Abstract
Background
Delirium is acute brain dysfunction associated with serious illness. Emerging data indicate that delirium occurs in greater than 20% of children in pediatric intensive care units. Cardiac bypass surgery is a known risk factor for delirium in adults, but has never been systematically studied in pediatrics.
Objectives
To describe the incidence of delirium in pediatric patients after cardiac bypass surgery, and explore associated risk factors and effect of delirium on in-hospital outcomes.
Design
Prospective observational single-center study.
Setting
Fourteen-bed pediatric cardiothoracic intensive care unit (PCICU).
Patients
One hundred and ninety four consecutive admissions following cardiac bypass surgery, age one day to 21 years.
Interventions
Subjects were screened for delirium daily using the Cornell Assessment of Pediatric Delirium.
Measurements and Main Results
Incidence of delirium in this sample was 49%. Delirium most often lasted 1–2 days, and developed within the first 1–3 days after surgery. Age less than two years, developmental delay, higher RACHS-1 score, cyanotic disease, and albumin less than three were all independently associated with development of delirium in a multivariable model (all p values <0.03). Delirium was an independent predictor of prolonged ICU LOS, with patients who were ever delirious having a 60% increase in ICU days compared to patients who were never delirious (p<0.01).
Conclusions
In our institution, delirium is a frequent problem in children after cardiac bypass surgery, with identifiable risk factors. Our study suggests that cardiac bypass surgery significantly increases children’s susceptibility to delirium. This highlights the need for heightened, targeted delirium screening in all PCICUs to potentially improve outcomes in this vulnerable patient population.
Key Words for indexing: pediatric, delirium, cardiac, bypass, post-operative, pediatric critical care
Introduction
Delirium is a syndrome that commonly occurs in critically ill children, characterized by acute onset of cerebral dysfunction with a fluctuation in mental status, awareness/attention, and disorganized thinking (1). A recent single-center observational study described a delirium prevalence rate of 21% in a general pediatric intensive care unit (PICU) (2). In adults, delirium is associated with poor prognosis reflected by longer hospital length of stay, prolonged mechanical ventilation, worse functional and cognitive outcome, and higher mortality rate (3,4). Prevalence of delirium in mechanically ventilated adult patients may be as high as 80% and ranges from 20–60% in non-ventilated adults (5).
Post-cardiotomy delirium is a subset of delirium that occurs after cardiac bypass surgery. It is associated with poor outcomes including: increased risk of mortality, increased risk of stroke, poor functional status, increased hospital readmissions, and substantial cognitive dysfunction for 1 year following surgery (6). It has been a known entity since the 1960’s with the advent of extracorporeal circulation for cardiac surgery and has a reported incidence ranging from 23%–70% in adult cardiothoracic ICUs (7,8,9,10,11). Post-cardiotomy delirium in adults typically develops during the first to second day after admission to the ICU and lasts for one to three days. (10). Published risk factors for delirium development in the adult population include: benzodiazepine use, increasing age, length of surgery greater than three hours, use of bypass, intra-operative blood transfusion, renal dysfunction, metabolic syndrome, low serum albumin, and prior history of stroke or depression (7,8,10,11).
The incidence of delirium in children after cardiac bypass surgery has never been systematically studied. The objective of this study was to prospectively evaluate children admitted to our pediatric cardiothoracic intensive care unit (PCICU) after cardiopulmonary bypass surgery, assess each child for delirium daily, and determine risk factors associated with its development. A secondary objective was to assess the effect of delirium on short-term outcome measures including ICU length of stay and time on mechanical ventilation.
Materials and Methods
Patient Selection
The Institutional Review Board of Columbia University Medical Center approved this observational study with a waiver of requirement for informed consent. From December 2014 to August 2015, we enrolled all consecutive patients admitted to our PCICU, a tertiary level critical care unit with an annual volume of 350 cardiopulmonary bypass surgeries. Enrollment criteria included PCICU admission following on-pump cardiac surgery and age ≤ 21 yrs.
