Abstract
Aims/Hypothesis
The increasing number of people with dementia and cognitive impairments makes it essential to detect and prevent modifiable risk factors of dementia. This study focuses on type 2 diabetes mellitus, especially on undiagnosed cases and their increased risk of cognitive impairment. Furthermore, the potential of physical activity and social integration to moderate the relation between diabetes and cognitive impairment is assessed.
Methods
We used cross-sectional data from 1299 participants of the Berlin Aging Study II (BASE-II) aged between 60 to 84 years and performed logistic regression models to analyze the association of diabetes status, physical activity, and cohabitation status with poor cognitive performance. Cognitive performance was measured with the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)-Plus test battery.
Results
Undiagnosed diabetes (odds ratio (OR) = 2.12, p = 0.031), physical inactivity (OR = 1.43, p = 0.008) and non-cohabiting (OR = 1.58, p = 0.002) were associated with an increased likelihood of poor cognitive performance. The highest odds were observed in participants who suffered from undiagnosed or insulin-dependent diabetes and, in addition, were inactive (undiagnosed diabetes: OR = 3.44, p = 0.003; insulin-dependent diabetes: OR = 6.19, p = 0.019) or lived alone (undiagnosed diabetes: OR = 4.46, p = 0.006; insulin-dependent diabetes: OR = 6.46 p = 0.052). Physical activity and cohabiting appeared to be beneficial.
Conclusions/Interpretation
Physical activity and cohabitation status moderate the link between diabetes mellitus and cognitive performance. Special attention should be paid to undiagnosed and insulin-dependent diabetes cases, which have a particularly high risk of poor cognitive performance.
Introduction
Cognitive impairments and dementia are among the leading risk factors for disability and death [1–3]. The increase of the number of people living to high ages, when cognitive deficits and related diseases are most prominent, will inevitably lead to an increase in the number of people who have cognitive impairments and dementia. Under the assumption of constant age-specific prevalence, the number of persons with dementia will multiply over the next decades [4]. However, a reduction of age-specific prevalence may substantially diminish the number of affected people [5]. In a meta-analysis Norton and colleagues [6] showed that about one third of all Alzheimer disease (AD) cases are attributable to modifiable risk factors, and that a considerable number of dementia cases could be prevented in the future. As shown by longitudinal analyses, the presence of type 2 diabetes is associated with cognitive dysfunction [7–9] which may be a precursor of mild cognitive impairment (MCI) and dementia. Diabetics have significant lower scores in cognitive test batteries [10] and moderate performance decrements compared to non-diabetics [7, 8]. Especially, the cognitive domains memory, executive function and psychomotor speed have been found to be negatively affected by type 2 diabetes mellitus [9]. Even among non-diabetics, higher glucose levels are associated with an increased dementia risk [11]. There is an increased risk of conversion to dementia in diabetes patients, with a higher risk of conversion to vascular dementia (VaD) than to AD [12, 13]. Diabetics with MCI are more likely to develop dementia or AD than are non-diabetics with MCI [14]. However, effective glycemic control is correlated with a reduced risk of cognitive dysfunction and dementia [15, 16]. The underlying mechanisms between diabetes and dementia do not seem to be monocausal. Pathways via atherosclerosis, microvascular diseases, and the impact of glucose toxicity and insulin resistance of diabetics are suspected of leading to brain pathologies which cause vascular dementia, AD, or mixed forms [17]. Studies reported that the prevalence of diabetes has been increasing over the last decades and a substantial number of people live with undiagnosed diabetes [18–20]. The resulting lack of glycemic control means that undiagnosed diabetes increases the risk of all dementias, AD, and VaD [16].
Another important aspect of a diabetes related life style factor is regular physical activity. Numerous studies have proven the beneficial effects of physical activity on cognition [21–23]. A meta-analysis reported a 1.82-fold increased risk of AD if people were physically inactive [6]. Risks of cognitive impairment or decline were significantly reduced for persons with high levels of physical activity [24].
In addition to physical activity, the cohabitation status and levels of social integration also affect the development of cognitive decline [22, 25]. People who cohabited and/or were married had the lowest risks for MCI, AD, and cognitive decline [26, 27].
In the present study we analyze whether the presence of diabetes mellitus, physical activity, and cohabitation status are correlated with cognitive performance for members of a community-dwelling elderly population. We differentiate between non-diabetics, diabetics treated with oral anti-diabetic medications (ADM), diabetics treated with insulin, untreated diabetics, and undiagnosed diabetics. We hypothesize that diabetics with effective glycemic control have comparable risks of poor cognitive performance when compared to non-diabetics, whereas insulin-dependent diabetics show increased risks of cognitive impairment as they are probably in a later and more severe stage of the disease [28]. In a longitudinal analysis of health claims data, insulin-dependent diabetics had a 60% increased risk of dementia [15]. To identify persons at high risk, we focus on undiagnosed diabetes, which is assumed to pose the highest risks for cognitive impairments as those affected do not know about their disease and do not have good glycemic control. Furthermore, we assume that regular physical activity and cohabiting may moderate the link between diabetes and cognitive performance.
Materials and methods
Data
The Berlin Aging Study II (BASE-II) is an ongoing joint project of various disciplines involving several institutions. The population-based sample of community-dwelling participants, living in the greater metropolitan area of Berlin, Germany, covers numerous ageing-relevant variables [29]. The sample consists of 600 younger individuals ages 20 to 35 and 1600 older individuals ages 60 to 84 (for a detailed description of the study see Bertram and colleagues and Gerstorf and colleagues [29, 30]). All participants gave written informed consent to participation and the Ethics Committee of the Charité-Universitätsmedizin Berlin approved this study (approval number EA2/029/09).
