Abstract
Background
Reduction of cancer-related disparities requires strategies that link medically underserved communities to preventive care. In this community-based participatory research project, a public library system brought together stakeholders to plan and undertake programs to address cancer screening and risk behavior.
Methods
This study was implemented over 48 months in 20 large urban neighborhoods, selected to reach diverse communities disconnected from care. In each neighborhood, Cancer Action Councils were organized to conduct a comprehensive dynamic trial, an iterative process of program planning, implementation and evaluation. This process was phased into neighborhoods in random, stepped-wedge sequence. Population-level outcomes included self-reported screening adherence and smoking cessation, based on street-intercept interviews.
Results
Event history regressions (n=9,374) demonstrated that adherence outcomes were associated with program implementation, as were mediators such as awareness of screening programs and cancer information-seeking. Findings varied by ethnicity, and were strongest among respondents born outside the U.S. or least engaged in care.
Conclusion
This intervention impacted health behavior in diverse, underserved and vulnerable neighborhoods. It has been sustained as a routine library system program for several years after conclusion of grant support. In sum, participatory research with the public library system offers a flexible, scalable approach to reduce cancer health disparities.
Keywords: community-based participatory research, comprehensive dynamic trial, cancer screening, diversity, sustainability, event history analysis, interactive systems framework
Introduction
Low-income populations are more likely to be diagnosed with preventable cancers at later stages than the general population. Although disparities in cancer mortality have decreased over the past two decades (American Cancer Society, 2017), there are still marked differences due to race (DeSantis et al., 2016; Krok-Schoen, Baltic, & Paskett, 2017), socioeconomic status (Singh & Jemal, 2017), neighborhood resources (Hashim et al., 2016), and, in some instances, immigration status (Consedine, Tuck, Ragin, & Spencer, 2015; Torre et al., 2016). Cancer disparities arise in part due to differences in screening, risk behaviors and access to high quality care (American Cancer Society, 2011, 2017; Bradley, Schlesinger, Webster, Baker, & Inouye, 2004; Bradley, Webster, et al., 2004; Doubeni, Laiyemo, Reed, Field, & Fletcher, 2009; Elk & Landrine, 2012; Emmons et al., 2011; Husaini et al., 2001; Johnson, Mues, Mayne, & Kiblawi, 2008; Miranda, Tarraf, & Gonzalez, 2011; Taplin et al., 2004).
Reduction of racial and ethnic disparities in cancer incidence, stage of presentation and outcome will require strategies that link medically underserved communities to preventive care. The National Cancer Institute’s Cancer Control Planet Research-Tested Intervention Programs (RTIPs) website lists 34 evidence-based approaches focused on cancer prevention and screening in urban community settings (Research Tested Intervention Programs, 2017). For example, Faith Moves Mountains was designed to promote cervical cancer screening among medically-underserved women through programs set in religious institutions (Studts et al., 2012). Shenson and colleagues (Shenson et al., 2001) demonstrated a program to promote mammography through influenza clinics. Several of these programs focus on specific ethnic groups, including Hmong (Kagawa-Singer, Tanjasiri, Valdez, Yu, & Foo, 2009), Filipinos (Maxwell et al., 2010) and Vietnamese (Taylor et al., 2010) populations. Many examples of evidence-based strategies are available for implementation by communities across the country, including guidance on suitable settings and required resources. However, it is not clear which if any, of these recommended approaches are best-suited to a particular community context.
The National Cancer Institute has developed programs like the Community Networks Project (Braun et al., 2015) and more recently, the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) Initiative (National Cancer Institute, 2017) to mobilize community-based cancer prevention and screening efforts using and adapting evidence-based strategies. A number of authors describe community-based participatory research efforts to engage local stakeholders in planning and adapting strategies to overcome cancer disparities (Gehlert & Coleman, 2010). Community-based participatory research is a partnership approach that equitably involves community members, local organizations and researchers in all aspects of the research, and in which all partners contribute expertise and share decision-making and ownership (Israel, Schulz, Parker, & Becker, 1998; Minkler & Wallerstein, 2011). Community-based participatory research is particularly suited to the development and adaptation of health promotion interventions that take into account ethnic and cultural variation, social instability and limited resources for preventive health. For example, Larkey and colleagues (Larkey, Gonzalez, Mar, & Glantz, 2009) discuss use of community-based participatory research to facilitate recruitment of Latinas to participate in a health promotion study. Strickland (Strickland, 2006) describes challenges encountered during a 10-year program of community-based participatory research to promote cancer prevention with Pacific Northwest American Indian Tribes. Nguyen and colleagues formed a coalition involving Vietnamese-Americans in Santa Clara County, California, to develop and implement a six-component campaign focused on promoting Pap testing to detect cervical cancer. More recently, Davis and colleagues (Davis, Darby, Moore, Cadet, & Brown, 2017) used community-based participatory research to promote awareness of breast cancer screening among medically underserved African American women. For the most part, these and other community-based participatory research studies have focused on developing or tailoring strategies to address a particular type of cancer in a given community setting. There is considerable potential to use community-based participatory research as a basis for wide-scale efforts to identify and resolve any variety of health needs (Rapkin, 2012; A. Wandersman et al., 2008)
Evaluation of the wide-scale impact of community-based participatory research is challenging because local stakeholders may choose to address different problems using different strategies. A method for evaluating the process and outcomes of community-based participatory research involves implementing a Comprehensive Dynamic Trial (Rapkin & Trickett, 2005) design. This design incorporates procedures for quality improvement by utilizing feedback about the intervention to refine and improves its performance (Rapkin & Trickett, 2005). As such, an intervention using the Comprehensive Dynamic Trial model constitutes an overarching process encompassing iterative cycles of implementation and adaptation (Rapkin et al., 2012; Tebes, Thai, & Matlin, 2014; Trickett et al., 2011). In a Comprehensive Dynamic Trial, intervention implementation must be examined and tracked continuously, to provide decision-makers with data about intervention process and performance. Intervention elements are represented as time-changing exposures that may vary across communities and participants. Decision-makers systematically use these data to identify possible modifications in order to support an ongoing process of problem-solving and quality improvement (Collins, Murphy, & Bierman, 2004). Behavioral measures of interest may be linked to differences in exposure to various intervention elements and program components, across settings and over time.
Queens, one of the five boroughs of New York City, is one of the most ethnically and culturally diverse counties in the country, comprising many distinct low-income communities, each with poor cancer outcomes. In order to address these disparities, our team organized a partnership to conduct community-based participatory research which included four core members: a medical research institution, Albert Einstein College of Medicine; a cancer center nested in a public hospital system, Queens Cancer Center of Queens Hospital; the Queens Regional Office of the American Cancer Society; and the Queens Public Library System. There were several reasons for health agencies to join forces with the library system. Libraries are widely trusted and provide safe, accessible space for public programs in many communities, making them ideal partners and venues for community-based participatory research activities. In Queens, local libraries offer materials and services tailored to the ethnic and linguistic mix of the communities they serve.
The objective of our project, Queens Library HealthLink (HealthLink), was to demonstrate whether and how community-based participatory research would lead to a measureable and sustainable impact on cancer screening and preventive health behaviors in diverse, medically-underserved communities. We conducted this work over 48 months with 20 panels of community stakeholders organized for this project, known as Cancer Action Council (Council). Consistent with community-based participatory research, each Council had the opportunity to identify community needs and to plan and test a number of programs specifically suited to local priorities and preferences. We were working on the premise that each community would be able to arrive at strategies and programs to meet identified cancer prevention and detection needs. There was, however, no expectation that communities would do the same thing in the same way to achieve these outcomes. Rather, through an iterative process of problem solving, we expected that the impact of strategies developed by communities would emerge over time.
