Abstract
Bacteria can be engineered to function as diagnostics or therapeutics in the mammalian gut but commercial translation of these technologies has been hindered by the susceptibility of synthetic genetic circuits to mutation and unpredictable function during extended gut colonization. Here we report stable, engineered bacterial strains that maintain their function for 6 months in the mouse gut. We engineered a commensal murine Escherichia coli strain to detect tetrathionate, which is produced during inflammation. Using our engineered diagnostic strain, which retains memory of exposure in the gut for analysis by fecal testing, we detected tetrathionate in both infection-induced and genetic mouse models of inflammation over 6 months. The synthetic genetic circuits in the engineered strain were genetically stable and functioned as intended over time. The robust, durable performance of these strains confirms the potential of engineered bacteria as living diagnostics.
Bacteria in the gut microbiome can sense, respond to and manipulate the mammalian gut environment. Recombinant probiotics that have been engineered to exploit these features show promise as therapeutics and diagnostics for inflammatory bowel disease (IBD), autoimmune diabetes, obesity and other conditions1. However, engineered, live, bacterial diagnostics and therapies have only been tested under non-colonizing conditions in the guts of model organisms and humans. Similarly, engineered bacteria in other applications such as tumor-targeted strains of Salmonella typhimurium were cleared from the body within approximately one month in mouse and non-human primate models2. If recombinant probiotic bacterial strains could colonize the mammalian gut and function for extended periods the potential for translation of such technologies to humans could be vastly improved1. Unfortunately, the burden that synthetic genetic circuits place on their bacterial hosts3 can result in compensatory genetic mutation4, loss of engineered function5, or lack of growth of the recombinant strain3 in a host and environment-dependent manner6, 7.
We focus on the application of live, engineered diagnostics to detect transient (or highly localized) molecules in the gut for non-invasive diagnostic purposes, or to be used as the basis for on-demand therapeutics. The gut environment is largely inaccessible, and products from digestion or released by the host or microbiome can be modified by the microbiome or absorbed by the host before excretion. Several bacterial strains have been engineered to detect transient gene expression both in the laboratory8–11 and in vivo for up to 12 days12, 13. We engineered E. coli to record exposure to tetracycline and its analogues12. Our engineered strain contained two circuits, an environmentally-responsive promoter driving Cro protein expression, which functioned as a ‘trigger’ and a Cro-inducible CI/Cro transcriptional switch derived from phage lambda that functioned as a ‘memory element’12. The engineered E. coli detected anhydrotetracycline in the mouse gut and retained memory of this for up to 7 days following removal of the signal. However, the engineered strain responded to tetracycline derivatives, which may alter the microbiome through toxicity, preventing long-term analysis of strain stability and function in a physiologically relevant environment.
Here, we report the design of a trigger circuit that can detect and respond to tetrathionate. Tetrathionate is a transient product of reactive oxygen species (ROS), which are produced during inflammation14. Our aim was to develop an engineered strain and study the ability of the strain to colonize and function in the murine gut over an extended timeframe.
The conversion of thiosulfate, which is produced from hydrogen sulfide, into tetrathionate in the mouse intestine has been reported during S. typhimurium14 and Yersinia enterocolitica15 induced inflammation (Supplementary Fig. 1). Tetrathionate is detected in these bacteria by the TtrR/TtrS two-component system16 and can be used as a terminal electron acceptor for anaerobic respiration providing a growth advantage to these pathogens during inflammation14, 15, 17, 18. A range of other bacteria, including pathogens, may also be able to grow preferentially using tetrathionate19. Increased production of tetrathionate in toll-like receptor-1 deficient mice15 and an enrichment of tetrathionate utilization genes in the microbiota of a Tbet−/− Rag−/− mouse ulcerative colitis (TRUC) model20 point to the potential of tetrathionate as a general marker of inflammation. However, non-invasive detection of tetrationate is not possible, which has limited further investigation.
We engineered a commensal mouse E. coli strain, NGF-1 to detect tetrathionate (Fig. 1a.). Briefly we constructed a trigger element using ttrR/S genes and the PttrBCA promoter from S. typhimurium to drive Cro expression and inserted it into the genome of strain PAS637, already containing our previously published memory circuit (see Online Methods)12, thereby creating strain PAS638 (Fig. 1a; Supplementary Table 1). All synthetic constructs were integrated into the chromosome of E. coli strain, NGF-1 to reduce burden and allow long-term circuit retention without selection. Engineered strain PAS638 exhibits memory upon tetrathionate exposure, driving Cro and β-galactosidase (β-gal) expression from the synthetic memory element and maintaining their expression in the absence of tetrathionate (Fig. 1c). We induced memory during anaerobic growth in liquid culture by adding tetrathionate, which should activate expression through PttrBCA in the trigger element and switch the memory element from the off (CI+/Cro and β-gal−) to on (Cro and β-gal+/CI−) state. We quantified the response of the circuit by plating tetrathionate activated cultures onto solid media containing 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (x-gal), which turns blue in the presence of β-gal. Plating in the absence of tetrathionate and under aerobic conditions allowed optimal maturation of the x-gal indicator plates along with ensuring only colonies retaining a record of tetrathionate exposure were counted. (Fig. 1b). PAS638 bacteria were effectively induced by tetrathionate (EC50:0.38–0.85μM 95% CI) whereas the control strain, PAS637 (Supplementary Table 1), which does not contain the PttrBCA-cro trigger, showed no switching even at saturating concentrations of 2mM tetrathionate (Fig. 1c).
Figure 1. Engineering a tetrathionate responsive memory device in E.coli NGF-1 a).
