Abstract
Background
Sepsis-induced immune dysfunction ranging from cytokines storm to immunoparalysis impacts outcomes. Monitoring immune dysfunction enables better risk stratification and mortality prediction and is mandatory before widely application of immunoadjuvant therapies. We aimed to develop and validate a scoring system according to patients’ immune dysfunction status for 28-day mortality prediction.
Methods
A prospective observational study from a cohort of adult sepsis patients admitted to ICU between August 2013 and June 2016 at Kaohsiung Chang Gung Memorial Hospital in Taiwan. We evaluated immune dysfunction status through measurement of baseline plasma Cytokine levels, Monocyte human leukocyte-DR expression by flow cytometry, and stimulated immune response using post LPS stimulated cytokine elevation ratio. An immune dysfunction score was created for 28-day mortality prediction and was validated.
Results
A total of 151 patients were enrolled. Data of the first consecutive 106 septic patients comprised the training cohort, and of other 45 patients comprised the validation cohort. Among the 106 patients, 21 died and 85 were still alive on day 28 after ICU admission. (mortality rate, 19.8%). Independent predictive factors revealed via multivariate logistic regression analysis included segmented neutrophil-to-monocyte ratio, granulocyte-colony stimulating factor, interleukin-10, and monocyte human leukocyte antigen-antigen D–related levels, all of which were selected to construct the score, which predicted 28-day mortality with area under the curve of 0.853 and 0.789 in the training and validation cohorts, respectively.
Conclusions
The immune dysfunction scoring system developed here included plasma granulocyte-colony stimulating factor level, interleukin-10 level, serum segmented neutrophil-to-monocyte ratio, and monocyte human leukocyte antigen-antigen D–related expression appears valid and reproducible for predicting 28-day mortality.
Introduction
In intensive care units (ICUs), infected patients had more than twice mortality rate that of noninfected patients [1]. Infection may cause local organ involvement without generating a dysregulated systemic host response [2]. Sepsis is defined as the presence of an infection together with systemic manifestations [3]. Sepsis complicated by organ dysfunction is termed severe sepsis. Previous study revealed 750,000 patients suffered from severe sepsis annually in the United States (0.3% of total population and 2.26% of hospital discharges)[4] Severe sepsis patients had ICU mortality rate ranged from 21.0–36.7%, and in hospital mortality ranged from 22.0–44.5% based on different patient population and definition of severe sepsis [4–8]. Sepsis, which can be considered a battle between pathogens and a host’s immune system [9], is a life-threatening organ dysfunction due to a dysregulated host response to infection [10]. Immune cells play a critical role in the host’s response to sepsis.
Previous study revealed some patients dying of sepsis have marked immunosuppression [11, 12]. In septic patients, immunological variables behave in a mixed and time-dependent manner [13].
There are several existing scoring systems developed for mortality prediction, such as APACHE II, Sequential Organ Failure Assessment score (SOFA score), and Charlson Index. However, none of these takk immune dysfunction status into account. With a substantial degree of heterogeneity in the sepsis response ranging from cytokines storm to immunoparalysis, better patient stratification is needed [14]. Here we aimed to develop and validate a scoring system that can determine patients’ immune dysfunction status related to outcomes.
Materials and methods
Patient enrollment
This prospective observational study was conducted at Kaohsiung Chang Gung Memorial Hospital, a 2,700-bed tertiary teaching hospital in southern Taiwan. The study evaluated patients with severe sepsis or septic shock who were admitted to medical ICUs between August 2013 and June 2016. All patients admitted to participating units were screened for eligibility. Patients were enrolled if they agreed to undergo blood sampling during ICU hospitalization. Patients were excluded if they met either of the following criteria: (1) <18 years of age; or (2) Those who had ICU waiting time longer than 24 hours after diagnosis of sepsis. 3) Those who received granulocyte-colony stimulating factor (G-CSF) 1 week prior to ICU admission. All patients received blood sampling at day 1 of ICU admission. The primary outcome was 28-day mortality (day 1 defined as ICU admission day). All patients were followed until discharge from or death in the hospital. Data of the first consecutive 106 septic patients comprised the training cohort, and of other 45 patients comprised the validation cohort.
This study’s design was approved by the institutional review board of Chang Gung Memorial Hospital, and written informed consent was obtained from each patient or a suitable family member.
Definitions
The definition of severe sepsis was first adapted from the 2001 International Sepsis Definitions Conference and the Surviving Sepsis Campaign [15]. All enrolled patients fulfilled the definition of sepsis from Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [16]. We than adapted the new definition. Day 1 was the day of arrival to the ICU.
Plasma and PBMC preparation
Whole blood samples were drawn from all patients on day 1 of ICU admission and collected in heparinized tube (BD, Franklin Lakes, NJ, USA). All patients received blood sampling on day 1 of ICU admission. Using a Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden), whole blood was centrifuged at 400 g × 30 min to separate the plasma and PBMCs. All of the PBMC samples were treated immediately and the plasma samples were stored at -80°C until use. Fresh PBMCs were aliquoted into two parts: one that was used for the monocyte human leukocyte antigen D related (HLA-DR) expression measurement and another that was used for the cell culture.
