Abstract
Brain networks for intranasal chemosensation have been shown to be intrinsically organized in humans [1]. However, little is known about how changes in the intrinsic functional connectivity (FC) in chemosensory networks are related to aging. We, therefore, investigated the impact of age on resting state functional connectivity in the olfactory and trigeminal networks (ON and TN) by combining two freely available resting state fMRI data sets (obtained from the NITRC.org; Atlanta and New York) with data collected in our lab to generate a large sample size (N=103; 51 females) spanning the age range of 20–61 years. Seed regions were defined using MNI coordinates that anchor olfactory and trigeminal networks in activation studies and meta-analyses. The olfactory network (ON) included the piriform cortex and oribtofrontal cortex. The trigeminal network (TN) included the anterior insula and cingulate cortex. Scanner site, sex, and age were used as covariates in group level analyses. The FC between the ON and parahippocampal gyrus was negatively correlated with age. The FC between the TN and parahippocampal gyrus, however, was positively correlated. Similarly, age was positively correlated with the ON FC to the ventral striatum, and the TN FC to the default mode network (DMN). These results reflect divergent age-related alterations of the intrinsic FC of the human chemosensory system.
Keywords: olfactory network, trigeminal network, resting state, functional connectivity
INTRODUCTION
Intranasal chemosensation involves sensation of both the olfactory and trigeminal networks (ON and TN) [2]. The ON is related to autobiographical memories [3]. In contrast, the TN conveys somatosensory information (such as burning, pungency, or stinging, etc.), and is closely aligned with salience detection [4,5]. These two systems interact to modify the encoding of olfactory information and plays an important role in everyday safety, physical well-being and quality of life.
Previously, employing functional Magnetic Resonance Imaging (fMRI) and a seed-based resting state functional connectivity (FC) technique, we showed that both the ON and TN are intrinsically organized against a common baseline [1,6,7]. The same work showed that the ON, but not the TN, included FC with the hippocampus and medial temporal lobe memory systems [1]. There is evidence suggesting that structural changes of these intranasal chemosensory is a natural consequence of aging, but how these structural changes related to aging affect functional characteristics remains poorly understood [8]. Understanding this relationship is important because many characteristics of functional brain networks are age dependent and disrupted by disease [9,10].
Normal aging results in decreased cognitive and sensory abilities, including olfaction, which is common in people between the ages of 65 and 80 [11–15]. Unusually high age-related olfactory decline, such as increased detection and discrimination thresholds [11], is a high risk factor for developing dementia [16–18]. Although not implicated in neurodegeneration, age related loss of intranasal trigeminal sensitivity has been shown to take place in the periphery of the TN [19].
The objective of this study was to investigate age-dependent resting state FC of the ON and TN. Olfactory function is closely linked with memory performance, therefore, we hypothesized an age-dependent FC between the ON and the hippocampal memory system. Because interactions between the ON and TN have been shown to occur at both peripheral and central nervous levels [20,21], we also investigated age-related changes in FC of the TN, which has not been previously investigated with resting state fMRI.
METHOD
Resting state fMRI data from three cohorts were processed and pooled to generate a large sample size for a multi-cohort analysis. Each cohort contained a resting state T2*-weighted GE-EPI time series and a skull-stripped T1 MPRAGE anatomical image.
Participants
Hershey cohort
16 healthy adults (ages 20–61; 6 female) participated in a resting state fMRI study (180 volumes GE-EPI; 2.8 x 2.8 × 4.0 mm voxels; TR = 2 sec; TE = 30 msec; flip angle = 90o). Study protocols were carried out in accordance with the Declaration of Helsinki and the local IRB.
Atlanta cohort
Data from 28 healthy adults (ages 22–57; 15 female) were collected with the following imaging parameters: (205 volumes of T2*-weighted GE-EPI; 3.4 × 3.4 × 4.0 mm voxels; TR=2 sec). These data were contributed by H.S. Mayberg and available for download from: www.nitrc.org/frs/downloadlink.php/1825.