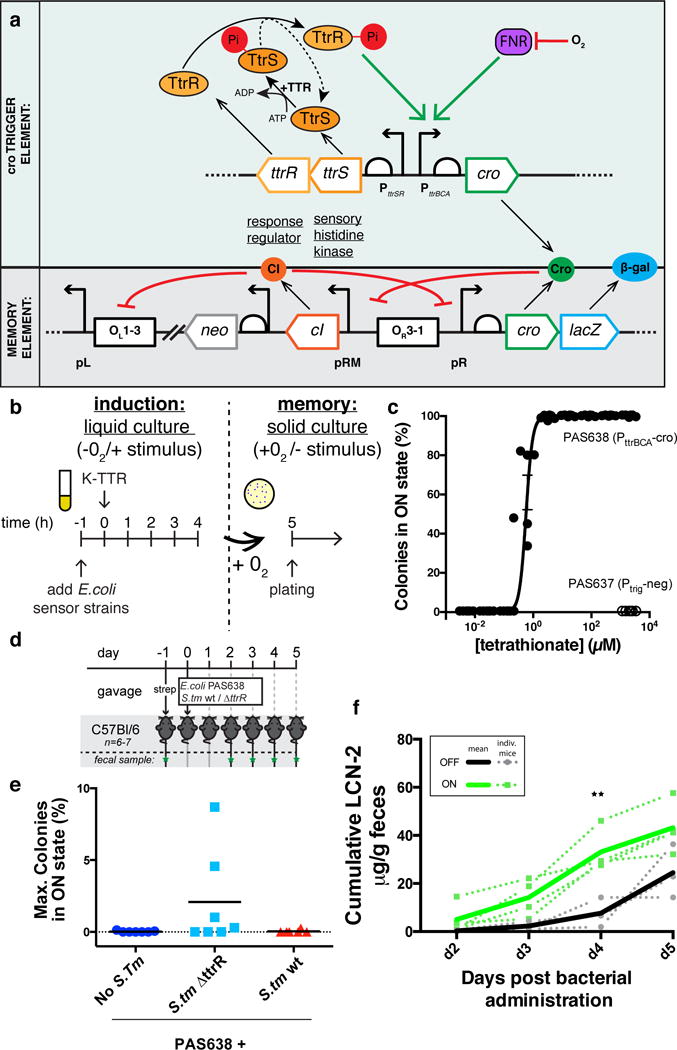
A bacterial memory device, PAS638, was constructed in mouse commensal E. coli NGF-1. S. typhimurium ttrR/S and PttrBCA drive Cro ‘trigger’ expression to switch a phage lambda-based memory circuit12. In the presence of tetrathionate, TtrS becomes phosphorylated, in turn phosphorylating TtrR, which activates expression through PttrBCA in anaerobic conditions. Cro protein expression switches memory ON, accompanied by lacZ reporter expression. b) Testing of in vitro memory showed. c) a dose-response curve of PAS638 (EC50: 0.38–0.85μM 95%CI) but no response of the triggerless control PAS637 strain. Graph shows individual values from 6 replicate colonies from 2 separate experiments, non-linear fit ± SEM. d) PAS638 and S. typhimurium 14028s (S. tm) bacteria were administered by oral gavage to mice one day after streptomycin treatment, and fecal samples were analyzed d2–5 post administration (green stars) e) showing specific response of PAS638 on day 4 and/or 5 (see also Supplementary Fig. 2a) when co-infected with S. typhimurium ΔttrR bacteria (n=7) but not control (n=7) or S. typhimurium wt bacteria (n=6). f) Cumulative LCN-2 levels in mice administered PAS638+ S. typhimurium ΔttrR were higher in mice with PAS638 response (green lines) than in those without (black lines). Graph shows plots for individual mice (dotted) and ON or OFF averages (solid lines). ** t-ratio(5) = 4.3, p=0.03 using multiple t-tests with Holm-Sidak multiple comparisons test and each timepoint analyzed individually without assuming a consistent SD. d2 (t-ratio(5) = 1.2, p = 0.3), d3 (t-ratio(5) = 2.5, p = 0.1) and d5 (t-ratio(5) = 2.3, p = 0.1) were not significantly different.
Having validated that PAS638 could record tetrathionate exposure in vitro, we next tested the specificity of PAS638 for tetrathionate in vivo using a murine S. typhimurium-induced colitis model21 (Fig. 1d). The presence of inflammation, specifically ROS, causes elevated levels of tetrathionate, which are rapidly reduced to thiosulfate by wild-type S. typhimurium strains but not by strains deficient in their tetrathionate reduction-capacity14. We pre-treated C57Bl/6 mice with streptomycin to reduce colonization resistance for S. typhimurium in the colon, and introduced PAS638, PAS638 with S. typhimurium, or PAS638 with a S. typhimurium ΔttrR variant which is unable to express tetrathionate reductase by oral gavage (Fig. 1d; Supplementary Table 1)18. Fecal samples were collected at days 2–5 post-administration. PAS638 did not switch on β-gal expression in control and S. typhimurium infected mice (Fig. 1e). However, when PAS638 was co-administered with S. typhimurium ΔttrR switching was increased, with 4/7 mice showing a higher proportion of memory-on colonies than any samples from control mice on days 4–5 post-infection (Fig. 1e, Supplementary Fig. 2a). Colony forming unit (CFU) counts for PAS638 E. coli (Supplementary Fig. 2b) and S. typhimurium variants (Supplementary Fig. 2c) were generally consistent between groups and showed no correlation with switching. We hypothesized that the mice in which PAS638 was switched on should also show signs of more acute inflammation.
We independently validated the presence of inflammation in the colitis model using quantification of the lipocalin-2 (LCN-2) biomarker (Supplementary Fig. 2d)22 and blinded histopathology scoring of cecum and colon sections (Supplementary Fig. 2e–f). Taken together these techniques identified inflammation in all S. typhimurium and S. typhimurium ΔttrR infected mice14. Of note, PAS638 + S. typhimurium ΔttrR mice in which memory-on state colonies were detected had significantly higher cumulative LCN-2 values at day 4 post-infection than those in which PAS638 remained off (Fig. 1f). These results show that our engineered memory strain specifically senses tetrathionate and that tetrathionate sensing corresponds to a more acute inflammatory response in vivo.
Next we set out to use PAS638 to further probe the pathways required for tetrathionate production. The Cytochrome B-245 Beta Chain gene (cybb; also known as gp91phox and Nox2), which encodes a subunit of the phagocyte NADPH-oxidase complex, has previously been implicated in the generation of tetrathionate14. We tested the response of PAS638 to S. typhimurium ΔttrR infection of cybb-deficient (cybb−/−) mice (Fig. 2a). Unexpectedly, PAS638 showed increased switching specifically in S. typhimurium ΔttrR infected mice (Fig. 2b). S. typhimurium ΔttrR -infected cybb−/− mice also had more acute weight loss and increased fecal LCN-2 levels compared with S. typhimurium ΔttrR -infected C57Bl/6 controls (Supplementary Fig. 3a–b). Uninfected cybb−/− mice also produced increased LCN-2 compared with uninfected C57Bl/6 suggesting a propensity to inflammation in this mouse strain (Supplementary Fig 3b). These results suggest that tetrathionate is produced during S. typhimurium infection, even in the absence of Cybb.