Analytical sample
For our analyses we used data of the older subsample and included only those individuals with complete information on diabetes status, physical activity, cohabitation status, and the neuropsychological test battery, resulting in an analytical sample of 1299 participants.
Measurement of cognitive performance
To measure cognitive performance we used the German version of the neuropsychological test battery CERAD-Plus (Consortium to Establish a Registry for Alzheimer's Disease) [31–33]. The complete test battery was administered to all 1299 participants studied here. The following tests were used to evaluate the cognitive performance of the subjects: Word list learning, word list recall, constructional praxis, recall of constructional praxis, verbal fluency, phonemic fluency, Trail Making Test A and Trail Making Test B. We applied the following standardization to all scales
and reversed the order of the values of Trail Making Tests A and B so that higher values correspond to better test performances. Creating an index that reflects the overall cognitive performance of the participants, we summed up the standardized values of all tests for each person. Previous studies showed that a total CERAD score may differentiate between normal controls and MCI subjects better than the Mini Mental Status Examination (MMSE) because of ceiling effects [34, 35]. In our sample, only two of the 1299 participants had a MMSE score of less than 24 points, which is an established cut-off point for a conspicuously impaired cognitive function. We dichotomized the total CERAD score index and labeled the lowest 25 percent of the distribution as ‘‘poor performance”.
Definition of diabetes mellitus
Study participants were defined as diabetic if one of the following criteria was fulfilled: (1) Subjects listed a diabetes diagnosis in the questionnaire; (2) Intake of an oral anti-diabetic drug or insulin; (3) Glycated haemoglobin (HbA1c) levels over 6.5%; (4) Fasting plasma glucose (FPG) over 126 mg/dL; (5) 2-hour glucose level over 200 mg/dL [36]. The 2-hour test was only administered to people who did not state a diagnosis of diabetes in the questionnaire. Because we know whether anti-diabetic medications were prescribed to participants, we divided them into five categories: Non-diabetics, diagnosed diabetics treated with oral anti-diabetic medications (ADM), diagnosed diabetics treated with insulin, diagnosed diabetics without any medical treatment, and persons with undiagnosed diabetes as of study participation.
Physical activity and cohabitation status
Physical activity was assessed with the Rapid Assessment of Physical Activity (RAPA) questionnaire [37, 38]. We defined subjects who stated “I do 30 minutes or more a day of moderate physical activities, 5 or more days a week” or “I do 20 minutes or more a day of vigorous physical activities, 3 or more days a week” as active.
The cohabitation status was assessed with the question “How do you live?”. We distinguished between people who lived together with a partner or a relative (labeled as “not alone”) and subjects living alone.
Covariates
The following covariates were entered into the statistical analyses: Sex; age in years as metric variable (ranging from 60 to 84); education (high = 12 or more years of education and/or having a higher education entrance qualification vs. low = less than 12 years of education); body mass index (less than 30 kg/m2 vs. 30 kg/m2 or more) [39]; current smoking (smoker vs. non-smoker); self-reported hypertension (yes vs. no), self-reported history of stroke (yes vs. no), self-reported cardiovascular diseases (none vs. at least one cardiovascular disease: coronary heart disease, cardiac insufficiency, peripheral arterial disease, impaired cerebral blood flow, or myocardial infarction); depression (yes vs. no: self-reported or 16 or more points on the CESD-scale [40]); and self-reported dyslipidemia (yes vs. no).
Statistical analyses
We compared the characteristics of the participants by their diabetes status and used one-way ANOVA for continuous variables and χ2 tests for categorical variables. We performed univariate logistic regression models with all used covariates and three multivariate logistic regression models for calculating the odds ratios (OR) of poor cognitive performance. With the exception of age, all independent variables were included as dummy variables. We extended our models by interaction terms in order to test for moderator effects of physical activity and cohabitation status with diabetes mellitus. All analyses were performed using STATA 12.1.