Monocyte HLA-DR expression measurement by flow cytometry
HLA-DR–related monocyte expression was measured by flow cytometry (Cytomics FC500; Beckman Coulter, Inc., Fullerton, CA, USA). Staining and cell acquisition for flow cytometry were performed within 1 hour after the blood sampling. Monoclonal antibodies were used as follows: CD14-PerCP/Cy5.5 (clone: HCD14; Biolegend, San Diego, CA, USA), HLA-DR-FITC (clone: L243; Biolegend) per 100 μL of PBMC blood. Negative controls were mouse monoclonal antibodies IgG1, PerCP/Cy5.5 (clone: MOPC-21), IgG2a, FITC (clone: MOPC-173), and IgG2a, PE (clone: MOPC-173), all of which were isotype-matched as recommended by the manufacturer. Monocytes were characterized based on their CD14 expression. At least 30,000 PBMCs were analyzed from each sample. The results are expressed as percentages of HLA-DR–positive monocytes of the total monocyte population.
Cell culture
PBMCs (1 × 106) were plated in a 5-mL round-bottom polystyrene test tube (BD Falcon, Bedford, MA USA) with 2 mL of sterile DMEM culture medium (Gibco, Grand Island, NY, USA) containing 1% heat-inactivated fetal bovine serum (Gibco), 1 mM L-glutamine, and 1 mM sodium pyruvate. Inflammation was induced using LPS 100 ng/mL (Sigma, St. Louis, MO, USA) or not. The tubes were incubated at 37°C in 5% CO2 for 4 hours. Samples of the conditioned media were analyzed for cytokine expression levels.
Milliplex assay
Cytokine levels of plasma and conditioned media including G-CSF, interleukin (IL)-10, IL-6, and tumor necrosis factor-α (TNF-α) were quantified using a Human Cytokine/Chemokine Magnetic Bead Panel customized Milliplex MAP kit (#HCYTOMAG-60K, EMD Millipore, Darmstadt, Germany). The assay was performed according to the manufacturer’s instructions. Standards and samples were analyzed on a MAGPIX System device (Millipore) by MILLIPLEX® Analyst 5.1 software using a five-parameter logistic curve fitting model.
Statistical analyses
Statistical analyses were performed using MedCalc (version 14.10.2). Categorical variables were compared using the chi-square test or Fisher’s exact test where appropriate, while continuous variables were analyzed using Student’s t-test or the Mann–Whitney U test where appropriate. A receiver operating characteristic (ROC) curve and Youden's index was used to determine the best cut-off values of the prognostic factors.
Multivariate analyses for independent prognostic factors selection were performed using backward elimination of logistic regression analysis.
These independent prognostic factors were included in the scoring system. Regression coefficient was used to weight the score of each factor. The Kaplan-Meier method and the log-rank test were used to determine the effect of the immune dysfunction scores on patient survival. Kruskal-Wallis test was used for assessing the association between post LPS stimulation immune response and immune dysfunction score. P values <0.05 were considered statistically significant.
Immune dysfunction score construction
The risk factors identified by multivariate analysis weighted points proportional to the β regression coefficient values were summed to calculate a risk score for each patient.
Patients were re-grouped into high, medium, and low immune dysfunction levels based on different value of immune dysfunction score.
Results
Patient characteristics
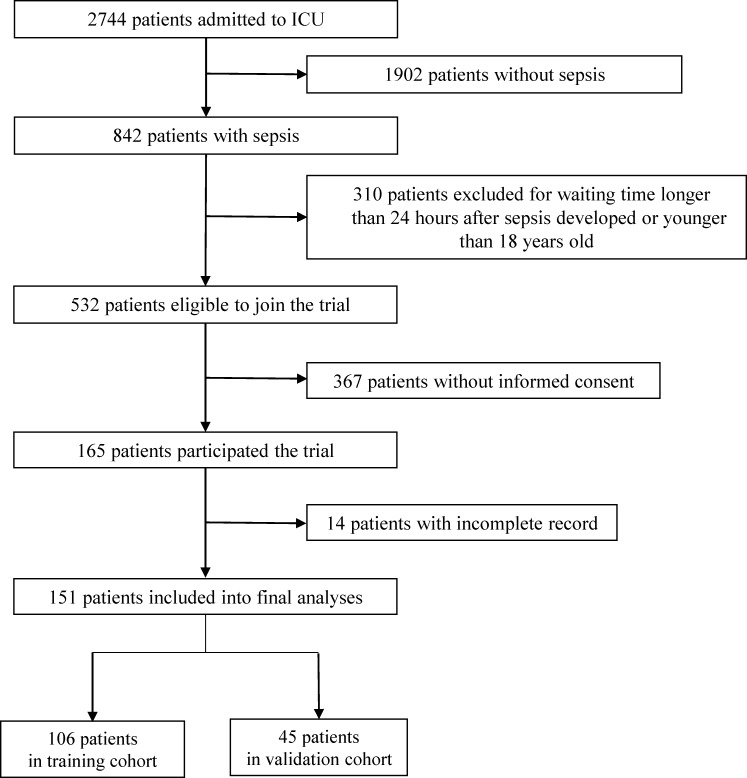
A total of 2744 patients were admitted into ICUs between August 2013 and June 2016. A total of 824 patients met the criteria for diagnosis with sepsis [16]; of them, 151 were enrolled (Fig 1). The first consecutive 106 septic patients were included in the training cohort to analyze the model construction, while the other 45 patients were assigned to the validation cohort. Among the two populations, 21 of 106 (19.8%) patients in the training cohort and 9 of 45 patients (20%) in validation cohort died within the first 28 days of ICU admission. The patients’ baseline characteristics are presented in Table 1. There were comparable severity levels and co-morbidity rates between survival and non-survival groups. However, there were significant intergroup differences regarding SOFA score as well as segmented neutrophil-to-monocyte ratio (SeMo ratio) (Table 1). Demographics and clinical characteristics between the training and validation cohorts are presented in S1 Table.