Figure 2. Tetrathionate-sensing of inflammation in vivo.
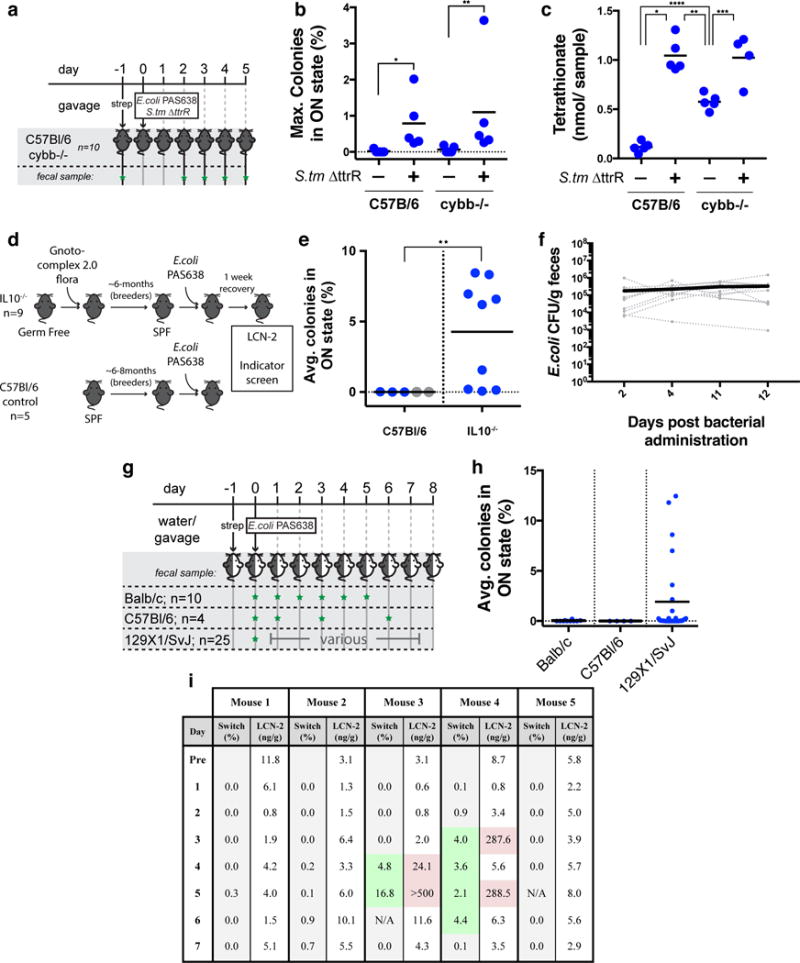
a) PAS638 and S. typhimurium ΔttrR bacteria were administered to cybb−/− and C57Bl/6 control mice (n= 5 per group) by oral gavage a day following streptomycin pre-treatment. b) Elevated PAS638 switching in the presence of S. typhimurium ΔttrR indicated the presence of tetrathionate in both strains. Graph shows max. percent over 5 day experiment, means are marked. *p=0.01 and ** p = 0.03 using Kruskal-Wallis test with Dunn’s multiple comparison correction. A comparison between the two S.tm ΔttrR samples was also done, p >0.99. c) Mass spectrometry from cecal extracts confirmed that tetrathionate was raised in cybb−/− and C57Bl/6 mice co-infected with S. typhimurium ΔttrR, along with intermediate levels in cybb−/− uninfected controls. Graph shows total tetrathionate detected per whole cecum; means are marked. *p<0.0001, **p0.0008, ***p=0.002 and **** p=0.0009; F(3,15) = 42.92 using one-way ANOVA with Tukey’s multiple comparison correction. Comparison of both infected groups (p=0.996) and C57Bl/6 control with cybb−/− infected mice (p<0.0001) were performed but not shown. d) PAS638 was administered to retired breeder IL10−/− mice raised in gnotobiotic and barrier SPF conditions and retired breeder C57Bl/6 control mice raised in SPF conditions. e) Indicator plating of fecal samples from C57Bl/6 and IL10−/− mice (> 1 week post administration) showed elevated tetrathionate response in IL10−/− mice. Blue values: average of 3–4 measurements from individual mice. Grey values: single measurement from individual mice. Means are marked ** p=0.009 between groups (including only blue values) using a two-tailed Mann-Whitney test. f) PAS638 stably colonized IL10−/− mice (n=10) even without streptomycin pre-treatment. Graph shows CFU counts for individual mice (dotted lines) and average of all mice (solid) g) Balb/c, C57Bl/6 and 129X1/SvJ mice were colonized with PAS638 and the bacterial memory state was analyzed (green star = fecal sample collected). h) Balb/c and C57Bl/6 mice showed no switching, while a subset of 129X1/SvJ mice showed elevated response. Graph shows averages from at >3 days for individual mice and means. i) PAS638 levels broadly correlated with increased LCN-2 levels in five 129X1/SvJ mice. Timepoints in which bacteria were more highly switched into the memory-on state (memory values >1% highlighted green) tended to correspond with higher LCN-2 levels (values >20ng/g highlighted red).
Mass spectrometry confirmed these results, directly detecting tetrathionate in cecum extracts from S.typhimurium ΔttrR-infected C57Bl/6 and all cybb−/− mice, with infected mice showing significantly higher tetrathionate levels than uninfected ones (Fig. 2c). We detect ~1 nanomole of tetrathionate from each inflamed mouse (Fig. 2c), translating to low micromolar concentrations if diluted across the approximate volume of the cecum (assuming 0.1–0.5cm3 cecum volume); this concentration is in the order of the in vitro EC50 of our sensor (Fig. 1c). Together these data show that the threshold of PAS638 sensing in vivo is in the order of 1 micromolar, and show that phagocyte NADPH-oxidase- independent tetrathionate production can be detected in the gut by our engineered E. coli strain. In contrast to previous reports we identified tetrathionate in cybb−/− mice using the S. typhimurium ΔttrR colitis model, both non-invasively by PAS638 response and by mass spectrometry detection14. These findings could foreseeably derive from sample measurement or microbiota differences between the studies.
