWNTs has also been utilized for efficient loading of chemotherapeutics [39,40]. However, further investigation into the potential cytotoxic effects of CNTs is necessary. In terms of the immune system, CNTs have been shown to be phagocytosed by B- and T-lymphocytes, without affecting cell viability or functionality [41]. However, high concentrations of SWNTs can arrest cell division and induce apoptosis [42]. Chronic inhalation of SWNTs can also be a hazard, and has been shown to induce inflammation and the appearance of epithelial granulomas [43].
2.5 Molecularly targeted nanoparticles
The development of targeted cancer therapies has led to major breakthroughs in the treatment of cancer over the past two decades. Notable examples of targeted therapeutics include bevacizumab (Avastin®), which targets VEGF to inhibit tumor angiogenesis [44,45], imatinib (Gleevec®) for inhibiting tyrosine kinases to treat chronic myelogenous leukemia [46], and trastuzumab (Herceptin®) to target human EGFR type 2 for the treatment of breast cancer [47,48]. The success of these therapies has led to extensive studies combining targeting molecules with therapeutic-containing nanoparticles. Ligands or antibodies conjugated to nanoparticles typically target proteins on the tumor cell surface, which can then be internalized by tumor cells to deliver therapeutics. This approach can partition more nanoparticles to targeted tissues, enhance the concentration of drug achieved within tumors, and limit off-target cytotoxic effects (Table 1). However, expression of the targeted protein on undesired cell populations can lead to off-target effects, and must be taken into account when choosing an appropriate molecule as the target for targeted nanoparticles.
Despite the recent technological advances in nanoparticle platforms for efficient loading and controlled release of therapeutics, local delivery to cancer sites remains a challenge. Most nanoparticle formulations, even targeted systems, rely on the EPR effect for delivery to tumor sites (Table 1). The EPR effect is a combination of: i) enhanced permeability due to a leaky, disorganized tumor vasculature, allowing nanoparticles to enter the tumor interstitial space; and ii) retention of nanoparticles due to a lack of effective lymphatic drainage [49]. This approach has been met with limited success, however, due to factors including elevated interstitial fluid pressure in tumors that can hinder the transport of nanoparticles across the vessel wall [4,49]. Additionally, there is little experimental data in patients that support this mechanism [4]. The majority of nanoparticle formulations injected systemically rapidly clear to the reticuloendothelial system [50].
In addition to these delivery obstacles, systemic delivery of nanoparticles is challenged by the lack of vasculature in hypoxic regions of tumors, and the ‘needle in a haystack’ problem of targeting and treating CTCs in the complex milieu of blood. Thus, new strategies need to be devised to guide nanoparticle therapeutics to target tumor cells.
3. Tumor cell and leukocyte similarities in function
Leukocytes and tumor cells share common physical properties and adhesive interactions with vascular components. Similarities including their transport within blood, adhesion to the vessel wall, and migration into inflammatory sites in soft tissue can potentially be exploited for efficient delivery of therapeutics.
3.1 Margination of CTCs and leukocytes
Despite the fact that CTCs within the bloodstream are exposed to a variety of factors in blood that affect their viability, including immunological stresses, blood cell collisions, and fluid shear stress [51,52], a small fraction of these cells are able to survive these conditions, and proliferate to form metastases in anatomically distant organs. CTCs within the bloodstream are very difficult to target and treat within the bloodstream, as the concentration of CTCs in patient blood is on the order of one in a million leukocytes [53] or one in a billion erythrocytes [54], creating what is known as a ‘needle in a haystack’ problem. However, what is known about rare CTCs is that they share similar migration characteristics with leukocytes in the bloodstream. Leukocytes tend to collect near the endothelial cell wall in blood vessels, rather than in the center of the vessel, in a passive rheological mechanism termed margination (Figure 1A) [55,56]. During this phenomenon, highly deformable, biconcave-shaped erythrocytes experience a drift velocity away from the vessel wall and collect in the center of vessels, displacing less deformable leukocytes toward the periphery [57–59]. CTCs, which are closer to the larger volume and spherical shape of leukocytes than the deformable erythrocytes, are also pushed toward the endothelial cell wall. Such margination phenomena can effectively surround CTCs within the circulating leukocyte population, thus making leukocytes a potentially attractive carrier of treatments to CTCs by exploiting their numerous adhesion receptors.
Figure 1. Tumor cell and leukocyte trafficking similarities.
(A) Margination in the vascular system. Deformable red blood cells drive leukocytes and CTCs to a marginated position near the vessel wall, due to their higher drift velocity away from walls. (B) Adhesion to inflamed blood vessels. Upon the onset of inflammation, endothelial cells upregulate E-selectin expression, which can cause free-flowing, selectin ligand-bearing leukocytes to adhesively interact with the endothelial cell wall. CTCs are also known to express sialylated carbohydrate ligands on their surface, which can adhere to selectins on the surface of the endothelium. (C) Transmigration into tissues. Upon firm adhesion to the endothelial cell wall, leukocytes are known to transmigrate into the extravascular space and migrate along chemoattractant gradients to the site of inflammation. CTCs also respond to chemoattractants and transmigrate to inflammatory sites, where they may then survive and form micrometastases.
CTC: Circulating tumor cell; ES: E-selectin.
3.2 Adhesion of tumor cells and leukocytes to the blood vessel wall
For free-flowing tumor cells and leukocytes to leave the bloodstream and migrate into soft tissues, they must first adhesively interact with endothelial cells that comprise the inner blood vessel wall (Figure 1B) [60]. The mechanisms behind leukocyte recruitment to the endothelial cell wall have been extensively studied over the past two decades [61,62]. Initially, free-flowing leukocytes in the post-capillary venules are captured along the endothelial cell wall, in the presence of wall shear stresses as low as 0.4–0.5 dyn/cm2 [63–65]. After initial tethering, leukocytes exhibit rolling adhesion on the receptor-bearing endothelial cell wall. Rolling adhesion is mediated by selectins expressed on the surface of inflamed endothelial cells, which possess rapid, force-dependent binding kinetics, and selectin ligands on the surface of leukocytes including L-selectin and P-selectin glycoprotein ligand-1 [66–69]. Leukocytes can subsequently transition from rolling to firm adhesion to the vessel wall, mediated via intercellular adhesion molecule-1 (ICAM-1) on the endothelial cell-surface binding to β2 integrins on the leukocyte surface, such as macrophage-1 antigen and lymphocyte function-associated antigen-1 [70–73].
CTCs can be captured on the endothelial cell wall in a manner similar to leukocytes (Figure 1B). Recent studies have shown that CTCs from various types of tumors possess sialylated carbohydrate ligands on their surface, which cause the tethering and rolling of CTCs on the selectin-bearing endothelial cell wall [74,75]. Under physiological flow conditions, E-selectin can induce tethering and rolling of cancer cells originating from prostate [76,77], breast [78,79], and colon [78,80]. Initial selectin-mediated rolling adhesion of CTCs has been shown to be an important prerequisite to metastasis formation in vivo [81,82]. For example, the number of spontaneous colon carcinoma metastases formed in vivo decreased by 84% in P- and E-selectin-deficient mice [83]. The remaining 16% of metastases formed within the pulmonary artery as CTCs were not able to transmigrate through the endothelium in the absence of E-selectin. It is not yet fully elucidated which receptors facilitate firm adhesion of CTCs to the endothelial cell wall; however, there is some evidence that the mucin MUC1 can ligate ICAM-1 to enable firm adhesion and subsequent transmigration [84]. Taken together, CTCs and leukocytes share striking similarities in terms of their initial adhesion to the vessel wall. This makes selectin receptors a potentially important mediator between leukocytes and CTCs for drug delivery purposes.