Results
Descriptive results
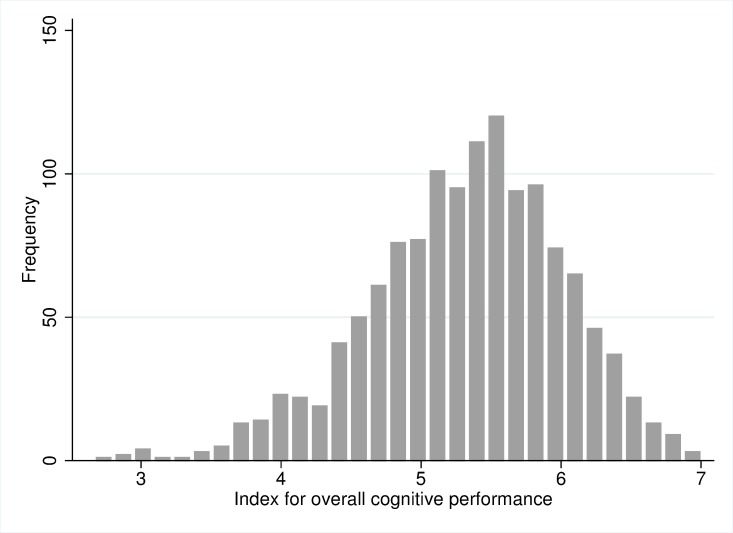
Fig 1 displays the distribution of the CERAD score index, which is approximately normally distributed. The index ranges from 2.6, indicating a low overall cognitive performance, to 7, indicating a high overall cognitive performance. In our sample, 325 persons had a poor cognitive performance (lowest 25%), 974 were defined to have a good cognitive performance. Table 1 portrays the distribution of all independent variables by the cognitive performance of the participants. Of the 659 men and 640 women, 12.7% were diabetics and about half of these (6%) were not being treated with any ADM. 3.1% of the participants did not know about their condition prior to the study participation. 48.3% had an active life style and 61.5% lived together with the partner or a relative. A distinction by diabetes status revealed that the five diabetes groups differ significantly regarding their HbA1c, FPG, and 2-hour glucose levels (Table 2). Post hoc tests using Bonferroni revealed significant mean differences in HbA1c levels between all treatment groups with the exception of untreated and undiagnosed diabetics. Regarding the FPG level, mean differences did not reach statistical significance between untreated diabetics, undiagnosed diabetics and diabetics treated with oral ADM (S1 Table–S3 Table). Insulin-dependent diabetics had highest average HbA1c and FPG levels. The 2-hour glucose level was significantly higher for undiagnosed diabetics compared to non-diabetics. Case numbers for these three parameters differ, as not all values were available for all participants. Untreated and undiagnosed diabetics were most often physically inactive. The five diabetes groups did not differ significantly regarding cohabitation status.
Fig 1. Distribution of the summary index of overall cognitive performance.
Source: BASE-II.
Table 1. Characteristics of the study population of BASE-II, N = 1299.
Source: BASE-II.
| Poor cognitive performance | Good cognitive performance | Total | |||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | |
| Diabetes mellitus | |||||||
| Non-diabetics | 266 | 81.9 | 868 | 89.1 | 1,134 | 87.3 | |
| Treated diabetics, oral ADM | 22 | 6.8 | 46 | 4.7 | 68 | 5.2 | |
| Treated diabetics, insulin | 7 | 2.2 | 12 | 1.2 | 19 | 1.5 | |
| Untreated diabetics | 13 | 4.0 | 25 | 2.6 | 38 | 2.9 | |
| Undiagnosed diabetics | 17 | 5.2 | 23 | 2.4 | 40 | 3.1 | |
| Physical activity | |||||||
| Active | 130 | 40.0 | 497 | 51.0 | 627 | 48.3 | |
| Inactive | 195 | 60.0 | 477 | 49.0 | 672 | 51.7 | |
| Cohabitation status | |||||||
| Not alone | 183 | 56.3 | 616 | 63.2 | 799 | 61.5 | |
| Alone | 142 | 43.7 | 358 | 36.8 | 500 | 38.5 | |
| Sex | |||||||
| Male | 187 | 57.5 | 472 | 48.5 | 659 | 50.7 | |
| Female | 138 | 42.5 | 502 | 51.5 | 640 | 49.3 | |
| Mean age in years (sd) | 68.98 (0.21) | 67.75 (0.11) | 68.06 (0.10) | ||||
| MMSE | |||||||
| <24 | 2 | 0.6 | 2 | 0.2 | 4 | 0.3 | |
| 24–30 | 323 | 99.4 | 970 | 99.6 | 1,293 | 99.5 | |
| Missing | 0 | 0.0 | 2 | 0.2 | 2 | 0.2 | |
| Education | |||||||
| Low | 216 | 66.5 | 471 | 48.4 | 687 | 52.9 | |
| High | 109 | 33.5 | 503 | 51.6 | 612 | 47.1 | |
| Hypertension | |||||||
| No | 159 | 48.9 | 545 | 56.0 | 704 | 54.2 | |
| Yes | 166 | 51.1 | 429 | 44.1 | 595 | 45.8 | |
| Stroke | |||||||
| No | 317 | 97.5 | 954 | 98.0 | 1271 | 97.8 | |
| Yes | 8 | 2.5 | 20 | 2.1 | 28 | 2.2 | |
| Cardiovascular diseases | |||||||
| None | 293 | 90.2 | 891 | 91.5 | 1184 | 91.2 | |
| At least one | 32 | 9.9 | 83 | 8.5 | 115 | 8.9 | |
| Depression | |||||||
| No | 190 | 58.5 | 602 | 61.8 | 792 | 61.0 | |
| Yes | 128 | 39.4 | 367 | 37.7 | 495 | 38.1 | |
| Missing | 7 | 2.2 | 5 | 0.5 | 12 | 0.9 | |
| Dyslipidemia | |||||||
| No | 207 | 63.7 | 608 | 62.4 | 815 | 62.7 | |
| Yes | 118 | 36.3 | 366 | 37.6 | 484 | 37.3 | |
| Body mass index | |||||||
| <30 kg/m2 | 258 | 79.4 | 809 | 83.1 | 1,067 | 82.1 | |
| > = 30 kg/m2 | 67 | 20.6 | 165 | 16.9 | 232 | 17.9 | |
| Current smoking | |||||||
| No | 290 | 89.2 | 887 | 91.1 | 1,177 | 90.6 | |
| Yes | 35 | 10.8 | 87 | 8.9 | 122 | 9.4 | |
sd = standard deviation
ADM = anti-diabetic medications
MMSE = Mini Mental Status Examination
Table 2. Characteristics of the study population by diabetes status, N = 1299.