Fig 1. Patient recruitment and assignment.
Table 1. Baseline clinical hematological and biochemical parameters, parameters, SOFA score, and immune profiles of the study patients.
| All (N = 106) |
Non-survivor (n = 21) |
Survivor (n = 85) |
P value |
|
|---|---|---|---|---|
| Clinical parameters | ||||
| Age (years), mean (SD)a | 68.22 (15.7) | 68.19 (14.5) | 68.23 (16.2) | 0.834 |
| APACHE II score, mean (SD) | 26.78 (8.5) | 28.55 (8.3) | 26.36 (8.6) | 0.269 |
| Male, n (%)b | 61 (57) | 11 (52) | 50 (58) | 0.593 |
| Body mass index, mean (SD) | 23.24 (5.1) | 24.75 (4.7) | 22.86 (5.5) | 0.078 |
| Charlson index, mean (SD) | 2.42 (1.5) | 2.7 (1.5) | 2.35 (1.6) | 0.104 |
| Cardiovascular disease, n (%) | 32 (30) | 6 (29) | 26 (31) | 0.857 |
| Hypertension, n (%) | 64 (60) | 15 (71) | 49 (58) | 0.248 |
| COPD, n (%) | 18 (17) | 2 (10) | 16 (19) | 0.309 |
| Asthma, n (%) | 5 (5) | 1 (5) | 4 (5) | 0.991 |
| Pulmonary tuberculosis, n (%) | 4 (4) | 1 (5) | 3 (4) | 0.791 |
| Cancer, n (%) | 15 (14) | 3 (14) | 12 (14) | 0.984 |
| Diabetes mellitus, n (%) | 55 (52) | 13 (62) | 42 (49) | 0.305 |
| Stroke, n (%) | 24 (23) | 5 (24) | 19 (22) | 0.886 |
| Chronic kidney disease, n (%) | 28 (26) | 8 (38) | 20 (24) | 0.175 |
| Baseline SOFS score, hematological and serum biochemical analyses | ||||
| SOFA score, mean (SD) | 9.45 (3.6) | 12.09 (4.4) | 8.8 (3.15) | 0.001 |
| WBC, 1000/μL, mean (SD) | 16.27 (8.5) | 18.61 (10.3) | 15.70 (7.9) | 0.290 |
| SeMo ratio, mean (SD) | 30.97 (50.3) | 23.75 (28.30) | 32.69 (54.2) | 0.046 |
| C-reactive protein, mg/L, mean (SD) | 171.79 (128.0) | 220.02 (152.0) | 160.61 (120.0) | 0.208 |
| Procalcitonin, ng/mL, mean (SD) | 23.93 (49.7) | 29.81 (49.0) | 22.43 (50.0) | 0.487 |
| Immune profiles | ||||
| G-CSF pg/mL, mean (SD) | 63.09 (104.8) | 94.74 (232.5) | 53.09 (99.13) | 0.033 |
| IL-10, pg/mL, mean (SD) | 15.25 (58.05) | 82.78 (147.26) | 10.62 (31.6) | 0.010 |
| IL-6, pg/mL, mean (SD) | 43.31 (84.3) | 91.16 (473.29) | 33.82 (80.8) | 0.004 |
| TNF-α pg/mL, mean (SD) | 31.15 (36.5) | 39.13 (87.8) | 28.46 (34.8) | 0.048 |
| HLA-DR expression %, mean (SD) | 90.30 (18.4) | 87.1 (39.9) | 92.05 (11.1) | 0.030 |
a continuous variables were analyzed using Mann–Whitney U test.
b Categorical variables were compared using the chi-square test or Fisher’s exact test where appropriate.
Abbreviations: COPD, Chronic obstructive pulmonary disease; G-CSF, granulocyte-colony stimulating factor; HLA-DR, human leukocyte antigen D–related; IL, interleukin; SeMo, segmented neutrophil-to-monocyte; SOFA, Sequential Organ Failure Assessment score.
Immune status and plasma cytokine expression
Among 106 patients in the training cohort, 2 patients had missing data about SeMo ratio, one had no baseline monocyte HLA-DR expression level. In non-survival patients, there were decreased median baseline HLA-DR monocyte expressions measured by flow cytometry compared to survival patients. Meanwhile, there were significant intergroup differences in G-CSF, IL-10, IL-6, and TNF-α expressions (Table 1).
