Abstract
Purpose
To determine the cumulative incidence of optic disc hemorrhage (ODH) before and after development of primary open angle glaucoma (POAG); determine the prognostic significance of ODH for the development of POAG; identify predictive factors for ODH.
Design
Prospective cohort study.
Methods
ODHs were evaluated in 3,236 eyes of 1618 Ocular Hypertension Treatment Study (OHTS) participants annually using stereoscopic optic disc photographs. The incidence of ODH before and after the development of POAG, the risk of ODH for POAG, and risk factors for ODH were determined using a multivariate proportional hazards regression models.
Results
After a median follow-up of 13 years, one or more ODHs were detected in 179 eyes of 169 participants. The incidence of ODH was 0.5% per year during an average of 13 years before the development of POAG and 1.2% per year during an average of 6 years after the development of POAG. The cumulative incidence of POAG in eyes with ODH was 25.6% compared to 12.9% in eyes without ODH. The occurrence of an ODH increased the risk of developing POAG 2.6-fold in the multivariate analysis (95% confidence interval, 1.7– 4.0; P<0.0001). Randomization to the observation group, older age, thinner central corneal thickness, larger vertical cup to disc ratio, higher intraocular pressure, and self-reported black race were identified as risk factors for ODH.
Conclusion
ODH is an independent predictive factor for the development of POAG in patients with ocular hypertension (OHT) and the predictive factors for ODH are very similar to those for POAG in OHT patients.
Introduction
Optic disc hemorrhages (ODH) can occur in a variety of ocular disease states, such as posterior vitreous detachment, optic neuropathy, retinal vascular disease, and glaucoma. They have been shown to be predictive of future glaucomatous damage in patients with primary open angle glaucoma (POAG)1–5 and normal tension glaucoma.5–10 More recently, ODHs have been shown to precede retinal nerve fiber layer thinning detected by Optical Coherence Tomography (OCT) in patients with glaucoma.11–15 There is also evidence that structural and functional glaucomatous changes may precede the occurrence of ODH.16–18 Whether ODHs are a precursor to, or marker of, glaucomatous damage, the literature supports that they are a predictive factor for progression in patients with glaucoma.19 However, fewer studies have addressed the issue of whether ODHs are a risk factor for the development of POAG in patients with ocular hypertension (OHT). In an interim report of the Ocular Hypertension Treatment Study after a total median follow-up of 8 years,20 we reported that identification of an ODH increased the risk of developing POAG 3.7-fold in a multivariate analysis that included baseline factors predictive of the development of POAG. Subsequently, the European Glaucoma Prevention Study confirmed that ODH was an independent risk factor for POAG with a hazard ratio of 1.97 in a similar multivariate analysis.21 Since there can be a delay between the appearance of an ODH and changes in the visual field or optic disc, additional participants who developed POAG after an ODH may have been identified later in the follow-up of the OHTS The purpose of the current report is to update results at 13 years (median) follow-up, which is an additional 5 years more than in our previous report.20 In this paper we report: 1. the cumulative incidence of ODH before and after development of POAG; 2. determine the prognostic significance of ODH for the development of POAG; 3. to identify factors predictive for ODH, including the development of POAG.
Subjects and Methods
Methods for the detection and definition of what constituted a glaucomatous ODH in OHTS1 have been described in detail previously20 and did not change between OHTS1 and OHTS2. An ODH was considered to be related to glaucoma if there was a flame- or splinter-shaped hemorrhage that was in the nerve fiber layer of the neuroretinal rim or retina and perpendicularly oriented to the edge of the optic disc either on or immediately adjacent to the optic disc. Hemorrhages associated with optic disc swelling or those accompanied by retinal hemorrhages consistent with retinal vascular occlusion or diabetic retinopathy were not considered glaucomatous hemorrhages. The presence of an ODH at the baseline visit was an exclusion criterion for potential OHTS participants, but was not considered a POAG endpoint.22 Annually, stereoscopic optic disc photographs were evaluated by two masked (non-MD) readers at the OHTS Optic Disc Reading Center (ODRC), and readers were asked to determine whether an ODH was present or not. If the two readers disagreed, the photographs were referred to a senior (MD) reader for adjudication. As part of the current report, all photographs that were determined to contain an ODH by the ODRC were re-reviewed by two of the study authors (DLB and SJG) to confirm the presence of an ODH and to determine whether the hemorrhage met the criteria for a glaucomatous hemorrhage as defined in the OHTS1 analysis.20 All evaluations were done with the readers and investigators masked to randomization group and diagnostic status. The current report used all of the data collected during the median 13 year follow-up of the OHTS. The analysis of whether ODH is a predictive factor for the onset of POAG used ODH prior to the development of POAG through OHTS 1 and 2. The analysis of predictive factors for ODH included time to development of ODH including subjects who developed ODH at the same visit or after the development of POAG.