Methods
Setting
Queens, the second largest borough of New York City has about 2.2 million residents, and is one of the most diverse counties in the United States, including households speaking over 108 different languages. The Queens Borough Public Library System is the largest circulating library system in the country, with 63 community locations each serving a distinct catchment area of Queens. There is a public library branch within five minutes of every address in Queens. Local branches tailor their staffing, holdings, and public programs to the particular cultural and linguistic mix of the communities they serve. We purposively selected 20 of 63 Queens neighborhoods to participate in the study, chosen to maximize ethnic and geographic variation and to reach low-income populations disconnected from health care. Based on U.S. Census and New York City data, selected neighborhoods had the largest proportion of residents living below the federal poverty line, ranging from 12% to 42% with a median of 18%. Between 16% and 33% of residents did not graduate high school, with a median of 29%. Neighborhoods varied considerably in terms of ethnic mix. Using median percentages to summarize across multiple neighborhoods, we selected six neighborhoods that were highly homogeneous, including five with majority African American residents (87%), and one neighborhood that was majority Hispanic – any race (80%). Nine neighborhoods were dominated by two ethnic groups, including five that were mixed Hispanic (35%) and White (31%), three that were mixed Asian (44%) and Hispanic (41%), and one that was predominately Hispanic (60%) and African American (33%). One neighborhood had an African American majority (47%) with large Hispanic (23%) and White (23%) minorities. The remaining four were highly heterogeneous with relatively similar proportions of Hispanic (31%), White (28%), Black (20%), and Asian (20%) residents. Percent of residents born outside the United States ranged from 22% to 72% with a median of 48%. In addition to English (42%) and Spanish (29%), these neighborhoods included speakers of many other languages including Chinese, French Creole, Korean, Russian, French, Italian, Greek, and various Indic and African languages. Neighborhoods were relatively young, with median proportions of 30% of residents under aged 20, 33% aged 21–39, 28% aged 40–64, and 11% aged 65 and over. There were also differences in health needs. Six of the neighborhoods had from 15% to 50% higher than expected rates of incidence of at least one preventable or detectable cancers. Between 10% and 19% of residents in each neighborhood lacked health insurance. Between 6% and 15% of residents reported needing health care but not receiving it, with a median of 13%.
Organization
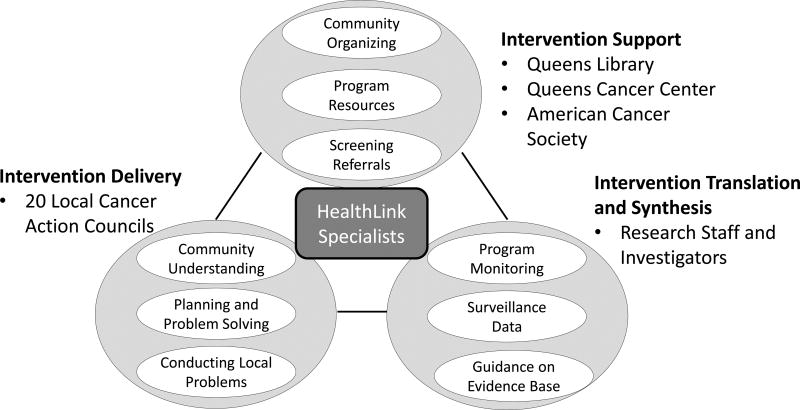
The HealthLink partnership constituted an interactive systems framework (Abraham Wandersman et al., 2008) for instituting population-level prevention, as defined by Wandersman and colleagues (Wandersman, Chien, & Katz, 2012; A. Wandersman et al., 2008) (Figure 1). The Intervention Support system was primarily housed within the Queens Borough Public Library System. Support was also provided by the Queens Cancer Center, a component of New York City’s safety net municipal health system, and by the American Cancer Society’s Queens Regional Office. Queens Cancer Center and the American Cancer Society ensured that referral to low or no-cost cancer screening services and follow-up care as well as services for smoking cessation could be offered through HealthLink. We created two full-time library staff positions for community organizers with master’s degrees in public health. These HealthLink Specialists worked to establish Councils in each of the selected neighborhoods and served as the main contact among all components of the HealthLink. Councils consisted of local community volunteers interested in cancer, including cancer survivors and family members, community library staff, and representatives of religious institutions, community service organizations, small businesses, and health care agencies. The Councils served as the Intervention Delivery System. Each Council conducted community-based participatory research in order to plan, implement and monitor programs tailored to address health disparities affecting their respective communities, with guidance and direct operational involvement from the HealthLink specialists. Study investigators and research staff from the Albert Einstein College of Medicine served as the “Intervention Translation and Synthesis” system, including management of research and evaluation activities, scientific guidance on strategies to promote cancer screening, and access to local data on cancer-related needs. Project management and coordination were provided by a committee composed of project leaders from the Library system, the Queens Cancer Center, the Queens Region of the American Cancer Society, and Albert Einstein College of Medicine. The project was reviewed and approved by the Albert Einstein College of Medicine Institutional Review Boards.
Figure 1. Structure of the HealthLink Partnership.
The HealthLink Partnership may be described using Wandersman and colleagues’ Interactive Systems Framework (Wandersman et al., 2012; A. Wandersman et al., 2008), including the delivery of community programs, support for community activities, and scientific synthesis and translation to inform planning and evaluation. The HealthLink Specialists ensured communication and coordination among these systems. (Permission pending to adapt from first author’s paper in this same journal.)
Design
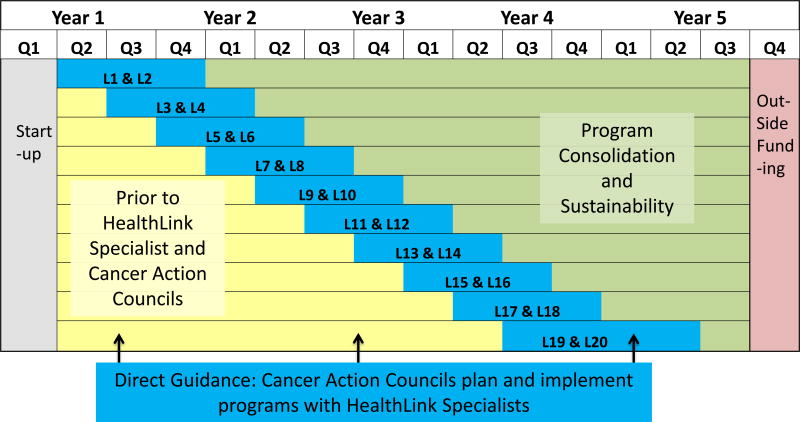
The study applied a “stepped-wedge” design to phase in Council activities over time across 20 communities (Figure 2). Our research design began with a six-month period of assessment before we began the intervention in any neighborhood. We then began to phase the intervention into two neighborhoods at a time. Every four months, the intervention was initiated in two new neighborhoods. It took ten four-month cycles to reach all neighborhoods.
Figure 2. Project Design and Timeline.
This Figure depicts the flow of activity in Queen Library HealthLink. Collection of surveillance data in all neighborhoods began about six months before the initiation of intervention activity in any neighborhood and continued through the duration of the project. The HealthLink intervention was phased in two communities at a time at four months intervals over 10 steps. The date of the first meeting of the neighborhood Council was used as the start date of the 12 months Direct Support Phase in each community. Twelve months after program initiation and for the duration of the project, Councils worked toward greater autonomy in the Sustainability phase.
Work was initiated in each neighborhood by two HealthLink specialists hired to work within the library system. HealthLink specialists had master’s degrees in public health with expertise in community organizing and health education. In each neighborhood, the HealthLink specialists conducted networking and key informant interviews to identify stakeholders that were interested in improving cancer prevention and screening. The stakeholders included cancer survivors and family members, representatives of religious organizations, health and social service agencies, small local businesses such as pharmacies and grocery stores, as well as library staff. These stakeholders formed the Councils for each of the 20 neighborhoods.