Immune dysfunction score
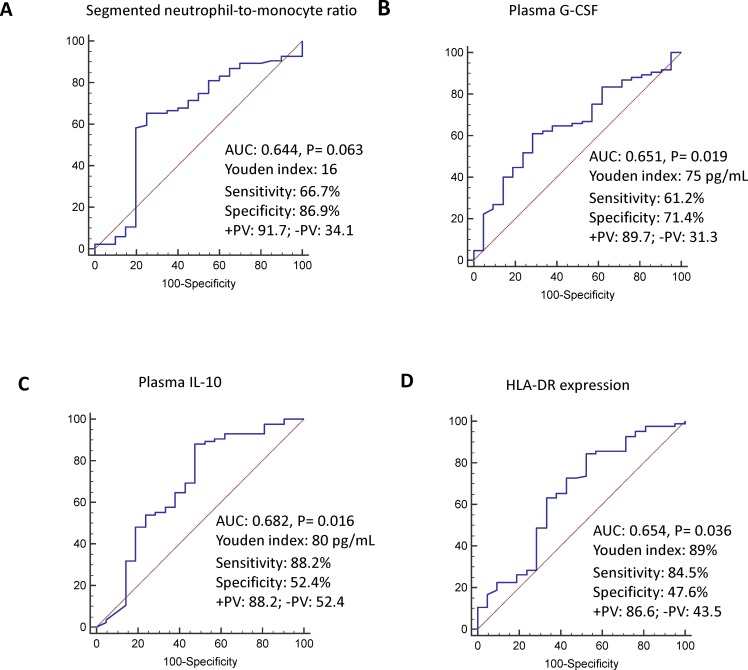
A ROC curve was used to examine the clinical and immune parameters that were statistically significant on univariate analysis to determine the best cut-off point for 28-day mortality prediction. The best cut-off points for SOFA score, SeMo ratio, and G-CSF, IL-10, IL-6, TNF-α, and monocyte HLA-DR expression were 10, 16, 75 pg/mL, 80 pg/mL, 41 pg/mL, 36 pg/mL, and 89%, respectively. Independent predictive factors identified on multivariate logistic regression analysis included SeMo ratio, G-CSF, IL-10, and monocyte HLA-DR expression (Table 2). The ROC curves for SeMo ratio, G-CSF level, IL-10 level, and monocyte HLA-DR expression had areas under the curve (AUC) of 0.644 (95%CI: 0.544–0.736), 0.651 (95%CI: 0.552–0.741), 0.682 (95%CI: 0.584–0.769), and 0.654 (95%CI: 0.555–0.744), respectively (Fig 2).
Table 2. Multivariate analyses for immune dysfunction score parameter selectiona.
| Regression coefficient | P value | Risk score | |
|---|---|---|---|
| SeMo ratio | 0.004 | ||
| >16 | Reference | 0 | |
| ≤16 | 1.337 | 1 | |
| G-CSF plasma level, pg/mL | 0.007 | ||
| >75 | 1.304 | 1 | |
| ≤75 | Reference | 0 | |
| IL-10 plasma level, pg/mL | <0.001 | ||
| >80 | 1.853 | 2 | |
| ≤80 | Reference | 0 | |
| HLA-DR expression % | 0.029 | ||
| >89 | Reference | 0 | |
| ≤89 | 1.248 | 1 |
aMultivariate analyses for independent prognostic factors selection were performed using backward elimination of logistic regression analysis.
Abbreviations: G-CSF, granulocyte-colony stimulating factor; IL, interleukin; HLA-DR, human leukocyte antigen D–related; SeMo, segmented neutrophil-to-monocyte
Fig 2.
Receiver operating characteristic curve for discriminating between 28-day survivors and non-survivors with sepsis in the intensive care unit using plasma segmented neutrophil-to-monocyte ratio (A), granulocyte-colony stimulating factor level (B), Interleukin-10 level (C), and monocyte human leukocyte antigen-antigen D–related expression (D). The sensitivity and specificity were determined at best cutoffs determined by Youden index.
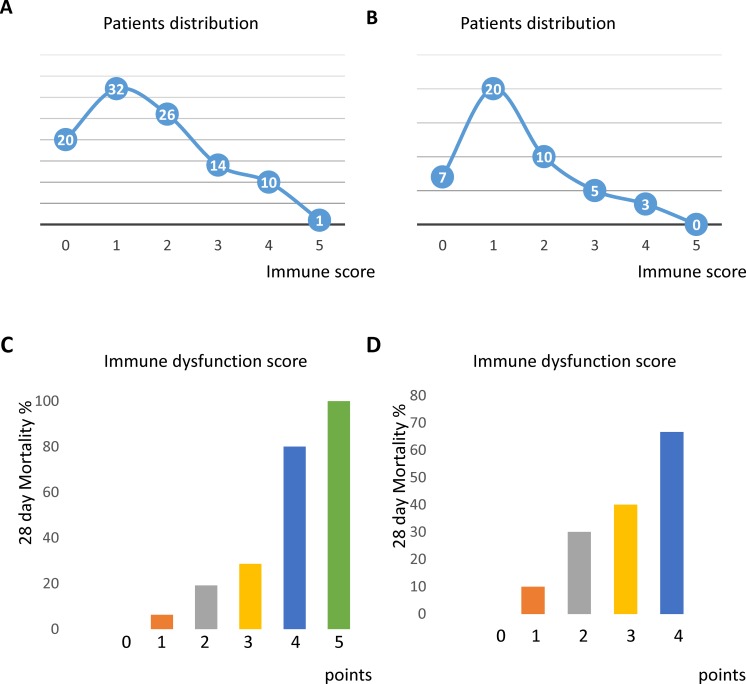
These independent risk factors associated with 28-day mortality were selected to construct the score in 103 patients in training cohort (Table 2). The patient distributions of immune dysfunction score of the 103 patients in the training cohort (Two patients had missing data about SeMo ratio, one had no baseline monocyte HLA-DR expression level) and 45 patients in the validation cohort are shown in Fig 3A and 3B, respectively. The 28-day mortality rates for patients at the dysfunction scores are shown in Fig 3C. (training cohort) and Fig 3D. (validation cohort).