Delirium Assessment
Delirium assessments were performed using the Cornell Assessment of Pediatric Delirium (CAPD), a reliable and simple bedside tool validated for use in children of all ages (2). The CAPD uses the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-V) criteria for the diagnosis of delirium (12). Specifically, the CAPD requires an acute and fluctuating change in awareness and cognition to diagnose delirium, and has been shown to discriminate between delirium and other causes of altered mental status in critically ill children (2). The CAPD was completed a minimum of once daily by the bedside nurse throughout the PCICU stay- unless the patient was unarousable to verbal stimulation (these patients were categorized as comatose) (13). As the CAPD is designed to take into account a period of extended observation (rather than being used as a point-in-time screen), time of assessment was towards the end of the nurse’s shift. A CAPD score of 9 or higher was considered diagnostic for delirium in developmentally-typical patients. In developmentally-delayed patients, a diagnosis of delirium required both a CAPD score of >9 and confirmation of change from neurologic baseline by an ICU practitioner (14). Developmental anchor points were available to be used in conjunction with the CAPD to provide point-of-care reference for the nurses (14). A patient with at least one delirious day during the PCICU stay was considered “ever delirious”. Bedside nurses received extensive education on delirium and delirium screening practices prior to study initiation.
Data Collection
Data was collected prospectively. Baseline demographic data were obtained. Putative risk factors for delirium development were extrapolated from a review of the pediatric and adult literature in medical and surgical ICU patients and adult post-cardiac surgical patients. Intraoperative data collected included: type of surgery, length of cardiopulmonary bypass, length of circulatory arrest, anesthetic medications used, and blood products transfused. Daily laboratory data and information regarding patients’ clinical course, including but not limited to respiratory support, transfusions, restraints, specific labs and medication exposure were collected throughout the patients’ PCICU stay. The Pediatric Index of Mortality 2 (PIM2) score was used to determine severity of illness upon admission to the PCICU (15). We calculated the Pediatric Logistic Organ Dysfunction Score (PELOD) as a measure of daily degree of multiple organ dysfunction syndrome (16). The PELOD score was modified to exclude the neurologic component (GCS), and we termed this modified PELOD the ‘PELOD minus GCS’, or ‘PELOD-GCS’. The GCS component was excluded because our primary outcome was mental status (i.e.: delirium) as measured by the CAPD, therefore we did not want to also include mental status as measured by the GCS. Risk Adjustment for Congenital Heart Surgery (RACHS-1) scores were determined for each patient surgery as an indicator of the risk of mortality imparted by the congenital heart surgery performed, with one reflecting the lowest risk of surgical mortality and six indicating the highest risk of surgical mortality (17).
Statistical Methods
Descriptive statistics were generated to describe demographic, baseline (including intraoperative), and daily data using N (%) or mean, median, standard deviation (SD), and range for categorical and continuous factors, respectively. Chi-square/Fisher’s Exact tests were performed as applicable to compare categorical factors between the ever delirious group and the never delirious group as well as between a delirious day and a non-delirious day. Independent sample t-tests/Wilcoxon rank-sum tests were performed as applicable to compare continuous factors. Multivariable logistic regression, including all factors that were significant at the 0.10 alpha level in bivariate analyses, was performed to predict development of delirium. Bi-directional stepwise selection based on Akaike information criterion produced the final model18. In addition, multivariable linear regression was used to predict number of PICU days. As the distribution for number of PICU days was skewed, the natural logarithm transformation was utilized for modeling. Relative number of PICU days and 95% confidence intervals were computed by exponentiation of the regression estimates with their respective lower and upper bounds. All statistical tests were two-sided with statistical significance evaluated at the 0.05 alpha level. Analyses were performed with R version 3.2.1 for Windows 64-bit.
Results
One hundred ninety four patients were enrolled and 1,342 patient days were evaluated. Each patient was assessed for delirium daily until discharge from the PCICU. The 1,342 patient days consisted of 380 delirious days, 313 coma days, and 649 non-delirious days. Delirium status was unknown for less than 1.4% of days (n= 19 days) because of missed opportunities for screening. These days were excluded from the analyses.