Source: BASE-II.
| Characteristics | N | Non-diabetics | Treated diabetics, oral ADM | Treated diabetics, insulin | Untreated diabetics | Un-diagnosed diabetics | p-value |
|---|---|---|---|---|---|---|---|
| HbA1c (mean; sd) | 1261 | 5.5; 0.4 | 6.6; 0.8 | 7.3; 1.1 | 6.2; 0.8 | 6.3; 0.6 | <0.001 |
| FPG in mg/dL (mean; sd) | 1278 | 91.3; 9.4 | 129.6; 31.7 | 151.2; 61.4 | 121.2; 27.0 | 123.2; 26.2 | <0.001 |
| 2-hour glucose in mg/dL (mean; sd) | 1115 | 103.7; 28.3 | — | — | — | 201.8; 66.2 | <0.001 |
| Physically inactive (%) | 1299 | 50.4 | 55.9 | 42.1 | 71.1 | 70.0 | 0.011 |
| Living alone (%) | 1299 | 39.3 | 30.9 | 26.3 | 31.6 | 40.0 | 0.407 |
| Men (%) | 1299 | 49.0 | 67.7 | 79.0 | 55.3 | 52.5 | 0.004 |
| Age in years (mean; sd) | 1299 | 68.1; 3.6 | 67.6; 3.5 | 68.1; 3.3 | 68.2; 4.1 | 68.1; 4.1 | 0.862 |
| High education (%) | 1299 | 48.0 | 35.3 | 52.6 | 42.1 | 45.0 | 0.305 |
| Hypertension (%) | 1299 | 42.0 | 73.5 | 79.0 | 84.2 | 55.0 | <0.001 |
| Stroke (%) | 1299 | 2.1 | 4.4 | 5.3 | 0.0 | 0.0 | 0.375 |
| Cardiovascular diseases (%) | 1299 | 8.2 | 10.3 | 42.1 | 13.2 | 5.0 | <0.001 |
| Depression (%) | 1299 | 37.7 | 45.6 | 47.4 | 34.2 | 37.5 | 0.701 |
| Dyslipidemia (%) | 1299 | 34.8 | 63.2 | 57.9 | 52.6 | 37.5 | <0.001 |
| BMI≥30 kg/m2 (%) | 1299 | 14.7 | 36.8 | 52.6 | 39.5 | 37.5 | <0.001 |
| Current smoking (%) | 1299 | 9.2 | 14.7 | 0.0 | 2.6 | 17.5 | 0.051 |
sd = standard deviation
ADM = anti-diabetic medications
HbA1c = glycated haemoglobin
BMI = body mass index
FPG = fasting plasma glucose
Model results
Univariate logistic regression analysis revealed that diabetics compared to non-diabetics had a statistically increased odds ratio (OR) of 1.82 (p = 0.001) of poor cognitive performance (Table 3). Table 4 presents our main results in the form of OR of poor cognitive performance dependent on the diabetes status. Model 1 is adjusted for sex, age, and education only. All other models are adjusted for sex, age, education, hypertension, stroke, cardiovascular diseases, depression, dyslipidemia, body mass index, and current smoking. Differentiation by the treatment of the diabetics revealed that undiagnosed diabetes is associated with a particularly high odds ratio of poor cognitive performance (Model 3: OR = 2.12, p = 0.031). Persons receiving oral ADM and untreated diabetics also showed elevated odds ratios, but estimates did not reach statistical significance. Although not statistically significant, the odds ratio for insulin-dependent diabetics was quite high (OR = 1.95, p = 0.193). The results are stable and do not change much when controlling for covariates. Being inactive was significantly correlated with poor overall cognitive performance (OR = 1.43, p = 0.008). People living alone had an increased odds ratio of 1.58 (p = 0.002).
Table 3. Univariate odds ratios of poor cognitive performance, N = 1,299.
Source BASE-II.
| Variable | OR | p-value | |
|---|---|---|---|
| Diabetes mellitus | |||
| Non-diabetics (RG) | 1.00 | ||
| Diabetics | 1.82 | 0.001 | |
| Diabetes mellitus | |||
| Non-diabetics (RG) | 1.00 | ||
| Treated diabetics, oral ADM | 1.56 | 0.097 | |
| Treated diabetics, insulin | 1.90 | 0.181 | |
| Untreated diabetics | 1.70 | 0.130 | |
| Undiagnosed diabetics | 2.41 | 0.007 | |
| Physical activity | |||
| Active (RG) | 1.00 | ||
| Inactive | 1.56 | 0.001 | |
| Cohabitation status | |||
| Not alone (RG) | 1.00 | ||
| Alone | 1.34 | 0.026 | |
| Sex | |||
| Men (RG) | 1.00 | ||
| Women | 0.69 | 0.005 | |
| Age in years | 1.10 | <0.001 | |
| Education | |||
| Low (RG) | 1.00 | ||
| High | 0.47 | <0.001 | |
| Hypertension | |||
| No (RG) | 1.00 | ||
| Yes | 1.33 | 0.028 | |
| Stroke | |||
| No (RG) | 1.00 | ||
| Yes | 1.20 | 0.661 | |
| Cardiovascular diseases | |||
| None (RG) | 1.00 | ||
| At least one | 1.17 | 0.467 | |
| Depression | |||
| No (RG) | 1.00 | ||
| Yes | 1.11 | 0.450 | |
| Missing | 4.44 | 0.012 | |
| Dyslipidemia | |||
| No (RG) | 1.00 | ||
| Yes | 0.95 | 0.682 | |
| Body mass index | |||
| <30 kg/m2 (RG) | 1.00 | ||
| > = 30 kg/m2 | 1.27 | 0.135 | |
| Current smoking | |||
| No (RG) | 1.00 | ||
| Yes | 1.23 | 0.326 | |
RG = Reference group
ADM = anti-diabetic medications
OR = odds ratio
Table 4. Odds Ratios of poor cognitive performance, N = 1299.