Fig 3.
Patient distributions in the training (n = 103) (A) and validation (n = 45) (B) cohort. Immune dysfunction score and 28-day mortality rate in the training (C) and validation (D) cohort.
The score predicted 28-day mortality with an AUC of 0.853 in the training cohort (Fig 4A) that was validated in the validation cohort (Fig 4B). Patients were re-grouped into high (score 4–5), medium (score 2–3), and low (score 0–1) immune dysfunction levels. The Kaplan-Meier survival analyses of the low, medium, and high immune dysfunction levels are shown in Fig 4C, and the results were validated in the validation cohort (Fig 4D).
Fig 4.
Receiver operating characteristic curve for discriminating between 28-day survivors and non-survivors with sepsis in the intensive care unit of the training (n = 103) (A) and validation (n = 45) (B) cohort. (C) Kaplan-Meier survival analyses of overall survival rates of patients with high, medium, and low immune dysfunction scores in the training cohort. (n = 103) (D) Overall survival rates of patients with high, medium, and low immune score in the validation cohort. (n = 45).
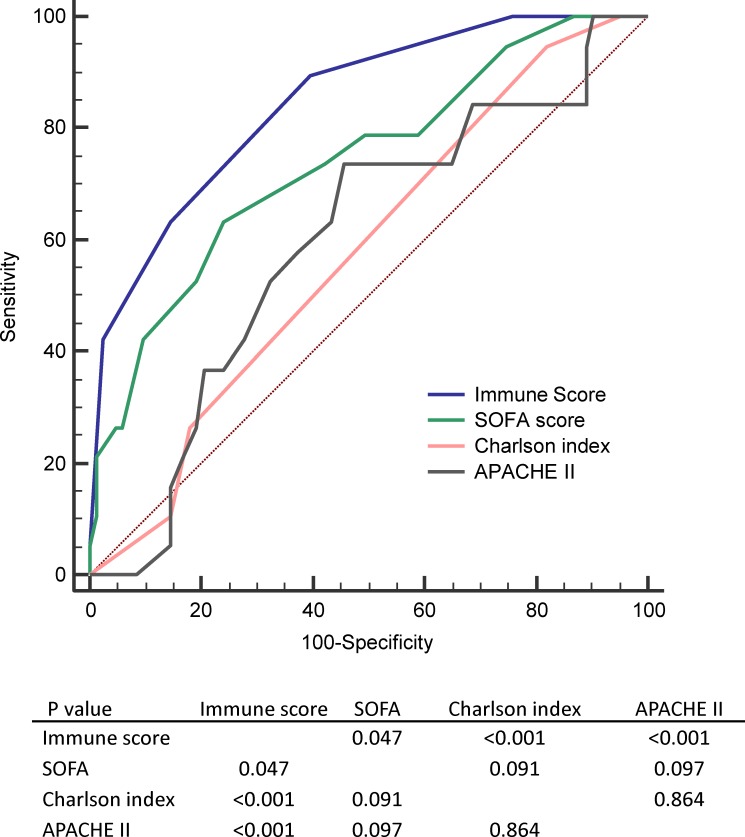
Performance measures of different scores including immune dysfunction score, SOFA score, Charlson index and APACHE II for 28-day mortality prediction were shown in Table 3 and Fig 5.
Table 3. Measure of performance predicting 28-day mortality by using different scoring systems(n = 102) a.
| Sensitivity | Specificity | +LR | -LR | AUC | |
|---|---|---|---|---|---|
| Immune score | 65.0 | 85.54 | 4.50 | 0.41 | 0.846 (0.751–0.940) |
| SOFA | 61.9 | 75.29 | 2.51 | 0.51 | 0.739 (0.611–0.867) |
| Charlson index | 95.0 | 18.82 | 1.17 | 0.27 | 0.578 (0.460–0.734) |
| APACHE II | 71.43 | 55.29 | 1.60 | 0.52 | 0.597 (0.446–0.710)) |
a One patient had no baseline Charlson index.
Fig 5. Receiver operating characteristic curve for comparing 28-day mortality prediction performance by using different scoring system in training cohort.
Stimulated immune response and immune dysfunction score
The stimulated immune response was evaluated using the cytokine elevation ratio, which was calculated by dividing the post–LPS-stimulated cytokine level by the pre–LPS-stimulated cytokine level. Of the 106 patients, 75 patients received stimulated immune response test.