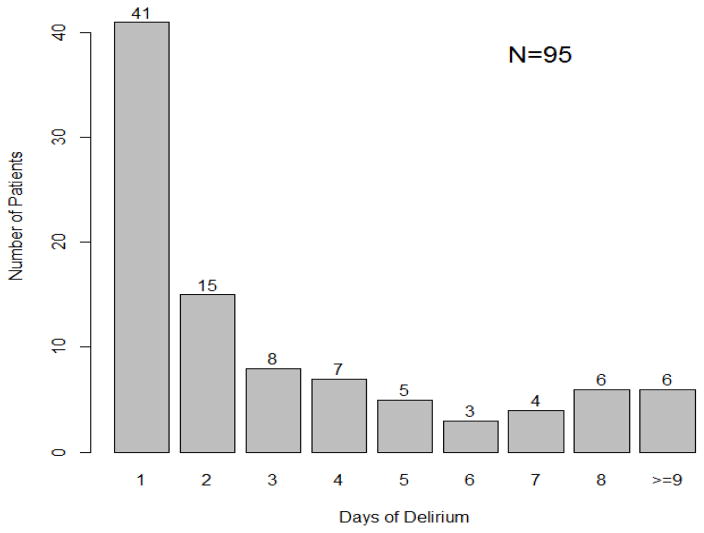
The incidence of delirium in this cohort was 49%, with a median of 2 delirious days per patient, SD ± 6.45 (Table 1, Figure 1). Delirium most often developed within the first 1–3 days after surgery, with 47% of patients who were ever delirious developing delirium for the first time on day 1, 20% on day 2, and 10.5% on day 3.
Table 1.
Demographic and Clinical Characteristics by Delirium Status (n=194)
| Characteristic | No. of Subjects (%) | Ever Delirious During PCICU Stay (%) | Never Delirious During PCICU Stay (%) | p Value |
|---|---|---|---|---|
| n | 194 | 95 (49.0) | 99 (51.0) | |
|
| ||||
| Gender | ||||
| Male | 114 (58.8) | 52 (54.7) | 62 (62.6) | 0.332 |
| Female | 80 (41.2) | 43 (45.3) | 37 (37.4) | |
|
| ||||
| Age, yr | ||||
| 0–2 | 81 (41.8) | 53 (55.8) | 28 (28.3) | |
| 3–5 | 48 (24.7) | 23 (24.2) | 25 (25.3) | <0.001 |
| 6–13 | 29 (14.9) | 11 (11.6) | 18 (18.2) | |
| >13 | 36 (18.6) | 8 (8.4) | 28 (28.3) | |
|
| ||||
| Developmental delaya | ||||
| No delay | 164 (84.5) | 74 (77.9) | 90 (90.9) | 0.021 |
| Delay | 30 (15.5) | 21 (22.1) | 9 (9.1) | |
|
| ||||
| Cyanotic Heart Disease | ||||
| No | 109 (56.2) | 36 (37.9) | 73 (73.7) | <0.0001 |
| Yes | 85 (43.8) | 59 (62.1) | 26 (26.3) | |
|
| ||||
| PIM2b, by median | ||||
| ≤1.6 | 102 (52.6) | 41 (43.2) | 61 (61.6) | 0.015 |
| >1.6 | 92 (47.4) | 54 (56.8) | 38 (38.4) | |
|
| ||||
| RACHS-1c Score | ||||
| 1 | 29 (14.9) | 5 (5.3) | 24 (24.2) | |
| 2 | 78 (40.2) | 40 (42.1) | 38 (38.4) | <0.001 |
| 3,4 | 80 (41.2) | 44 (46.3) | 36 (36.4) | |
| Heart Transplant | 7 (3.6) | 6 (6.3) | 1 (1.0) | |
|
| ||||
| Baseline Albumin | ||||
| ≤3 mg/dl | 16 (8.2) | 13 (13.7) | 3 (3.0) | |
| >3 mg/dl | 167 (86.1) | 78 (82.1) | 89 (89.9) | 0.009 |
| NA | 11 (5.7) | 4 (4.2) | 7 (7.1) | |
|
| ||||
| Cardiac Bypass Time, by quartile | ||||
| ≤59.3 | 49 (25.3) | 18 (18.9) | 31 (31.3) | |
| >59.3–88.5 | 48 (24.7) | 20 (21.1) | 28 (28.3) | 0.042 |
| >88.5–126.5 | 48 (24.7) | 30 (31.6) | 18 (18.2) | |
| >126.5 | 49 (25.3) | 27 (28.4) | 22 (22.2) | |
|
| ||||
| Received Steroids Intra-Op | ||||
| No | 106 (54.6) | 48 (50.5) | 58 (58.6) | 0.326 |
| Yes | 88 (45.4) | 47 (49.5) | 41 (41.4) | |
|
| ||||
| Intra-Op Blood Transfusion | ||||
| No | 151 (77.8) | 69 (72.6) | 82 (82.8) | 0.089 |
| Yes | 43 (22.2) | 26 (27.4) | 17 (17.2) | |
|
| ||||
| Circulatory Arrest | ||||
| No | 188 (96.9) | 94 (98.9) | 94 (94.9) | 0.212 |
| Yes | 6 (3.1) | 1 (1.1) | 5 (5.1) | |
Developmental delay defined as severe impairment in age-appropriate ability to communicate with caregiver at pre-hospital baseline.