Increased ROS levels are also associated with IBD in humans23 and may be present in patients even during subclinical inflammation. Interleukin-10 deficient mice (IL10−/−) have features of human IBD, including the effect of microbiota composition on disease progression24. To test whether PAS638 could detect subclinical inflammation in this model we colonized ~6 month-old, retired-breeder IL10−/− mice with PAS638 E. coli (Fig. 2d). These mice do not have acute colitis because they have been raised in gnotobiotic and barrier SPF conditions. The engineered bacteria (≥3 measurements over ≥2-weeks) detected tetrathionate in 8/9 IL10−/− mice, but not in separately-reared retired C57Bl/6 breeder controls (Fig. 2e). Measurement of fecal LCN-2 showed that this marker of inflammation was temporarily increased in IL10−/− mice immediately following administration but reduced after a week (Supplementary Fig. 3c). Switching levels in IL10−/− mice were therefore measured >1-week after bacterial administration, which means that the inflammation sensed was not caused by the process of administration. To confirm that PAS638 did not cause inflammation, we administered PAS638 by oral gavage without antibiotic pre-treatment into IL10−/− mice. PAS638 successfully colonized (Fig. 2f) and detected tetrathionate in 2/10 mice (2 measurements over 9 days) (Supplementary Fig. 3d). LCN-2 levels were not raised in contrast to streptomycin treated IL10−/− mice (Supplementary Fig. 3e), suggesting that streptomycin rather than PAS638 caused the previously observed post-administration LCN-2 increase This means that PAS638 can detect elevated tetrathionate in a physiologically relevant subclinical inflammatory environment.
To evaluate PAS638 in different mouse backgrounds we administered the bacteria by oral gavage to Balb/c, C57Bl/6 or 129X1/SvJ mice, after overnight provision of streptomycin in their drinking water to assist in colonization, and measured the response over 5–8 days (≥3 measurements) (Fig. 2g). PAS638 did not respond in Balb/c or C57Bl/6 mice; surprisingly, however, bacteria from a subset (~20–40%) of 129X1/SvJ mice showed elevated switching (Fig. 2h). PAS638 gavage in the absence of streptomycin pre-treatment, while producing successful colonization (Supplementary Fig. 3f), also showed similar switching results (Supplementary Fig. 3g). Interestingly, histology did not reveal consistent signs of inflammation (Supplementary Fig. 3h). However, the presence of memory-on state PAS638 bacteria correlated with the onset of elevated LCN-2 levels in 5 mice that were tested daily for 7 days (Fig. 2i), despite absolute LCN-2 levels being orders of magnitude lower than those measured during Salmonella infection (Supplementary Fig. 2d). While a definitive cause for the inflammation remained unclear, the 129X1/SvJ strain has documented defects in macrophage recruitment to sites of inflammation25, 26. Detection of tetrathionate in the presence of fluctuating and low-level inflammation that remained below histological detection further demonstrates the ability of PAS638 to detect a subclinical inflammatory environment.
Finally, we tested whether PAS638 could be used for long-term monitoring of tetrathionate. We used the PAS638-inducing environment of the 129X1/SvJ mouse to continually induce memory, thus allowing the fitness costs associated with both on- and off- states to be assessed. Five mice were colonized with PAS638 by oral gavage, after overnight provision of streptomycin in their drinking water, and maintained for 200 days, which corresponds to >1600 bacterial generations.27. We used intermittent fecal testing to analyze for the presence and function of PAS638 (Fig. 3a). Throughout this experiment PAS638 remained colonized at detectable levels without continued antibiotic selection (Fig. 3b) and colonies in the memory-on state were detected at each timepoint (Fig. 3c). Remarkably, colonies that were in the memory-off state in fecal samples at day 159 and day 200 retained the ability to respond to tetrathionate ex vivo (Fig. 3d). Similarly, colonies that were in the memory-on state in fecal samples retained an ability to turn off memory under repeat streaking on agar plates (Fig. 3d). Together these results indicate that PAS638 had not acquired mutations to prevent its correct function.
Figure 3. PAS638 does not accrue mutations in synthetic elements over long periods.
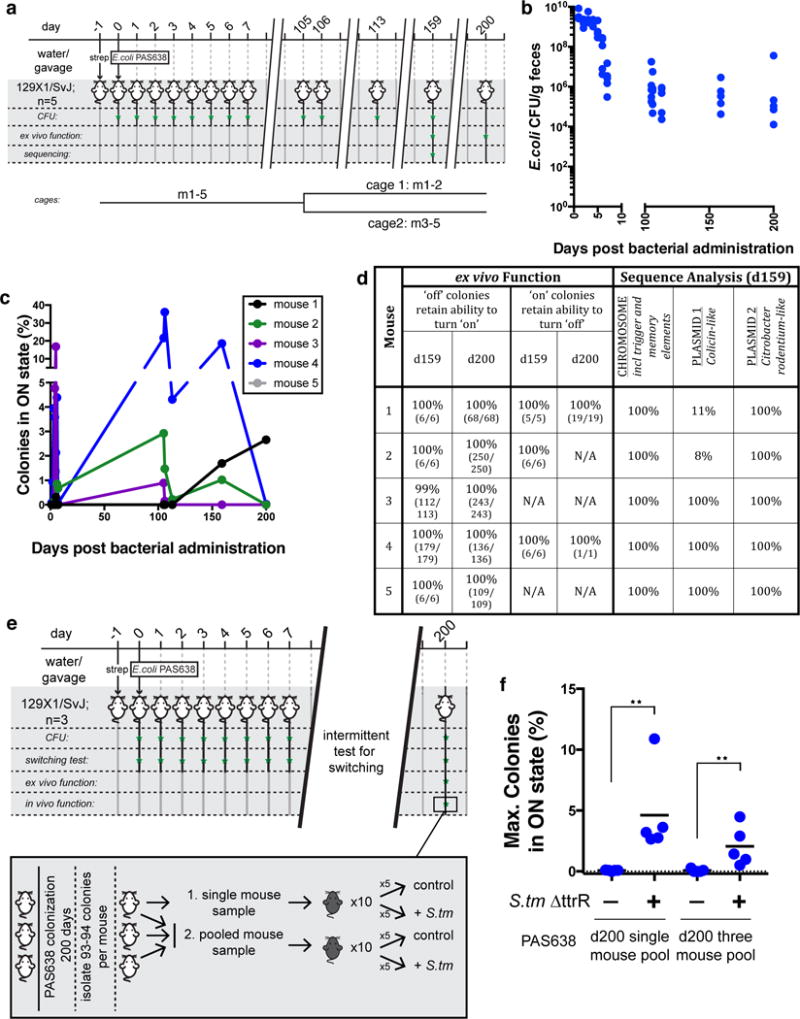
a) 129X1/SvJ mice were colonized with PAS638 for a 200-day period, >1600 bacterial generations. Mice were housed together for days 1–105, and then separated into two cages. b) PAS638 titers remained detectable following establishment in the streptomycin pre treated gut, without further antibiotic selection. c) Function was evident by switching at timepoints throughout the 200-day experiment. d) Ex vivo functional tests and next-generation sequencing analysis of PAS638 colonies following 159 and 200 days colonization confirmed that synthetic circuits did not mutate in any way over this period. Only one mutational insertion in the Colicin-like plasmid was identified in two cohoused mice, m1 and m2. e) In vivo function was further confirmed through administration of PAS638 and S. typhimurium ΔttrR to C57Bl/6 mice (n=5 per group), using PAS638 colonies isolated after colonization of three separate 129X1/SvJ mice for 200 days. Pooled colonies from a single mouse (94 colonies), or all three mice (280 colonies) were tested. f) Infection specific PAS638 switching response confirmed retained functionality in vivo. Graph shows max. switching percent measured from d3–5 post–infection; means are marked. ** p=0.008 using a two-tailed Mann-Whitney test of each experiment separately.
PAS638 can colonize the mouse gut and retain the ability to respond to inflammation in vivo for at least 6-months. Colonies that retained ex vivo function were pooled from either one or three mice in a repeat long-term experiment, after 200 days colonization (Supplementary Fig. 4a). Bacteria from these colonies were administered to C57Bl/6 mice in the presence or absence of S. typhimurium ΔttrR (Fig. 3e). Blinded scoring of histopathology, weight loss, and LCN-2 concentrations all indicated successful infection (Supplementary Fig. 4b–d). The post-colonization PAS638 showed elevated switching specifically in S. typhimurium ΔttrR co-infected mice (Fig. 3f), confirming the ability of the engineered bacteria to function in vivo after extended growth in the gut.
Whole genome sequencing further demonstrates circuit stability. To analyze whether mutations had accrued in PAS638 during colonization, at day 159 PAS638 colonies from five mice (Fig. 3a) were pooled at the individual mouse level and these were compared to the ancestral PAS638 by whole genome sequencing. The sequencing depth that was achieved (average chromosomal fold-coverage of 80–152) can identify mutations that occur in more than ~10% of the population (see Online Methods). The only unique mutation identified from the 5 mouse samples was a repeat expansion in an intergenic region of the E. coli NGF-1 colicin-like plasmid, which was detected in 89% and 92% of reads from two mice (Fig. 3d). No mutations or rearrangements were detected on the chromosome, including the synthetic gene elements, or the NGF-1 Citrobacter rodentium-like plasmid (see Online Methods). Further, junction prediction indicated that the synthetic element remained in the same genomic location in all samples. The detected mutation, (A)9→10 in an intergenic region, is not expected to affect the function of the bacteria in any way. Detection of a single mutant is consistent with the number expected from wild-type E. coli over the approximate 1200 generations in the gut28, 29. The two mice carrying bacteria with the detected mutations were housed together, while other mice were separated at day 105 (Fig. 3a). Given this mutation spread and fixed amongst the cohoused mice, we are confident that other beneficial mutations would have been detected at the sequencing depth achieved. Together these findings indicate that these synthetic circuits place sufficiently low burden on the bacteria carrying them to allow robust function over >6-months in the complex gut environment.
These long-term experiments were facilitated by the use of E. coli NGF-1, which has high colonization capacity (Fig. 3b)12. Further, colonization by this strain does not require pre-administration of antibiotics which means that the models are more physiologically relevant. Our results confirm the association of increased tetrathionate concentrations with inflammation both in the presence and absence of pathogen infection. We detected low-level inflammation in uninfected IL10−/− and 129X1/SvJ mice. Further, in at least one case the PAS638 response provided more consistent results over a multiple day period than LCN-2 measurement (Fig. 2i) due to the memory capacity of the engineered bacterial strain. In summary, these results show that synthetic bacterial devices can colonize the complex host mammalian gut and be used to monitor and understand disease.
Online Methods
Strain construction
Details of strains constructed for this study are provided (Supplementary Table 1). All synthetic constructs were integrated chromosomally in the mouse commensal Escherichia coli NGF-1 strain12 or Salmonella typhimurium 14028s.
The lambda derived CI/Cro memory element was originally inserted between the mhpR and lacZ loci of E. coli TB1030 and transferred to E. coli NGF-1 by P1vir transduction31 as previously described12. The tetrathionate responsive trigger circuit, consisting of ttrR, ttrS genes and the PttrBCA promoter was amplified directly from S. typhimurium LT2. Overlap PCR was used to append the cro gene downstream of PttrBCA and add ~36bp flanks for insertion into the araB-araC locus of E. coli TB10. The primer sets used were as follows: set 1: FW: ttatggataaaaatgctatggcatagcaaagtgtga, REV: accagaatacgaacaacgtatgagccatgatgtgacggaagatcacttcgcagaataaat; set 2: FW atttattctgcgaagtgatcttccgtcacatcatggctcatacgttgttcgtattctggt, REV: ataatctttcagggttatgcgttgttccatattgactcccgtccacattgccaacaatga; and set 3: FW tcattgttggcaatgtggacgggagtcaatatggaacaacgcataaccctgaaagattat, REV: acggcagaaaagtccacattgattatttgcacggcgttatgctgttgtttttttgttactcgggaaggg. The locus was ultimately transferred to E. coli NGF-1 by P1vir transduction.
S. typhimurium strains were derived from S. typhimurium 14028s. To confer tetracycline resistance to all S. typhimurium strains a zhj-1401::Tn10 construct from S. typhimurium LT2 SA2700 (Salmonella Genetic Stock Center) transferred to both S. typhimurium strains used in this study by P22 transduction32. To prevent tetrathionate reduction capacity, a ttrR knock-out TT2247018 construct was transferred to S. typhimurium by P22 transduction.
In vitro induction with tetrathionate
Tetrathionate-based memory was induced under in vitro conditions in either liquid culture or during growth on plates. For liquid culture induction, strains were backdiluted 1:1000 from overnight culture in SOC broth into pre-reduced anaerobic SOC broth and grown at 37°C in an anaerobic chamber (Coy Lab Products) using 7%H2/ 20% CO2/63% N2 culture gas. After 1h, potassium tetrathionate (Sigma) dissolved in pre-reduced SOC media was added to cultures and induction was undertaken at 37°C in the anaerobic chamber for 4h. Memory was assayed by plating and growth in aerobic conditions of serial dilutions of the bacteria on luria-broth (LB) + 300μg/mL streptomycin sulfate (Sigma) + 60μg/mL 5-Bromo-4-chloro-3-indolyl b-D-galactopyranoside (x-gal) (Santa Cruz Biotechnology) agar plates. Switching levels were estimated through counting greater than 250 blue and white colonies unless otherwise noted. In vitro testing on solid plates involved plating on LB + 300μg/mL streptomycin sulfate (Sigma) + 60μg/mL x-gal (Santa Cruz Biosciences) + 10 mM sodium tetrathionate (Sigma) agar. Growth was maintained in anaerobic conditions for approximately 8–12 hours using the Gaspak system (BD Biosciences), followed by further growth in aerobic conditions to allow development of memory and the x-gal reporter.
In vivo testing of tetrathionate memory
The Harvard Medical School Animal Care and Use Committee approved all animal study protocols.
General analysis
For most experiments, female, Balb/c (Charles River), C57Bl/6 (Charles River/ Jackson laboratories), 129X1/SvJ (Jackson laboratories), B6.129P2-Il10tm1Cgn/J (Jackson laboratories) or B6.129S-Cybbtm1Din/J (Jackson laboratories) mice of 8–12 weeks, including ~2 weeks acclimatization to our mouse facility, were administered PAS638 E. coli NGF-1 bacteria (1× 107–8 CFU/ mouse) by oral gavage. Except where specifically stated this occurred following pre-treatment with USP-grade streptomycin sulfate (Gold Biotechnology; 0.5g/L in drinking wrater or 20mg per mouse by oral gavage). Bacteria were pelleted and resuspended at a dilution of ~1/10 from overnight culture in sterile PBS prior to gavage of 100uL/ mouse.
Fecal samples were collected from mice under study and homogenized at 50 or 100 mg/mL in sterile PBS by vortexing in 1.5mL eppendorf tubes for ~5 minutes. Large debris was pelleted from homogenized feces by centrifugation at 200rpm (4g) for 20min in a benchtop centrifuge. Bacteria were cultured on agar plates following serial dilution of the resulting supernatant. Enumeration by colony counting and analysis of switching by comparison of blue (lac+, ON) and white (lac−, OFF) colony counts was achieved by plating on LB + 60μg/mL x-gal (Santa Cruz Biosciences) agar with 300μg/mL streptomycin sulfate (Sigma), 34 μg/mL chloramphenicol (Sigma) or a combination of both drugs. All mice were pre-screened for the presence of resistant colonies in feces pre bacterial administration. At least 250 colonies were counted per sample and where this was not possible results were excluded from switching quantification.
LCN-2 quantification was undertaken using the Mouse lipocalin-2/NGAL DuoSet ELISA kit (R&D Systems), using the manufacturer recommended protocol. Samples for ELISA were prepared as previously described22, briefly, involving homogenization of 100mg/mL fecal pellets in PBS + 0.1% Tween20 by vortexing for 20 minutes at 4°C, followed by spinning at 12,000g for 10 minutes at 4°C. Clear supernatant was diluted at least 10 fold for quantification by ELISA. ELISA results were obtained on a Victor3V plate reader (Perkin Elmer) with 450/8nm and 540/8nm absorbance filters. For analysis, absorbance corrected values were interpolated from a sigmoidal four parameter logistic standard curve using Prism 6 for Mac OS X software (GraphPad).
At the conclusion of the experiments, where required, mice were sacrificed and dissected, their bowel removed and fixed whole in Bouin’s fixative (Sigma). Fixed tissues were embedded in paraffin, sectioned and stained with hematoxylin and eosin. Scoring of severity of inflammation was undertaken on single longitudinal sections of whole gut in a blinded fashion by a trained rodent histologist (RTB).
At least 4 mice were used per experimental group, to which mice were assigned randomly where relevant. This sample size was estimated as a minimum based on its power to detect whether PAS638 had an overall detection capacity (a factor of both bacterial sensitivity and variability of mouse models) of ~47% with 95% confidence. These calculations treated the occurrence of above-threshold tetrathionate as a dichotomous variable given PAS638’s low false positive rate in vitro and in pilot in vivo experiments. For inflammation models determined to have higher variability or lower penetrance, larger sample sizes were used accordingly.
Streptomycin treated Salmonella colitis model
The streptomycin-treated Salmonella colitis model was undertaken in C57Bl/6 (Charles River or Jackson laboratories) and B6.129S-Cybbtm1Din (Jackson laboratories) mice as previously described21. Briefly, mice were administered with 20mg USP grade streptomycin sulfate (Gold Biotechnology) by oral gavage following 4h nil per os (NPO). 24 hours later, again following 4h NPO, mice were administered bacterial strains, resuspended from overnight culture in phosphate buffered saline (Gibco), by oral gavage. Bacteria were administered at ~1×107 E. coli and ~1×108 S. typhimurium per mouse as previously described21
Selective plating for enumeration of S. typhimurium strains was achieved on M9 + 0.4% glucose + 30μg/mL tetracycline (Sigma) agar. Enumeration and analysis of switching was achieved using plating on LB + 300μg/mL streptomycin sulfate (Sigma) + 60μg/mL x-gal (Santa Cruz Biosciences) agar.