The analysis data set included the complete OHTS database, which includes data from baseline randomization through March 2009. To determine whether ODH was predictive of the development of POAG, a univariate Cox proportional hazards model was performed with detection of ODH during follow-up as a time-dependent factor. Only ODHs prior to the time of the 1st suspicious date of the development of POAG were included in Cox model. The robust sandwich variance estimate option was used to account for the correlation between the 2 eyes of participants. To determine if ODH was an independent factor predictive of the development of POAG, ODH was added as a time-dependent factor to the published OHTS Cox prediction model that includes age, vertical cup-to-disc ratio, pattern standard deviation index on visual field testing, intraocular pressure (IOP), and central corneal thickness (CCT). 23
To identify factors, including the development of POAG, that were predictive of ODH through 13 years of follow-up, univariate and multivariate Cox proportional hazards models were performed. All data from randomization through the development of ODH or the last optic disc photograph of the participant were included. Predictors in univariate Cox models with p<0.05 were tested in the multivariate Cox proportional hazard model and retained in the multivariate model if statistically significant at P=0.05. Covariates in Cox models included POAG status as a time-dependent variable. Robust sandwich variance estimate option was used to account for the correlation between the 2 eyes of participants. The cumulative incidence of ODH was assessed with Kaplan-Meier analyses to account for variable follow-up time.
Results
The OHTS data files from February 1994 through March 2009 were used in this report. The median follow-up was 13 years for 1,636 participants. POAG developed in 362 eyes of 279 participants, including 213 eyes (59%) by optic disc criteria alone, 129 eyes (36%) by visual field criteria alone, and 20 eyes (5%) by both. The cumulative incidence of eyes with at least one ODH before POAG by follow-up years 1 through 14 was 0.37% (n =12), 0.92% (n =29), 1.6% (n = 49), 2.2% (n = 66), 2.5% (n =75), 2.9% (n = 85), 3.6% (n= 104), 4.0% (n = 115), 4.6% (n=129), 5.2% (n=143), 5.7% (n=154), 6.4% (n=168), and 7.0% (n=179), respectively, or 0.5% per year.
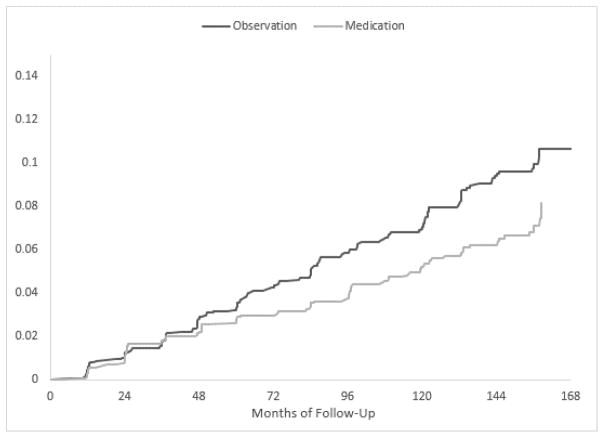
Overall, and by randomization group, the cumulative incidence of eyes with at least one ODH at 13 years of follow up was 8.5%, including 7.4 % in the medication group and 9.8% in the observation group. The Figure shows the cumulative incidence of ODHs in the medication and observation groups over the median 13 years of follow-up. ODHs occurred less frequently in the medication group compared to the observation group, the hazard ratio was statistically significant (HR= 0.70, 95% CI 0.52 – 0.95, P = 0.02, Cox proportional hazards model). Table 1 displays a frequency distribution of ODHs detected in 179 eyes of the 169 participants prior to POAG and in non-POAG eyes. 3093 eyes had no disc hemorrhages and, of those, 322 developed POAG, 300 had no subsequent ODHs but 42 did. Of the 179 eyes with ODH, 139 (78%) did not develop POAG during a median follow-up of 49 months after the disc hemorrhage and 40 eyes (21.6%) subsequently developed a POAG endpoint after 36 months (median) – 20 eyes (50%) by optic disc criteria after 37 months (median), 18 eyes (45%) by visual field criteria after 20.4 months (median), and 2 eyes (5%) by both after 90.7 months (median).
Figure.