PIM2 = Pediatric Index of Mortality II, see text and reference for explanation of score (15).
RACHS-1 score = Risk Adjustment for Congenital Heart Surgery score, see text and reference for explanation of score (17)
Figure 1.
Duration of delirium in the PCICU. A histogram displaying the duration of delirium in PCICU.
Demographic data and baseline characteristics are presented in Table 1. Nearly half the patients were less than 2 years old. Sixteen percent of patients were characterized as developmentally delayed (defined as severe impairment in ability to communicate in an age-appropriate way with caregiver prior to hospitalization). Cyanotic heart disease was identified in 44% of patients. Children with a RACHS-1 score of two comprised 40% of the patient population. The PIM-2 score on admission (median score 1.6, inter-quartile range [IQR] 0.6–2.8) and daily PELOD-GCS (median score 3, IQR 0–5) reflect a critically ill population, often with multiple organ dysfunction. Thirty-six percent of patients were extubated in the immediate post-operative period, prior to admission to the ICU. During daily assessments, 37% were mechanically ventilated and 13% were on non-invasive mechanical ventilation (Table 2).
Table 2.
Clinical Characteristics by Encounter and Delirium Status (n = 1029a)
| Characteristic | No. of Observations (%) | Ever Delirious During PCICU Stay (%) | Never Delirious During PCICU Stay (%) | p Value |
|---|---|---|---|---|
| n | 1029 | 380 (36.9) | 649 (63.1) | |
|
| ||||
| Restraints | ||||
| No | 630 (61.2) | 115 (30.3) | 515 (79.4) | <0.0001 |
| Yes | 399 (38.8) | 265 (69.7) | 134 (20.6) | |
|
| ||||
| Opiates | ||||
| No | 149 (14.5) | 20 (5.3) | 129 (19.9) | <0.0001 |
| Yes | 880 (85.5) | 360 (94.7) | 520 (80.1) | |
|
| ||||
| Benzodiazepines | ||||
| No | 670 (65.1) | 179 (47.1) | 491 (75.7) | <0.0001 |
| Yes | 359 (34.9) | 201 (52.9) | 158 (24.3) | |
|
| ||||
| Vasopressors | ||||
| No | 868 (84.4) | 286 (75.3) | 582 (89.7) | <0.0001 |
| Yes | 161 (15.6) | 94 (24.7) | 67 (10.3) | |
|
| ||||
| Dexmedetomidine | ||||
| No | 704 (68.4) | 189 (49.7) | 515 (79.4) | <0.0001 |
| Yes | 325 (31.6) | 191 (50.3) | 134 (20.6) | |
|
| ||||
| Respiratory Supportb | ||||
| Room Air | 286 (27.8) | 31 (8.2) | 255 (39.3) | |
| Nasal Cannula | 173 (16.8) | 26 (6.8) | 147 (22.7) | |
| HFNCc | 57 (5.5) | 13 (3.4) | 44 (6.8) | <0.0001 |
| NIPPVd | 133 (12.9) | 54 (14.2) | 79 (12.2) | |
| MVe | 380 (36.9) | 256 (67.4) | 124 (19.1) | |
Coma days excluded from analysis.