IL10 knockout model
For initial experiments, male retired breeder mice (~6 months age) from an ILIO−/− background33 were transferred to barrier SPF conditions, following growth under gnotobiotic conditions. The mice were colonized with a complex set of human commensal microbes, Gnotocomplex 2.0 (Supplementary Table 2), which is an expanded version of the Gnotocomplex designed to capture additional functionality and phylogenetic diversity34. Control mice were male retired breeder mice (typically 6–8 months age) from a C57Bl/6 background raised in SPF conditions (Charles river). Mice were administered PAS638 bacteria (~3×10^7 per mouse) by oral gavage following USP streptomycin sulfate treatment (Gold Biotechnology; 0.5g/L in drinking water). In mice where colonization was lower than 2.5×104 CFU/g following gavage, streptomycin was re-administered within the first week following bacterial administration for up to 48h to assist robust colonization.
Analysis of fecal samples was undertaken as described above. To avoid adverse influence of streptomycin and bacterial administration in IL10−/− mice, which are sensitive to aberrant inflammatory responses (as evident from LCN-2 quantification in week 1 post administration (Fig. 4b)), blue-white colony screening was not undertaken in the week following administration. Results were generated from 3-4 fecal samples taken over the subsequent 2 weeks for control C57Bl/6 mice and ~1 month for IL10−/− mice.
Il10−/− studies were also undertaken in B6.129P2-Il10tm1Cgn/J with administration of PAS638 occurring without streptomycin pre treatment.
Tetrathionate quantification by mass spectrometry
Tetrathionate was quantified from extracts prepared from whole mouse cecum. Following dissection, the cecum was cut open and cecal contents were carefully removed from the underlying tissue and mucous layer. The mucosa was scraped with a blunt spatula and this was added to 500uL of water, which was additionally used to wash the cecal tissue by pipetting vigorously over the surface approx. 10 times. The remaining cecal tissue was then added to the same tube, and tissue and debris was removed by centrifugation at 10,000 g for 10 minutes. Supernatants were then filter sterilized using 13mm 0.20 μm, Hydrophilic, PTFE syringe filters (Millex-LG) and frozen at −80°C prior to quantification.
HPLC-MS/MS analysis of standards and extracts was carried out using an Agilent 1260 Infinity HPLC system equipped with an Agilent Eclipse Plus C18 column (100 × 4.6 mm, particle size 3.5 mm, flow rate: 0.5 mL/min, solvent A: 10 mM ammonium acetate in dd.H2O, solvent B: acetonitrile, injection volume: 5 mL) connected to an Agilent 6530 Accurate-Mass Q-TOF instrument. The following gradient was used (time/min, %B): 0, 0; 1, 0; 2, 95; 6, 95; 7, 0, 9, 0. The mass spectrometer was operated in negative mode and the autosampler was kept at 4°C. MS-fragmentation experiments were carried out using collision induced dissociation (CID) fragmentation with nitrogen as the collision gas at 10 eV. By comparing fragmentation patterns of standards and samples, tetrathionate could be unequivocally identified.
The following fragments could be detected: 224.8660 ([S4O6H]-, parent ion), 144.9078 ([S3O3H]-) and 80.9652 ([SO3H]-) (Supplementary Fig. 5).
To quantitate tetrathionate levels in extracts, a standard curve was recorded using freshly prepared Sodium Tetrathionate (Sigma) standards in distilled water (0.0001, 0.001, 0.01, 0.1 and 1 mM). Samples were kept on ice or at 4°C. After HPLC-MS/MS analysis, EIC peaks (224.84-224.88 Da) were automatically integrated using the MassHunter Workstation Software (version: B.07.00). A log-log plot of peak area versus tetrathionate concentration was used to generate a linear fit.
Whole Genome Sequencing and analysis
Bacterial samples were selected by plating from feces, as described above, on LB + 300μg/mL streptomycin sulfate (Sigma) + 60μg/mL x-gal (Santa Cruz Biosciences) agar. Colonies were scraped from plates, resuspended and prepared for sequencing as a pool using a small-volume modification of the Illumina Nextera XP kit as described previously35. Sequencing was performed on the Illumina Miseq platform, using paired end 50-bp reads. Raw sequence reads are available with links to NCBI BioProject accession number PRJNA380756.
Alignment and base calling of the ancestral sample was done via BreSeq36 using default options, aligning to E.coli NGF-1 (NCBI references NZ_CP016007.01, NZ_CP016008.01 and NZ_CP016009.01) and E. coli MG1655 (NCBI reference NC_000913.3) for sequences flanking engineered elements, which were cloned in E. coli MG1655 and transferred to the strain by P1 transduction. Breseq’s gdtools package was used to modify the ancestral genbank file to eliminate mutations in the ancestral strain. We then ran breseq at high sensitivity (settings-consensus-minimum-coverage-each-strand 3 –consensus-frequency-cutoff 0.1) to call mutations in the passaged strains. Genomic coverage is as reported by breseq, As the number of reads at any given point is high, the chance of not seeing a mutation that occurs at x% of the population in at least k reads can be computed directly via Poisson approximation, i.e. , so for the case of a mutation in 10% of the population, at 100× coverage, there is a 99% chance it will appear in at least three independent reads.
Statistical analysis
All statistical analyses were undertaken in Prism 6 or 7 for Mac OS X (GraphPad). Details of individual tests and resulting p-values are included in figure legends and text where appropriate.
Supplementary Material
Acknowledgments
We thank A. Graveline and L. Bry for discussions and assistance with mouse experiments and A. Verdegaal for experimental assistance. S. typhimurium TT22470 was a gift from J. Roth. We thank Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the Rodent Histopathology Core, which provided histology preparation service. Dana-Farber/Harvard Cancer Center is supported in part by a NCI Cancer Center Support Grant # NIH 5 P30 CA06516. DTR was supported by a Human Frontier Science Program Long-Term Fellowship and an NHMRC/RG Menzies Early Career Fellowship from the Menzies Foundation through the Australian National Health and Medical Research Council. TWG was supported by a Leopoldina Research Fellowship (LPDS 2014-05) from the German National Academy of Sciences Leopoldina. The research was funded by Defense Advanced Research Projects Agency Grant HR0011-15-C-0094 (PAS) and the Wyss Institute for Biologically Inspired Engineering.
Footnotes
Author Contributions
D.T.R., T.W.G., M.B., S.J.K., G.K.G., J.C.W. and P.A.S. designed experiments. D.T.R. and M.J.N. performed and analyzed in vitro characterization. D.T.R. performed and analyzed all mouse experiments. T.W.G. performed and analyzed tetrathionate mass spectrometry. M.B. performed and analyzed whole genome sequencing. S.J.K. and J.W.K. generated strains and collected preliminary data for the study. R.T.B. performed all histology scoring. D.T.R. and P.A.S. wrote the manuscript.