Cumulative incidence of participants who developed an optic disc hemorrhage in the Ocular Hypertension Treatment Study. The hazard ratio for developing a disc hemorrhage in the medication group was 0.70 with a 95% CI 0.52 – 0.95, P=0.02.
Table 1.
Optic Disc Hemorrhages Prior to and After the Development of Primary Open Angle Glaucoma during 13 years of follow-up
| Number of disc hemorrhages Prior to POAG or in non POAG eyes per eye |
Number of eyes 3,272eyes of 1636 pts. |
Number (%) of eyes reaching POAG endpoint N = 362 eyes of 279 pts. |
POAG eyes: Number of Optic Disc Hemorrhages after POAG |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | |||
| 0 | 3093 | 322(10.4%) | 300 | 19 | 1 | 2 | 0 |
| 1 | 145 | 28 (19.3%) | 24 | 0 | 4 | 0 | 0 |
| 2 | 21 | 5 (23.8%) | 3 | 1 | 0 | 0 | 1 |
| 3 | 10 | 4 (40.0%) | 1 | 0 | 2 | 0 | 1 |
| 4 | 3 | 3 (100%) | 2 | 0 | 1 | 0 | 0 |
| All | 3272 | 362 | 330 | 20 | 8 | 2 | 2 |
Pts. = Participants
Left side of Table 1 shows number and percentage of eyes that reached a POAG endpoint after documentation of 0, 1, 2, 3, or for optic disc hemorrhages. The right side of the table shows the number of optic disc hemorrhages documented after the development of a POAG endpoint in eyes who had 0, 1, 2, 3, or 4 hemorrhages before a POAG endpoint.
Of the 362 eyes in which POAG developed, 32 eyes of 30 participants had documented ODHs after the development of POAG after a median follow-up of 53.7 months. Table 1 also displays the frequency distribution of ODHs in 32 eyes of 30 participants after a diagnosis of POAG. Twenty-two (69%) of these 32 eyes had no documented ODHs before the POAG endpoint and 10 (31%) had at least one pre-POAG ODH. Among the 22 eyes that had no documented ODHs prior to POAG, the cumulative incidence of the post-POAG ODHs from 1 to 6 years was 2.8% (n= 8), 4.9% (n=13), 6.5% (n=16), 7.8% (n=18), 9.2% (n=20), and 10.0 % (n=21), respectively, or approximately 1.2% per year. There was one eye with the initial ODH 11 years after the first POAG endpoint. The maximum number of POAG ODHs after reaching a POAG endpoint was 6 in a single eye. No difference was found in the incidence of post- POAG ODHs between participants who had reached POAG by disc criteria versus field criteria (P= 0.17).
To assess whether ODH increased the risk of POAG, ODH was included as a time-dependent covariate in a Cox proportional hazards model of time to POAG. The risk of developing POAG after occurrence of an ODH was 3.98 times higher than without an ODH (hazard ratio, 3.98; 95% confidence interval, 2.7 –5.9; P<0.0001). After adjusting for baseline factors in the published OHTS Cox prediction model of POAG,21,23 and randomization assignment, the independent contribution of ODH to risk was less (hazard ratio, 2.6; 95% confidence interval, 1.7– 4.0; P<0.0001).
In univariate Cox proportional hazards models, baseline variables significantly predictive (p<0.05) of ODH were age, CCT, vertical cup to disc ratio, PSD, IOP, history of heart disease, gender, randomization group and self-reported race (Table 2). POAG status as a time dependent variable in the univariate model did not statistically significantly increase the risk of ODH (p=0.09). The final multivariate model for ODH showed that increased age, thinner CCT, larger vertical CD ratio, higher IOP, self-reported black race and randomization to observation were predictive of ODH (Table 3). Factors that were not statistically significant in the multivariate model included history of heart disease (p=0.30), female gender (p=0.64) and PSD (p=0.08).
Table 2.