Highest level of respiratory support was recorded for each day
HFNC = High Flow Nasal Cannula
NIPPV = Non-Invasive Positive Pressure Ventilation
MV = Mechanical Ventilation
Bivariate analyses of baseline and intraoperative risk factors associated with delirium development are shown in Table 1. When compared to patients who were never delirious during their ICU stay, children who were delirious were more likely to be under two years of age, have cyanotic heart disease, developmental delay, a baseline albumin less than or equal to three mg/dL, a higher RACHS-1 surgical score, and a PIM-2 score above the median. A longer bypass time, when compared by quartiles, was associated with development of delirium (all p<0.05; Table 1). Children who were ever delirious were also more likely to have been in a coma at some point during their ICU stay (49.5% v. 31.3%, p=0.015).
When assessed by time-dependent variables each PCICU day, children who were physically or pharmacologically restrained (on opiates and/or benzodiazepines) were more likely to be delirious. Need for vasopressors and respiratory support were associated with delirium (all p<0.0001; Table 2). A higher PELOD-GCS score was associated with delirium as well (median 3 v. 2, p<0.0001).
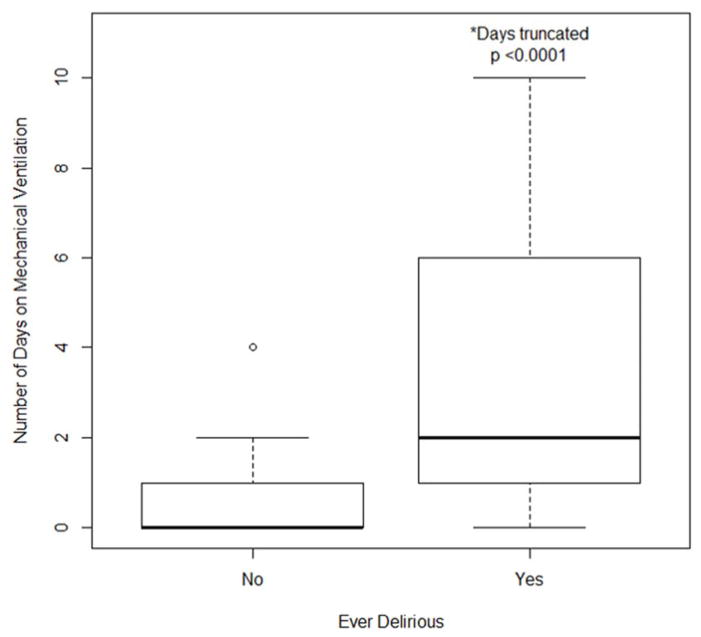
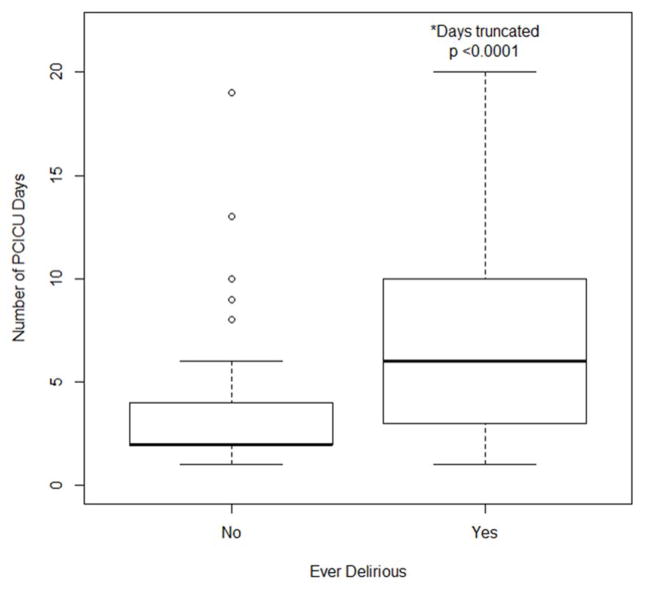
The median number of days mechanically ventilated was higher in those who were ever delirious than in those who were never delirious (2 vs. 0, SD ± 12.2 and 1.5 respectively; p <0.0001, Figure 2). Patients who developed delirium had a longer median length of ICU stay compared to those who were never delirious (6 vs. 2 days, SD ± 15.4 and 2.8 respectively; p < 0.0001, Figure 3).
Figure 2.
Associations between subjects diagnosed with delirium during this study and number of days on mechanical ventilation.
