Abstract
Innate lymphoid cells (ILCs) are a new class of immune cells that include NK cells and appear to be the innate counterparts to CD4+ helper T cells and CD8+ cytotoxic T cells based on developmental and functional similarities. Like T cells, both NK cells and other ILCs also show connections to the MHC. In human and mouse, NK cells recognize and respond to classical and nonclassical MHC I molecules as well as structural homologues, whereas mouse ILCs have recently been shown to express MHC II. We describe the history of MHC I recognition by NK cells and discuss emerging roles for MHC II expression by ILC subsets, making comparisons between both mouse and human when possible.
Keywords: innate lymphoid cells, ILCs, HLA, MHC, innate, human, mouse, cytokines
Introduction
Over the past several years, the universe of lymphocytes has rapidly expanded with the recognition and subsequent investigation of a new major class of immune cells called innate lymphoid cells (ILCs)(1–8). Like other lymphocytes, ILCs develop from the common lymphoid progenitor(2, 4). However, the lack of recombined antigen-specific loci and other lineage markers distinguish ILCs from conventional T and B cells, as well as innate-like T and B cell subsets with restricted repertoires such as Natural Killer T (NKT), γδT, B1a, and B1b cells(9). Thus, unlike other mature lymphocytes, ILCs are present and even expanded in Rag1 and Rag2-deficient mice, which lack DNA recombination machinery. With regard to localization, ILCs are more similar to innate-like T and B cell subsets than conventional T and B cell populations as they are predominately a tissue-resident population(9), located in greatest number and frequency at mucosal barriers such as the small intestine lamina propria(2–4, 10). But with regard to functional programs, ILCs appear to be more similar to conventional T cells(2). Here, we introduce the major classes of ILCs, and focus on the connection between NK cells and ILCs and the major histocompatibility complex (MHC). We discuss from a historical perspective the more well-known mechanisms by which NK cell interact with MHC class I (MHC I) and the emerging mechanisms by which ILCs modulate the adaptive immune response via MHC class II expression (MHC II), in both mouse and human.
What are ILCs?
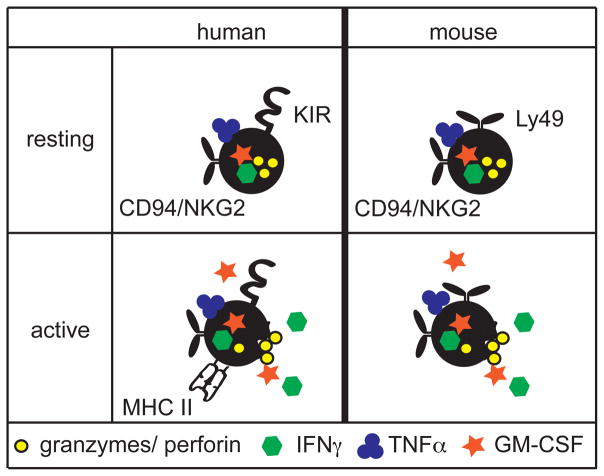
Before 2008, two members of the ILC class had already been discovered, namely natural killer (NK) cells and lymphoid tissue inducer (LTi) cells. NK cells recognize virally infected cells and tumor cells through germline-encoded receptors and kill them via release of lytic granules containing perforin and granzymes(11–13). In addition to their cytolytic activity, NK cells also secrete cytokines such as IFN-γ, GM-CSF and TNF-α(11–13) (Figure 1). Another type of ILC, the LTi cell, was known for some time to orchestrate the generation of lymphoid organs during fetal development(14, 15). LTi cells express the cell surface heterotrimer lymphotoxin-α1β2 (LTα1β2). This acts on LTβR+ stromal organizer cells to induce lymph node and Peyer’s patch organogenesis by stimulating the production of chemokines, which in turn attract lymphocytes to form secondary lymphoid organs(16). After 2008, additional ILC subtypes were identified in human and mouse and grouped together based on shared developmental requirements and functional cytokine production(1): ILC1 produce interferon γ (IFNγ), like NK cells; ILC2 produce IL-5, IL-13, IL-9 and amphiregulin; and ILC3 produce IL-22 and/or IL-17. ILC3 also include a cellular subset phenotypically similar to fetal LTi cells, referred to as LTi-like cells(17, 18) or NKp46−CCR6+ ILC3 cells(19), which seem to be the adult counterpart of fetal LTi cells. Compared to NK cells, ILCs appear to have no or limited cytolytic activity (2, 10, 18, 20). Remarkably, the functional specializations of ILCs as well as their developmental programs resemble those previously recognized in CD4+ T helper cell subsets(2). Therefore, ILC1, ILC2 and ILC3 are considered the innate counterparts of Th1, Th2 and Th17, while NK cells are the innate counterpart to CD8+ cytotoxic T cells(2, 21).