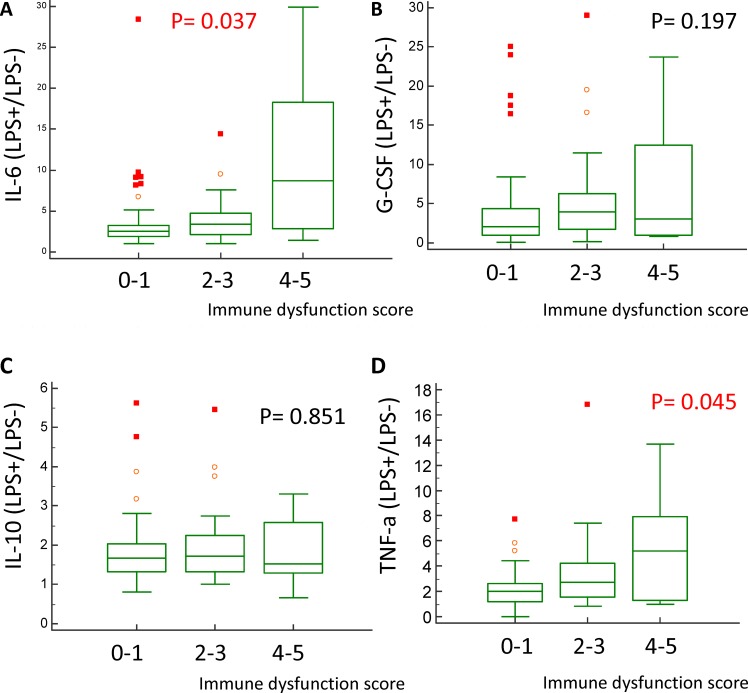
The 28-day survivors had lower IL-6 and TNF-α elevation ratios after LPS stimulation than the non-survivors (Table 4). Patients with a higher immune dysfunction score also had higher IL-6 and TNF-α elevation ratios after LPS stimulation (Fig 6). Stimulated IL-10 and G-CSF ratios were not associated with 28-day mortality and immune dysfunction score.
Table 4. Ratio of immune response after LPS stimulation and 28-day mortality a.
| All (N = 75) Median (IQR) |
Non-survivors (n = 14) Median (IQR) |
Survivors (n = 61) Median (IQR) |
P value |
|
|---|---|---|---|---|
| G-CSF | 2.06 (3.85) | 3.70 (4.99) | 2.01 (2.87) | 0.230 |
| IL-10 | 1.60 (0.78) | 1.42 (1.17) | 1.65 (0.73) | 0.669 |
| IL-6 | 2.68 (2.69) | 4.60 (5.98) | 2.62 (1.70) | 0.017 |
| TNF-α | 2.09 (1.95) | 2.85 (4.56) | 2.05 (1.55) | 0.027 |
a Kruskal-Wallis test was used for assessing the association between post LPS stimulation immune response and immune dysfunction score.
G-CSF, granulocyte-colony stimulating factor; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor-α
Fig 6. Association between post lipopolysaccharide stimulation immune response and immune dysfunction score 0–1, 2–3, 4–5 in training cohort using Kruskal-Wallis test (n = 75).
Interleukin -6 (A), granulocyte-colony stimulating factor (B), interleukin -10 (C), and tumor necrosis factor-α (D).
Discussion
In our study, baseline immune parameters including decreased monocyte HLA-DR expression, higher plasma G-CSF level, higher plasma IL-10 level, and lower serum SeMo ratio were independent predictors of 28-day mortality in sepsis patients.
The immune response in patients with sepsis ranges from an exuberant pro-inflammatory cascade to a profoundly immunosuppressed phenotype [17]. The proper balance between the competing pro- and anti-inflammatory pathways determines the outcomes of patients with sepsis. The identification of immunoparalysis from immune storm is potentially important before widely application of immunomodulation therapies [18].
Decreased monocyte HLA-DR expression measured by flow cytometry is currently the best marker for monitoring immune alterations in critically septic patients [19]. The maladaptive immune dysfunction in patients with sepsis manifests across a range of cellular actions and functions that involve the innate and adaptive arms of the immune system. Defects have been noted in neutrophils and monocytes [20]. The effects of protracted sepsis on the innate immune system in macrophages include increased anti-inflammatory cytokine secretion, decreased anti-inflammatory cytokine secretion, decreased HLA-DR expression, and decreased pathogen killing. Higher monocyte counts contribute to improved outcomes after both PBMC and umbilical cord blood transplantations [21]. Monocytes may contribute to secondary injury after intracerebral hemorrhage [22]. In neutrophils, sepsis-related effects include immature neutrophil release, increased IL-10 secretion, and others [9]. Neutrophils are essential to the early control of invading pathogens. Neutrophil apoptosis is delayed during sepsis [23]. Infection control requires the efficient migration of neutrophils to the site of infection. The insufficient number of neutrophils recruited to the site of infection does not control the infection locally, contributing to the systemic spread of the pathogen [24].