Figure 3.
Associations between subjects diagnosed with delirium during this study and PCICU length of stay (LOS).
In multivariable analysis, when controlling for age, RACHS-1 score, albumin level, developmental delay, and cyanotic disease patients of increasing age demonstrated a lower odds of developing delirium, with teenagers having the lowest delirium rates (OR 0.06; p<0.001). Patients with a pre-operative albumin level greater than 3 mg/dL (used as a surrogate marker of good nutritional status) demonstrated a lower odds of delirium development (OR 0.19; p=0.028). Presence of developmental delay (OR 3.38; p=0.023), cyanotic disease (OR 2.43; p=0.019), and a RACHS-1 score of 3 or 4 (OR 2.93; p=0.008) were strongly associated with delirium (Table 3). Of note, PIM-2 score was left out of the final predictive model, as it was directly collinear with many risk factors already included in the model.
Table 3.
Multivariable Logistic Regression Analysis Predicting Delirium (N = 177)
| Predictor Variable | Adjusted OR (95% CI) | p Value |
|---|---|---|
| Age (yr) | ||
| 0–2 | Reference | |
| >2–5 | 0.30 (0.12 – 0.72) | 0.008 |
| >5–13 | 0.30 (0.10 – 0.87) | 0.027 |
| >13 | 0.06 (0.02 – 0.22) | <0.001 |
|
| ||
| Baseline Albumin | ||
| ≤3 mg/dl | Reference | |
| >3 mg/dl | 0.19 (0.04 – 0.84) | 0.028 |
|
| ||
| Developmental delay | ||
| No delay | Reference | |
| Delay | 3.38 (1.18 – 9.66) | 0.023 |
|
| ||
| RACHS-1a Score | ||
| 1,2 | Reference | |
| 3,4 | 2.93 (1.32 – 6.50) | 0.008 |
|
| ||
| Cyanotic Heart Disease | ||
| No | Reference | |
| Yes | 2.43 (1.16 – 5.09) | 0.019 |
RACHS-1 score = Risk Adjustment for Congenital Heart Surgery score, see text and reference for explanation of score (17)
After controlling for previously defined confounders, as listed above, a diagnosis of delirium was associated with a 60% increase in PCICU LOS. In fact, delirium contributed more to LOS than presence of cyanotic heart disease, which conferred only a 34% increase in number of PICU days. Only mechanical ventilation rivaled delirium as a risk factor for prolonged ICU LOS (Table 4).
Table 4.
Multivariable Linear Regression Analysis Predicting PICU LOS
| Predictor Variable | Relative Number of PICU Days (95% CI) | p Value |
|---|---|---|
| Ever Delirious | ||
| No | Reference | |
| Yes | 1.59 (1.25 – 2.02) | <0.001 |
|
| ||
| Ever Mechanically Ventilated | ||
| No | Reference | <0.001 |
| Yes | 1.63 (1.28 – 2.07) | |
|
| ||
| Cyanotic Heart Disease | ||
| No | Reference | |
| Yes | 1.34 (1.07 – 1.68) | 0.010 |
|
| ||
| RACHS-1 Scorea | ||
| 1,2 | Reference | |
| 3,4 | 1.33 (1.05 – 1.68) | 0.018 |
|
| ||
| Age Category (yr) | ||
| 0–2 | Reference | |
| 3–5 | 0.84 (0.64 – 1.10) | 0.197 |
| 6–13 | 0.95 (0.70 – 1.31) | 0.774 |
| >13 | 0.84 (0.60 – 1.19) | 0.323 |
|
| ||
| Baseline Albumin | ||
| ≤3 mg/dl | Reference | |
| >3 mg/dl | 0.72 (0.49 – 1.04) | 0.085 |
|
| ||
| Developmental delay | ||
| No delay | Reference | |
| Delay | 0.86 (0.65 – 1.15) | 0.308 |
Results are from a multivariable linear regression with natural log-transformed number of PCICU days as outcome. Ratios >1.0 for relative number of PICU days and respective 95% confidence intervals indicate increased number of days over reference group.