New York cohort
Data from 59 healthy adult participants (ages 21–49; 31 female) were collected with the following imaging parameters: (197 volumes of T2*-weighted GE-EPI; 3.0 mm isotropic voxels; TR = 2 sec). These data were contributed by M.P. Milham and F.X. Castellanos and available for download from www.nitric.org/frs/downloadlink.pho/1632, and www.nitric.org/fsr/downloadlink.pho/1633.
The Atlanta and New York cohorts are freely available for research purposes without restriction, however, the data sets did not include slice timing information for fMRI data.
Data preprocessing
Preprocessing and analyses were completed with AFNI. Except for a slice timing correction step for the Hershey cohort, preprocessing steps were the same for all thee cohorts and included: motion correction, third order polynomial detrending and band pass filter (0.008–0.08 Hz) via linear regression, smoothing to a target 8 mm3 FWHM, and transformation into MNI space with 2mm isotropic voxels. Volumes with greater than 1mm interscan head movements were censored from further analyses. T1 anatomical images were aligned to the first functional image for each participant and were then warped to a standard MNI template.
Functional connectivity analysis
Seed time courses were extracted in MNI space as the average time course within a three voxel radius (6mm) centered on coordinates that anchor fMRI activation of the olfactory [22] and trigeminal [23] systems, as in our previous publication [1]. Coordinates reported by Iannilli et al were in Talairach space and were converted to MNI space via the Brett transform using GingerALE software [24]. Seeds comprising the ON included left and right piriform cortex ([−22 0 −14], [22 2 −12]) and oribtofrontal cortex (OFC; [−24 30 −10], [28 34 −12]), and were deemed most likely to be activated by a meta-analysis of olfactory stimulation [22]. Seeds comprising the TN included left and right anterior insula ([−37 23 −7], [42 6 −20]) and cingulate cortex ([−12 18 30], [12 30 30]). The seed locations for the cingulate cortex were obtained from activation patterns contrasting chemical stimulation to baseline. Insula seeds were obtained from the conjunction of chemical/mechanical (right insula) and chemical/electrical (left insula) activation.
Second level analyses of the group of combined cohorts were conducted with a series of one-sample and two-sample t-tests, and included age, sex and scanner site as covariates. A cluster threshold of 20 voxels was used to correct for multiple comparisons at the group level [25]. The coordinates for select peak voxels in significant clusters are given by MNI coordinates [x y z] in radiological convention.
RESULTS
The top panel in Figure 1 shows the locations where FC of the ON was significantly stronger than the TN. These regions include the ventromedial PFC (vmPFC), subcallosal gyrus, retrosplenial cortex, and the hippocampus, each bilaterally. The bottom panel in Figure 1 shows where FC of the TN was significantly stronger than FC of the ON. These regions include the insula, thalamus, medial occipital cortex, middle temporal gyrus, cingulate gyrus, brain stem (red nucleus and pons), and cerebellar cortex. Table 1 tabulates the MNI coordinates and t-values for peak voxels from the contrast of the ON and TN. The unique brain regions belonging to the ON and TN, against a common baseline, have been reported in Tobia et al., 2016 [1]. Figure 2 shows regions where either the ON or TN was significantly correlated with age. For the ON, age was negatively correlated with FC to the parahippocampal gyrus (Figure 2 top left), but was positively correlated with FC to the caudate and nucleus accumbens (Figure 2, bottom left). For the TN, age was positively correlated with FC to the parahippocampal gyrus, which overlapped with the age-related decline in FC with the ON (Figure 2 top right), and age was also positively correlated with FC between the TN and both the anterior and posterior cingulate cortex (Figure 2 bottom right).
Figure 1.

Intrinsic network contrasts. Top: The olfactory network showed significantly stronger functional connectivity than the trigeminal network in anterior and posterior midline regions including the vmPFC (white circle), posterior OFC (red circle) and retrosplenial cortex (green circle), as well as the hippocampus (bottom). Bottom (left panel): The trigeminal network showed significantly stronger functional connectivity than the olfactory network in the thalamus (red circle), insula (white circles), medial occipital cortex (yellow circle), basal ganglia (green circle), and middle temporal gyrus (orange circle). Bottom (right panel): trigeminal network showed significantly stronger functional connectivity than the olfactory network in the cingulate gyrus (red circle), brain stem (yellow circle), and cerebellar cortex (green circle). Images show functional connectivity superimposed on the MNI template in radiological convention (left = right).