Competing Financial Interests Statement
The authors declare no competing financial interests.
Accession Codes
PAS638 sequencing reads are available at NCBI SRA (BioSample accession numbers SAMN06671873 - SAMN06671878) linking to BioProject PRJNA380756.
Data Availability
Sequence reads are available at NCBI SRA (BioSample accession numbers SAMN06671873 - SAMN06671878) linking to BioProject PRJNA380756. E. coli NGF-1 assemblies for chromosome and plasmids are available with NCBI references NZ_CP016007.01, NZ_CP016008.01 and NZ_CP016009.01.
References
- 1.Mimee M, Citorik RJ, Lu TK. Microbiome therapeutics - Advances and challenges. Adv Drug Deliv Rev. 2016;105:44–54. doi: 10.1016/j.addr.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clairmont C, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 3.Ceroni F, Algar R, Stan GB, Ellis T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat Methods. 2015;12:415–418. doi: 10.1038/nmeth.3339. [DOI] [PubMed] [Google Scholar]
- 4.Sleight SC, Sauro HM. Visualization of Evolutionary Stability Dynamics and Competitive Fitness of Escherichia coli Engineered with Randomized Multigene Circuits. ACS Synth Biol. 2013;2:519–528. doi: 10.1021/sb400055h. [DOI] [PubMed] [Google Scholar]
- 5.Sleight SC, Bartley BA, Lieviant JA, Sauro HM. Designing and engineering evolutionary robust genetic circuits. J Biol Eng. 2010;4:12. doi: 10.1186/1754-1611-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser F, et al. Genetic circuit performance under conditions relevant for industrial bioreactors. ACS Synth Biol. 2012;1:555–564. doi: 10.1021/sb3000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorochowski TE, van den Berg E, Kerkman R, Roubos JA, Bovenberg RAL. Using Synthetic Biological Parts and Microbioreactors to Explore the Protein Expression Characteristics of Escherichia coli. ACS Synth Biol. 2014;3:129–139. doi: 10.1021/sb4001245. [DOI] [PubMed] [Google Scholar]
- 8.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 9.Archer EJ, Robinson AB, Süel GM. Engineered E. coli that detect and respond to gut inflammation through nitric oxide sensing. ACS Synth Biol. 2012;1:451–457. doi: 10.1021/sb3000595. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, et al. Permanent genetic memory with >1-byte capacity. Nat Methods. 2014;11:1261–1266. doi: 10.1038/nmeth.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farzadfard F, Lu TK. Synthetic biology. Genomically encoded analog memory with precise in vivo DNA writing in living cell populations. Science. 2014;346:1256272. doi: 10.1126/science.1256272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotula JW, et al. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc Natl Acad Sci USA. 2014;111:4838–4843. doi: 10.1073/pnas.1321321111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimee M, Tucker AC, Voigt CA, Lu TK. Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst. 2015;1:62–71. doi: 10.1016/j.cels.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamdar K, et al. Genetic and Metabolic Signals during Acute Enteric Bacterial Infection Alter the Microbiota and Drive Progression to Chronic Inflammatory Disease. Cell Host Microbe. 2016;19:21–31. doi: 10.1016/j.chom.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensel M, Hinsley AP, Nikolaus T, Sawers G, Berks BC. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol Microbiol. 1999;32:275–287. doi: 10.1046/j.1365-2958.1999.01345.x. [DOI] [PubMed] [Google Scholar]
- 17.Winter SE, Lopez CA, Bäumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14:319–327. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price-Carter M, Tingey J, Bobik TA, Roth JR. The alternative electron acceptor tetrathionate supports B12-dependent anaerobic growth of Salmonella enterica serovar typhimurium on ethanolamine or 1,2-propanediol. J Bacteriol. 2001;183:2463–2475. doi: 10.1128/JB.183.8.2463-2475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YW, Denkmann K, Kosciow K, Dahl C, Kelly DJ. Tetrathionate stimulated growth of Campylobacter jejuni identifies a new type of bi-functional tetrathionate reductase (TsdA) that is widely distributed in bacteria. Mol Microbiol. 2013;88:173–188. doi: 10.1111/mmi.12176. [DOI] [PubMed] [Google Scholar]
- 20.Rooks MG, et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014;8:1403–1417. doi: 10.1038/ismej.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chassaing B, et al. Fecal lipocalin 2, a sensitive and broadly dynamic noninvasive biomarker for intestinal inflammation. PLoS ONE. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmonds NJ, Rampton DS. Inflammatory bowel disease–a radical view. Gut. 1993;34:865–868. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keubler LM, Buettner M, Häger C, Bleich A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm Bowel Dis. 2015;21:1967–1975. doi: 10.1097/MIB.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White P, Liebhaber SA, Cooke NE. 129X1/SvJ mouse strain has a novel defect in inflammatory cell recruitment. J Immunol. 2002;168:869–874. doi: 10.4049/jimmunol.168.2.869. [DOI] [PubMed] [Google Scholar]
- 26.Hoover-Plow JL, et al. Strain and model dependent differences in inflammatory cell recruitment in mice. Inflamm Res. 2008;57:457–463. doi: 10.1007/s00011-008-7062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myhrvold C, Kotula JW, Hicks WM, Conway NJ, Silver PA. A distributed cell division counter reveals growth dynamics in the gut microbiota. Nat Commun. 2015;6:10039. doi: 10.1038/ncomms10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Popodi E, Tang H, Foster PL. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc Natl Acad Sci USA. 2012;109:E2774–2783. doi: 10.1073/pnas.1210309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Davis RW, Botstein D, Roth JR. A Manual for Genetic Engineering: Advanced Bacterial Genetics. Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 33.Devkota S, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucci V, et al. MDSINE: Microbial Dynamical Systems INference Engine for microbiome time-series analyses. Genome Biol. 2016;17:121. doi: 10.1186/s13059-016-0980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baym M, et al. Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS ONE. 2015;10:e0128036. doi: 10.1371/journal.pone.0128036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deatherage DE, Barrick JE. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol. 2014;1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