RACHS-1 score = Risk Adjustment for Congenital Heart Surgery score, see text and reference for explanation of score (17)
Discussion
In this study cohort, nearly 1 in 2 children developed delirium after cardiac bypass surgery. This is consistent with reported post-cardiotomy delirium rates in adults (19,20,21,22,23), and considerably higher than the 10–30% delirium rates reported in the general population of critically ill children (1,24,25). Children undergoing cardiac bypass surgery are likely among those at highest risk for delirium development and would benefit from routine screening to allow for early detection and intervention. Importantly, and consistent with previous research in this topic, our data reveals an independent association between increasing PCICU length of stay and the development of delirium (1).
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-V), delirium has an organic cause and is therefore a direct consequence of a general medical condition or its treatment (12). Although the pathophysiology of pediatric post-cardiotomy delirium has not yet been clarified, it is likely a syndrome resulting from a multifactorial process involving pre-operative patient vulnerability, perioperative insults, and iatrogenic factors related to post-operative PCICU care (26,27). This study suggests that pre-operative patient vulnerability is a risk factor, as children with poor nutrition (represented in this cohort as those patients with albumin <3 mg/dL) and atypical brains (developmental delay) were at higher risk for delirium. These vulnerable children may have been more susceptible to the effects of cardiac bypass, anesthesia, and surgery. Putative mechanisms for peri-operative insults that predispose to delirium include increased inflammatory mediators, subclinical cerebral infarctions, alterations in neurotransmitters, and generalized hypo-perfusion and cellular hypoxia- all of which can be exacerbated by a prolonged bypass time (28,29). Finally, consistent with both adult and pediatric research in delirium, we demonstrated an association with delirium development and iatrogenic factors including benzodiazepines, opiates and physical restraints (2,7,11). Granular details regarding particular medications used (rather than drug categories), and their doses, was beyond the scope of this study. This is especially important area for future research, as these are potentially modifiable risk factors.
In our PCICU, there are no protocols in place for sedation or early mobilization. Anecdotally, with increased awareness of delirium in our unit, we have seen a progression from use of primarily fentanyl and midazolam infusions as first-line sedation, to the use of fentanyl and dexmedetomidine infusions. In critically ill adults, delirium rates have been successfully reduced with lower benzodiazepine exposure, protocolized sedation, and early mobilization (30,31). This remains a necessary area for investigation in pediatric cardiac critical care.
Study strengths and limitations
This study has notable strengths. To our knowledge, it is the first to prospectively describe delirium in a PCICU using a standardized delirium-screening tool and includes a large number (n=194) of subjects. These subjects were followed throughout their ICU stay with daily detailed data collection and analysis of multiple risk factors.
There are several limitations. For the most part, delirium screening was only performed once per day. As the CAPD was usually completed by the day-shift nurse, we often do not have a delirium screen to reflect the overnight period, and may have missed children who were delirious only at night. This may have falsely lowered the delirium incidence and prevalence measured in this study. Future studies, with multiple daily delirium assessments may provide a more robust representation of delirium in the PCICU. In addition, we did not determine delirium subtype (hypoactive, hyperactive, or mixed); this is an important area of focus for future studies.
Once children in this study were diagnosed with delirium, it was addressed and treated appropriately, primarily with a non-pharmacologic approach (investigation for inciting causes, minimization of deliriogenic medication, and with environmental modification). On occasion, in refractory cases with severe behavioral disturbance, we added an atypical antipsychotic. Treatment of delirium in our cohort may have decreased its duration, and lead to an underestimation of the true duration of PCICU delirium. This is a necessary study limitation, as we could not ethically withhold treatment once delirium was diagnosed.
Although our data collection included many possible risk factors for delirium development, identified from the adult post-cardiotomy delirium literature, it is possible that other unknown and important risk factors were missed. Importantly, as this is an observational study, we do not attempt to establish causality; instead we describe associations between risk factors and delirium development. This should serve as a basis for future interventional study designs.
Finally, this study was performed at a single institution. Therefore widespread extrapolation of results to all institutions may not be appropriate; similar findings in other units are necessary to corroborate these findings. The time has come for a multi-institutional trial to further explore modifiable risk factors and treatment options for delirium in children after cardiac bypass surgery.