Our results revealed that the SeMo ratio is an independent risk factor that can predict 28-day mortality. Monocytes from septic patients typically exhibit a diminished capacity to release pro-inflammatory cytokines such as TNF-α and IL-6, whereas the release of anti-inflammatory mediators such as IL-10 is neither impaired nor enhanced [9]. Although anti-inflammatory therapy (e.g., IL-10) makes sense during the initial hyperinflammatory phase, immune stimulation by the administration of monocyte-activating cytokines (interferon-γ, GM-CSF) may be useful during “immunoparalysis” [25]. GM-CSF therapy facilitates rapid recovery of immunoparalysis and prevents nosocomial infection [26, 27]. Clinical trials aimed at downregulating these mediators using antibodies against endotoxin have been uniformly disappointing. One of the reasons for such failure may be the lack of precise immunological parameters [28].
IL-10 overexpression on sepsis day 1 is suggestive of the overt anti-inflammation that is predictive of poor outcome. There are divergent subtypes within the heterogeneous syndrome of sepsis [17]. Most sepsis deaths in the ICU occur after a prolonged course, which is difficult to reproduce in animal models [29]. Sepsis-induced immune suppression leads to increased susceptibility to secondary infections with associated late mortality [30].
We also used immunologic monitoring by evaluating the monocyte cytokine production in patients with sepsis after endotoxin stimulation. Although these techniques involve in vitro analysis and are not widely available, this may help us understand immune dysfunction severity [31, 32]. In our series, patients with higher monocyte IL-6 and TNF-α production after re-exposure to LPS had higher 28-day mortality rates and immune dysfunction score. There was no association between IL-10, G-CSF expression and 28-day mortality rates as well as immune dysfunction score. As pro-inflammatory cytokines, serum and plasma TNF-α and IL-6 levels have been shown to increase significantly among patients with sepsis, particularly in those who are culture-positive [33, 34]. Some previous studies revealed immunosuppression consequent to monocyte desensitization to endotoxin that led to fatal outcomes [35]. However, these studies were mainly conducted in animals. Our study, unlike previous studies, revealed that patients with proinflammatory cytokine overproduction after LPS re-exposure had higher 28-day mortality rates [13, 36–38]. Inappropriately suppressed baseline levels of IL-6 and TNF-a in the unstimulated PBMCs may consequent to higher mortality rate.
The main strength of this study is its relatively large sample size with complete cytokine data and clinical correlation. However, there are several limitations worth noting. These limitations include selection bias, since all patients were recruited from a single medical center, and patient heterogeneity with regards to the sepsis source. Although patients with different sepsis sources are included in this analysis, the heterogeneity is reflective of the diverse phenotype of sepsis patients in the clinical setting. Furthermore, despite the differences in sepsis source among the patients studied, our immune dysfunction was able to be validated in a separate cohort, albeit relatively small. It is clear that a larger, more robust sample of patients will be required in the future for validation purposes to ensure the generalizability of the immune dysfunction scoring system to a broader patient population. In this study, patients with ICU waiting times longer than 24 hrs were excluded. Therefore, whether our results can be applied to patients with longer ICU waiting times has yet to be determined. Finally, the current inaccessibility to plasma cytokine levels and monocyte HLA-DR expression in the routine clinical setting prevents the widespread applicability of the immune dysfunction score. However, with further technological advancements and decreased costs in the future, it may be possible to incorporate these biomarkers into the clinical setting for risk stratification.
We are now also exploring the effects of dynamic immune status on other important outcomes in addition to 28-day mortality that are beyond the scope of this study.
Conclusion
The immune dysfunction scoring system developed here incorporates plasma G-CSF level, IL-10 level, serum SeMo ratio, and monocyte HLA-DR expression and appears valid and reproducible for predicting 28-day mortality.
Supporting information
(DOCX)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work is supported in part by grants from the Chang Gung Memorial Hospital Grant (CMRPG8B1061, CMRPG8B1062, CMRPG8B1063, CMRPG8C0551, and CMRPG8C0052 to WF Fang; CMRPG8B1071 to 73 to YH Wang; CMRPG8B1081 to 83 to CC Wang) and a grant from Taiwan Ministry of Science and Technology (MOST 104-2314-B-182A-123-) to WF Fang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. Epub 2009/12/03. doi: 10.1001/jama.2009.1754 . [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. Epub 2016/02/24. doi: 10.1001/jama.2016.0287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af . [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. . [DOI] [PubMed] [Google Scholar]
- 5.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342:d3245 Epub 2011/06/15. doi: 10.1136/bmj.d3245 ; PubMed Central PMCID: PMC3113333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, Abraham E. The last 100 years of sepsis. Am J Respir Crit Care Med. 2006;173(3):256–63. doi: 10.1164/rccm.200510-1604OE . [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. Epub 2015/09/29. doi: 10.1164/rccm.201504-0781OC . [DOI] [PubMed] [Google Scholar]