Figure 1.
Resting human and mouse NK cells are similar. MHC I recognition is encoded by genes in the KIR family of immunoglobulin-like receptors in human, and the Ly49 family of lectin-like receptors in mouse. Both human and mouse NK cells express members of the NKG2/CD94 lectin-like family, which recognize non-classical MHC I molecules. Upon activation, both human and mouse NK cells lyse targets through the release of granzymes and perforin and share production of the cytokines IFNγ, TNFα, and GM-CSF. However, activated human NK cells also can express MHC II.
In the absence of antigen-specific receptors, ILCs primarily respond to cytokine signals, including an IL-1 family danger signal and an additional STAT-activating cytokine: IL-12, IL-18 and IL-15 stimulate ILC1; IL-25, thymic stromal lymphopoietin (TSLP) and IL-33 trigger ILC2; and IL-23 and IL-1β stimulate ILC3 (2–4). Thus, ILCs are an innate source of cytokines that are sensitive to, and respond to, changes in the cytokine microenvironment. This principle explains three key roles for ILCs that have emerged: 1) homeostatic functions, likely due to constitutive cytokine production and/or effective antigen-independent responses to subtle homeostatic perturbations; 2) robust and emergent responses to danger stimuli, such as early infection, leading to temporary control; and 3) crosstalk with other innate immune cells and regulation of the adaptive immune response. Interestingly, both NK cells and ILCs also show connections to the MHC, though in different ways. While NK cell receptors have long been known to bind classical and nonclassical MHC I molecules similar to CD8+ T cells in human and mouse(11), more recently, mouse ILC2(22) and NKp46−CCR6+ ILC3(23–25) have been demonstrated to express MHC II and interact with CD4+ T cells. This raises the intriguing possibility that NK cells and other ILCs coevolved with T cells to utilize non-redundant, complementary mechanisms of recognizing and responding to self as well as to directly modulate the T cell response through the presentation of non-self antigens.
NK cells: a historical perspective
NK cells were first discovered in the late 1960s and early 1970s based on functional properties. Specifically, early studies demonstrated the “natural,” or non-immunized, ability of immune cells to recognize and kill tumor cell lines (26, 27). In the transplant field, investigators further identified that F1 hybrid mice rejected bone marrow from inbred parental grafts, called “hybrid resistance,” which failed to be explained by other known immune mechanisms(28). In the mid-1980s, NK cells were proposed to be the unique lineage of cells responsible for these properties, initially identified by lymphocyte size and the markers CD3−CD16+ in human and CD3−NK1.1+ in C57BL/6 mice(29), and later refined to include CD56+ in human(30) and NKp46+ in mice(31). But how were the phenomena of tumor cell killing and hybrid resistance unified?
The first insight into how NK cells mediated cytotoxicity emerged in 1986 with the hypothesis of “missing self” (32, 33). This hypothesis developed from the discovery that syngeneic hosts inoculated with a tumor line expressing low levels of the MHC I H-2b resulted in less tumor growth in vivo and better NK cell lysis in vitro compared with tumors expressing higher levels of H-2b (32). The authors extrapolated from these results that hybrid resistance might be similarly due to 50% lower expression of “self-H-2” found on inbred parental grafts(32, 33). At the time, this finding was somewhat perplexing, as NK cell lysis had been demonstrated not to be MHC restricted, unlike T cell killing, through the study of athymic nude mice. However, several rapid-fire publications subsequently supported the hypothesis of “missing self”(34, 35), though a mechanism remained elusive. In the early 1990s, work from the Yokoyama lab provided the first molecular mechanism of “missing self” by demonstrating that the mouse NK cell receptor Ly49, now known as Ly49A, specifically blocked NK cell killing of a susceptible tumor cell line transfected with H2-Dd but not H2-Kd or H2-Ld, suggesting Ly49 directly recognized H2-Dd(36). Perhaps most surprisingly, this finding was also the first to demonstrate that receptor ligation by MHC I led to NK cells inhibition, and not activation. Shortly thereafter, the Moretta lab similarly described the first human inhibitory NK cell receptor, p58, which is now known as KIR2DL(37), presumed to similarly bind MHC I. This was definitively demonstrated when the KIR gene family was later cloned(38, 39).