OHTS: Factors predictive of optic disc hemorrhage in Univariate Cox models
| Predictor | # eyes (for categorical variables) | Hazard Ratio | P-value | 95% Confidence Intervals
|
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
|
| |||||
| POAG Status as time dependent covariate | |||||
| POAG | 362 | 1.89 | 0.09 | 0.91 | 3.92 |
| Not POAG* | 2874 | ||||
|
| |||||
| Randomization Group | |||||
| Medication | 1612 | 0.70 | 0.006 | 0.54 | 0.90 |
| Observation * | 1624 | ||||
|
| |||||
| Age (per decade increase) | 1.47 | <0.0001 | 1.29 | 1.68 | |
|
| |||||
| Corneal thickness (per 40μ decrease) | 1.19 | 0.0110 | 1.04 | 1.36 | |
|
| |||||
| Vertical cup/disc ratio (per 0.1 DD increase) | 1.19 | <0.0001 | 1.11 | 1.27 | |
|
| |||||
| Pattern Standard Deviation (1 unit increase) | 2.37 | 0.0005 | 1.45 | 3.86 | |
|
| |||||
| IOP (per 10 mmHg increase) | 2.09 | 0.0002 | 1.42 | 3.09 | |
|
| |||||
| Myopia Spherical Equivalent | 0.99 | 0.76 | 0.94 | 1.05 | |
|
| |||||
| Peripapillary Crescent | |||||
| Small | 2121 | 1.28 | 0.16 | 0.94 | 1.75 |
| Medium | 197 | 1.59 | 0.08 | 0.95 | 2.66 |
| Large | 43 | 1.98 | 0.16 | 0.77 | 5.10 |
| None * | 875 | ||||
|
| |||||
| Smoked at least 100 cigarettes | |||||
| Yes | 1252 | 1.18 | 0.24 | 0.90 | 1.54 |
| No * | 1532 | ||||
|
| |||||
| Gender | |||||
| Female | 1842 | 0.73 | 0.014 | 0.56 | 0.94 |
| Male * | 1394 | ||||
|
| |||||
| Ethnicity | |||||
| Black | 798 | 0.57 | 0.002 | 0.40 | 0.82 |
| Other* | 2438 | ||||
|
| |||||
| Family history of glaucoma | |||||
| Yes | 1106 | 0.88 | 0.34 | 0.67 | 0.15 |
| No * | 2130 | ||||
|
| |||||
| History of asthma | |||||
| Yes | 224 | 0.98 | 0.95 | 0.58 | 1.66 |
| No * | 3006 | ||||
|
| |||||
| History of cancer | |||||
| Yes | 188 | 1.2 | 0.50 | 0.71 | 2.04 |
| No * | 3048 | ||||
|
| |||||
| History of diabetes mellitus | |||||
| Yes | 382 | 0.86 | 0.50 | 0.55 | 1.34 |
| No * | 2848 | ||||
|
| |||||
| History of high blood pressure | |||||
| Yes | 1222 | 1.0 | 0.98 | 0.77 | 1.31 |
| No * | 2004 | ||||
|
| |||||
| History of low blood pressure | |||||
| Yes | 142 | 0.86 | 0.65 | 0.44 | 1.67 |
| No * | 3088 | ||||
|
| |||||
| History of heart disease | |||||
| Yes | 202 | 1.57 | 0.053 | 0.99 | 2.48 |
| No * | 3030 | ||||
|
| |||||
| History of lung disease | |||||
| Yes | 78 | 0.79 | 0.63 | 0.30 | 2.05 |
| No * | 3152 | ||||
|
| |||||
| History of stroke | |||||
| Yes | 38 | 0.57 | 0.31 | 0.08 | 4.10 |
| No * | 3198 | ||||
|
| |||||
| Migraine Headaches | |||||
| Yes | 356 | 0.94 | 0.78 | 0.62 | 1.43 |
| No * | 2844 | ||||
Reference Group
Table 3.
OHTS: Factors predictive of optic disc hemorrhage in Multivariate Cox proportional hazard model.
| Predictor | # eyes (for categorical variables) | Hazard Ratio | P-value | 95% Confidence Intervals
|
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
|
| |||||
| Randomization Group | |||||
| Medication | 1612 | 0.70 | 0.02 | 0.52 | 0.95 |
| Observation * | 1624 | ||||
|
| |||||
| Age (per decade increase) | 1.39 | <0.0001 | 1.20 | 1.61 | |
|
| |||||
| Corneal thickness (per 40μ decrease) | 1.18 | 0.048 | 1.00 | 1.38 | |
|
| |||||
| Vertical cup/disc ratio (per 0.1 DD increase) | 1.21 | <0.0001 | 1.12 | 1.30 | |
|
| |||||
| IOP (per 10 mmHg increase) | 2.00 | 0.001 | 1.32 | 3.06 | |
|
| |||||
| Self-Reported Race | |||||
| Black | 798 | 0.48 | 0.0003 | 0.52 | 0.95 |
| Other* | 2438 | ||||
Reference Group
Discussion
Bjerrum first reported the association between ODH and glaucoma in 1889.24 However, it was not until 1970 that Drance and Begg further described the relationship between ODH in open-angle glaucoma.25 Numerous studies have subsequently explored ODH as a risk factor for the development and progression of glaucoma.1–15 More recently, several studies have demonstrated abnormalities in the optic disc and retinal nerve fiber layer and visual field that precede the development of an ODH in glaucoma patients. 16–18
ODH typically appear as a flame- or splinter-shaped hemorrhage extending radially from the optic nerve head to the adjacent retina, and this shape is a result of the orientation of the axons in the retinal nerve fiber layer. ODH may be subtle and mistaken for a blood vessel on cursory examination. Even experienced clinicians may miss an ODH,20 and they are not detected with devices that image the optic nerve and RNFL such as optical coherence tomography. Therefore, optic disc photographs are considered the most sensitive detection method for ODH.