- 8.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–2. Epub 2014/05/20. doi: 10.1001/jama.2014.5804 . [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. Epub 2013/11/16. doi: 10.1038/nri3552 ; PubMed Central PMCID: PMCPMC4077177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762–74. Epub 2016/02/24. doi: 10.1001/jama.2016.0288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–605. Epub 2011/12/22. doi: 10.1001/jama.2011.1829 ; PubMed Central PMCID: PMCPMC3361243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–8. doi: 10.1016/S1473-3099(13)70001-X ; PubMed Central PMCID: PMCPMC3798159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez HG, Gonzalez SM, Londono JM, Hoyos NA, Nino CD, Leon AL, et al. Immunological characterization of compensatory anti-inflammatory response syndrome in patients with severe sepsis: a longitudinal study*. Crit Care Med. 2014;42(4):771–80. Epub 2013/12/25. doi: 10.1097/CCM.0000000000000100 . [DOI] [PubMed] [Google Scholar]
- 14.Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med. 2016;4(4):259–71. Epub 2016/02/27. doi: 10.1016/S2213-2600(16)00046-1 ; PubMed Central PMCID: PMCPMC4820667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. Epub 2003/04/12. doi: 10.1097/01.CCM.0000050454.01978.3B . [DOI] [PubMed] [Google Scholar]
- 16.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775–87. Epub 2016/02/24. doi: 10.1001/jama.2016.0289 ; PubMed Central PMCID: PMCPMC4910392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee CS. Opening the Debate on the New Sepsis Definition. Precision Medicine: An Opportunity to Improve Outcomes of Patients with Sepsis. Am J Respir Crit Care Med. 2016;194(2):137–9. Epub 2016/05/12. doi: 10.1164/rccm.201604-0697ED . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med. 2013;187(12):1287–93. doi: 10.1164/rccm.201301-0036CP . [DOI] [PubMed] [Google Scholar]
- 19.Monneret G, Venet F. Monocyte HLA-DR in sepsis: shall we stop following the flow? Crit Care. 2014;18(1):102 Epub 2014/01/08. doi: 10.1186/cc13179 ; PubMed Central PMCID: PMCPMC4056426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conway Morris A, Datta D, Shankar-Hari M, Weir CJ, Rennie J, Antonelli J, et al. Predictive value of cell-surface markers in infections in critically ill patients: protocol for an observational study (ImmuNe FailurE in Critical Therapy (INFECT) Study). BMJ open. 2016;6(7):e011326 Epub 2016/07/20. doi: 10.1136/bmjopen-2016-011326 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Bourgeois A, Peterlin P, Guillaume T, Delaunay J, Duquesne A, Le Gouill S, et al. Higher Early Monocyte and Total Lymphocyte Counts Are Associated with Better Overall Survival after Standard Total Body Irradiation, Cyclophosphamide, and Fludarabine Reduced-Intensity Conditioning Double Umbilical Cord Blood Allogeneic Stem Cell Transplantation in Adults. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2016;22(8):1473–9. Epub 2016/04/28. doi: 10.1016/j.bbmt.2016.04.015 . [DOI] [PubMed] [Google Scholar]
- 22.Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2015;46(8):2302–4. Epub 2015/07/02. doi: 10.1161/strokeaha.115.009880 ; PubMed Central PMCID: PMCPMC4519364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamayo E, Gomez E, Bustamante J, Gomez-Herreras JI, Fonteriz R, Bobillo F, et al. Evolution of neutrophil apoptosis in septic shock survivors and nonsurvivors. J Crit Care. 2012;27(4):415 e1–11. Epub 2011/11/15. doi: 10.1016/j.jcrc.2011.09.001 . [DOI] [PubMed] [Google Scholar]
- 24.Sonego F, Castanheira FV, Ferreira RG, Kanashiro A, Leite CA, Nascimento DC, et al. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Frontiers in immunology. 2016;7:155 Epub 2016/05/21. doi: 10.3389/fimmu.2016.00155 ; PubMed Central PMCID: PMCPMC4844928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, et al. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22 Suppl 4:S474–81. . [DOI] [PubMed] [Google Scholar]
- 26.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37(3):525–32. Epub 2010/12/15. doi: 10.1007/s00134-010-2088-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–8. Epub 2009/07/11. doi: 10.1164/rccm.200903-0363OC . [DOI] [PubMed] [Google Scholar]
- 28.Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000;26 Suppl 1:S124–8. . [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Sherwood ER. Immunology. Getting sepsis therapy right. Science (New York, NY. 2015;347(6227):1201–2. Epub 2015/03/15. doi: 10.1126/science.aaa8334 ; PubMed Central PMCID: PMCPMC4398343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016;315(14):1469–79. Epub 2016/03/16. doi: 10.1001/jama.2016.2691 . [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34(8):1084–93. doi: 10.1086/339549 . [DOI] [PubMed] [Google Scholar]
- 32.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3(6):678–81. . [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Rizvi M. Serum tumor necrosis factor alpha and C-reactive protein in pediatric patients with sepsis and its correlation with microbiologic findings. Indian J Pathol Microbiol. 2010;53(3):494–7. doi: 10.4103/0377-4929.68290 . [DOI] [PubMed] [Google Scholar]
- 34.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119(8):771–8. . [DOI] [PubMed] [Google Scholar]
- 35.Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24(3):273–87. doi: 10.1385/IR:24:3:273 . [DOI] [PubMed] [Google Scholar]
- 36.Skrupky LP, Kerby PW, Hotchkiss RS. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology. 2011;115(6):1349–62. doi: 10.1097/ALN.0b013e31823422e8 ; PubMed Central PMCID: PMCPMC3433833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bone RC, Grodzin CJ, Balk RA. et al. Chest. 1997;112(1):235–43. . [DOI] [PubMed] [Google Scholar]
- 38.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101(1):36–47. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.