3.3 Tumor cell and leukocyte migration toward chemoattractant gradients
Following firm adhesion to the blood vessel wall, leukocytes and tumor cells can transmigrate through the endothelium and migrate along gradients of extracellular matrix-bound or soluble chemoattractants to inflammatory sites and solid tumors (Figure 1C). Chemoattractants bind to G-protein–coupled receptors on the surface of leukocytes to activate downstream effectors, which initiate receptor internalization and signal transduction [85,86]. Such signaling induces the activation of β2 integrins on the leukocyte surface, which induces cell adhesion, and polarization of the actin cytoskeleton, which facilitates directional sensing and cell polarization [87,88]. Polarization allows small GTPases, Rac, Cdc42, and PI3K to accumulate at the leading edge of the leukocyte, PTEN tyrosine/PIP3 phosphatases at the posterior edges, and Rho GTPases and its effectors at the trailing edge [85]. These collectively initiate actomyosin contraction and tail retraction, which induce leukocyte migration [89]. The ability of leukocytes to respond and migrate in the presence of chemoattractants is essential in the response to inflammation, infection, and lymphocyte homing to tissues, to name a few [73,90,91].
Chemoattractant gradients also play a major role in tumor cell and leukocyte migration in the context of cancer progression [92,93]. In the tumor microenvironment, cancer epithelial cells produce higher levels of chemokines than their normal counterparts, in addition to increased expression of chemokine receptors [94]. This creates a highly inflammatory micro-environment that promotes the recruitment of leukocytes such as neutrophils, lymphocytes, and macrophages, which can influence the progression of cancer and its ability to metastasize [95–98]. Additionally, tumor cells can acquire chemoattractant receptors during transformation, and migrate along gradients typically utilized by leukocytes to form metastases in anatomically distant organs [86]. Leukocyte and tumor cell localization within difficult-to-reach tumor sites, along with their similarities in terms of migration to tissues, can be utilized to locally deliver agents such as therapeutics to induce cancer cell death, or anti-inflammatory agents to suppress the ability of leukocytes to promote the tumor microenvironment.
4. Methods to attach nanoparticles to leukocytes
The drug delivery vehicles described above can be attached to leukocytes using a variety of methods, ranging from cell-surface functionalization to internalization within leukocytes. Depending on the type of therapeutic delivered, the type of leukocyte targeted, and the site of delivery, one or a combination of functionalization methods may be needed for nanoparticle attachment.
4.1 Receptor-mediated adhesion
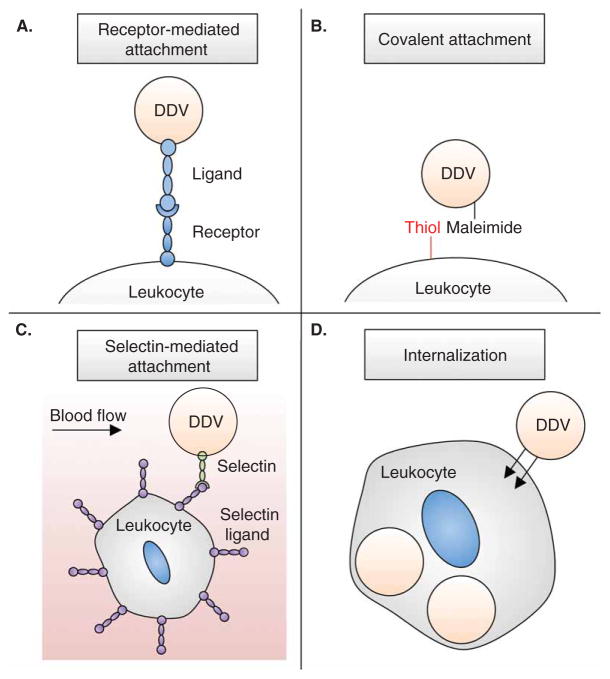
Receptor–ligand interactions are a potentially advantageous method to bind nanoparticles to the surface of leukocytes (Figure 2A), due to their reliability, reproducibility, and ability to trigger potentially desired cell-receptor activation and signal transduction in vivo. For example, B-cells expressing CD44 have been utilized to bind cellular ‘backpacks’ via interactions with its natural ligand, hyaluronic acid [99]. This approach has also been utilized to bind delivery vehicles to T-cells and macrophages [100–102]. Natural variations in receptor–ligand affinities must be taken into account, and could affect the binding strength of a nanoparticle to the leukocyte surface. Certain receptors on the leukocyte surface can also undergo matrix metalloproteinase-mediated shedding [103,104], which can act to cleave nanoparticles bound to the leukocyte surface. Receptors can also be internalized by leukocytes due to a variety of factors, one being fluid shear stress exposure [69,105], and must be taken into account if surface presentation of nanoparticles is desired rather than loading within the cell. Additionally, potential nonspecific receptor–ligand interactions with off-target cells, along with subsequent receptor activation and signaling, must be considered for in vivo applications. If utilized properly, however, multiple receptor types may be conjugated to a single particle to mediate the delivery of therapeutics between two cell types, such as leukocytes and cancer cells.
Figure 2. Approaches to functionalize leukocytes with drug delivery vehicles (DDVs).
(A) Receptor–ligand interactions to attach DDVs to the leukocyte surface. (B) Covalent binding of maleimide-bearing DDVs to thiol groups on the protein-covered leukocyte surface. (C) Selectin-coated DDVs bind to leukocytes within the circulation under flow conditions. (D) Internalization of DDVs by phagocytic leukocytes.
4.2 Covalent binding
Covalent coupling of nanoparticles to the surface of leukocytes provides potentially stronger and more stable binding than receptor-mediated adhesion (Figure 2B). Traditionally utilized to bind maleimide-functionalized nanoparticles to amino (lysine-NH2) or thiol (cysteine-SH) groups on proteins [106,107], similar covalent binding strategies have been used to stably bind nanoparticles to the surface of cells. Leukocytes in particular have many functional groups on their surface, such as thiols and amines, due to their large number of cell-surface proteins [108,109]. This approach has been utilized to stably attach nanoparticles, composed of materials ranging from liposomes to polymers, to the surface of T-lymphocytes without cytotoxic effects or loss of cell function [110]. In contrast to nanoparticles adsorbed to the surface, which can be removed during simple washing, covalent binding promoted stable attachment that can withstand several washing steps. In addition to prolonged surface retention, covalent binding of nanoparticles to the cell surface can resist nanoparticle internalization, along with potentially undesired receptor activation that could be triggered by receptor-mediated attachment.
4.3 Selectin-mediated adhesion
Selectins provide a unique and effective way to rapidly attach nanoparticles to the surface of cells bearing selectin ligands (Figure 2C). The rapid, force-dependent binding kinetics of selectins allow for nanoparticles to rapidly bind to the surface of selectin ligand-bearing cells under flow conditions. In particular, in vitro surfaces with immobilized P-selectin–coated liposomes attached themselves to the surface of free-flowing HL60 leukocytes. Utilizing E-selectin–coated liposomes containing the chemotherapeutic doxorubicin, this strategy has also been utilized to attach drug-loaded particles to the surface of free-flowing cancer cells as a form of CTC neutralization [111,112]. Most recently, selectin-coated nanoparticles have been shown to attach to the surface of leukocytes directly within human blood and within the circulation of mice [113]. This method is currently being employed to deliver apoptosis-inducing ligands to cancer cells in the circulation. Given that selectin ligands are expressed on the surface of most circulating leukocytes [67,90], selectin-mediated adhesion is advantageous for functionalizing a broad range of cells for therapeutic delivery within the circulation. In contrast to other forms of receptor-mediated attachment, selectins can be used for direct binding to leukocytes within the blood, eliminating the need to isolate leukocytes from blood, with subsequent incubation and culture steps needed for nanoparticle attachment.
4.4 Internalization
Nanoparticles can also be internalized by leukocytes for efficient delivery of therapeutics. One method to internalize particles is through the use of the natural phagocytic properties of certain leukocyte subpopulations to ingest particles (Figure 2D) [114,115]. Subpopulations of neutrophils, monocytes, and macrophages all possess phagocytic properties; however, monocytes and macrophages are particularly appropriate due to their longer lifespan and ability to be cultured ex vivo. Known as the ‘Trojan Horse’ method, this approach has been used to internalize particles such as LP-Dox, gold nanoshells, fluorescent microspheres, and nanozymes within leukocytes [30,116–119]. It is important to note that this approach has been limited to certain subpopulations of leukocytes, and thus requires careful ex vivo isolation from blood. Intracellular trafficking of nanoparticles must be taken into account, as internalized nanoparticles can remain trapped within endosomes and either be targeted to lysosomes for degradation [120], or recycled from the cell [121]. Nanoparticle size, shape, material, surface chemistry, and charge must be taken into account to escape endosomes for release into the leukocyte cytoplasm. Once there, however, biodegradable nanoparticles such as polymers and lipids can potentially release therapeutic within the cell, thus reducing the amount of drug delivered to the target site, and potentially causing unwanted side effects to the leukocyte carrier. The ‘Trojan Horse’ approach has unique advantages, however, in that it eliminates the need to occupy the cell membrane and potentially interfere with receptor–ligand interactions. Additionally, this approach avoids potential cell-surface receptor shedding, signaling, and internalization that can be encountered with receptor-mediated attachment of nanoparticles.
5. Leukocytes as carriers of nanoparticles to metastatic cells and tumors: initial results
In recent years, initial results have shown that leukocytes are effective at carrying drugs and nanoparticles to difficult-to-target sites for cancer therapy. Nanoparticles can either be stably attached to the leukocyte surface, or internalized within the cell, to efficiently deliver therapeutics to tumors and to rare CTCs within the bloodstream.
5.1 Leukocyte ‘trojan horses’ for nanoparticle delivery to solid tumors
Initial studies focused on utilizing leukocytes as ‘Trojan Horses’ for delivery of therapeutics to solid tumors. As tumors grow rapidly, the tumor core becomes distanced from the nearest capillaries, causing tumor cells to become necrotic and/or hypoxic [5,6]. Additionally, the lack of a vasculature hinders the delivery of nanoparticle-based therapeutics to the core region of the tumor. While nanoparticles may lack the ability to reach these sites, monocytes can be recruited from the peripheral blood to sites of tumors due to chemoattractant gradients. Additionally, monocytes have an innate phagocytic ability, which can be utilized to internalize nanoparticles as a means to carry therapeutics to tumors (Figure 3A).
Figure 3. Cellular ‘Trojan Horse’ mechanism to deliver nanoparticle-based therapeutics to solid tumors.
(A) Monocytes and/or macrophages that typically infiltrate solid tumors internalize therapeutic and/or diagnostic nanoparticles for delivery into tumor sites. (B) Monocytes and macrophages containing gold nanoshells can be photoablated by near-infrared (NIR) light, inducing cell death and releasing therapeutics and/or nanoshells for uptake into tumor cells. (C) Tumor cell death then occurs via (1) therapeutic delivery in tumor cells or (2) heating of nanoshell-containing tumor tissue by NIR.
hv: Near-infrared light; NIR: Near-infrared.
Gold nanoshells have been suggested as an ideal therapeutic to be utilized within cellular ‘Trojan Horses’ [30], given that they can be uptaken by tumor cells and induce cell death by photoablation via NIR light, which can increase tumor tissue temperature by over 30°C in the presence of nanoshells [122]. Initial work has shown that monocytes successfully phagocytose gold nanoshells over a period of 24 h. Macrophages, which are differentiated from monocytes upon migration into tumors, also successfully phagocytosed gold nanoshells over similar incubation periods. Cell death was induced in nanoshell-containing macrophages via photoablation (Figure 3B), which induces the release of nanoshells within the tumor to allow for tumor cell uptake, and subsequent heating and killing of tumor tissue via NIR (Figure 3C). In an in vitro model of the macrophage infiltration of the tumor microenvironment, macrophages and gold nanoshells were co-cultured with breast tumor spheroids. Macrophages were found to infiltrate tumor spheroids in vitro, and cell death of macrophages was induced by photoablation.
The ‘Trojan Horse’ approach has also been exploited to deliver nanoparticles to experimental brain metastases. Nanoparticle uptake in the brain is hindered by physical barriers such as the blood–brain barrier (BBB) and blood–cerebrospinal fluid barrier [123,124]. However, macrophages of peripheral blood monocyte origin are able to infiltrate brain metastases despite the presence of an intact BBB [125], and are also present in clinical brain tumor specimens at contents ranging from 4 to 70% [126]. Thus, macrophages have been utilized as ‘Trojan Horses’ to cross the BBB and deliver therapeutics to metastatic deposits in the brain. Both nanoshells and fluorescent microspheres, utilized for therapeutic and diagnostic purposes, respectively, were successfully phagocytosed and localized within vacuoles in the cytoplasm of monocytes and macrophages [117]. Utilizing brain tumor xenografts in mice, both macrophages and internalized microspheres were found within brain metastases, 24 h post-injection into mice.
The ‘Trojan Horse’ approach can also be used to deliver chemotherapeutics to tumors in vivo [116]. Utilizing mouse peritoneal macrophages, which were shown to migrate into A549 subcutaneous tumors in vivo, LP-Dox was internalized within macrophages. These remained viable for over 12 h, even at high concentrations of LP-Dox. LP-Dox was released from macrophages and induced cell death in A549 cancer cells in vitro. Upon injection in vivo, macrophages containing LP-Dox infiltrated the interior of A549 subcutaneous tumors, and their migration was characterized via the intrinsic fluorescence of doxorubicin. Additionally, tumor treatment with systemic injections of macrophages containing LP-Dox induced A549 subcutaneous tumor reduction over a 35-day span, compared to controls. Taken together, the ability of monocytes and macrophages to migrate along chemoattractant gradients and penetrate the BBB can be utilized in the ‘Trojan Horse’ approach to deliver a variety of nanoparticle-based therapeutics and imaging agents to tumor tissues that are typically difficult to target via systemic injection.
Most recently, SWNTs were found to be uptaken almost exclusively by a single subpopulation of leukocytes [127]. SWNTs were injected into the tail vein of mice, and intravital microscopy was used to observe that nanotubes were uptaken into circulating blood cells. Upon blood draw and subsequent Fluorescence-activated cell sorting analysis of SWNT+ blood cells, only a specific monocyte subset, known as Ly-6Chi lymphocytes, displayed substantial SWNT uptake. Interestingly, only < 3% of neutrophils, < 1% of lymphocytes, and < 1% of Ly-6Clow lymphocytes took up SWNTs, while nearly 100% of Ly-6Chi lymphocytes displayed SWNT uptake. The sub-population of monocytes containing SWNTs were able to enter the tumor interstitium, and SWNTs functionalized with an arginylglycylaspartic acid (RGD) peptide significantly enhanced the number of monocytes reaching the tumor site. While the mechanisms behind SWNT selectivity to specific leukocyte subpopulations and increased monocyte targeting to tumors in the presence of RGD are not yet understood, identification of specific circulating immune cell populations for the delivery of nanoparticles may have important implications for both cancer therapeutics and diagnostics. Furthermore, the enhanced infiltration of monocytes due to the presence of RGD extends the ‘Trojan Horse’ approach, demonstrating that the delivery of nanotube-containing monocytes is not merely reliant on the innate homing abilities of monocytes.
5.2 Lymphocyte surface engineering for nanoparticle delivery to solid tumors
In addition to uptake and internalization, nanoparticles can also be conjugated to the extensive surface area of leukocytes for delivery into solid tumors. Exploiting the thiol-rich surface of T-lymphocytes, liposomes and lipid-coated polymer nanoparticles with thiol-reactive maleimide headgroups were stably attached to the cell surface, without affecting key cellular functions [110]. Additionally, nanoparticle conjugation to the surface of T-cells was observed to have no effect on their ability to traffic to tumors, thus allowing nanoparticles to be effectively carried to the tumor site. Particles were loaded with cytokines IL-15 and IL-21 to amplify the therapeutic functions of T-cells to treat lung and bone metastases. While systemic injections of free cytokines and T-cells did not have a significant effect on T-cell proliferation within tumors, systemic injection of T-cells carrying cytokine-loaded nanoparticles was able to localize them within tumors and cause robust proliferation in vivo. All mice receiving nanoparticle-conjugated T-cells were able to completely eradicate lung and bone tumors.
Further investigation of maleimide-functionalized nanoparticle conjugation to thiols on the T-cell surface has shown the ability to deliver compounds into the T-cell synapse, as a means to boost antitumor immunity [128]. While migrating T-cells carried surface-linked nanoparticles at the uropod, they were rapidly redistributed to the immunological synapse during target tumor cell recognition. To exploit this redistribution, nanoparticles were used to deliver an inhibitor of key phosphatases to downregulate T-cell receptor activation at the synapse, blocking suppressive signals from tumor cells that typically restrain antitumor activity. In vitro, conjugation of inhibitor-encapsulated nanoparticles to the T-cell surface significantly enhanced their proliferation, compared to controls. Conjugation of the loaded nanoparticles also promoted T-cell expansion in orthotopic prostate tumors in vivo, reduced tumor burden, and enhanced survival compared to controls. These results show that leukocyte-mediated delivery of therapeutics can even serve to enhance cancer immunotherapy, by locally delivering the cytokines and inhibitors necessary to enhance the T-cell response with tumors for anti-tumor immunity in vivo.
5.3 Natural killer cell-surface engineering to target lymph node micrometastases
Approaches using functionalized leukocytes have been assessed in vitro for the therapeutic targeting of metastasis, which can occur via the vascular or lymphatic circulation [129]. Cancer cells traveling through the lymphatic circulation can lodge within sentinel lymph nodes (SLNs), where they lie dormant for a period of time before forming micrometastases [130]. Also present within the lymphatic circulation and SLNs are many immune cells that typically respond to tumor antigens, such as lymphocytes, macrophages, and antigen-presenting cells. In particular, natural killer (NK) cells have been shown to activate apoptotic pathways in cancer cells [131], kill most tumor cells within the circulation [132], and reside within lymph nodes [133]. Despite this, cancer cells continue to evade the host immune response [134]. Evidence of morphological and functional variation in cancer patient SLNs suggests potential immune suppression, which can result in the failure to eliminate micro-metastases in SLNs. Thus, the presence of micrometastases within SLNs typically signals a poor prognosis in cancer patients after surgical resection of the primary tumor [135–137].
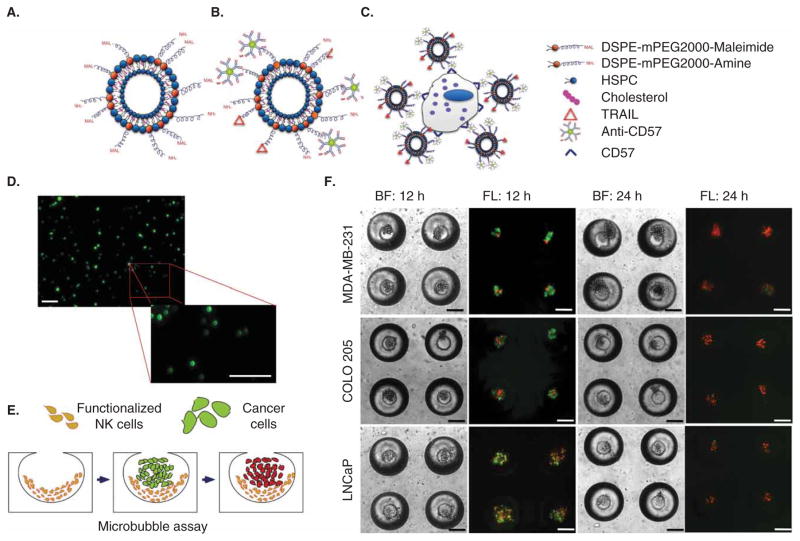
In an attempt to functionalize NK cells to overcome immune suppression, the surface of NK cells was functionalized with TNF-related apoptosis-inducing ligand (TRAIL) liposomes to kill cancer cells in in vitro models of lymph node micrometastases. TRAIL binds to death receptors DR4 and DR5 on the surface of a variety of cancer cell types, which induces apoptosis through intrinsic and extrinsic pathways [138–140]. Thiolated TRAIL protein and anti-CD57 were covalently bound to maleimide groups on the surface of liposomes (Figure 4A and B). Conjugation of liposomes to the surface of NK cells was facilitated by antibody binding to CD57 (Figure 4C and D), a marker found on a subpopulation of NK cells [141]. As an in vitro model of SLNs, functionalized NK cells were seeded into microbubbles comprised of polydimethylsiloxane, which mimic both the size and elastic modulus of lymph nodes (Figure 4E) [142]. MDA-MB-231, COLO 205, and LNCaP cancer cells, which typically metastasize to lymph nodes, were then cultured within microbubbles containing functionalized NK cells. After 24 h in culture, functionalized NK cells successfully induced cancer cell apoptosis in the in vitro model of SLNs (Figure 4F). This approach provides not only an in vitro model to assess the sensitivity of lymph node micrometastases to therapeutics, but also a potential means to enhance the NK cell therapeutic response to micrometastases within lymph nodes, which can become immune suppressive in cancer patients. Further studies will be required to assess successful in vivo functionalization and subsequent localization of NK cells to SLNs, as a means to target metastases within lymph.
Figure 4. Functionalized natural killer (NK) cells to target lymph node micrometastases.
(A,B) Liposomes with maleimide groups (A) react with thiolated apoptosis-inducing ligand TRAIL and anti-CD57 antibodies. (C) Liposomes functionalized to surface of NK cells via antibody binding to CD57. (D) Micrographs of fluorescent liposomes functionalized to the NK cell surface. Scale bar = 100 μm. (E) Schematic of functionalized NK cell intervention in an in vitro model of lymph node micrometastasis. (F) Brightfield and fluorescent micrographs of functionalized NK cells inducing cancer cell apoptosis in an in vitro model of lymph node micrometastases comprised of MDA-MB-231, COLO 205, and LNCaP cancer cells. Cells are labeled with Annexin-V (green) and propidium iodide (red) after 12 and 24 h to assess cell viability. Scale bar = 100 μm.
Figure parts reproduced by permission of The Royal Society of Chemistry [156].
DSPE: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; HSPC: hydrogenated soy phosphatidylcholine; NK: Natural killer.
5.4 Targeting metastatic cells in the bloodstream
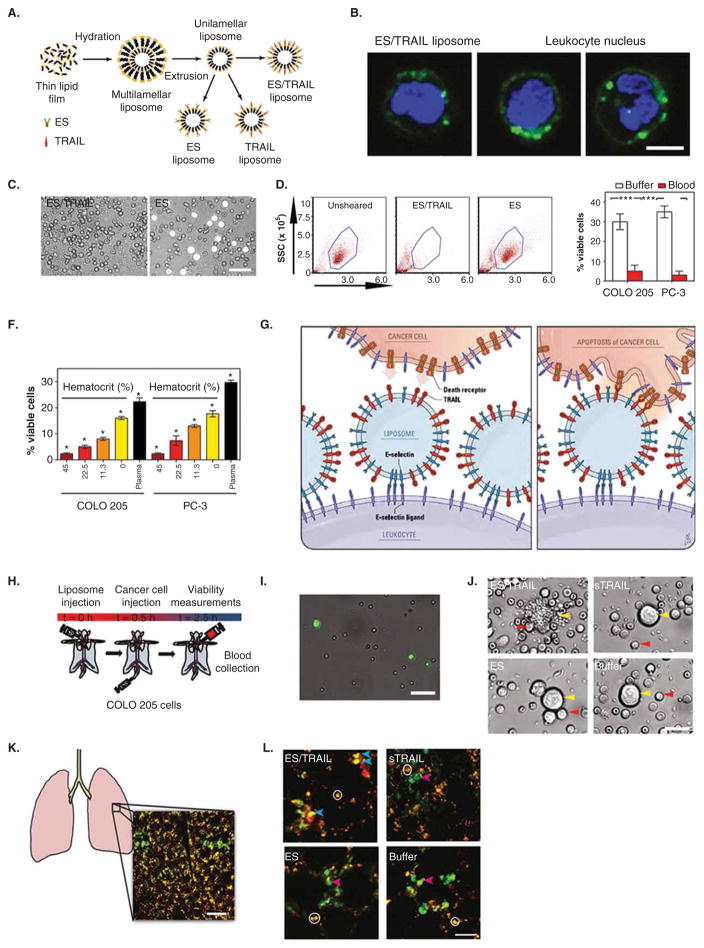
Recently, a unique approach termed ‘unnatural killer cells’, leukocytes functionalized with nanoparticles to target and kill cancer cells within blood, was developed as a means to neutralize CTCs with the potential to form new metastases [113]. To target and kill cancer cells, nanoscale liposomes were functionalized with the adhesion receptor E-selectin (ES) and the apoptosis-inducing ligand TRAIL (Figure 5A). Selectins facilitate rapid, force-dependent adhesion to selectin ligands on tumor cells and leukocytes in blood (Figure 5A), which then allows TRAIL ligands to come within a reactive distance of death receptors on the cancer cell surface, signaling for cell apoptosis. TRAIL is an ideal therapeutic for this delivery method due to the fact that it preferentially induces apoptosis in cancer cells, while exerting minimal cytotoxic effects on most normal cells [143]. Interestingly, ES/TRAIL liposomes bound remarkably well to the surface of many types of leukocytes in blood under flow conditions (Figure 5B), with minimal cytotoxic effects. Upon treatment of cancer cells with ES/TRAIL liposomes in human blood under flow conditions, negligible viable cancer cells remained after only 2 h of treatment (Figure 5C and D). While blood typically reduces therapeutic efficacy through cellular internalization and non-specific binding of the therapeutic to plasma proteins, the ability of ES/TRAIL liposomes to target and kill cancer cells was enhanced in human blood, compared to conditions in buffer alone (Figure 5E). Alteration in hematocrit levels (Figure 5F), in addition to removal of all ES/TRAIL liposomes unbound to leukocytes in blood, demonstrated that blood cells are essential in the enhanced apoptotic response of cancer cells in blood. Upon addition of ES/TRAIL liposomes to cancer cell-spiked blood, liposomes attach to the surface of leukocytes and are available for inducing apoptosis in cancer cells that they come into contact with (Figure 5G). Liposome tethering to the leukocyte surface can also enhance cancer cell apoptosis due to the compressive forces between cancer cells and leukocytes under flow. Compressive forces act to flatten the cancer cell glycocalyx [144], composed of biologically inert macromolecules, thus allowing TRAIL to come within a reactive distance to the cancer cell death receptors and form bonds. This approach is intended to neutralize CTCs as rare as 1–100 cells per ml in blood [53,145], and margination of leukocytes and CTCs along the vessel wall allows CTCs to essentially become surrounded by the circulating leukocyte population. Thus, upon functionalization of leukocytes in blood, CTCs can essentially be surrounded by both adhesion receptors and therapeutic ligands upon entering the bloodstream, thus increasing the probability of neutralizing rare CTCs before they are able to form new metastases.
Figure 5. Unnatural killer cells to target and kill cancer cells in flowing human blood in vitro and in the peripheral circulation of mice in vivo.
(A) Schematic of E-selectin (ES) and TRAIL (ES/TRAIL) functionalized liposome synthesis. (B) Confocal micrographs of fluorescent ES/TRAIL liposomes functionalized to the surface of leukocytes after exposure to blood flow. Scale bar = 5 μm. Green: ES/TRAIL liposome. Blue: leukocyte nucleus. (C) Micrographs of COLO 205 cells (white) after treatment with ES/TRAIL liposomes (left) or ES-conjugated liposomes in blood under shear flow for 2 h. Scale bar = 50 μm. (D) Flow cytometry of COLO 205 cancer cells after treatment with ES/TRAIL or ES liposomes in blood under shear flow in a cone-and-plate viscometer (shear rate: 188 s−1) for 2 h. Unsheared: viable untreated cancer cell control. (E) Comparison of fraction of viable COLO 205 and PC-3 cancer cells after treatment with ES/TRAIL liposomes in buffer versus blood. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. ***p < 0.0001 (unpaired t test). (F) Fraction of viable COLO 205 and PC-3 cancer cells after treatment with ES/TRAIL liposomes in blood with varying percentages of hematocrit. Hematocrit was varied, whereas other blood components remained constant, based on a normal hematocrit of 45%. Plasma indicates removal of all blood cells. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. *p < 0.05 (one-way ANOVA with Tukey’s post-test). (G) Schematic of two-step mechanism involving functionalization of leukocytes with liposomes (left), which then contact circulating cancer cells and activate the death receptor (right). (H) Schematic of in vivo mouse experiment to functionalize leukocytes via systemic delivery of ES/TRAIL liposomes, followed by targeting of circulating cancer cells in the bloodstream. (I) Representative micrographs of COLO 205 cancer cells removed from mouse circulation after treatment with ES/TRAIL liposomes (upper left), sTRAIL (upper right), ES liposomes (lower left), and buffer (lower right) injections. Scale bar = 20 μm. (J) Leukocytes functionalized with fluorescent ES/TRAIL liposomes (green) upon removal from mouse circulation 2.5 h after systemic injection. Scale bar = 50 μm. (K) Schematic of mouse lung and example two-photon excited fluorescence (2PEF) image stack from mouse lung where Hoechst-labeled COLO 205 cells (green) are arrested in vasculature of lung (visible by autofluorescence, yellow). Scale bar = 80 μm. (L) 2PEF images of Hoescht-labeled COLO 205 cells (green) with Alexa Fluor 568-labeled Annexin-V apoptosis probe (red) for each experimental group. Red arrows point to apoptotic COLO 205 cells (red and green colocalized), and blue arrows indicate non-apoptotic COLO 205 cells (green only). White circles indicate regions of autofluorescence from lung tissue. Scale bar = 30 μm.
Significant progress has been made in utilizing ‘unnatural killer cells’ to target and kill cancer cells in the peripheral circulation of mice in vivo. ES/TRAIL liposomes were injected into the peripheral circulation of mice (Figure 5H), where they successfully tethered to the surface of leukocytes (Figure 5I). Following ES/TRAIL liposome injection, tail vein injection of cancer cells was utilized to model leukocyte/CTC interactions within the mouse circulation, representing a common model of lung metastasis [146–149] since the early work of Fidler et al. [150–152]. Upon removal of the peripheral blood from the circulation after 2 h via cardiac puncture, negligible viable cancer cells were found remaining compared to controls (Figure 5J). Upon examination of the remaining cancer cells within the mouse vasculature using multiphoton microscopy (Figure 5K), a decreased number of cancer cells was found in the lungs of treated mice, with the majority of remaining cancer cells labeled as apoptotic in treated mice but not in the control group (Figure 5L).
In addition to the advantages of this approach discussed above, tethering liposomes to the surface of leukocytes in blood is beneficial for increasing liposome circulation time, by avoiding renal clearance mechanisms. By focusing the therapeutic effects to within the vascular microenvironment, reduced dosages are needed to target metastatic cells, as the dosages of TRAIL used in this current study were approximately two orders of magnitude lower than the dosages used in human clinical trials of TRAIL protein [153–155]. Representing an important first step in targeting CTCs in the bloodstream, the ‘unnatural killer cells’ approach can potentially be utilized as a preventative measure upon diagnosis of highly metastatic hematogenous cancers that originate from epithelial tissues including breast, prostate, and lung.
6. Conclusion
Due to their shared ability to marginate in blood, adhesively interact with the blood vessel wall, and migrate along chemoattractant gradients to tumors and sites of inflammation, leukocytes have the potential to guide advanced nanoparticle platforms directed at CTCs and malignant tissues that have not previously been targeted successfully. Nanoparticles can be conjugated to the surface of leukocytes via methods such as receptor-mediated adhesion, covalent coupling, and selectin-mediated adhesion. Nanoparticles can also be internalized within phagocytic leukocytes, for efficient drug delivery while leaving the cell membrane unoccupied. Leukocyte carriers have proven effective in delivering drugs to solid tumors, models of lymph node metastasis, and cancer cells in the circulation in vivo. The promise of leukocytes as carriers of nanoparticle therapeutics warrants further investigation for applications including drug delivery to CTCs, metastatic tumor sites, and hypoxic regions of tumors, to name a few.
7. Expert opinion
Over the last several decades, major breakthroughs have been made in the development of advanced nanoparticle platforms for cancer therapy. The size, shape, and porosity of nanoparticles can now be controlled by a variety of methods. Additionally, nanoparticles can be functionalized with various proteins, small-molecule drugs, and nucleic acids, and drug release from nanoparticles can be controlled in a sophisticated manner. While further investigation into the synthesis of such platforms is still warranted, methods to deliver nanoparticles to poorly accessible anatomical regions will be needed for these systems to reach their full therapeutic potential.
New strategies need to be pioneered to guide nanoparticles particularly in the field of cancer, where portions of tumor tissue lack an accessible vasculature, and single CTCs are surrounded by billions of cells within blood. Since traditional systemic delivery of nanoparticles can be inefficient for such purposes, leukocytes can provide a new means to direct drugs to target tumor cells while minimizing systemic toxicity that is traditionally observed. Leukocytes have the innate ability to migrate similarly to tumor cells both in blood and within tissues, and utilize chemoattractant gradients to infiltrate solid tumors, making these cells an ideal carrier of nanoparticles. While most nanoparticle formulations typically do not reach the tumor site due to clearance via the reticuloendothelial system, attachment of nanoparticles to leukocytes can act to potentially bypass this mechanism. Carrier leukocytes can also be advantageous in the field of cancer immunotherapy, as adjuvant-loaded nanoparticles can be attached to the surface of tumor-infiltrating lymphocytes, which can facilitate T-cell therapy directly at the tumor site. By utilizing leukocytes as carriers of adjuvants, systemic toxicity encountered due to multiple adjuvant injections required for T-cell therapies can be minimized.
To assess the potential of leukocytes as carriers for targeted cancer drug delivery, further investigations in vivo are required, particularly those utilizing spontaneous metastasis models. Additionally, studies assessing the effects of nanoparticle attachment and internalization on innate leukocyte functions will be needed, as the long-term effects of such functionalization are not well understood. These emerging studies, however, show that leukocytes are a promising carrier for targeted cancer drug delivery, and can be combined with sophisticated nanoparticle platforms to provide a unique means to target hypoxic regions of tumors, metastases, and rare CTCs with potential to form new metastases.
Article highlights.
A variety of nanoparticle formulations have been developed for cancer therapy, but barriers remain in delivering such platforms to circulating tumor cells and metastatic sites.
Leukocytes are an ideal carrier of nanoparticles for the treatment of cancer, due to their abilities to circulate in blood and migrate along chemoattractant gradients to tumor and inflammatory sites.
Different techniques can be utilized to attach nanoparticles to leukocytes, including surface functionalization approaches and those intended to achieve internalization.
Attachment of nanoparticles to leukocytes can potentially minimize nanoparticle clearance via the reticuloendothelial system, and subsequently enhance therapeutic efficacy.
Adjuvant-loaded nanoparticles can be attached to the surface of tumor-infiltrating lymphocytes, which facilitate T-cell therapy directly at the tumor site.
Leukocytes can be functionalized with therapeutic nanoparticles, directly in the bloodstream, to target and kill circulating cancer cells.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The authors were supported by the Cornell Center on the Microenvironment and Metastasis through Award Number U54CA143876 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2•.Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12(1):39–50. doi: 10.1038/nrc3180. This article broadly reviews current research, opportunities, and challenges in combining aspects of engineering, cancer biology, and medicine to develop nanotechnology platforms to treat metastatic cancer. [DOI] [PubMed] [Google Scholar]
- 3.Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–33. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–17. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–50. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM. The hypoxic cell. Cancer Res. 1999;59:5863–70. [PubMed] [Google Scholar]
- 7.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98. doi: 10.1146/annurev-med-040210-162544. This article reviews clinical data of approved nanoparticle delivery systems, as well new platforms that are currently undergoing clinical trials. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Jeong JH, Chun KW, Park TG. Target-specific cellular uptake of PLGA nanoparticles coated with poly (L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir. 2005;21(19):8852–7. doi: 10.1021/la0502084. [DOI] [PubMed] [Google Scholar]
- 11.Farokhzad OC, Jon S, Khademhosseini A, et al. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64(21):7668–72. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Gu FX, Chan JM, Wang AZ. Nanoparticles in Medicine: therapeutic Applications and Developments. Clin Pharmacol Ther. 2008;83:761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 13.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu F, Zhang L, Teply BA, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA. 2008;105(7):2586–91. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2012;64:206–12. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 17.Calvo P, Remuñan-López C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14(10):1431–6. doi: 10.1023/a:1012128907225. [DOI] [PubMed] [Google Scholar]
- 18.Hrkach JS, Peracchia MT, Bomb A, et al. Nanotechnology for biomaterials engineering: structural characterization of amphiphilic polymeric nanoparticles by 1H NMR spectroscopy. Biomaterials. 1997;18(1):27–30. doi: 10.1016/s0142-9612(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 19.Rancan F, Todorova A, Hadam S, et al. Stability of polylactic acid particles and release of fluorochromes upon topical application on human skin explants. Eur J Pharm Biopharm. 2012;80(1):76–84. doi: 10.1016/j.ejpb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Muzzarelli R, Baldassarre V, Conti F, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988;9(3):247–52. doi: 10.1016/0142-9612(88)90092-0. [DOI] [PubMed] [Google Scholar]
- 21.Felt O, Buri P, Gurny R. Chitosan: a Unique Polysaccharide for Drug Delivery. Drug Dev Ind Pharm. 1998;24(11):979–93. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74(1–3):317–23. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Jiang X, Ding Y, et al. Synthesis and characterization of chitosan–poly (acrylic acid) nanoparticles. Biomaterials. 2002;23:3193–201. doi: 10.1016/s0142-9612(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 24.Guo R, Zhang L, Jiang Z, et al. Synthesis of alginic acid-poly[2-(diethylamino)ethyl methacrylate] monodispersed nanoparticles by a polymer-monomer pair reaction system. Biomacromolecules. 2007;8(3):843–50. doi: 10.1021/bm060906i. [DOI] [PubMed] [Google Scholar]
- 25.Bangham AD. Liposomes: the Babraham connection. Chem Phys Lipids. 1993;64(1–3):275–85. doi: 10.1016/0009-3084(93)90071-a. [DOI] [PubMed] [Google Scholar]
- 26.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 27.Barenholz Y. Doxil® – The first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Leonard RCF, Williams S, Tulpule A, et al. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet™) Breast. 2009;18(4):218–24. doi: 10.1016/j.breast.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–51. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 30.Choi M-R, Stanton-Maxey KJ, Stanley JK, et al. A Cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7(12):3759–65. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 31.Park J-H, Maltzahn von G, Xu MJ, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci USA. 2010;107(3):981–6. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minelli C, Lowe SB, Stevens MM. Engineering nanocomposite materials for cancer therapy. Small. 2010;6(21):2336–57. doi: 10.1002/smll.201000523. [DOI] [PubMed] [Google Scholar]
- 33.Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16(24):6139–49. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44(10):936–46. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 36.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 37.Yang W, Thordarson P, Gooding JJ, et al. Carbon nanotubes for biological and biomedical applications. Nanotechnology. 2007;18(41):412001. [Google Scholar]
- 38.Chakravarty P, Marches R, Zimmerman NS, et al. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc Natl Acad Sci USA. 2008;105(25):8697–702. doi: 10.1073/pnas.0803557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsher K, Liu Z, Daranciang D, Dai H. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules. Nano Lett. 2008;8(2):586–90. doi: 10.1021/nl072949q. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–60. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumortier H, Lacotte S, Pastorin G, et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6(7):1522–8. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 42.Cui D, Tian F, Ozkan CS, et al. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155(1):73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Lam C-W, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77(1):126–34. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 44.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang SX. Bevacizumab and breast cancer: current therapeutic progress and future perspectives. Expert Rev Anticancer Ther. 2009;9(12):1715–25. doi: 10.1586/era.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 47.Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 48.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11:4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 49.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 51.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:1–11. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20(1):96–9. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu M, Stott S, Toner M, et al. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid-Schönbein GW, Usami S, Skalak R, Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980;19(1):45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- 56.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–42. [PubMed] [Google Scholar]
- 57.Goldsmith HL, Spain S. Margination of leukocytes in blood flow through small tubes. Microvasc Res. 1984;27(2):204–22. doi: 10.1016/0026-2862(84)90054-2. [DOI] [PubMed] [Google Scholar]
- 58.Firrell JC, Lipowsky HH. Leukocyte margination and deformation in mesenteric venules of rat. Am J Physiol Heart Circ Physiol. 1989;256(6 Pt 2):H1667–74. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]
- 59.Nobis U, Pries AR, Cokelet GR, Gaehtgens P. Radial distribution of white cells during blood flow in small tubes. Microvasc Res. 1985;29(3):295–304. doi: 10.1016/0026-2862(85)90020-2. [DOI] [PubMed] [Google Scholar]
- 60.Cheung LSL, Raman PS, Balzer EM, et al. Biophysics of selectin-ligand interactions in inflammation and cancer. Phys Biol. 2011;8(1):015013. doi: 10.1088/1478-3975/8/1/015013. [DOI] [PubMed] [Google Scholar]
- 61.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 62.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 63.Sundd P, Pospieszalska M, Cheung L, et al. Biomechanics of leukocyte rolling. Biorheology. 2011;48:1–35. doi: 10.3233/BIR-2011-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundd P, Pospieszalska MK, Ley K. Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol Immunol. 2013;55(1):59–69. doi: 10.1016/j.molimm.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finger E, Puri K, Alón R, et al. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–8. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 66.Cao TM, Mitchell MJ, Liesveld J, King MR. Stem cell enrichment with selectin receptors: mimicking the pH environment of trauma. Sensors (Basel) 2013;13:12516–26. doi: 10.3390/s130912516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 68.Mitchell MJ, Lin KS, King MR. Fluid shear stress increases neutrophil activation via platelet-activating factor. Biophys J. 2014;106(10):2243–53. doi: 10.1016/j.bpj.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitchell MJ, King MR. Shear-induced resistance to neutrophil activation via the formyl peptide receptor. Biophys J. 2012;102:1804–14. doi: 10.1016/j.bpj.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding ZM, Babensee JE, Simon SI, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–38. [PubMed] [Google Scholar]
- 71.Diamond MS. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111(6):3129–39. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes B, Hollers J, Crockett-Torabi E, Smith CW. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Investig. 1992;90:1687–96. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 74.van Ginhoven TM, van den Berg JW, Dik WA, et al. Preoperative dietary restriction reduces hepatic tumor load by reduced E-selectin-mediated adhesion in mice. J Surg Oncol. 2010;102(4):348–53. doi: 10.1002/jso.21649. [DOI] [PubMed] [Google Scholar]
- 75.McDonald B, Spicer J, Giannais B, et al. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- 76.Barthel SR, Wiese GK, Cho J, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci USA. 2009;106(46):19491–6. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok J. Rolling of Human Bone-Metastatic Prostate Tumor Cells on Human Bone Marrow Endothelium under Shear Flow Is Mediated by E-Selectin. Cancer Res. 2004;64(15):5261–9. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 78.Tözeren A, Kleinman HK, Grant DS, et al. E-selectin-mediated dynamic interactions of breast-and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995;60:426–31. doi: 10.1002/ijc.2910600326. [DOI] [PubMed] [Google Scholar]
- 79.Myung JH, Gajjar KA, Pearson RM, et al. Direct measurements on CD24-mediated rolling of human breast cancer MCF-7 cells on E-selectin. Anal Chem. 2011;83(3):1078–83. doi: 10.1021/ac102901e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem. 2006;281(20):13899–905. doi: 10.1074/jbc.M513617200. [DOI] [PubMed] [Google Scholar]
- 81.Brodt P, Fallavollita L, Bresalier RS, et al. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71(4):612–19. doi: 10.1002/(sici)1097-0215(19970516)71:4<612::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 82.Biancone L, Araki M, Araki K, et al. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996;183(2):581–7. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Köhler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer. 2010;102(3):602–9. doi: 10.1038/sj.bjc.6605492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahn JJ, Chow JW, Horne GJ, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22(6):475–83. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 85.Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–89. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balkwill FR. The chemokine system and cancer. J Pathol. 2011;226(2):148–57. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 87.Kishimoto T, Jutila M, Butcher E. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–8. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kishimoto T, Jutila M, Berg E, Butcher E. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245(4923):1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 89.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 90.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270(19):11025–8. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 91.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 92.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Bajpai S, Mitchell MJ, King MR, Reinhart-King CA. A microfluidic device to select for cells based on chemotactic phenotype. Technology. 2014;02(02):101–5. doi: 10.1142/S2339547814200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salsman VS, Chow KKH, Shaffer DR, et al. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment. PLoS One. 2011;6(5):e20267. doi: 10.1371/journal.pone.0020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinet N, Beck G, Bernard V, et al. Mechanism for the recruitment of macrophages to cancer site. In vivo concentration gradient of monocyte chemotactic activity. Cancer. 1992;70(4):854–60. doi: 10.1002/1097-0142(19920815)70:4<854::aid-cncr2820700422>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 97.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–7. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 98.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 99.Swiston AJ, Gilbert JB, Irvine DJ, et al. Freely suspended cellular “backpacks” lead to cell aggregate self-assembly. Biomacromolecules. 2010;11(7):1826–32. doi: 10.1021/bm100305h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swiston AJ, Cheng C, Um SH, et al. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008;8(12):4446–53. doi: 10.1021/nl802404h. [DOI] [PubMed] [Google Scholar]
- 101.Vasconcellos FC, Swiston AJ, Beppu MM, et al. Bioactive polyelectrolyte multilayers: hyaluronic acid mediated B lymphocyte adhesion. Biomacromolecules. 2010;11(9):2407–14. doi: 10.1021/bm100570r. [DOI] [PubMed] [Google Scholar]
- 102.Doshi N, Swiston AJ, Gilbert JB, et al. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv Mater. 2011;23(12):H105–9. doi: 10.1002/adma.201004074. [DOI] [PubMed] [Google Scholar]
- 103.Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leucocytes. J Biol Chem. 1996;271(20):11634–40. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- 104.Lee D, Schultz JB, Knauf PA, King MR. Mechanical shedding of L-selectin from the neutrophil surface during rolling on Sialyl Lewis x under flow. J Biol Chem. 2007;282(7):4812–20. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 105.Su SS, Schmid-Schönbein GW. Internalization of formyl peptide receptor in leukocytes subject to fluid stresses. Cel Mol Bioeng. 2010;3(1):20–9. doi: 10.1007/s12195-010-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loughrey HC, Choi LS, Cullis PR, Bally MB. Optimized procedures for the coupling of proteins to liposomes. J Immunol Methods. 1990;132(1):25–35. doi: 10.1016/0022-1759(90)90394-b. [DOI] [PubMed] [Google Scholar]
- 107.Nobs L, Buchegger F, Gurny R, Allémann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93(8):1980–92. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 108.Donoghue N, Yam PT, Jiang XM, Hogg PJ. Presence of closely spaced protein thiols on the surface of mammalian cells. Protein Sci. 2000;9(12):2436–45. doi: 10.1110/ps.9.12.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawrence DA, Song R, Weber P. Surface thiols of human lymphocytes and their changes after in vitro and in vivo activation. J Leukoc Biol. 1996;60(5):611–18. doi: 10.1002/jlb.60.5.611. [DOI] [PubMed] [Google Scholar]
- 110••.Stephan MT, Moon JJ, Um SH, et al. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–41. doi: 10.1038/nm.2198. This research article describes an approach to attach adjuvant drug-loaded nanoparticles to the surface of therapeutic T-cells to enhance tumor elimination in a model of adoptive T cell therapy for cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitchell MJ, Chen CS, Ponmudi V, et al. E-selectin liposomal and nanotube-targeted delivery of doxorubicin to circulating tumor cells. J Control Release. 2012;160:609–17. doi: 10.1016/j.jconrel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mitchell MJ, Castellanos CA, King MR. Nanostructured surfaces to target and kill circulating tumor cells while repelling leukocytes. J Nanomater. 2012;2012(3):1–10. doi: 10.1155/2012/831263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113••.Mitchell MJ, Wayne E, Rana K, et al. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc Natl Acad Sci USA. 2014;111(3):930–5. doi: 10.1073/pnas.1316312111. This research article describes a new approach in which leukocytes in the circulation are functionalized with liposomes bearing an adhesion receptor (E-selectin), and an adhesion receptor (TRAIL), to target and kill cancer cells in the bloodstream that have potential to form new metastases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Badwey JA, Karnovsky ML. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- 115.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–45. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 116.Choi J, Kim HY, Ju EJ, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 117.Choi M-R, Bardhan R, Stanton-Maxey KJ, et al. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3(1–6):47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brynskikh AM, Zhao Y, Mosley RL, et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine (Lond) 2010;5(3):379–96. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Batrakova EV, Li S, Reynolds AD, et al. A Macrophage-nanozyme delivery system for parkinson’s disease. Bioconjug Chem. 2007;18(5):1498–506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sahay G, Querbes W, Alabi C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–8. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100(23):13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graff CL, Pollack GM. Drug transport at the blood-brain barrier and the choroid plexus. Curr Drug Metab. 2004;5(1):95–108. doi: 10.2174/1389200043489126. [DOI] [PubMed] [Google Scholar]
- 124.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. Neurotherapeutics. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schackert G, Simmons RD, Buzbee TM, et al. Macrophage infiltration into experimental brain metastases: occurrence through an intact blood-brain barrier. J Natl Cancer Inst. 1988;80:1027–34. doi: 10.1093/jnci/80.13.1027. [DOI] [PubMed] [Google Scholar]
- 126.Morantz RA, Wood GW, Foster M, et al. Macrophages in experimental and human brain tumors. J Neurosurg. 1979;50(3):305–11. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 127•.Smith BR, Ghosn E, Rallapalli H, et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat Nanotechnol. 2014;9:481–7. doi: 10.1038/nnano.2014.62. This research article describes an advanced immune cell-based delivery system of nanotubes, in which single-walled carbon nanotubes are taken up almost exclusively by a single immune cell subset, Ly-6hi monocytes, and that functionalization of nanotubes with an arginylglycylaspartic acid (RGD) ligand significantly enhances tumor penetration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stephan MT, Stephan SB, Bak P, et al. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–87. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812–17. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. APMIS. 2008;116:754–70. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 131.Zamai L, Ponti C, Mirandola P, et al. NK cells and cancer. J Immunol. 2007;178(7):4011–16. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 132.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300. [PubMed] [Google Scholar]
- 133.Bajénoff M, Breart B, Huang AYC, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203(3):619–31. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 135.Hartkopf AD, Banys M, Krawczyk N, et al. Bone marrow versus sentinel lymph node involvement in breast cancer: a comparison of early hematogenous and early lymphatic tumor spread. Breast Cancer Res Treat. 2012;131:501–8. doi: 10.1007/s10549-011-1802-x. [DOI] [PubMed] [Google Scholar]
- 136.Gerber B, Krause A, Müller H, et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol. 2001;19:960–71. doi: 10.1200/JCO.2001.19.4.960. [DOI] [PubMed] [Google Scholar]
- 137.Kubuschok B, Passlick B, Izbicki JR, et al. Disseminated tumor cells in lymph nodes as a determinant for survival in surgically resected non–small-cell lung cancer. J Clin Oncol. 1999;17:19–24. doi: 10.1200/JCO.1999.17.1.19. [DOI] [PubMed] [Google Scholar]
- 138.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26(21):3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 139.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 140.Mitchell MJ, King MR. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. N J Phys. 2013;15(1):015008. doi: 10.1088/1367-2630/15/1/015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wing-Han Yuen Q, Zheng YP, Huang YP, et al. In-vitro strain and modulus measurements in porcine cervical lymph nodes. Open Biomed Eng J. 2011;5:39–46. doi: 10.2174/1874120701105010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang XD, Nguyen T, Thomas WD, et al. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482(3):193–9. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell MJ, King MR. Theme: physical Biology in Cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am J Physiol Cell Physiol. 2014;306:C89–97. doi: 10.1152/ajpcell.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 146.Yan L, Cai Q, Xu Y. The Ubiquitin-CXCR4 Axis Plays an Important Role in Acute Lung Infection-Enhanced Lung Tumor Metastasis. Clin Cancer Res. 2013;19(17):4706–16. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tucci P, Agostini M, Grespi F, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci USA. 2012;109(38):15312–17. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang C-Y, Lin S-C, Su W-H, et al. Somatic LMCD1 mutations promoted cell migration and tumor metastasis in hepatocellular carcinoma. Oncogene. 2012;31(21):2640–52. doi: 10.1038/onc.2011.440. [DOI] [PubMed] [Google Scholar]
- 149.Kim S, Takahashi H, Lin W-W, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–24. [PubMed] [Google Scholar]
- 151.Fidler IJ. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res. 1974;34:1074–8. [PubMed] [Google Scholar]
- 152.Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57:1199–202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell MJ, King MR. Unnatural killer cells to prevent bloodborne metastasis: inspiration from biology and engineering. Expert Rev Anticancer Ther. 2014;14(06):641–4. doi: 10.1586/14737140.2014.916619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Subbiah V, Brown RE, Buryanek J, et al. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther. 2012;11(11):2541–6. doi: 10.1158/1535-7163.MCT-12-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herbst RS, Eckhardt SG, Kurzrock R, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;17:2839–46. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 156.Chandrasekaran S, McGuire MJ, King MR. Sweeping lymph node micrometastases off their feet: an engineered model to evaluate natural killer cell mediated therapeutic intervention of circulating tumor cells that disseminate to the lymph nodes. Lab Chip. 2014;14(1):118–27. doi: 10.1039/c3lc50584g. [DOI] [PubMed] [Google Scholar]