Molecular mechanisms of NK cell function
Though MHC I-recognizing inhibitory NK cell receptors were the first to be characterized, it is now known that NK cell cytotoxicity requires not only loss of inhibition but also an activating signal (11). In the past 15 years, the germline-encoded molecules expressed by NK cells that recognize MHC I have become increasingly well understood. The mouse and human inhibitory NK cell receptors first described by the Yokoyama and Moretta groups are encoded by single genes within larger families that are unique to each respective species. Sequence and structural homology within each family, but not between species, suggest that NK cell receptors that bind MHC I independently arose in both mice and human from gene duplication events and became specialized (11), an example of convergent evolution. In human, these receptors are encoded in the KIR gene family of the Ig superfamily, while in mouse, they are encoded by the C-type lectin Ly49 family. Other NK cell receptors outside of the KIR and Ly49 families include the CD94/NKG2 family members, which are conserved between human and mouse, and bind non-classical MHC I molecules, such as HLA-E in human and Qa-1 in mouse (40–43) (Figure 1). Despite structural differences, each receptor family has evolved both inactivating and activating receptors, which signal through conserved mechanisms. Inhibitory receptors, such as the prototypical receptor Ly49A, contain ITIM motifs that recruit and activate phosphatases such as SHP-1 upon receptor ligation to downregulate signal transduction(44, 45). Meanwhile, activating receptors associate with the adaptors, such as DAP12 and CD3ζ which act through ITAM motifs to recruit and activate the tyrosine kinases Syk and ZAP70(46). Therefore, when both activating and inhibitory stimuli are present, it is the balance between these pathways that modulates NK cell function(47, 48).
But what was the specificity and the physiologic role of activating NK cell receptors, which were unlikely to have evolved to mediate transplant rejection? This was clarified by the finding that some activating receptors bind to pathogen-encoded ligands. One of the best-studied pathogen-binding activating NK cell receptors is Ly49H, which is ligated by the murine cytomegalovirus (MCMV) protein m157, a structural homologue of MHC I. Functionally, mice that express Ly49H effectively mediate host-protection to MCMV infection compared to those that do not(49, 50). Another activating NK cell receptor in the NKG2 family, NKD2D, was also discovered to be conserved between human and mouse, and to bind stress-induced ligands, such as MIC and ULBP in human and Rae1 and H-60 in mouse, which have conformational similarity to MHC I(51, 52). Combined with the evidence that some viruses(53, 54) and tumors(55) selectively downregulate MHC I to escape immune control, these findings suggest that NK cells act as both a first line of defense before adaptive immunity develops, as well as a second line of defense after T cell-focused immunoevasion strategies progress.
Species differences in MHC II expression
Unlike MHC I, there is little evidence that NK cells or ILCs recognize MHC II(11, 56, 57); however, there is increasing evidence that some mouse ILCs express MHC II and this modulates the adaptive immune system(22–25). One important consideration for studying MHC II using mouse models is that expression significantly differs between human and mouse. In mouse, MHC II expression is relatively restricted, found predominantly on professional antigen-presenting cells (APCs), including dendritic cells, macrophages, and B cells, an in some contexts, on atypical APCs such as eosinophils(58, 59). In human, many activated cells express MHC II, including CD4+ T cells and NK cells (Figure 1), perhaps as well as non-immune cells such as epithelial cells(58, 59). For T cells, the mechanism leading to species differences in MHC II expression is known (58). This is caused by differences in the regulation of the master transcription factor for MHC II, the major histocompatibility class II transactivator or CIITA, which uses three promoters in both human and mouse(60). Upon activation, human T cells specifically engage the pIII promoter, leading to CIITA and subsequently MHC II expression; in mouse T cells, this promoter is constitutively methylated and thus inaccessible to transcriptional machinery(58, 61). While some reports have described that human NK cells present antigen(62) and others have suggested immunoregulatory roles for MHC II+ activated T cells(58), the role of MHC II expression by human lymphocytes remains controversial.