Conclusion
Children undergoing cardiac bypass surgery are at high risk for delirium development in the post-operative period. Widespread delirium screening in PCICUs is necessary to detect this prevalent problem and allow for necessary interventions. With a systematic approach to detecting delirium, we can improve the quality of care we provide to critically ill children in the PCICU.
Footnotes
This study was performed at New York Presbyterian Hospital, Columbia University Medical Center.
All authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Silver G, Traube C, Gerber LM, et al. Pediatric Delirium and Associated Risk Factors: A Single-Center Prospective Observational Study. Pediatric Critical Care Medicine. 2015;16:303–309. doi: 10.1097/PCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: A Valid, Rapid, Observational Tool for Screening Delirium in the PICU*. Critical Care Medicine. 2014;42:656–663. doi: 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 4.Van den Boogaard M, Schoonhoven L, Evers AWM, et al. Delirium in critically ill patients: Impact on long-term health-related quality of life and cognitive functioning. Critical Care Medicine. 2012;40:112–118. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical Care Medicine. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 6.Cropsey C, Kennedy J, Han J, et al. Cognitive Dysfunction, Delirium, and Stroke in Cardiac Surgery Patients. Semin Cardiothorac Vasc Anesth. 2015;19:309–317. doi: 10.1177/1089253215570062. [DOI] [PubMed] [Google Scholar]
- 7.Burkhart CS, Dell-Kuster S, Gamberini M, et al. Modifiable and Nonmodifiable Risk Factors for Postoperative Delirium After Cardiac Surgery With Cardiopulmonary Bypass. Journal of Cardiothoracic and Vascular Anesthesia. 2010;24:555–559. doi: 10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kazmierski J, Kowman M, Banach M, et al. Incidence and predictors of delirium after cardiac surgery: Results from The IPDACS Study. J Psychosom Res. 2010;69:179–185. doi: 10.1016/j.jpsychores.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Sadler PD. Incidence, degree, and duration of postcardiotomy delirium. Heart Lung. 1981;10:1084–1092. [PubMed] [Google Scholar]
- 10.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the Cardiovascular ICU: Exploring Modifiable Risk Factors. Critical Care Medicine. 2013;41:405–413. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- 13.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 14.Silver G, Kearney J, Traube C. Delirium screening anchored in child development: The Cornell Assessment for Pediatric Delirium. Palliat Support Care. 2014:1–7. doi: 10.1017/S1478951514000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater A, Shann F, Pearson G, et al. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 16.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 18.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1976;19:716–723. [Google Scholar]
- 19.Burkhart CS, Dell-Kuster S, Gamberini M, et al. Modifiable and Nonmodifiable Risk Factors for Postoperative Delirium After Cardiac Surgery With Cardiopulmonary Bypass. Journal of Cardiothoracic and Vascular Anesthesia. 2010;24:555–559. doi: 10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Kazmierski J, Kowman M, Banach M, et al. Incidence and predictors of delirium after cardiac surgery: Results from The IPDACS Study. J Psychosom Res. 2010;69:179–185. doi: 10.1016/j.jpsychores.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Sadler PD. Incidence, degree, and duration of postcardiotomy delirium. Heart Lung. 1981;10:1084–1092. [PubMed] [Google Scholar]
- 22.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the Cardiovascular ICU: Exploring Modifiable Risk Factors. Critical Care Medicine. 2013;41:405–413. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith HAB, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit*. Critical Care Medicine. 2011;39:150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith HAB, Gangopadhyay M, Goben CM, et al. The Preschool Confusion Assessment Method for the ICU: Valid and Reliable Delirium Monitoring for Critically Ill Infants and Children. Crit Care Med. 2016;44:592–600. doi: 10.1097/CCM.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CH. Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol. 2014;27:117–122. doi: 10.1097/ACO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrøder Pedersen S, Kirkegaard T, Balslev Jørgensen M, et al. Effects of a screening and treatment protocol with haloperidol on post-cardiotomy delirium: a prospective cohort study. Interact Cardiovasc Thorac Surg. 2014;18:438–445. doi: 10.1093/icvts/ivt501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sockalingam S, Parekh N, Bogoch II, et al. Delirium in the postoperative cardiac patient: a review. J Card Surg. 2005;20:560–567. doi: 10.1111/j.1540-8191.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 30.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 31.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomized controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]