Development of an ODH may be related to biomechanical forces influencing the lamina cribrosa and surrounding tissues including the IOP, cerebrospinal fluid pressure, arterial pressure, and venous pressure.26 Cigarette smoking and a history of heart disease were associated with an increased risk of ODH in OHTS, providing supporting evidence that vascular factors are important in the pathogenesis of ODH. It is unclear whether ODH play a causative role in glaucoma progression or whether they are simply a marker for the disease. Blood is known to be neurotoxic as a result of extravasated inflammatory cells, hemoglobin, and thrombin.27,28
Baseline factors associated with ODH in the multivariate Cox proportional hazards model were older age, larger vertical cup to disc ratio, thinner central corneal thickness, IOP and self-reported race. Participants randomized to the medication group had lower incidence of ODH than participants randomized to the observation group. Many of the baseline factors that were associated with an increased risk of ODH were also associated with an increased risk of developing POAG.23 However, ODH remained an independent predictor of glaucoma in the multivariate analysis.
ODH occur more commonly in eyes with glaucoma or OHT than in normal eyes, and their frequency is higher in normal-tension glaucoma than high-tension glaucoma. The cross sectional prevalence of ONH has been reported to range from 0 to 0.4% in normal eyes, 0.4% to 10% in eyes with OHT, 4.2% to 17.6% in eyes with high-tension glaucoma, and 20.5% to 33.3% in eyes with normal-tension glaucoma in population- and clinic-based studies.29–31 The incidence of ODH has ranged from 0.04 to 0.34 times per year in eyes with high-tension glaucoma and 0.11 to 0.46 times per year in eyes with normal-tension glaucoma in clinic-based studies. 31–33 The incidence of ODH was approximately 0.5% per year in the setting of this multicenter randomized clinical trial of participants with OHT prior to development of POAG and with follow-up spanning more than a decade. This is likely an underestimate for two reasons. First, because participants with ODH at baseline were excluded from the OHTS cohort due to uncertainty at the time as to whether ODH was a sign of early POAG or a risk factor, the participants enrolled in OHTS only included those who did not have ODH at baseline and were probably less likely to have ODH during follow-up. Second, ODHs are transient, usually resolving within 2 months.29 Since photographs were only taken annually and ODHs may appear and resolve undetected between photographs, the incidence is likely higher than reported here.
Numerous studies have described an increased risk of visual field progression1–10,34–36 and neuroretinal rim/RNFL thinning 11–15,37,38 in glaucoma patients with ODH. However, the relationship between ODH and progression to glaucoma in patients with OHT is less well described. Drance and colleagues1 studied 29 patients with OHT and ODHs to 29 ocular hypertensive patients without ODHs and found 10 patients who progressed to glaucoma in the ODH group compared to only one in the group without ODHs. Airaksinen and coauthors looked retrospectively at 25 patients with OHT and found that 8 developed glaucomatous optic nerve or visual field changes over a follow-up of 1 to 2 years; however, no control group was studied.39 In a retrospective study of 17 OHT patients, Siegner and Netland5 found that those with ODHs were more likely to develop glaucomatous optic nerve or visual field changes compared to OHT patients without ODHs. The European Glaucoma Prevention Study was a prospective randomized trial similar to OHTS, comparing treatment to observation in patients with OHT. That study found that intercurrent ODH increased the risk of developing POAG by 1.97,21 similar to the magnitude of increased risk of 2.8 in this report.
Although the presence of an ODH was associated with an increased risk of developing POAG in the OHTS, the majority of eyes (78%) with an ODH did not progress to glaucoma over a median follow-up of 49 months after ODH. ODH may occur with other ocular conditions, including posterior vitreous detachment, optic nerve drusen, retinal vascular occlusion, ischemic optic neuropathy, and papilledema.26,40 ODH may also develop in association with a systemic condition, such as diabetes mellitus, hypertension, leukemia, or systemic lupus erythematosus. We attempted to exclude participants with these conditions in the present analysis.
The long-term analysis of the OHTS data shows a strong relationship between randomization to treatment group and ODH. Participants in the treatment group had a 30% lower hazard ration for an ODH. This was not found in the OHTS 1 analysis20 but there was a trend in this direction (risk ratio 0.75; 95% CI 0.53 – 1.09, P = 0.13). It is likely that the increased number of ODHs and the longer follow-up in the longitudinal analysis resulted in this trend becoming statistically significant. Several studies have examined the relationship between IOP lowering treatment and the frequency of ODH. Hendrickx et al observed that reduction of IOP significantly decreased the number of ODH in patients with high-tension glaucoma, but not in patients with normal-tension glaucoma.32 Miyake et al found a significant reduction in the frequency of ODH after trabeculectomy with mitomycin C compared to preoperative rates in both high-tension and normal-tension glaucoma patients,33 suggesting that those patients with profound IOP lowering have a reduced risk of ODH. Medeiros et al found that, in eyes with ODH, IOP-lowering therapy slowed the velocity of VF progression compared to pre-treatment.41 In contrast, the Early Manifest Glaucoma Trial (EMGT) demonstrated that the presence or frequency of ODH was not related to IOP lowering therapy.42
Although the OHTS was the largest and longest study to look at the predictive factors associated with the development of POAG in people with OHT, there are several limitations to the current analysis. First, while surveillance for ODH was performed every 6 months by study investigators and annually using stereoscopic disc photos, we only used data from annual photographs because we could not verify that ODHs identified by investigators fit the strict definition of “glaucomatous” optic disc hemorrhages. It is possible that ODHs were observed in between annual photographs and had time to resolve since they are known to be transient. This would result in observation bias resulting in an underestimate of the incidence of optic disc hemorrhages. Second, ODH was the only intercurrent, or non-baseline predictive factor, considered in this analysis. It is possible that other intercurrent predictive factors such as level of IOP, fluctuations in IOP, compliance with medical therapy, and other factors increase the risk of developing POAG and might affect the predictive risk of ODH on POAG if they were included in a multivariate risk analysis model. It is hoped that future analysis of the OHTS dataset will incorporate these potential predictive factors into a comprehensive risk calculator. OHTS 3, which is a new study to re-examine the original OHTS cohort seven and eight years after completion of OHTS 2, may add refinement to the current estimates of hazard ratios as well as help develop a comprehensive risk calculator that considers all measured intercurrent predictive factors.
In summary, the present study demonstrates a low incidence of ODH in participants with OHT alone (0.5%/year) but a doubling of the incidence of ODH after POAG has developed (1.2%/year). Intercurrent ODH increased the risk of developing POAG in participants almost three fold. Since the occurrence of an ODH is a strong predictor of the subsequent development of POAG in patients with OHT, we recommend careful examination of the optic nerve at all follow-up visits to detect ODH. More frequent monitoring should be undertaken if an ODH is detected in a patient with OHT and institution or escalation of medical therapy may be advisable in some cases. Older age, larger vertical cup:disc ratio, thinner central corneal thickness, higher IOP and self-reported African-American race were factors predictive of ODH in multivariate models.
Supplementary Material
Acknowledgments
Funding Support: Supported by National Eye Institute, National Institutes of Health, Bethesda, MD (P30-EY014801, EY09341, EY09307, EY009307-16S1) and Research to Prevent Blindness, New York, NY.
Footnotes
- D. Budenz: Consulting fees – Alcon Labs, Ft. Worth, TX, USA; Inotek, Lexington, MA, USA; Novartis, Cambridge, MA, USA; Travel grant – New World Medical, Rancho Cucamonga, CA, USA; Data Safety Monitoring Board fees – Ivantis, Irvine, CA, USA. Research grants – New World Medical Inc., Rancho Cucamonga, CA, Abbott Medical Optics, Abbott Park, IL, USA; National Eye Institute (NIH), Bethesda, MD, USA.
- M. Kass, M. Gordon: Research Grants – National Eye Institute (NIH), Bethesda, MD, USA.