The baseline data collection period began simultaneously in all communities and continued until the initiation of formal Council meetings in each community. HealthLink Specialists’ efforts to organize local Councils began several months in advance of the Council’s planned start-date in each community. The date of the first Council meeting in a given neighborhood was considered the date of the initiation of the HealthLink intervention in that community. Initial council meetings were held in two new communities every four months, stepped-in for three years. Council activities were initiated in random sequence with the exception of the Central Library branch, which was included in the first pair at the request of the Library System.
Intervention
The HealthLink intervention entailed work with Councils to identify and understand local health needs in order to formulate and implement tailored education and screening programs (Roy et al., 2014). Councils in the 20 selected neighborhoods included over 100 community stakeholders representing 65 organizations. Councils generally included 3 to 7 members.
In each community, the intervention was conducted in two phases (Figure 2). The first phase, Direct Guidance, lasted for 12 months and included regular meetings of the Councils, organized by the HealthLink specialists. During that time, the specialists provided education on cancer screening, epidemiological data on local health statistics, and examples of programs that might be attempted in the community. Discussion at Council meetings in this phase focused on ensuring members’ familiarity with cancer-related issues and their familiarity with community partners, including the local safety-net hospital system, the American Cancer Society, and the research team. HealthLink specialists guided Councils in setting priorities and brainstorming ways to address identified needs. Councils could also draw on the four core HealthLink partner organizations to aid in planning and carrying out programs, and to identify evidence-based approaches to consider. Consistent with Comprehensive Dynamic Trial methods, program results including attendance, participant feedback, and Council’s own observations were taken into account when planning subsequent programs.
Twelve months after initiation, each Council entered into the Program Consolidation and Sustainability phase (Figure 2). Councils continued in this phase for the remainder of the study. During this phase, HealthLink specialists worked with Councils to transfer leadership roles and to enable them to plan and implement programs more independently. Note that it was neither feasible nor desirable for HealthLink specialists to step away from Councils completely. Their continued involvement was necessary to help Councils gain access to program space and resources at local libraries, to draw on minimal seed funding available for local programs, and to answer questions about program plans and results. However, HealthLink specialists reduced their logistical support and their responsibility for organizing and leading monthly meetings during the Sustainability phase. HealthLink specialists worked with Councils prior to and after the initiation of the Sustainability phase to help develop and support Councils members in leadership roles. This approach proved to make implementation of the study feasible because it allowed HealthLink specialists to focus efforts on new neighborhood Councils as older Councils were assuming greater autonomy.
Councils were encouraged to mount any variety of programs that they deemed would best address local needs. They were encouraged to talk about any preventive-health issues related to cancer, as long as all information and materials provided conformed to National Cancer Institute guidance. Councils were always encouraged to review each of their programs and to make modifications to improve reach, impact and efficiency. Each Council had a budget of $1500 that could be used to offset any expenses associated with conducting programs (e.g., translation, purchase of intervention materials, incentives, and videography). Councils could also seek monetary or in-kind donations associated with offering programs.
Evaluation
Programs implemented by Councils were intended to have community-wide impact through increased awareness, improved access to preventive care, and diffusion of accurate cancer information. Although we tracked the numbers of people attending Councils’ various programs, we chose not to focus our evaluation on the health behavior of program attendees and participants. In order to evaluate the broad impact of the HealthLink intervention, it was necessary to institute a program of community surveillance. Prior to initiation of the intervention in any community, the research team began to conduct anonymous street intercept interviews with people who lived, worked, or otherwise spent time in each neighborhood. Intercept interviews were carried out completely separately from the Councils and the programs that they planned. Interviews were conducted in multiple public spaces (e.g., near parks, libraries, malls, major business districts) within the 20 selected communities for about 48 months. The research team was systematic in terms of approaching each neighborhood at different times of the day, ranging from mid-morning to early evening. Data collection occurred in different blocks of time on different weekdays and occasional weekends. We worked with local police precincts in each community to identify high-traffic areas that were safe for staff to approach people for these interviews. We were often on public main streets but we were also in malls and shopping centers when it was particularly cold weather. Members of local communities were hired as temporary staff to serve as interpreters, so that we could obtain interviews in multiple languages. Each community was sampled in 15 waves over four years, beginning six months before Council meetings began in the first two communities, and continuing about once per quarter until one year after initiation of Council meetings in the final two communities. At each wave in each community, interviewers used random number tables to decide which passers-by to approach, stratified by apparent age (above and below 40) and gender. At least thirty people were sampled per neighborhood per wave.
Measures
Dependent variables obtained from the intercept interviews included questions adopted from the Behavioral Risk Factor Surveillance Survey (Mokdad, Stroup, & Giles, 2003; Remington et al., 1988; Zhang et al., 2015) to track self-reported rates of breast, colorectal and cervical cancer screening, attempts to quit smoking and tobacco cessation. It was necessary to gather data on all of these different behaviors because Cancer Action Councils had the latitude to address one or more cancer health disparity. Mediators potentially associated with the influence of HealthLink on these dependent measures included cancer knowledge, cancer information seeking, awareness of low/no cost cancer screening, health insurance, and time since most recent medical check-up. Moderators of intervention effects included respondents’ reported frequency of local library use. Demographic covariates included race/ethnicity, country of origin, length of residence in current neighborhood. Health system covariates were also included to account for hospital closures and reduced access to low-cost screening due to the economic downturn as well as changes in mammography recommendations (Weiss et al., 2012). Individuals’ exposure to these covariates was determined by the dates of their survey interview relative to the timing of the health-system level events.
Modeling HealthLink Program Effects
We hypothesized that HealthLink’s impact on cancer-related behaviors and mediators would emerge over time in each participating community, in direct relationship to the cumulative frequency and reach of programs implemented by the local Council. In order to examine this hypothesis, we derived a set of five independent variables to describe survey respondents’ potential exposures to programmatic activity that had occurred within each neighborhood up to a given point in time: cumulative number of local programs, up to the month prior to a respondent’s completion of the street-intercept survey; cumulative community-wide attendance at those local programs; and number of months since the most recent local program to examine whether program influence decayed over time. We also included a measure of likely personal awareness of programs, based on the cumulative number of local programs weighted by respondents’ frequency of library use. In addition to local activity, we included the cumulative number of outside programs implemented by HealthLink elsewhere in Queens, inversely weighted by distance, to examine whether respondents received any benefits from HealthLink programs implemented by other Councils. These five indicators of program implementation and activity served as the independent variables representing HealthLink effects in event-history regression analyses to explain outcomes.
Statistical Analysis
Event-history analysis was used to model how discrete occurrences and cumulative activities that unfolded over time affected indicators of interest (Allison, 1984). This application of multiple logistic regression analysis shows temporal associations between Councils’ implementation of programs and the rates of cancer-related health behaviors and mediators across the 20 participating neighborhoods. Event-history analysis allowed us to test the cumulative influence of programs and community attendance at programs, as well as decay of effects that occurred during time intervals between programs. HealthLink effects were tested after controlling for significant demographic and health systems covariates. In addition to overall program effects, we examined interactions between measures of program activity and patient demographics (i.e., age, gender [when applicable], race/ethnicity, U.S. nativity, education). Analyses of cancer screening and prevention behaviors were conducted including and excluding mediators in the model, to examine the hypothesis that HealthLink’s effects on screening were due to improved awareness or access to preventative care. We also conducted analysis to examine HealthLink’s impact of on cancer screening and prevention within the subgroup of respondents most disengaged from care (i.e., greater than two years since most recent check-up or without insurance).
Results
Council Programs
HealthLink specialists identified volunteers to participate as Council stakeholders in 20 communities. Active Councils were established in 19 of 20 communities. In one community, Council members met sporadically and ultimately did not undertake any programs. Data from this community remains in the analysis (consistent with an “intention to treat”). Over the course of 48 months, the 19 active Councils implemented 192 programs.
Programs took place at local libraries (62%), various community (23%) and religious settings (9%) and the Queens Cancer Center (6%). Programs reflected the ethnic and cultural characteristics of the local community. In terms of content, most Council programs focused on a mix of health and wellness issues (38%) or general cancer information (19%). Other programs addressed specific screening topics including breast and cervical (23%), colorectal (11%), and cervical cancer only (3%), Cancer risk reduction was addressed in about one third of programs, including diet and exercise (26%), smoking cessation (6%), and sexually-transmitted infections (4%). Across all content areas, programs employed one or more modalities including education (e.g., lectures to promote cancer awareness by survivors or healthcare providers) (79%), entertainment or recreational activity (e.g., dance performance, quilting bee) (33%), direct linkage to care (e.g., referral to New York State Cancer Services Program, poolside skin cancer screening) (11%), and an on-site screening van (19%). Programs were delivered by a mix of Council members (27%), core HealthLink partners (26%) and HealthLink specialists (5%), as well as outside community organizations (29%), advocate and survivor organizations (25%), and medical and public health experts (18%). Programs were mainly conducted in English (97%) with some bilingual in Spanish (19%) or Chinese (2%).
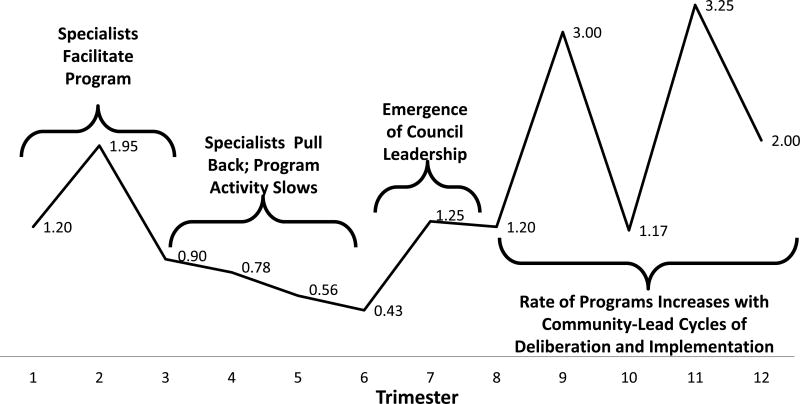
Phasing HealthLink into different neighborhoods over time allowed us to observe neighborhood variance in cumulative program activity over time. Figure 4 shows the mean number of programs implemented during every four-month trimester after initiation of the Council in each neighborhood. The first three trimesters of Direct Guidance showed a moderate level of program activity, corresponding to the initial year that HealthLink specialists worked intensively with Councils. After HealthLink specialist involvement was intentionally pulled back at the start of each Council’s second year Sustainability Phase, the average number of programs implemented by each Council tended to diminished for the next three trimesters. It seemed to take about a year of working independently for Councils to find their footing. At the beginning of Year 3, Councils’ rate of program implementation increased to levels about 70% greater than Year 1, when HealthLink specialists were involved. Of particular interest, number of programs over the last six trimesters shows alternating periods of low and high program activity, consistent with the iterative cycles of deliberation-implementation that would be expected in a Comprehensive Dynamic Trial.
Figure 4. Mean Number of Programs per Council by Trimester of Activity.
Over the course of the implementation, Councils were assisted in planning interventions by HealthLink Specialists in the first year (trimesters 1–3), leading to a moderate average rate of program activity. After one year, our design required Specialists to shift activities from a given Council to start-up in new communities. During each Council’s second year (trimesters 4–6), the number of programs tended to diminish. By the third year (trimesters 7–9), activity picked up due to the emergence of local leadership and council’s increased experience. By the fourth year, average program activity increased substantially. Consistent with the comprehensive dynamic trial model, activities seemed to settle in to patterns of planning (lower rates) followed by implementation (higher rates). Note that the calendar for each Council began during different months, controlling for seasonal affects. This pattern of activity suggests that the HealthLink process fostered a sustainable mechanism for program planning and implementation.
Consistent with the Comprehensive Dynamic Trial model, we hypothesized that HealthLink’s impact on cancer-related behaviors in each participating community would emerge over time, in direct relationship with the cumulative frequency and reach of programs implemented by local Councils. In order to test this hypothesis, we examined how health behavior was associated with our five indicators of intervention effects number of local programs (overall or by topic); cumulative attendance months since the most local recent program, activity weighted by personal awareness of programs and the number of outside programs implemented elsewhere in Queens. These variables served to capture the ebb and flow of program activities evident in Figure 4 as they pertained to each neighborhood at any given point in time. These five indicators served as the independent variables representing HealthLink effects in event-history regression analyses to explain outcomes. Thus, the intervention “condition” to which any survey respondent was exposed is defined by the values on the aforementioned indicators of HealthLink program effects for each participant’s neighborhood up to the point in time that that individual was accrued to the study.
Sample Characteristics
Table 1 shows the characteristics of the 9,374 respondents to the brief street intercept interview. Demographic variables: As intended, the sample is balanced by gender (53% female) and age above and below 40 years. The mix of different races and ethnicities, country of origin, and levels of education reflect the diversity of Queens. Three quarters of sample have some form of health insurance, and a similar proportion have had a check-up in the past year. Over 60% of the sample report that they make at least some use of their local libraries. Mediators: Cancer knowledge was mixed, with 39% missing at least one of three basic questions. About 30% of the sample had sought information about cancer in the past year, and 19% stated that they were aware of free screening. Overall adherence to age- and gender appropriate screening recommendations in place at the time of the study was low. Among men and women 50 years of age or older, 59% were up to date on any recommended screening (colonoscopy in the past 10 years or stool tests in the past year). About 66% of women over age 40 reported having had a mammogram in the past year; 61% of women of all ages had had an annual Pap test. Among 20% of the sample who said they were current or recent tobacco smokers, 59% had attempted to quit in the past year and 19% said that they were no longer smoking.
Table 1.
Demographic Characteristics of Study Sample
| N | Value | ||
|---|---|---|---|
| Gender: % female | 9366 | 53% | |
| Mean Age (sd) | 9356 | 46.1 (50.0) | |
| Education: | 8915 | ||
| % High school/GED | 83% | ||
| % College Graduate | 32% | ||
| Race | 9075 | ||
| Caucasian | 24% | ||
| African-American | 31% | ||
| Asian | 16% | ||
| % Hispanic | 32% | ||
|
| |||
| Other characteristics | |||
| Born in the USA: N (%) | 4031 | 43% | |
| Years in neighborhood: mean (sd) | 15.8 | 16% | |
| Have a regular doctor: N (%) | 7189 | 78% | |
| Have health insurance: N (%) | 6958 | 76% | |
| % Primarily uses library in intercept neighborhood | 9299 | 56% | |
| % Secondarily uses library in intercept neighborhood | 9298 | 5% | |
|
| |||
| Baseline values of dependent variables | |||
| Cancer Knowledge: mean (sd) | 9131 | 2.5 (0.7) | |
| % Ever looked for information specifically about cancer | 9189 | 29% | |
| % Adhere to annual pap smear | 4776 | 61% | |
| % Annual mammogram | 2799 | 66% | |
| % Adhere to recommended colorectal cancer screening | 3797 | 59% | |
| % Current smoker | 7205 | 20% | |
| % Tried to quit smoking in past year | 1470 | 59% | |
| % Successfully quit smoking in past year | 1074 | 19% | |
Impact of HealthLink on Mediators
Event history logistic regression analyses used measures of cumulative HealthLink program activity to account for variation in rates of health behaviors across neighborhoods over the course of implementation. Table 2 reports details of analyses on hypothesized mediators of HealthLink’s impact on cancer-related behaviors, including significant effects of demographic and health system covariates. The summary below focuses on observed overall and sub-group specific impact of HealthLink programs on mediators.
The impact of HealthLink on awareness of free/low cost screening differed according to respondents’ race and ethnicity. Among Latinos, awareness was associated with cumulative attendance and impact tended to be sustained months after the most recent program, suggesting community-level diffusion. Awareness depended on personal exposure to the library among women and non U.S-born participants. HealthLink programs from nearby outside communities also influenced awareness among non-U.S. born, White and Asian respondents.
Knowledge about cancer was associated with total number of outside HealthLink programs. This effect appears slightly stronger among U.S. born but weaker among Black respondents.
Reported Cancer Information Seeking was more prevalent after recent programs and was also associated with personal exposure to the library, particularly among White and non-U.S. born respondents.
Having health insurance was associated with the cumulative number of local programs. Beyond this overall effect, likelihood of having health insurance was associated with cumulative programs weighted by personal among female, younger, non-U.S. born and Latino respondents. Reported rates of health insurance were negatively associated with the cumulative number of outside programs, except among Asians respondents.
Reporting a medical check-up in the past year was associated with cumulative number of local programs and was associated with sustained increases after programs.
Table 2.
Relationship of program activity, effects, and content on mediators, adjusted for demographics and systems events
| Mediator | Awareness of Free Screening (N= 8716) |
Cancer Knowledge (N= 8784) |
Information Seeking (N= 8783) |
Health Insurance (N= 8747) |
Annual Physical (N= 8802) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| OR | 95% CI for OR | OR | 95% CI for OR | OR | 95% CI for OR | OR | 95% CI for OR | OR | 95% CI for OR | |||||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Indicators of Program Activity | ||||||||||||||||
|
| ||||||||||||||||
| Cumulative Local Programs | 0.98 | 0.97 | 1.00 | 1.03 | 1.02 | 1.05 | 1.02 | 1.01 | 1.04 | |||||||
| Cumulative Attendance | ||||||||||||||||
| Cumulative Outside | 1.00 | 1.00 | 1.01 | 1.00 | 0.99 | 1.00 | ||||||||||
| Time since most Recent | 0.93 | 0.85 | 1.00 | 1.13 | 1.00 | 1.26 | ||||||||||
| Likelihood of Awareness | 1.01 | 0.98 | 1.04 | 1.05 | 1.03 | 1.08 | ||||||||||
|
| ||||||||||||||||
| Sub-Group-Specific Program Effects | ||||||||||||||||
|
| ||||||||||||||||
| Attendance × Latino | 1.08 | 1.01 | 1.16 | |||||||||||||
| Recent × Latino | 1.18 | 1.06 | 1.32 | |||||||||||||
| Outside × US Born | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.01 | ||||||||||
| Outside × White | 1.00 | 1.00 | 1.01 | |||||||||||||
| Outside × Asian | 1.00 | 1.00 | 1.01 | 1.01 | 1.00 | 1.01 | ||||||||||
| Outside × Black | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | ||||||||||
| Awareness × US Born | 0.98 | 0.96 | 1.00 | 0.97 | 0.95 | 0.98 | 0.98 | 0.96 | 1.00 | |||||||
| Awareness × Gender | 1.02 | 1.00 | 1.04 | 1.02 | 1.00 | 1.03 | ||||||||||
| Awareness × White | 1.02 | 1.00 | 1.04 | |||||||||||||
| Awareness × Age | 0.99 | 0.99 | 1.00 | |||||||||||||
| Awareness × Latino | 1.02 | 1.00 | 1.05 | |||||||||||||
|
| ||||||||||||||||
| Demographics | ||||||||||||||||
|
| ||||||||||||||||
| Age | 1.25 | 1.21 | 1.29 | 0.93 | 0.91 | 0.96 | 1.04 | 1.01 | 1.08 | 1.44 | 1.39 | 1.50 | 1.23 | 1.18 | 1.27 | |
| Female Gender | 1.31 | 1.15 | 1.49 | 0.74 | 0.68 | 0.81 | 1.61 | 1.47 | 1.78 | 1.49 | 1.33 | 1.67 | 1.82 | 1.61 | 2.04 | |
| Asian | 0.74 | 0.63 | 0.87 | 0.92 | 0.71 | 1.20 | ||||||||||
| U.S. Black | 1.37 | 1.17 | 1.59 | 1.79 | 1.42 | 2.24 | ||||||||||
| White | 1.24 | 1.10 | 1.40 | 1.09 | 0.94 | 1.27 | ||||||||||
| Latino | 1.11 | 1.00 | 1.24 | 1.07 | 0.89 | 1.29 | 0.62 | 0.53 | 0.71 | |||||||
| African | ||||||||||||||||
| Native American | 1.53 | 1.07 | 2.19 | |||||||||||||
| Pacific Islander | 0.47 | 0.28 | 0.79 | 2.29 | 0.98 | 5.35 | ||||||||||
| Born in US | 1.66 | 1.39 | 1.97 | 1.08 | 0.93 | 1.27 | 1.69 | 1.50 | 1.92 | 2.63 | 2.28 | 3.04 | 1.19 | 1.05 | 1.35 | |
| HS Grad | 1.50 | 1.27 | 1.77 | 1.81 | 1.52 | 2.15 | 1.61 | 1.41 | 1.85 | |||||||
| College Grad | 1.32 | 1.19 | 1.46 | 1.84 | 1.66 | 2.05 | ||||||||||
|
| ||||||||||||||||
| Systems Events | ||||||||||||||||
|
| ||||||||||||||||
| Mammography Recommendations | 1.05 | 0.91 | 1.22 | 0.74 | 0.64 | 0.86 | ||||||||||
| Hospital Closures | 1.08 | 0.94 | 1.23 | |||||||||||||
OR=Odds Ratio; Bolded values are statistically significant with a Type I error rate of 0.05.
Impact of HealthLink on Cancer Screening and Prevention
Table 3 depicts the complete event history regression model for colorectal, breast and cervical screening and smoking quit attempts. We found no effect on smoking cessation. Each regression model is controlled for demographic and health system covariates as well as mediators of health behavior. In general, mediators were all significantly associated with screening and smoking quit attempts in the expected directions. To better understand the influence of HealthLink on outcomes, we repeated analyses excluding mediators (Supplemental Table 1) and in a subsample of patients who lacked either health insurance or routine check-ups visits (Supplemental Table 2). Relevant results of these supplemental analyses are reported in the text below.
Colorectal Cancer Screening. Among Latinos, adherence to colorectal screening was associated with cumulative local programs. This effect remained significant after controlling for significant associations of colorectal screening with mediators. In the disengaged subgroup, cumulative attendance was also associated with increased screening among U.S. born respondents (OR = 1.24, 95% CI = 1.03 to 1.49).
Breast Cancer Screening. After controlling for mediators, we found that screening among immigrants was most prevalent after recent programs. Inclusion of mediators masked an association between mammography and cumulative programs among Asian women only (OR = 1.03, 95% CI =1.00 to 1.07). In analysis of the subgroup of women most disengaged from care, programs weighted by library use was associated with mammography among non-U.S. born (OR = .95, 95% CI =.90 to .99) while Latina were influenced by programs in adjacent neighborhoods (OR = 1.13, 95% CI = 1.04 to 1.23).
Cervical Cancer Screening. Adherence to Pap screening was positively associated with the number of programs focused specifically on breast and cervical screening, particularly among White women. However, discussion of sexual risk behavior per se appeared to attenuate this affect. In analysis without mediators, it was also evident that the number of programs focused on breast and cervical cancer had a positive affect (OR = .91, 95% CI = .87 to .97) but cumulative attendance was negatively associated with cervical screening (OR = 1.05, 95% CI = 1.01 to 1.10). This may suggest that smaller group programs to address cervical cancer have greater impact. Among Latinas only, rates of cervical cancer screening increased in the months after recent programs (similar to reported awareness of low/no-cost screening). For women in the disengaged subgroup, the effect of programs focused on sexual risk depended on women’s level education, apparently having greater benefit for non-high school graduates (OR = .41, 95% CI = .20 to .83).
Attempts to Quit Tobacco Use were associated with cumulative number of local programs for all groups, and particularly among Latinos. However, this effect was attenuated for programs specific to risks of tobacco use. In analyses excluding mediators, the aforementioned effects remained strongest among Latinos. Asian respondents showed a different pattern, with quit attempts associated with longer delays after programs, suggesting an effect that emerged over time. Effects were similar in the subgroup analysis of people disengaged from care, with additional evidence that Latinos responded to greater attendance at local programs in their communities (OR = 1.82, 95% CI = 1.11 to 2.96), that programs focused on tobacco unexpectedly attenuated this effect (OR = .13, 95% CI = .03 to .65), and that effects of program increased over time for immigrants (OR = 1.87, 95% CI = 1.22 to 2.85).
Table 3.
Impact of cancer-related program activity, effects, and content on screening outcomes in the past year, adjusted for mediators and demographics
| Outcomes with Mediators | Colorectal Screening (N=3551) | Mammograms (N=2604) | Cervical Screening (N=4461) | Tobacco Quit Attempts (N=1376) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| OR | 95% CI for OR | OR | 95% CI for OR | OR | 95% CI for OR | OR | 95% CI for OR | ||||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | ||||||
| Indicators of Program Activity | |||||||||||||
|
| |||||||||||||
| Cumulative Local Programs | 0.92 | 0.86 | 0.97 | 1.02 | 1.00 | 1.05 | |||||||
| Cumulative Attendance | |||||||||||||
| Cumulative Outside Programs | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.00 | |||||||
| me since most Recent Program | |||||||||||||
| Likelihood of Awareness | |||||||||||||
|
| |||||||||||||
| Group-Specific Program Effects | |||||||||||||
|
| |||||||||||||
| Local Programs × Latino | 1.03 | 1.01 | 1.06 | 1.11 | 1.04 | 1.19 | |||||||
| Most Recent × Born in US | 0.85 | 0.74 | 0.98 | ||||||||||
| Most Recent × Latino | 1.13 | 1.00 | 1.28 | ||||||||||
|
| |||||||||||||
| Program Content Areas | |||||||||||||
|
| |||||||||||||
| Breast/Cervical CA | 1.06 | 1.00 | 1.12 | ||||||||||
| Sexual Risk × White | 0.49 | 0.27 | 0.86 | ||||||||||
| Cervical CA × White | 1.65 | 0.95 | 2.87 | ||||||||||
| Tobacco Risk | 0.55 | 0.37 | 0.84 | ||||||||||
|
| |||||||||||||
| Demographics | |||||||||||||
|
| |||||||||||||
| Age | 1.39 | 1.28 | 1.51 | 1.11 | 1.03 | 1.20 | 0.88 | 0.85 | 0.92 | 0.88 | 0.81 | 0.95 | |
| Gender | |||||||||||||
| Asian | 0.73 | 0.59 | 0.90 | 0.66 | 0.50 | 0.86 | 0.44 | 0.36 | 0.54 | ||||
| U.S. Black | |||||||||||||
| White | 0.66 | 0.53 | 0.81 | 0.61 | 0.51 | 0.73 | |||||||
| Latino | |||||||||||||
| African | 3.96 | 1.11 | 14.07 | ||||||||||
| Born in US | |||||||||||||
| High School Grad | 1.10 | 0.91 | 1.32 | ||||||||||
| College Grad | 1.17 | 1.01 | 1.35 | 0.57 | 0.43 | 0.75 | |||||||
|
| |||||||||||||
| Mediators | |||||||||||||
|
| |||||||||||||
| Awareness of Free Screening | 1.22 | 1.00 | 1.50 | 1.22 | 1.04 | 1.44 | |||||||
| Cancer Knowledge | 1.19 | 1.02 | 1.38 | 1.33 | 1.17 | 1.52 | 1.83 | 1.45 | 2.30 | ||||
| Information Seeking | 1.44 | 1.22 | 1.69 | 1.32 | 1.10 | 1.59 | 1.26 | 1.09 | 1.45 | 1.49 | 1.16 | 1.93 | |
| Health Insurance | 1.82 | 1.47 | 2.25 | 1.94 | 1.51 | 2.50 | 1.49 | 1.25 | 1.77 | 1.51 | 1.15 | 1.99 | |
| Annual Physical | 4.24 | 3.31 | 5.44 | 7.84 | 5.64 | 10.89 | 7.05 | 5.62 | 8.83 | ||||
OR=Odds Ratio; Bolded values are statistically significant with a Type I error rate of 0.05.
Discussion
This study is among the first to demonstrate methods to capture temporal associations between community-based participatory research program implementation and behavior change at the population level across multiple, diverse neighborhoods. Results indicate that public libraries can be an important partner in community-based participatory research, to reduce excess burden of cancer in medically-underserved communities. This intervention facilitated changes in mediators of cancer screening, suggestive of contemplation (awareness), preparation (information seeking) and action steps (obtaining insurance), as well as maintenance in terms of more routine use of primary care. (Prochaska & DiClemente, 1982) Rates of cancer screening were most affected among those at greatest risk, and were associated with cumulative programming or program attendance. For the most part, we did not observe differences among library users and non-users, suggesting broad diffusion. The Councils have remained active three years after the end of grant-funding, with Queens Public Library System support. As such, HealthLink was able to engender a stable and sustainable process for generating programs to address community health needs.
The results of this study support a vision for public health research and practice that utilizes on-going community-based problem-solving to build and sustain programs. This approach orients public health programs and policies toward continuous quality improvement. As observed here, such an approach can improve outcomes for more vulnerable and marginalized segments of the community. There is potential to achieve greater efficiency and, ultimately, institutionalization of successful approaches. A dynamic planning process makes it possible to respond to changing needs, unpredictable circumstances and emerging evidence. The delivery of interventions can be guided by the latest findings on effectiveness and tailoring along with emerging local evidence, as stakeholders gain experience in program planning and implementation. Rather than emphasize the dissemination of static intervention protocols, efforts to promote evidence-based public health practice should encourage community-engaged problem solving as the most viable way to improve reach, effectiveness, and performance in light of local circumstances. This approach would also facilitate the introduction of new scientific findings, as clinical advances in screening and prevention could readily be accommodated. By partnering with the library system as a mediating structure, it has been possible to sustain local stakeholders’ ability to address health problems with increasing autonomy.
By capturing data on program development and implementation over time, the Comprehensive Dynamic Trials design allowed us to observe the associate measures of intervention process and performance measures with increasing influence on target behaviors. This approach made it possible to empirically address the complexity of implementing programs in multiple different communities, as dictated by independent community councils. Roy et al. (Roy et al., 2014) details the process followed in our study, and describes the difficulty of some sites compared to others. Consistent with tenets of community-based participatory research, each group had the latitude to prioritize problems and address them in their own manner in the sequence and time frame of their choosing. The Comprehensive Dynamic Trial Design’s ability to examine the unfolding influence of programmatic activity is fundamental to the evaluation of any intervention that involves on-going planning and enhancement over the course of implementation.