ILC3 and the regulation of effector T cells
Thus far, most research regarding ILC expression of MHC II has focused on the NKp46−CCR6+ subset of ILC3 (23–25) found in mouse. NKp46− CCR6+ ILC3 are found predominantly in the small and large intestine lamina propria and lymph nodes but also in the spleen(24, 63–65). These cells are functionally similar to NKp46−CCR6− and NKp46+CCR6− mouse ILC3 subsets in that they produce IL-22 and/or IL-17 in response to IL-1β and IL-23(64, 66). Interestingly, lineage tracing and cell adoptive transfer studies have demonstrated that NKp46−CCR6+ ILC3 develop from a different progenitor from other ILCs(67) and, unlike NKp46−CCR6−ILC3 and human tonsil ILC3, do not appear to upregulate the transcription factor T-bet upon IL-23/IL-15 stimulation(19, 68, 69). Without T-bet, NKp46−CCR6+ ILC3 never become NKp46+ and do not transition to an IFNγ-producing ILC1-like population, also called “ex-RORγt ILC3” (20, 69). Therefore, there is sufficient evidence in mouse to consider NKp46−CCR6+ILC3 a distinct population. But given the many MHC II-expressing APCs found in the same locations as NKp46−CCR6+ ILC3, could there be a unique role for MHC II expressed by these cells?
Whereas professional APCs upregulate co-stimulatory molecules following activation, atypical APCs do not(59); thus, given their lack of co-stimulatory molecules, ILCs are considered to belong to the latter category(24, 59). Surprisingly, mice that genetically lacked MHC II expression specifically in ILC3 and T cells via Rorc(γt)Cre-induced deletion of floxed H2-Ab1 were demonstrated to have increased responses to commensal bacteria by two independent groups(23, 24). Findings differed between the groups whether there were gross signs of intestinal inflammation, as presented by rectal prolapse in in Rorc(γt)Cre/H2-Ab1fl/fl mice, possibly due to differences in the microbiota between facilities. However, both groups found an overall increase in the frequency of Th17 cells, which was independent of colonization by the microbe segmented filamentous bacteria (SFB) (23), a known major driver of mouse Th17 development(70). In mouse, NKp46−CCR6+ ILC3 were able to process, to a limited extent, and present class II antigens, but were unable to cause T cell proliferation(24). Instead, the loss of MHC II-expression by NKp46−CCR6+ ILC3 in commensal-specific TCR transgenic mouse model led to an increased number of activated CD4+ T cells in the colonic lamina propria. This was antigen dependent, as activated T cell frequencies decreased upon antibiotic treatment in commensal-specific TCR transgenic mice and were normal in OVA-specific TCR transgenic mice(25). Although it is unclear whether this similarly occurs in humans, colonic lamina propria ILC3 from children with Crohn’s disease, a condition known to be associated with deregulated responses to the microbiota, on average had significantly less expression of MHC II(25). This result linked inflammation with reduced ILC3 MHC II expression, although caution is needed, as many cells can variably express human MHC II depending on activation status(58). Collectively, these data suggest that ILC3 have an immunoregulatory role mediated by MHC II to constrain the T cell repertoire, reminiscent of T-reg functions.
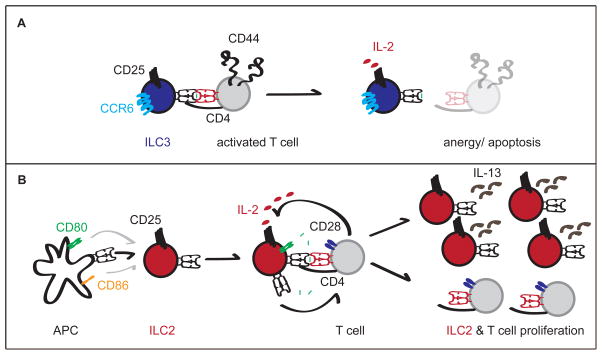
Very recently, the manner by which NKp46−CCR6+ ILC3 suppress T cell responses to the microbiota has been more mechanistically elucidated. First, NKp46−CCR6+ ILC3 expression of MHC II was shown to be regulated by the pIV promoter elements of CIITA in an IFNγ-independent manner(25)– a mode of regulation previously only recognized in the thymic epithelial cells (TEC) involved in T cell selection(71–73). This led to the hypothesis that NKp46−CCR6+ ILC3 share a role with TEC in controlling T cell reactivity in the periphery. Second, in line with the lack of costimulatory molecules by NKp46−CCR6+ILC3, regulation of T cells appears to be a negative selection (Figure 2A). Co-cultured activated TCR transgenic T cells with peptide-pulsed NKp46−CCR6+ILC3 led to T cell apoptosis(25), which was linked to increased relative use of IL-2 by cognate NKp46−CCR6+ILC3 compared to the T cell (Figure 2A). Thus, NKp46−CCR6+ILC3 appear to constrain the anti-commensal T cell repertoire using a hybrid mechanism that takes advantage of both TEC (MHC II expression) and T-reg (IL-2 sequestration) strategies.