Source: BASE-II.
| Model 1 * | Model 2 ** | Model 3** | |||||
|---|---|---|---|---|---|---|---|
| Variable | OR | p-value | OR | p-value | OR | p-value | |
| Diabetes mellitus | |||||||
| Non-diabetics (RG) | 1.00 | 1.00 | 1.00 | ||||
| Treated diabetics, oral ADM | 1.42 | 0.210 | 1.34 | 0.314 | 1.34 | 0.317 | |
| Treated diabetics, insulin | 1.88 | 0.202 | 1.80 | 0.247 | 1.95 | 0.193 | |
| Untreated diabetics | 1.61 | 0.189 | 1.51 | 0.270 | 1.48 | 0.299 | |
| Undiagnosed diabetics | 2.43 | 0.009 | 2.22 | 0.020 | 2.12 | 0.031 | |
| Physical activity | |||||||
| Active (RG) | 1.00 | ||||||
| Inactive | 1.43 | 0.008 | |||||
| Cohabitation status | |||||||
| Not alone (RG) | 1.00 | ||||||
| Alone | 1.58 | 0.002 | |||||
| Hosmer-Lemeshow-Chi² (df = 8) | 5.13 | 12.77 | 4.25 | ||||
| p-value(Hosmer-Lemeshow-Chi²) | 0.743 | 0.120 | 0.834 | ||||
*Adjusted for sex, age, education
**Adjusted for sex, age, education, hypertension, stroke, cardiovascular diseases, depression, dyslipidemia, body mass index, current smoking
RG = Reference group
ADM = anti-diabetic medications
OR = odds ratio
df = degrees of freedom
Model results with interaction terms
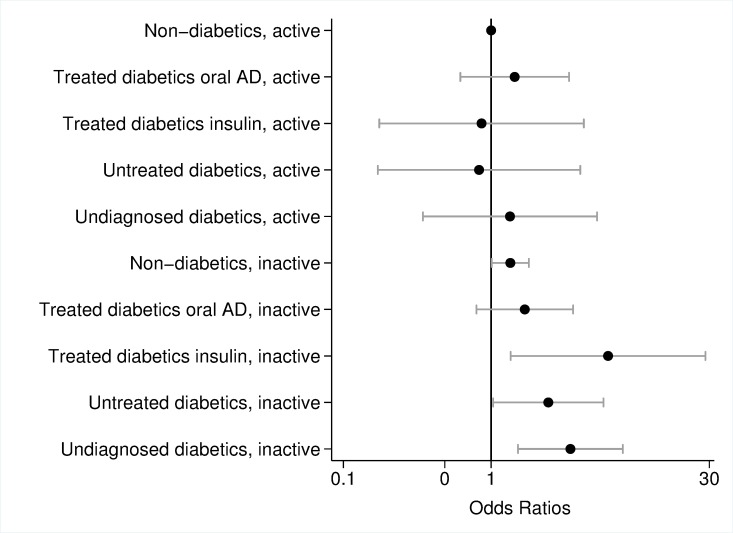
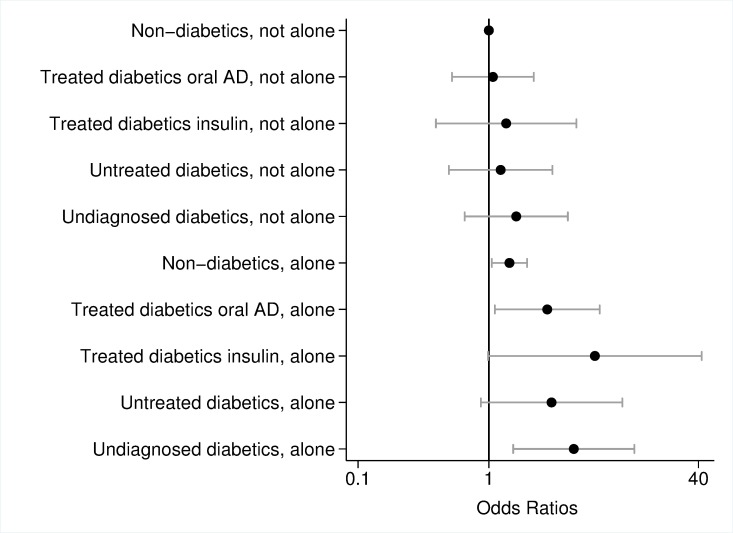
Models with interaction terms revealed that the likelihood of poor overall cognitive performance was particularly high if people suffered from undiagnosed diabetes (OR = 3.44, p = 0.003) or were treated with insulin (OR = 6.19, p = 0.019) and were also inactive (Fig 2). Also inactive non-diabetics and inactive untreated diabetics had increased odds ratios (non-diabetics: OR = 1.35, p = 0.042; untreated diabetics: OR = 2.44, p = 0.042) compared to active non-diabetics. However, physically active persons did not have an increased likelihood of poor overall cognitive performance independent of their diabetes status. Fig 3 presents model results of the interaction between diabetes and cohabitation. Living alone increased the risk of poor overall cognitive performance for non-diabetics (OR = 1.44, p = 0.022) as well as for diabetics treated with oral ADM (OR = 2.79, p = 0.029) and undiagnosed diabetics (OR = 4.46, p = 0.006), whereas effects were highest for insulin-dependent diabetics who lived alone (OR = 6.46, p = 0.052). Diabetic people who cohabitate had an odds ratio of poor cognitive performance comparable to non-diabetics.