- J. Huecker, S. Gedde– Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drance SM, Fairclough M, Butler DM, Kottler MS. The importance of disc hemorrhage in the prognosis of chronic open angle glaucoma. Arch Ophthalmol. 1977;95(2):226–228. doi: 10.1001/archopht.1977.04450020028004. [DOI] [PubMed] [Google Scholar]
- 2.Shihab ZM, Lee P-F, Hay P. The significance of disc hemorrhage in open-angle glaucoma. Ophthalmology. 1982;89(3):211–213. doi: 10.1016/s0161-6420(82)34805-8. [DOI] [PubMed] [Google Scholar]
- 3.Diehl DL, Quigley HA, Miller NR, Sommer A, Burney EN. Prevalence and significance of optic disc hemorrhage in a longitudinal study of glaucoma. Arch Ophthalmol. 1990;108(4):545–550. doi: 10.1001/archopht.1990.01070060093056. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996;103(7):1014–1024. doi: 10.1016/s0161-6420(96)30572-1. [DOI] [PubMed] [Google Scholar]
- 6.Chumbley LC, Brubaker RF. Low-tension glaucoma. Am J Ophthalmol. 1976;81(6):761–767. doi: 10.1016/0002-9394(76)90359-7. [DOI] [PubMed] [Google Scholar]
- 7.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129(6):707–714. doi: 10.1016/s0002-9394(00)00441-4. [DOI] [PubMed] [Google Scholar]
- 8.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 9.Kono Y, Sugiyama K, Ishida K, Yamamoto T, Kitazawa Y. Characteristics of visual field progression in patients with normal-tension glaucoma with optic disk hemorrhages. Am J Ophthalmol. 2003;135(4):499–503. doi: 10.1016/s0002-9394(02)02056-1. [DOI] [PubMed] [Google Scholar]
- 10.Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120(3):512–519. doi: 10.1016/j.ophtha.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Suh MH, Park KH, Kim H, et al. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. J Glaucoma. 2012;21(6):358–366. doi: 10.1097/IJG.0b013e3182120700. [DOI] [PubMed] [Google Scholar]
- 12.Hwang YH, Kim YY, Kim HK, Sohn YH. Changes in retinal nerve fiber layer thickness after optic disc hemorrhage in glaucomatous eyes. J Glaucoma. 2014;23(8):547–552. doi: 10.1097/IJG.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 13.Niles PI, Greenfield DS, Sehi M, et al. Detection of progressive macular thickness loss using optical coherence tomography in glaucoma suspect and glaucomatous eyes. Eye (Lond) 2012;26(7):983–991. doi: 10.1038/eye.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi F, Park KH, Kim DM, Kim TW. Retinal nerve fiber layer thickness evaluation using optical coherence tomography in eyes with optic disc hemorrhage. Ophthalmic Surg Lasers Imaging. 2007;38(2):118–125. doi: 10.3928/15428877-20070301-06. [DOI] [PubMed] [Google Scholar]
- 15.Kernstock C, Dietzsch J, Januschowski K, Schiefer U, Fischer MD. Optical coherence tomography shows progressive local nerve fiber loss after disc hemorrhages in glaucoma patients. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(4):583–587. doi: 10.1007/s00417-011-1825-3. [DOI] [PubMed] [Google Scholar]
- 16.Law SK, Choe R, Caprioli J. Optic disk characteristics before the occurrence of disk hemorrhage in glaucoma patients. Am J Ophthalmol. 2001;132(3):411–413. doi: 10.1016/s0002-9394(01)01009-1. [DOI] [PubMed] [Google Scholar]
- 17.Jeoung JW, Park KH, Kim JM, et al. Optic disc hemorrhage may be associated with retinal nerve fiber loss in otherwise normal eyes. Ophthalmology. 2008;115(12):2132–2140. doi: 10.1016/j.ophtha.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes CGV, Prata TS, Liebmann CA, Tello C, Ritch R, Liebmann JM. Spatially consistent, localized visual field loss before and after disc hemorrhage. Investig Ophthalmol Vis Sci. 2009;50(10):4727–4733. doi: 10.1167/iovs.09-3446. [DOI] [PubMed] [Google Scholar]
- 19.Uhler TA, Piltz-Seymour J. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Curr Opin Ophthalmol. 2008;19(2):89–94. doi: 10.1097/ICU.0b013e3282f3e6bc. [DOI] [PubMed] [Google Scholar]
- 20.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113(12):2137–2143. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114(1):3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MO, Kass Ma. The Ocular Hypertension Treatment Study: Design and baseline description of the participants. 1999;117(5):573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 23.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 24.Bjerrum J. Om en tilfojelse til den saedvanlige synsfelfundersogelse samt om synfelet ved glaukom. Nord Ophthalmol Tskr (Copenh) 1889;2:141–85. [Google Scholar]