Figure 2.
A. NKp46−CCR6+ ILC3 engage CD44hiCD62Llo activated T cells in an antigen-dependent manner via MHC II. In the absence of costimulatory molecules expression by ILC3, T cells become anergic or apoptotic, which is enhanced by sequestration of IL-2 by ILC3. B. ILC2 express MHC II transcript, but also trogocytose it perhaps as well as costimulatory molecules, most likely from professional APC. In the presence of exogenous peptides or trogocytosed MHC II bound to antigen, ILC2 interact with CD4+ T cells and can induce their proliferation in vitro. CD4+ T cell-produced IL-2 may similarly lead to increased ILC2 proliferation and IL-13 production in vitro and in vivo.
ILC2 and T cell help
Besides ILC3, mouse ILC2 have also been demonstrated to express MHC II(22), though unlike ILC3, results have yet to be confirmed by multiple groups. Similar to ILC3, ILC2 express MHC II transcripts and demonstrate cell-surface expression by flow cytometry. Unlike ILC3, mouse ILC2 were also shown to express costimulatory molecules CD80 and, to a more limited extent, CD86, a phenotype also reported for human CRTH2+ IL7R+ ILC2. Interestingly, cell-surface expression of MHC II is at least partially due to trogocytosis(22), likely acquired from other professional APC (Figure 2B). It remains unclear if costimulatory molecules are also acquired via trogocytosis or transcribed by ILC2. In co-culture experiments, OVA-peptide but not OVA loaded ILC2 lead to proliferation of OTII T cells and type-two polarization. These data suggest that ILC2 are unlikely to drive T cell response directly through phagocytosis and presentation of antigen but perhaps may do so through surface exchange of antigen or trogocytosis (Figure 2B).
While ILC2 present antigens to T cells, there is also evidence that T cells can help ILC2. In vitro, T cells were shown to enhance ILC2 proliferation in an antigen-dependent manner, which correlated with T cell expression of IL-2. Functionally, in the context of helminth infection with N. brasiliensis, adoptive transfer of MHC II-deficient ILC2 to IL13-deficient mice resulted in less parasite clearance than transfer of MHC II-sufficient ILC2, though this was independent of changes to T cell populations. However, IL-2 was able to rescue N. brasiliensis clearance in Rag deficient mice, which correlated with enhanced ILC2 numbers (Figure 2B). Collectively, these data demonstrate that T cell may provide IL-2 help to ILC2 via MHC II interactions.
Perspectives for human health and disease
The discovery of NK cells and other ILCs were originally described in mouse, and later extended to human. Over 40 years after the initial description of NK cell activity, it is now well accepted that NK cells physiologically mediate anti-viral immunity in both mouse and human. This is supported by the study of rare individuals with defects in NK cell development or function, who frequently demonstrate increased susceptibility to viral infection, particularly to herpesviruses(74). However, investigation of NK cells for therapeutic purposes has mirrored the history of the field, focusing more on anti-tumor and transplantation biology. The most promise thus far has been in donor NK-cell-mediated graft-versus-leukemia in some allogeneic stem cell transplant, particularly those with KIR/KIR ligand mismatch (75). Other therapeutic strategies involving NK cells in both solid and liquid tumors remain an active area of research. In recent years, our understanding of mouse ILC biology has rapidly expanded, but research efforts are still in early stages. Less is currently known about human ILCs, and the least about species differences between human and mouse ILCs. Similar to what has been described in the NK cell literature, species differences among ILCs are likely to be an important consideration in the future, especially with regard to ILC expression of MHC II. Presently, we know that patients with bare lymphocyte syndrome type II who share missing expression of MHC II due various genetic causes commonly have chronic diarrhea. It is possible that these patients could have enhanced responses to commensals in addition to persistent infections. Over time, data from rare, selectively immunocompromised patients may increasingly emerge to better define the role for ILCs in human. Meanwhile, continued study of basic biology of both mouse and human ILCs will be a requisite first step to more completely understand disease pathogenesis and identify new targets and strategies for disease modification.