Table 1.
MNI coordinates for peak voxels in the ON and TN
| Anat. Label | MNI: x y z | t-val |
|---|---|---|
| Olfactory > Trigeminal | ||
| ** Right OFC | 28 34 −12 | 87.47 |
| ** Left OFC | −24 30 −10 | 80.82 |
| ** Left Piriform | −22 0 −14 | 61.12 |
| ** Right Piriform | 22 3 −12 | 51.46 |
| Right vmPFC | 4 39 −16 | 10.16 |
| Right Parahippocampal/Hippocampus | 22 −19 −11 | 7.38 |
| Left Hippocampus | −23 −18 −13 | 6.72 |
| Left Posterior Cingulate | −6 53 16 | 6.44 |
| Trigeminal > Olfactory | ||
| ** Left Cingulate Gyrus | −12 −19 30 | −99.0 |
| ** Right Insula/STG | 42 −6 −20 | −71.52 |
| ** Left Insula | −36 −21 0 | −99.0 |
| ** Right Cingulate Gyrus | 12 30 30 | −82.61 |
| Right Precentral Gyrus | 38 9 40 | −10.49 |
| Left Thalamus | −10 −15 1 | −7.88 |
| Right Lingual Gyrus | 22 −57 −1 | −4.35 |
| Left Lingual Gyrus | −16 −70 −1 | −4.47 |
| Left Lentiform Nucleus | −20 −2 −1 | −4.39 |
| Brainstem | 4 −28 −33 | −5.36 |
peak coordinate is seed region.
vmPFC = ventromedial prefrontal cortex; STG=superior temporal gyrus. The ON and TN, against a common baseline, have been reported in Tobia et al., 2016 [1].
Figure 2.
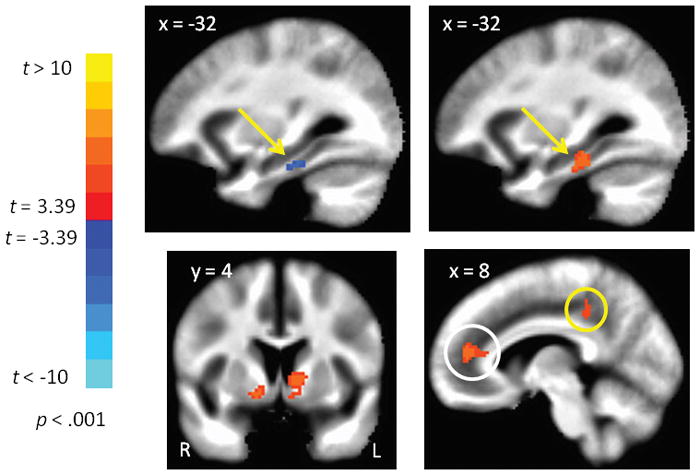
Network functional connectivity correlated with age. Top panel: Functional connectivity of the olfactory network and the parahippocampal gyrus was negatively correlated with age (left), but this correlation was reversed for the trigeminal network in an overlapping swath of gray matter (right). Bottom panel: Functional connectivity of the olfactory network and the ventral striatum (basal ganglia) was positively correlated with age (left). Functional connectivity of the trigeminal network and the anterior and posterior cingulate cortex gyrus (white and yellow circles respectively) was positively correlated with age (right). Images show functional connectivity superimposed on the MNI template in radiological convention (left = right).
DISCUSSION
The main finding of this study was the significant negative correlation between age and the strength of FC between the ON and the parahippocampal gyrus. This finding is consistent with the known link between olfaction and the hippocampal memory system [12, 26], and concurrent age-related decline of both olfactory processing and hippocampal-related memory functioning [6–9]. Moreover, FC between the ON and the hippocampus was significantly stronger than FC between the TN and the hippocampus, even after controlling for age effects. These findings suggest fMRI may be a sensitive biomarker to detect alterations in cognitive and memory network functioning via the olfactory system pathology. While this has been suggested for Alzheimer’s disease (AD [18]), ours is the first demonstration that the integrity of these two systems can be assessed as a function of age in the absence of sensory stimulation. Additionally, compared to the ON, TN showed stronger FC with visual areas, however, this difference in FC has a caveat. This is because neither the ON nor the TN showed significant FC with the visual system known to influence olfactory processing [27]. Since, the ON and TN are not spatially independent, this result suggests that brain regions part of the TN must be taken into account when investigating the innate connection between the olfactory and visual systems. Taken together, this study extended the findings of Tobia et al., 2016 (in which the unique brain regions belonging to the ON and TN against a common baseline were reported) by investigating the relative position of the ON and TN against each other [1].