Limitations
The caveats of the present research should be noted. In any research where it is not possible to isolate respondents, there is always the potential for outside factors to change over time, and to exert an influence upon dependent variables of interest that can confound intervention effects. Midway through the implementation of HealthLink, Queens began to experience significant impacts of the economic recession (Weiss et al., 2012). These challenges included but were not limited to: hospital closures, staff layoffs at partnering organizations, cuts in early detection screening programs, and reduction in insurance coverage in the population as a result of increased unemployment. In addition, changes to clinical guidelines for breast cancer screening occurred (U.S. Preventive Services Task Force, 2009). These disruptions individually and collectively reduced access to screening programs and interfered with Councils’ abilities to offer certain programs and referrals. Confusion and controversy arising from the change in US Prevention Services Task Force recommendations regarding the initiation and frequency of mammography screening presented another potential confounder. While our regression models adjusted for these confounders, it is likely that the odds ratio estimates of the program’s effect were attenuated by these factors.
In some instances, we noted that the number of concurrent programs in outside communities detracted from the benefits of local programs. Combined with the dip in program activity apparent in the second year of Council meetings, at the start of the Sustainability phase, this unexpected finding suggests that our project may have been under-resourced. Having a greater number of staff or allowing HealthLink specialists to spend more time with each Council might have mitigated this “sophomore slump” and this negative aspect of wide-scale implementation.
Other than a general problem solving framework, we did not require Councils to maintain fidelity to a specific group process or procedure. Future research using Comprehensive Dynamic Trial Methods would benefit from measurement of the process of deliberation and effectiveness of decision makers. In participatory intervention models such as ours, stakeholders have considerable influence on the focus, pace and impact of selected strategies to promote health behavior. Such groups may be organized, trained and supported in a variety of ways. Research to link community outcomes to decision-making bodies’ fidelity to intended processes of deliberation and problem-solving would be most valuable. The Comprehensive Dynamic Trial Design suggests a way to achieve this, by using event history analyses to test the bi-directional temporal influence of on-going stakeholder deliberation and problem-solving with markers of program activity and effectiveness. Better decision-making processes should contribute to greater impact on behavior while greater community response to intervention efforts should encourage and reinforce decision-makers.
Conclusions
The HealthLink project had a measureable and sustainable impact on cancer prevention and screening. This effect was particularly strong among those with the most need and least resources. Community-based participatory research implemented and evaluated using a Comprehensive Dynamic Trial design has great potential for better meeting public health needs. This model offers a context for basic discovery concerning the nature of modifications and adaptations needed to make interventions work better for different people in different settings. Findings of the present study can help to facilitate similar processes for public health interventions, challenging and aiding community members to partner with public, private and academic partners to address unique local health issues.
Supplementary Material
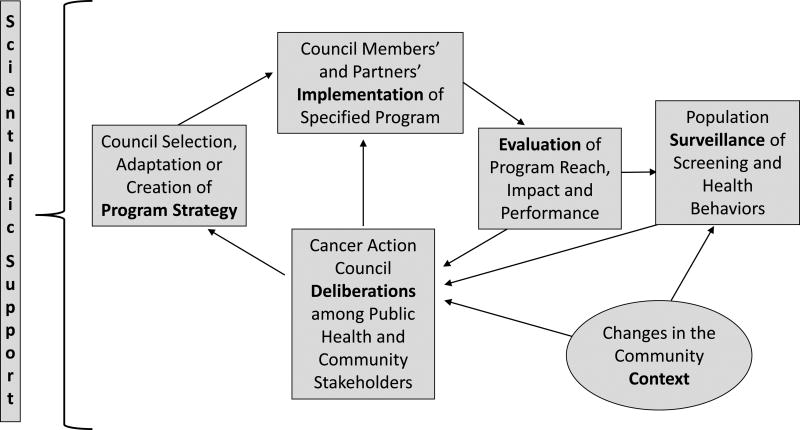
Figure 3. The HealthLink Intervention Process.
As a Comprehensive Dynamic Trial, HealthLink employs an iterative, quality-improvement strategy, carried out as stakeholder-engaged community-based participatory research. Council members deliberate about aspects of local program performance -- reach, acceptability, fidelity, efficacy, and efficiency – in order to identify modifications to program strategies as well as new health promotion goals. Programs are optimized in each locale through continued cycles of deliberation, adaptation, implementation and evaluation.
Acknowledgments
We acknowledge the commitment of the Queens Library HealthLink Cancer Action Councils to improving access to cancer prevention, screening and treatment in their communities; and Elliott Goytia, M.D., M.A., for his contribution to the conceptualization of this project and for his support of the work of the Cancer Action Councils. This work was funded by the National Cancer Institute (Grant Number: R01 CA119991-01, Rapkin PI). BDR, AS, and AC had full access to all of the data in the study. BDR takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Allison PD. Event history analysis: Regression for longitudinal event data. Sage; 1984. [Google Scholar]
- American Cancer Society. Colorectal Cancer Facts & Figures 2011–2013. Atlanta, Georgia: American Cancer Society; 2011. [Google Scholar]
- American Cancer Society. 2017 Retrieved from https://www.cancer.org/latest-news/cancer-statistics-report-death-rate-down-23-percent-in-21-years.html.
- Bradley EH, Schlesinger M, Webster TR, Baker D, Inouye SK. Translating research into clinical practice: making change happen. Journal of the American Geriatrics Society. 2004;52:1875–1882. doi: 10.1111/j.1532-5415.2004.52510.x. [DOI] [PubMed] [Google Scholar]
- Bradley EH, Webster TR, Baker D, Schlesinger M, Inouye SK, Barth MC, Koren MJ. Translating research into practice: speeding the adoption of innovative health care programs. Issue Brief (Commonwealth Fund) 2004 Jul;(724):1–12. [PubMed] [Google Scholar]
- Braun KL, Stewart S, Baquet C, Berry-Bobovski L, Blumenthal D, Brandt HM, Cooper LC. The National Cancer Institute’s community networks program initiative to reduce cancer health disparities: outcomes and lessons learned. Progress in community health partnerships: research, education, and action. 2015;9:21. doi: 10.1353/cpr.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prevention Science. 2004;5:185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consedine NS, Tuck NL, Ragin CR, Spencer BA. Beyond the black box: a systematic review of breast, prostate, colorectal, and cervical screening among native and immigrant African-descent Caribbean populations. Journal of immigrant and minority health. 2015;17(3):905–924. doi: 10.1007/s10903-014-9991-0. [DOI] [PubMed] [Google Scholar]
- Davis C, Darby K, Moore M, Cadet T, Brown G. Breast care screening for underserved African American women: Community-based participatory approach. Journal of psychosocial oncology. 2017;35(1):90–105. doi: 10.1080/07347332.2016.1217965. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA: a cancer journal for clinicians. 2016;66(4):290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(8):2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elk R, Landrine H. Cancer disparities: causes and evidence-based solutions. Springer Publishing Company; 2012. [Google Scholar]
- Emmons KM, Cleghorn D, Tellez T, Greaney ML, Sprunck KM, Bastani R, Puleo E. Prevalence and implications of multiple cancer screening needs among Hispanic community health center patients. Cancer causes & control : CCC. 2011;22(9):1343–1349. doi: 10.1007/s10552-011-9807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert S, Coleman R. Using community-based participatory research to ameliorate cancer disparities. Health & social work. 2010;35(4):302–309. doi: 10.1093/hsw/35.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim D, Farhat Z, Wallenstein S, Manczuk M, Holcombe RF, Thorpe L, Boffetta P. Standardized cancer incidence disparities in Upper Manhattan New York City neighborhoods: the role of race/ethnicity, socioeconomic status, and known risk factors. European Journal of Cancer Prevention. 2016;25(4):349–356. doi: 10.1097/CEJ.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husaini BA, Sherkat DE, Bragg R, Levine R, Emerson JS, Mentes CM, Cain VA. Predictors of breast cancer screening in a panel study of African American women. Women & health. 2001;34(3):35–51. doi: 10.1300/J013v34n03_03. [DOI] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annual review of public health. 1998;19(1):173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- Johnson CE, Mues KE, Mayne SL, Kiblawi AN. Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the Health Belief Model. Journal of lower genital tract disease. 2008;12(3):232–241. doi: 10.1097/LGT.0b013e31815d8d88. [DOI] [PubMed] [Google Scholar]
- Kagawa-Singer M, Tanjasiri SP, Valdez A, Yu H, Foo MA. Outcomes of a breast health project for Hmong women and men in California. American journal of public health. 2009;99(S2):S467–S473. doi: 10.2105/AJPH.2008.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krok-Schoen JL, Fisher JL, Baltic RD, Paskett ED. Abstract B73: Trends in racial disparities in cancer incidence among white and black older adults in the United States; Paper presented at the American Association for Cancer Research Meeting; Philadelphia, PA. 2017. [Google Scholar]
- Larkey LK, Gonzalez JA, Mar LE, Glantz N. Latina recruitment for cancer prevention education via community based participatory research strategies. Contemporary Clinical Trials. 2009;30(1):47–54. doi: 10.1016/j.cct.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Maxwell AE, Bastani R, Danao LL, Antonio C, Garcia GM, Crespi CM. Results of a community-based randomized trial to increase colorectal cancer screening among Filipino Americans. American journal of public health. 2010;100(11):2228–2234. doi: 10.2105/AJPH.2009.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M, Wallerstein N. Community-based participatory research for health: From process to outcomes. John Wiley & Sons; 2011. [Google Scholar]
- Miranda PY, Tarraf W, Gonzalez HM. Breast cancer screening and ethnicity in the United States: implications for health disparities research. Breast cancer research and treatment. 2011;128(2):535–542. doi: 10.1007/s10549-011-1367-8. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Stroup DF, Giles WH. Public health surveillance for behavioral risk factors in a changing environment recommendations from the behavioral risk factor surveillance team. Morbidity and mortality weekly report recommendations and reports RR. 2003;52(9) [PubMed] [Google Scholar]
- National Cancer Institute. 2017 Retrieved from https://healthcaredelivery.cancer.gov/prospr/
- Prochaska JO, DiClemente CC. Transtheoretical therapy: Toward a more integrative model of change. Psychotherapy: Theory, Research and Practice. 1982;19(3):276–288. [Google Scholar]
- Rapkin BD. Paths for the Future: Using what we have learned to eliminate cancer disparities. In: Elk R, Landrine H, editors. Cancer disparities: causes and evidence-based solutions. New York: Springer Publishing Company; 2012. pp. 497–524. [Google Scholar]
- Rapkin BD, Trickett EJ. Comprehensive dynamic trial designs for behavioral prevention research with communities: Overcoming inadequacies of the randomized controlled trial paradigm. In: Trickett EJ, editor. Community intervention and AIDS: Targeting the community context. Cary, NC: Oxford University Press; 2005. pp. 249–277. [Google Scholar]
- Rapkin BD, Weiss ES, Lounsbury DW, Thompson HS, Goodman RM, Schechter CB, Padgett DK. Using the Interactive Systems Framework to Support a Quality Improvement Approach to Dissemination of Evidence-Based Strategies to Promote Early Detection of Breast Cancer: Planning a Comprehensive Dynamic Trial. American Journal of Community Psychology. 2012;50(3–4):497–517. doi: 10.1007/s10464-012-9518-6. [DOI] [PubMed] [Google Scholar]
- Remington PL, Smith MY, Williamson DF, Anda RF, Gentry EM, Hogelin GC. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981–87. Public health reports. 1988;103(4):366–375. [PMC free article] [PubMed] [Google Scholar]
- Research Tested Intervention Programs. 2017 Retrieved from https://rtips.cancer.gov/rtips/index.do.
- Roy UB, Michel T, Carpenter A, Lounsbury DW, Sabino E, Stevenson AJ, Rapkin BD. Community-Led Cancer Action Councils in Queens, New York: Process Evaluation of an Innovative Partnership With the Queens Library System. Preventing chronic disease. 2014;11:130176. doi: 10.5888/pcd11.130176. doi: http://dx.doi.org/10.5888/pcd11.130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenson D, Cassarino L, DiMartino D, Marantz P, Bolen J, Good B, Alderman M. Improving access to mammograms through community-based influenza clinics: A quasi-experimental study. American journal of preventive medicine. 2001;20(2):97–102. doi: 10.1016/s0749-3797(00)00281-6. [DOI] [PubMed] [Google Scholar]
- Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of environmental and public health. 2017;2017 doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland CJ. Challenges in community-based participatory research implementation: Experiences in cancer prevention with Pacific Northwest American Indian tribes. Cancer Control. 2006;13(3):230. doi: 10.1177/107327480601300312. [DOI] [PubMed] [Google Scholar]
- Studts CR, Tarasenko YN, Schoenberg NE, Shelton BJ, Hatcher-Keller J, Dignan MB. A community-based randomized trial of a faith-placed intervention to reduce cervical cancer burden in Appalachia. Preventive medicine. 2012;54(6):408–414. doi: 10.1016/j.ypmed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin SH, Ichikawa L, Yood MU, Manos MM, Geiger AM, Weinmann S, Barlow WE. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? Journal of the National Cancer Institute. 2004;96(20):1518–1527. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- Taylor VM, Jackson JC, Yasui Y, Nguyen TT, Woodall E, Acorda E, Ramsey S. Evaluation of a cervical cancer control intervention using lay health workers for Vietnamese American women. American journal of public health. 2010;100(10):1924–1929. doi: 10.2105/AJPH.2009.190348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebes JK, Thai ND, Matlin SL. Twenty-First Century Science as a Relational Process: From Eureka! To Team Science and a Place for Community Psychology. American Journal of Community Psychology. 2014;53(3–4):475–490. doi: 10.1007/s10464-014-9625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Sauer AMG, Chen MS, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA: a cancer journal for clinicians. 2016;66(3):182–202. doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett EJ, Beehler S, Deutsch C, Green LW, Hawe P, McLeroy K, Schulz AJ. Advancing the science of community-level interventions. American journal of public health. 2011;101(8):1410–1419. doi: 10.2105/AJPH.2010.300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- Wandersman A, Chien VH, Katz J. Toward an evidence-based system for innovation support for implementing innovations with quality: tools, training, technical assistance, and quality assurance/quality improvement. American Journal of Community Psychology. 2012;50(3–4):445–459. doi: 10.1007/s10464-012-9509-7. [DOI] [PubMed] [Google Scholar]
- Wandersman A, Duffy J, Flaspohler P, Noonan R, Lubell K, Stillman L, Saul J. Bridging the gap between prevention research and practice: the interactive systems framework for dissemination and implementation. American Journal of Community Psychology. 2008;41(3–4):171–181. doi: 10.1007/s10464-008-9174-z. [DOI] [PubMed] [Google Scholar]
- Wandersman A, Duffy J, Flaspohler P, Noonan R, Lubell K, Stillman L, Saul J. Bridging the gap between prevention research and practice: The interactive systems framework for dissemination and implementation. American journal of community psychology. 2008;41(3–4):171–181. doi: 10.1007/s10464-008-9174-z. [DOI] [PubMed] [Google Scholar]
- Weiss ES, Stevenson AJ, Erb-Downward J, Combs S, Sabino EE, Michel TA, Rapkin B. Sustaining CBPR partnerships to address health disparities in times of economic instability. Journal of health care for the poor and underserved. 2012;23(4):1527–1535. doi: 10.1353/hpu.2012.0170. [DOI] [PubMed] [Google Scholar]
- Zhang X, Holt JB, Yun S, Lu H, Greenlund KJ, Croft JB. Validation of multilevel regression and poststratification methodology for small area estimation of health indicators from the behavioral risk factor surveillance system. American journal of epidemiology. 2015;182(2):127–137. doi: 10.1093/aje/kwv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.