Acknowledgments
The Colonna laboratory is supported by grants from the US National Institutes of Health (1U01AI095542, R01DE021255, and R21CA16719). The authors thank M. Cella and J. Bando for critical comments.
References
- 1.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–65. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–74. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Miller H, Song C. Beyond NK cells: the expanding universe of innate lymphoid cells. Front Immunol. 2014;5:282. doi: 10.3389/fimmu.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–7. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermijlen D, Prinz I. Ontogeny of Innate T Lymphocytes - Some Innate Lymphocytes are More Innate than Others. Front Immunol. 2014;5:486. doi: 10.3389/fimmu.2014.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinette ML, Fuchs A, Cortez VS, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–17. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 12.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32:317–25. doi: 10.1385/IR:32:1-3:317. [DOI] [PubMed] [Google Scholar]
- 14.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 15.Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol. 1997;9:507–14. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- 16.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–74. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 17.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–90. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonarbourg C, Mortha A, Bui VL, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klose CS, Flach M, Mohle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–56. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–57. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliphant CJ, Hwang YY, Walker JA, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–95. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto Y, Panea C, Nakato G, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hepworth MR, Fung TC, Masur SH, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348:1031–5. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 27.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 28.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971;134:1513–28. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137:2735–9. [PubMed] [Google Scholar]
- 30.Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–8. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walzer T, Blery M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–9. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 33.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 34.Hoglund P, Ohlen C, Carbone E, et al. Recognition of beta 2-microglobulin-negative (beta 2m−) T-cell blasts by natural killer cells from normal but not from beta 2m− mice: nonresponsiveness controlled by beta 2m− bone marrow in chimeric mice. Proc Natl Acad Sci U S A. 1991;88:10332–6. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 36.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 37.Moretta A, Vitale M, Bottino C, et al. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colonna M, Brooks EG, Falco M, Ferrara GB, Strominger JL. Generation of allospecific natural killer cells by stimulation across a polymorphism of HLA-C. Science. 1993;260:1121–4. doi: 10.1126/science.8493555. [DOI] [PubMed] [Google Scholar]
- 39.Wagtmann N, Biassoni R, Cantoni C, et al. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–49. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 40.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 41.Lee N, Llano M, Carretero M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95:5199–204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salcedo M, Bousso P, Ljunggren HG, Kourilsky P, Abastado JP. The Qa-1b molecule binds to a large subpopulation of murine NK cells. Eur J Immunol. 1998;28:4356–61. doi: 10.1002/(SICI)1521-4141(199812)28:12<4356::AID-IMMU4356>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 43.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–8. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burshtyn DN, Scharenberg AM, Wagtmann N, et al. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell KS, Dessing M, Lopez-Botet M, Cella M, Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J Exp Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–14. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 47.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–6. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 50.Smith HR, Heusel JW, Mehta IK, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–31. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Gazzar A, Groh V, Spies T. Immunobiology and conflicting roles of the human NKG2D lymphocyte receptor and its ligands in cancer. J Immunol. 2013;191:1509–15. doi: 10.4049/jimmunol.1301071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen GB, Gandhi RT, Davis DM, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 54.Petersen JL, Morris CR, Solheim JC. Virus evasion of MHC class I molecule presentation. J Immunol. 2003;171:4473–8. doi: 10.4049/jimmunol.171.9.4473. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 56.Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol. 2004;65:282–90. doi: 10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–30. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 60.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–16. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schooten E, Klous P, van den Elsen PJ, Holling TM. Lack of MHC-II expression in activated mouse T cells correlates with DNA methylation at the CIITA-PIII region. Immunogenetics. 2005;57:795–9. doi: 10.1007/s00251-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 62.Roncarolo MG, Bigler M, Haanen JB, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147:781–7. [PubMed] [Google Scholar]
- 63.Kim MY, Rossi S, Withers D, et al. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–74. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magri G, