Abstract
Doehl et al. combine empirical data with computer simulation to demonstrate that RAG-2 mice intravenously infected with Leishmania donovani form heterogeneous skin parasite patches that govern infectiousness to sand flies. This model provides a much-needed tool to explore the relevance of asymptomatic and symptomatic visceral leishmaniasis patients as infection reservoirs
Keywords: Leishmania donovani, anthroponotic visceral leishmaniasis, vector sand flies, skin parasite patches, outward transmission, bite site
One of the burning questions facing programs aimed at control and elimination of anthroponotic visceral leishmaniasis (AVL) concerns identification of the main source of infection for sand fly vectors [1, 2]. To date, the comparative contribution of asymptomatic, symptomatic or post-kala azar dermal leishmaniasis patients to outward transmission of Leishmania donovani, the causative agent of AVL, to vector sand flies remains unknown and represents a serious obstacle to control efforts. In Doehl et al. [3], the authors develop a model using RAG-2 immunodeficient mice, that lack the rag-2 gene and cannot generate mature T or B lymphocytes, in combination with red fluorescent L. donovani parasites to address questions pertaining to host infectiousness for vector sand flies. The authors demonstrate that in addition to invading visceral organs, L. donovani parasites injected into the tail vein of RAG-2 mice reproducibly form skin patches of variable size six months into the infection. Distinct from recent reports of atypical dermotropic strains of L. donovani where the infection is confined to skin ulcers [4], Doehl et al. [3] demonstrate the involvement of intact-looking skin as a common clinical manifestation of viscerotropic strains, that disseminate to visceral organs and cause AVL. This is important as contribution of intact skin to sand fly infection is highly contested even in Leishmania infantum infections where cutaneous lesions are frequently reported as a source of infectiousness to vector sand flies [5–7].
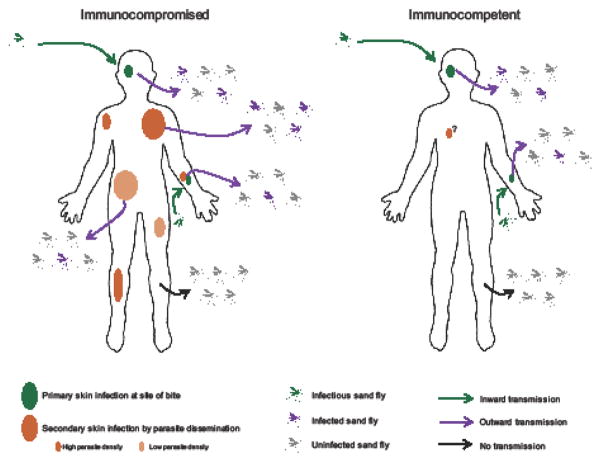
To address the relevance of parasite patchiness within intact skin to outward transmission to sand flies, Doehl et al. [3] proceed to compare scenarios of patchiness in infected animals at both macro- (parasite patches throughout the skin of an animal) and micro- (heterogeneous parasite distribution in one patch) scales. Analyses based on historical data, the authors’ own data, and simulations using fixed or fluid model parameters for sand fly midgut blood volume and blood pool or proboscis volumes, show that, for example if 1000 or 500 amastigotes are set as a threshold to infect sand flies, the model combining both macro- and micro-scale patchiness best fit the observed rates of experimental outward transmission success; models of homogenous ‘non-patchiness’, that best simulate blood parasitemia, do not. Further, the macro- and micro-scale model of heterogeneity of parasite patches restrict the number of successful outward transmissions but enhance the chance that a particular sand fly feeding on a patch will develop a mature transmissible infection, a prediction that also fits well with empirical data. Hence, the authors find that mice with larger and/or dense patches are more infectious to sand flies compared to those with smaller or less dense patches (Figure 1) despite having similar blood parasite densities. This further supports the conclusion that under the conditions modeled skin and not blood is the primary source of parasites accessible to vector sand flies. Together, these data expose the relevance of a skewed but pervasive skin parasite landscape to infectiousness of a L. donovani-infected host for vector sand flies.
Figure 1. Modeling outward transmission implicates skin of immunocompromised Leishmania donovani-infected hosts as a major source of parasites for vector sand flies.
In both immunocompromised and immunocompetent hosts, inward transmission by infectious sand flies results in persistence of sand fly-accessible parasites in host skin. If immunocompromised, parasites disseminate to the skin forming patches that vary in size and density giving rise to a heterogeneity in host infectiousness to sand flies, with large and/or more dense patches transmitting at higher rates than small and/or less dense patches. Dissemination of L. donovani parasites to the skin in an immunocompetent host remains to be determined (?).