Fig 2. Odds ratios and 95% confidence interval of poor cognitive performance, interaction effects of diabetes mellitus and physical activity adjusted for sex, age, education, hypertension, stroke, cardiovascular diseases, depression, dyslipidemia, body mass index, current smoking; logarithmic scale.
Error bars represent 95% confidence interval. Source: BASE-II.
Fig 3. Odds ratios and 95% confidence interval of poor cognitive performance, interaction effects of diabetes mellitus and cohabitation status adjusted for sex, age, education, hypertension, stroke, cardiovascular diseases, depression, dyslipidemia, body mass index, current smoking; logarithmic scale.
Error bars represent 95% confidence interval. Source: BASE-II.
Discussion
In the current study undiagnosed diabetics without effective glycemic control were shown to have the highest risks of poor cognitive performance, which is in line with a previous study by Xu and colleagues [16]. A considerable proportion, almost one quarter, of all diabetes cases did not know about their disease prior to participation in this study. Insulin-dependent diabetics also showed highly elevated odds ratios of poor cognitive performance, but most likely due to the small case numbers these estimates did not reach statistical significance. Intake of insulin seems to be an indicator for the severity of the disease, with insulin-dependent diabetics at a more severe stage [28]. Both undiagnosed and insulin-dependent diabetics had the highest HbA1c, FPG, or 2-hour glucose levels, which indicates insufficient or no glycemic control. In contrast, diabetics treated with oral ADM and untreated diabetics had comparable risks of poor cognitive performance compared to non-diabetics. These persons are probably still in a mild stage of the disease, and effective glycemic control seems to be achieved either by oral ADM or by nutrition. Previous studies have shown that treatment with oral ADM is beneficial for cognition and may attenuate the harmful effects of diabetes in patients [15, 16]. A meta-analysis revealed that diets of low-carbohydrates, a low-glycemic index, Mediterranean, and high in protein are effective in glycemic control [41] and may therefore reduce the risk of cognitive impairment in early stages of the disease as compared to diabetics with insufficient or no glycemic control.
Along with the status of diabetes, life style variables had an influence on the likelihood of poor cognitive performance. People who were inactive and living alone have significantly elevated risks, which is in line previous studies [21–26]. Fratiglioni and colleagues [22] proposed three hypotheses about how social integration and physical activity might offer protection from cognitive impairment and dementia. First, the cognitive-reserve hypothesis postulates that physical activity and social interactions enhance the plasticity of the brain and compensatory functions, therefore perhaps preventing cognitive decline. Second, the vascular hypothesis describes the beneficial effect of physical activity and social integration on the pathogenesis of cardiovascular diseases, which are in turn risk factors for cognitive impairment and dementia. Third, the stress hypothesis assumes that physically active and socially integrated people show lower levels of stress and can better cope with stress. A failure of stress adaption does indeed matter in the development of cognitive decline and dementia [22].
The combination of undiagnosed or insulin-dependent diabetes and inactivity or non-cohabitation was particularly deleterious. However, if participants were regularly physically active or lived together with a partner or relative, the risks of poor cognitive performance did not differ from those of active or cohabiting non-diabetics. This result was independent of the treatment status of the diabetics. Still, the enhanced risk due to undiagnosed or insulin-dependent diabetes could partly be compensated for by physical activity and social integration. Both factors most likely counteract the harmful effects of diabetes and insufficient glycemic control. This finding is in line with a recent a study indicating that moderate-to-high intensity aerobic exercise may have a disease-modifying effect in terms of reduced levels of tau proteins in cerebrospinal fluid, increases in blood flow in the brain, and improvements of executive functions in elderly people with MCI and prediabetes [42].
The present study does have some limitations. First, the cross-sectional design does not allow us to draw causal conclusions. There is always the possibility of reverse causation, meaning that cognitive impairment is the cause of an unhealthy life style which leads to diabetes or inactivity. Second, social integration was assessed in conjunction with the cohabitation status. Living alone does not necessarily mean that people are not socially integrated. Nevertheless, cohabiting is one of the main components of social interaction [43] and we were able to demonstrate the positive effect of living with a partner or relative. Third, we used a relatively strict definition of physical activity. However, a sensitivity analysis with a wider definition that also classified people as active who report doing”moderate physical activities every week, but less than 30 minutes a day or 5 days a week” or “vigorous physical activities every week, but less than 20 minutes a day or 3 days a week” showed similar effects. Fourth, in a life-course perspective, untreated diabetics may receive drug treatment sooner or later and would then belong to the group of treated diabetics. There might also be social selection forces, such as education or income, which would increase the likelihood of receiving a diabetes diagnosis and thus interplay with the positive effect of the treatment and compliance of the patients. Especially, the group of undiagnosed diabetics may have a generally lower health literacy and the lack of their diabetes diagnosis may potentially be associated with also undiagnosed cardiovascular diseases or dyslipidemia which were self-reported. This should be in mind when interpreting estimates of cognitive performance of this group.
The strength of this study is the large number of cases with available information on their cognitive status measured with a total score of the CERAD-Plus test battery, which is superior to the MMSE in detecting MCI [34, 35]. The advantage of a summary score is that every subtest carries the same weight. The score gives a full picture of a participant’s cognitive performance. On the other hand, the total score does not allow an evaluation of the performance in special cognitive domains. The in-depth medical anamnesis and the numerous laboratory values allow us to reliably identify undiagnosed diabetes cases, which in turn enabled us to differentiate between diagnosed diabetics receiving no drug treatment and undiagnosed diabetics who also were untreated. A comparison of demographic characteristics with the German general population revealed that the BASE-II sample is relatively healthy and well educated [29]. The effects of diabetes, physical activity, and cohabitation status on cognitive performance are therefore potentially underestimated.
Several studies have indicated a decreasing trend of dementia prevalence and incidence [44–50], primarily due to higher educational levels and a reduction of vascular risk factors, especially stroke [51]. Nevertheless, it is questionable whether these trends will continue as the rising diabetes prevalence could counteract successes in the prevention of other cardiovascular diseases and thus the observed trends. Consequently, it is essential to detect and treat type 2 diabetes mellitus as early as possible, not only to prevent cognitive decline and subsequent dementia but also other complications which might be caused by diabetes, for example retinopathy, nephropathy, or polyneuropathy [20]. In addition, physical activity and social integration play an important role in helping to overcome the problem of an increasing prevalence of diabetes. An early screening for diabetes, especially in patients with overweight or familial history of diabetes, may be useful. Beyond screening and providing comprehensive information to the population targeted life style intervention strategies for diabetic patients are valuable [52]. Interventions such as an increased physical activity, dietary education and counseling for treatment adherence showed beneficial effects on risk factors such as high BMI and HbA1c values in diabetic patients [53]. In the case of frequent hyperglycemic phases, diabetic patients could receive targeted trainings in outpatient clinics. If such courses were held in small groups, this would also promote social interaction.
A healthy life style and goal-directed detection and treatment of diabetes might contribute to the continuation of decreasing incidence and prevalence of dementia. Further research is needed to analyze to what extent these factors influence the development of cognitive impairments and dementia.
Supporting information
Source: BASE-II.
(DOCX)
Source: BASE-II.
(DOCX)
Source: BASE-II.
(DOCX)
Acknowledgments
Thanks to all employees and participants of BASE-II who supported this study by their contributions, discussions, and cooperation.
Data Availability
Due to concerns for participant privacy, data are available only upon request. External scientists may apply to the Steering Committee of BASE-II for data access. Please contact Katrin Schaar, scientific coordinator, at schaar@mpib-berlin.mpg.de.
Funding Statement
This study was funded by the German Federal Ministry of education and Research [BMBF (grant number #16SV5536K)] and the German Center of Neurodegenerative Diseases (DZNE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88(10):1452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ. Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2010;24(1):90–5. doi: 10.1097/WAD.0b013e31819fe7d1 [DOI] [PubMed] [Google Scholar]
- 3.Barberger-Gateau P, Alioum A, Pérès K, Regnault A, Fabrigoule CC, Nikulin M, et al. The contribution of dementia to the disablement process and modifying factors. Dement Geriatr Cogn Disord. 2004;18(3–4):330–7. doi: 10.1159/000080127 [DOI] [PubMed] [Google Scholar]
- 4.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Doblhammer G, Fink A, Fritze T, Günster C. The demography and epidemiology of dementia. GMHC. 2013;1(2):29–33. doi: 10.1016/j.gmhc.2013.04.002 [Google Scholar]
- 6.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 7.van den Berg E, Reijmer YD, Bresser J, Kessels RPC, Kappelle LJ, Biessels GJ. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(1):58–65. doi: 10.1007/s00125-009-1571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of Cognitive Dysfunction in Adults with Type 2 Diabetes: A Meta-analysis of Six Cognitive Domains and the Most Frequently Reported Neuropsychological Tests Within Domains. J Int Neuropsychol Soc. 2014;20(03):278–91. doi: 10.1017/S1355617713001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodl CT, Seaquist ER. Cognitive Dysfunction and Diabetes Mellitus. Endocr Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grodstein F, Chen J, Wilson RS, Manson JE. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24(6):1060–5. [DOI] [PubMed] [Google Scholar]
- 11.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose Levels and Risk of Dementia. N Engl J Med. 2013;369(6):540–8. doi: 10.1056/NEJMoa1215740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Intern Med J. 2012;42(5):484–91. doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 13.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–50. doi: 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable Predictors of Dementia in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Am J Psychiatry. 2015;172(4):323–34. doi: 10.1176/appi.ajp.2014.14070878 . [DOI] [PubMed] [Google Scholar]
- 15.Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol. 2015;78(2):284–94. doi: 10.1002/ana.24439 [DOI] [PubMed] [Google Scholar]
- 16.Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia. 2009;52(6):1031–9. doi: 10.1007/s00125-009-1323-x [DOI] [PubMed] [Google Scholar]
- 17.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 18.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. J Am Med Assoc. 2015;314(10):1021–9. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 19.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 20.Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2014;103(2):150–60. doi: 10.1016/j.diabres.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 21.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718–26. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 22.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–53. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- 23.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67(1):80–6. doi: 10.1001/archneurol.2009.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18(2):57–64. [DOI] [PubMed] [Google Scholar]
- 25.Helmer C. Dementia and marital status at midlife and late life. BMJ. 2009;339 doi: 10.1136/bmj.b1690 [DOI] [PubMed] [Google Scholar]
- 26.van Gelder BM, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Marital Status and Living Situation During a 5-Year Period Are Associated With a Subsequent 10-Year Cognitive Decline in Older Men: The FINE Study. J Gerontol B Psychol Sci Soc Sci. 2006;61(4):P213–P9. [DOI] [PubMed] [Google Scholar]
- 27.Håkansson K, Rovio S, Helkala E-L, Vilska A-R, Winblad B, Soininen H, et al. Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ. 2009;339 doi: 10.1136/bmj.b2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. [DOI] [PubMed] [Google Scholar]
- 29.Bertram L, Böckenhoff A, Demuth I, Düzel S, Eckardt R, Li S-C, et al. Cohort profile: the Berlin Aging Study II (BASE-II). Int J Epidemiol. 2014;43:703–12. doi: 10.1093/ije/dyt018 [DOI] [PubMed] [Google Scholar]
- 30.Gerstorf D, Bertram L, Lindenberger U, Pawelec G, Demuth I, Steinhagen-Thiessen E, et al. Editorial Gerontology. 2016;in press; doi: 10.1159/000441495 [DOI] [PubMed] [Google Scholar]
- 31.Morris J, Heyman A, Mohs R, Hughes J, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's disease (CERAD): Part I—Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–65. [DOI] [PubMed] [Google Scholar]
- 32.Memory Clinic Basel. CERAD-Plus 2005 [July 8, 2015]. Available from: http://www.memoryclinic.ch/.
- 33.Lezak MD. Neuropsychological Assessment: Oxford University Press; 2004. [Google Scholar]
- 34.Chandler M, Lacritz L, Hynan L, Barnard H, Allen G, Deschner M, et al. A total score for the CERAD neuropsychological battery. Neurology. 2005;65(1):102–6. doi: 10.1212/01.wnl.0000167607.63000.38 [DOI] [PubMed] [Google Scholar]
- 35.Seo EH, Lee DY, Lee JH, Choo IH, Kim JW, Kim SG, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry. 2010;18(9):801–9. doi: 10.1097/JGP.0b013e3181cab764 [DOI] [PubMed] [Google Scholar]
- 36.NCD Risk Factor Collaboration. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331 288 participants. Lancet Diabetes Endocrinol. 2015;3(8):624–37. doi: 10.1016/S2213-8587(15)00129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MMB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):1–8. [PMC free article] [PubMed] [Google Scholar]
- 38.University of Washington Health Promotion Research Center. How Physically Active are You?–An Assessment of Level and Intensity of Physical Activity 2014 [September 10, 2015]. Available from: http://depts.washington.edu/hprc/docs/rapa_03_06.pdf.
- 39.World Health Organization. Body mass index—BMI 2017 [September 18, 2017]. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 40.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 41.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–16. doi: 10.3945/ajcn.112.042457 [DOI] [PubMed] [Google Scholar]
- 42.Baker LD, Skinner J, Craft S, Cholerton B, Callaghan M, Hanson A, et al. Aerobic exerecise reduces phosphorylated tau protein in cerebrospinal fluis in older adults with mild cognitive impairment. 8th Clinical Trials on Alzheimer's Disease (CTAD) 2015. [Google Scholar]
- 43.Victor C, Scambler S, Bond J, Bowling A. Being alone in later life: loneliness, social isolation and living alone. Rev Clin Gerontol. 2000;10(04):407–17. [Google Scholar]
- 44.Doblhammer G, Fink A, Fritze T. Short-term trends in dementia prevalence in Germany between the years 2007 and 2009. Alzheimers Dement. 2015;11(3):291–9. doi: 10.1016/j.jalz.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 45.Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ, Hofman A, Ikram MA, Breteler MMB. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456–63. doi: 10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- 46.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U. S. elderly population. Adv Gerontol. 2005;16:30–7. [PubMed] [Google Scholar]
- 47.Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4(2):134–44. doi: 10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doblhammer G, Fink A, Zylla S, Willekens F. Compression or expansion of dementia into old age in Germany? An observational study of short-term trends in incidence and death rates of dementia between 2006/07 and 2009/10 based on German health insurance data. Alzheimers Res Ther. 2015;7(1):66–76. doi: 10.1186/s13195-015-0146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80(20):1888–94. doi: 10.1212/WNL.0b013e318292a2f9 [DOI] [PubMed] [Google Scholar]
- 50.Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–12. doi: 10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. New Engl J Med. 2013;369(24):2275–7. doi: 10.1056/NEJMp1311405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clinical Diabetes: a publication of the American Diabetes Association; 2016;34(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Pei J-H, Kuang J, Chen H-M, Chen Z, Li Z-W, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis. Metabolis. 2015;64(2):338–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source: BASE-II.
(DOCX)
Source: BASE-II.
(DOCX)
Source: BASE-II.
(DOCX)
Data Availability Statement
Due to concerns for participant privacy, data are available only upon request. External scientists may apply to the Steering Committee of BASE-II for data access. Please contact Katrin Schaar, scientific coordinator, at schaar@mpib-berlin.mpg.de.