An unexpected result of this study is the age dependent increase in FC between the ON and ventral striatum. The ventral striatum is typically associated with dopaminergic-mediated reward processing [28], which is negatively impacted by aging [29]. This finding, therefore, suggests that the ON resting state FC might be capable of providing an index for the intrinsic functionality of dopaminergic processing. Such information may be useful to further investigate neurodegenerative diseases, such as Parkinson’s disease (PD), where olfactory and dopamine dysfunctions are cardinal symptoms [17]. Moreover, there is an established link between aging and an increase in dopaminergic cells in the olfactory bulb with concomitantly reduced olfactory sensation [30], suggesting that advanced fMRI techniques capable of imaging the olfactory bulb activation and its FC may provide a direct assessment of the dopamine system.
The trigeminal system is closely aligned with salience detection and it has been reported that the TN overlaps with the salience network [1,4,5]. The TN showed increased FC to the parahippocampal gyrus (overlapping with the negative correlation of the ON), as well as to the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) nodes of the default mode network (DMN) as a function of increasing age. Previous research has shown that age-related increase in intranasal trigeminal sensation thresholds was specifically related to peripheral processing [19]. On the other hand, our findings showed that the intrinsic organization of the TN does not deteriorate with age, but is enhanced with increasing age.
Trigeminal processing is not typically associated with the hippocampal memory system and, therefore, our unexpected finding of a stronger FC of the TN to the parahippocampal gyrus is intriguing. It is possible that this finding could be related to compensatory mechanisms of aging such that the central processing of the trigeminal sensation is enhanced in order to offset the observed decline in peripheral trigeminal sensation [19]. This interpretation, however, should be considered with caution because the parahippoampal gyrus did not show significant FC with the TN [1]. Thus, the age-related increase in FC of the TN with the parahippocampal gyrus could be another compensatory mechanism to offset age-related olfactory deficits. In addition, there was enhanced FC of the TN to the ACC and PCC nodes of the default mode network (DMN) as a function of increasing age. Both AD and PD are associated with altered DMN activity [31, 32]. Thus, the observed age-related resting state FC of the TN may provide an additional avenue to dissect the prodromal manifestation of these dissimilar neurodegenerative pathologies.
All participants in the combined data set were considered healthy, but none contained information about olfactory functioning. Such information is important for studies of chemosensory networks because a considerable number of people with olfactory impairment reported having experienced an olfaction-related hazardous event at some point in their lives [33]. As such, longitudinal studies are needed to carefully determine age-related changes that occur over time from factors that damage the olfactory system. Nevertheless, in this study we provide a well-grounded methodology for understanding the neural underpinnings of age-related changes in chemosensory networks.
In summary, this study established the age-related changes in the resting state FC of the intrinsic ON and TN in a large normal cohort, spanning the adult age range, with fMRI data collected from three different population locations. Results corroborate our previous findings using a smaller sample size [17], and further elaborated the age-related changes on resting state FC of the intrinsic ON and TN. Although not spatially independent, a differential pattern of FC was observed between the ON and TN. The seed-based approach we used in this paper, therefore, can be more informative than a whole-brain Independent Component Analysis (ICA), where spatial independence has a higher emphasis. As such, the ON, in combination with the TN resting state FC, provided a more comprehensive understanding of age-related behavior of intranasal chemosensation that may be useful in assessing early onset of neurodegenerative pathology. Future studies should seek to discriminate between ON and TN resting state FC markers for neurodegenerative diseases such as PD, versus AD and other forms of dementia. The age-related effects reported here is an important step in this direction.
Acknowledgments
The study was supported by a grant from the Department of Radiology, Pennsylvania State University College of Medicine.
References
- 1.Tobia MJ, Yang QX, Karunanayaka P. Intrinsic intranasal chemosensory brain networks shown by resting-state functional MRI. Neuroreport. 2016;27(7):527–531. doi: 10.1097/WNR.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 2.Brand G. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev. 2006;30(7):908–917. doi: 10.1016/j.neubiorev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Gottfried JA, Smith AP, Rugg MD, Dolan RJ. Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42(4):687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- 4.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walla P, Mayer D, Deecke L, Lang W. How chemical information processing interferes with face processing: a magnetoencephalographic study. Neuroimage. 2005;24(1):111–117. doi: 10.1016/j.neuroimage.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez B, Karunanayaka P, Wang J, Tobia MJ, Vasavada M, Eslinger PJ, et al. Different patterns of age-related central olfactory decline in men and women as quantified by olfactory fMRI. Oncotarget. 2017 doi: 10.18632/oncotarget.16977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 10.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 11.Murphy C. Age-related effects on the threshold, psychophysical function, and pleasantness of menthol. J Gerontol. 1983;38(2):217–222. doi: 10.1093/geronj/38.2.217. [DOI] [PubMed] [Google Scholar]
- 12.Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986(1–2):39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60(4):510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- 14.Luzscz MA, Bryan J. Toward understanding age-related memory loss in late adulthood. Gerontology. 1998;45:2–9. doi: 10.1159/000022048. [DOI] [PubMed] [Google Scholar]
- 15.Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 17.Hummel T, Fliessbach K, Abele M, Okulla T, Reden J, Reichmann H, et al. Olfactory FMRI in patients with Parkinson’s disease. Front Integr Neurosci. 2010;4:125. doi: 10.3389/fnint.2010.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010;1357:184–194. doi: 10.1016/j.brainres.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasnelli J, Hummel T. Age-related decline of intranasal trigeminal sensitivity: is it a peripheral event? Brain Res. 2003;987(2):201–206. doi: 10.1016/s0006-8993(03)03336-5. [DOI] [PubMed] [Google Scholar]
- 20.Frasnelli J, Schuster B, Zahnert T, Hummel T. Chemosensory specific reduction of trigeminal sensitivity in subjects with olfactory dysfunction. Neuroscience. 2006;142(2):541–546. doi: 10.1016/j.neuroscience.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Iannilli E, Gerber J, Frasnelli J, Hummel T. Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res. 2007;1139:235–244. doi: 10.1016/j.brainres.2006.12.082. [DOI] [PubMed] [Google Scholar]
- 22.Seubert J, Freiherr J, Djordjevic J, Lundstrom JN. Statistical localization of human olfactory cortex. Neuroimage. 2013;66:333–342. doi: 10.1016/j.neuroimage.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Iannilli E, Del Gratta C, Gerber JC, Romani GL, Hummel T. Trigeminal activation using chemical, electrical, and mechanical stimuli. Pain. 2008;139(2):376–388. doi: 10.1016/j.pain.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saive AL, Royet JP, Plailly J. A review on the neural bases of episodic odor memory: from laboratory-based to autobiographical approaches. Front Behav Neurosci. 2014;8:240. doi: 10.3389/fnbeh.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karunanayaka PR, Wilson DA, Vasavada M, Wang J, Martinez B, Tobia MJ, Kong L, Eslinger P, Yang QX. Rapidly acquired multisensory association in the olfactory cortex. Brain and Behavior. 2015;5:1–14. doi: 10.1002/brb3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 29.Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision making in old age. Front Hum Neurosci. 2009;3:34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alizadeh R, Hassanzadeh G, Soleimani M, Joghataei MT, Siavashi V, Khorgami Z, et al. Gender and age related changes in number of dopaminergic neurons in adult human olfactory bulb. J Chem Neuroanat. 2015;69:1–6. doi: 10.1016/j.jchemneu.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao N, Shek-Kwan Chang R, Cheung C, Pang S, Lau KK, Suckling J, et al. The default mode network is disrupted in Parkinson’s disease with visual hallucinations. Hum Brain Mapp. 2014;35(11):5658–5666. doi: 10.1002/hbm.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130(3):317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]