Understandably, Doehl et al. [3] focused on one timepoint after infection to address the implication of skin parasite patches to outward transmission to sand flies. Nevertheless, it would be prudent and informative to determine the kinetics of the appearance of parasites in the skin and the rate of patch evolution. Perhaps the high variability in the size of parasite patches is temporal, with larger patches referencing the earliest parasites to disseminate to the skin. Gathering such information can be highly valuable to model the potential infectiousness of the skin of AVL-infected individuals during early asymptomatic versus late symptomatic stages.
Having chosen RAG-2 mice as a basis for their model, the data obtained by Doehl et al. [3] could be interpreted as mimicking what occurs in an immunocompromised individual (Figure 1), including symptomatic VL patients that are known to become immunosuppressed. Though the evidence is clear that without hindrance by the immune system L. donovani parasites disseminate to the skin, it is not clear that they can do so in an immunocompetent environment. Moreover, it is not clear whether the parasites are simply disseminating everywhere in the immunocompromised animal or they show preferential tropism to the skin to become accessible to the vector. The authors’ use immune reconstitution to assess if outward transmission is affected, but that is difficult to interpret. At best, it probably reflects changes in infection features upon immunotherapy. Nevertheless, finding that T cell-reconstituted mice become more infectious to sand flies compared to their pre-T cell transfer state is interesting and worthy of further study.
Though highly pertinent to the transmission dynamics of AVL, this model shows only one face of a coin. The natural route of infection with L. donovani is by bite of an infectious sand fly. This means that the infection is always initiated in the skin. Our group has previously reported that a proportion of parasites remains in the skin after transmission by sand flies [8, 9]. These primary skin parasites are present at bite sites of symptomatic and asymptomatic animals, remain viable long term, and can be taken up by sand flies (Figure 1). Potentially, a larger surface of the skin may be parasitized by a combination of primary and secondary populations of skin-resident amastigotes. Alternatively, primary parasites at bite sites, that are potentially less prevalent, may represent a rare but primary source of parasites for vectors in asymptomatic individuals, while secondary and more prominent skin-resident parasite populations would represent the major source of infection for sand fly vectors in symptomatic subjects. These are exciting questions to ask that will advance our knowledge of how L. donovani is spread and maintained in vector populations. Needless to say, obstacles that face Leishmania as it completes its development within the sand fly midgut remains the overriding determinant of sand fly infectiousness to mammalian hosts [10].
Doehl et al. [3] have pioneered a study that establishes the involvement of intact-looking skin of L. donovani-infected immunocompromised animals as a major source of infection for vector sand flies. To comprehend the full implication of their findings, the development of the parasite landscape in skin over time after vector-transmission of L. donovani to immunocompetent animals should be determined. Building on the model established by Doehl et al. [3] should provide invaluable information regarding the infectiousness of immunocompetent versus immunocompromised or suppressed hosts to vector sand flies, overcoming a major impediment to current efforts to eliminate AVL.
Acknowledgments
This work was funded by the Intramural Research Programs at the NIAID, NIH. We thank Jesus G. Valenzuela for reading the manuscript, and Ryan Kissinger for help with the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cameron MM, et al. Understanding the transmission dynamics of Leishmania donovani to provide robust evidence for interventions to eliminate visceral leishmaniasis in Bihar, India. Parasit Vectors. 2016;9:25. doi: 10.1186/s13071-016-1309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mo AXP, Hall BF. Visceral Leishmaniasis Control and Elimination: Is There a Role for Vaccines in Achieving Regional and Global Goals? Am J Trop Med Hyg. 2016;95(3):514–521. [Google Scholar]
- 3.Doehl JSP, et al. Skin parasite landscape determines host infectiousness in visceral leishmaniasis. Nat Commun. 2017;8(1):57. doi: 10.1038/s41467-017-00103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karunaweera ND. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep’s clothing? Trends Parasitol. 2009;25(10):458–463. doi: 10.1016/j.pt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Courtenay O, et al. Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Negl Trop Dis. 2014;8(1):e2583. doi: 10.1371/journal.pntd.0002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurenti MD, et al. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet Parasitol. 2013;196(3–4):296–300. doi: 10.1016/j.vetpar.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Solano-Gallego L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan H, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J Infect Dis. 2013;207(8):1328–1338. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslan H, et al. New Insights Into the Transmissibility of Leishmania infantum From Dogs to Sand Flies: Experimental Vector-Transmission Reveals Persistent Parasite Depots at Bite Sites. J Infect Dis. 2016;213(11):1752–1761. doi: 10.1093/infdis/jiw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dostalova A, Volf P. Leishmania development in sand flies: parasite-vector interactions overview. Parasit Vectors. 2012;5:276. doi: 10.1186/1756-3305-5-276. [DOI] [PMC free article] [PubMed] [Google Scholar]