- 25.Drance SM, Begg IS. Sector hemorrhage: A probable acute ischemic disc change in chronic simple glaucoma. Can J Ophthalmol. 1970;5(2):137–41. [PubMed] [Google Scholar]
- 26.Suh MH, Park KH. Pathogenesis and clinical implications of optic disk hemorrhage in glaucoma. Surv Ophthalmol. 2014;59(1):19–29. doi: 10.1016/j.survophthal.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Calingasan NY, Gibson GE. Vascular endothelium is a site of free radical production and inflammation in areas of neuronal loss in thiamine-deficient brain. Ann N Y Acad Sci. 2000;903(914):353–356. doi: 10.1111/j.1749-6632.2000.tb06386.x. [DOI] [PubMed] [Google Scholar]
- 28.Vollaard NBJ, Reeder BJ, Shearman JP, Menu P, Wilson MT, Cooper CE. A new sensitive assay reveals that hemoglobin is oxidatively modified in vivo. Free Radic Biol Med. 2005;39(9):1216–1228. doi: 10.1016/j.freeradbiomed.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology. 1986;93(6):853–857. doi: 10.1016/s0161-6420(86)33658-3. [DOI] [PubMed] [Google Scholar]
- 30.Healey P. Optic disc haemorrhage: The more we look the more we find. Clin Experiment Ophthalmol. 2011;39(6):485–486. doi: 10.1111/j.1442-9071.2011.02648.x. [DOI] [PubMed] [Google Scholar]
- 31.Suh MH, Park KH. Period prevalence and incidence of optic disc haemorrhage in normal tension glaucoma and primary open-angle glaucoma. Clin Experiment Ophthalmol. 2011;39(6):513–519. doi: 10.1111/j.1442-9071.2010.02482.x. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickx KH, van den Enden A, Rasker MT, et al. Cumulative incidence of patients with disc hemorrhages in glaucoma and the effect of therapy. Ophthalmology. 1994;101(7):1165–1172. doi: 10.1016/s0161-6420(94)31192-4. [DOI] [PubMed] [Google Scholar]
- 33.Miyake T, Sawada A, Yamamoto T, Miyake K, Sugiyama K, Kitazawa Y. Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma. 2006;15(2):164–171. doi: 10.1097/00061198-200604000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol. 1997;115(10):1257–1262. doi: 10.1001/archopht.1997.01100160427006. [DOI] [PubMed] [Google Scholar]
- 35.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129(5):562–568. doi: 10.1001/archophthalmol.2011.72. [DOI] [PubMed] [Google Scholar]
- 36.Kim JM, Kyung H, Azarbod P, Lee JM, Caprioli J. Disc haemorrhage is associated with the fast component, but not the slow component, of visual field decay rate in glaucoma. Br J Ophthalmol. 2014;98(11):1555–1559. doi: 10.1136/bjophthalmol-2013-304584. [DOI] [PubMed] [Google Scholar]
- 37.Ahn JK, Park KH. Morphometric change analysis of the optic nerve head in unilateral disk hemorrhage cases. Am J Ophthalmol. 2002;134(6):920–922. doi: 10.1016/s0002-9394(02)01791-9. [DOI] [PubMed] [Google Scholar]
- 38.Chung E, Demetriades AM, Christos PJ, Radcliffe NM. Structural glaucomatous progression before and after occurrence of an optic disc haemorrhage. Br J Ophthalmol. 2014:21–25. doi: 10.1136/bjophthalmol-2014-305349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Airaksinen PJ, Mustonen E, Alanko HI. Optic disc haemorrhages precede retinal nerve fibre layer defects in ocular hypertension. Acta Ophthalmol. 1981;59(5):627–641. doi: 10.1111/j.1755-3768.1981.tb08728.x. [DOI] [PubMed] [Google Scholar]
- 40.Soares AS, Artes PH, Andreou P, Leblanc RP, Chauhan BC, Nicolela MT. Factors associated with optic disc hemorrhages in glaucoma. Ophthalmology. 2004;111(9):1653–1657. doi: 10.1016/j.ophtha.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Medeiros FA, Alencar LM, Sample PA, Zangwill LM, Susanna R, Weinreb RN. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology. 2010;117(11):2061–2066. doi: 10.1016/j.ophtha.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Bengtsson B, Leske MC, Yang Z, Heijl A. Disc hemorrhages and treatment in the Early Manifest Glaucoma Trial. Ophthalmology. 2008;115(11):2044–2048. doi: 10.1016/j.ophtha.2008.05.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.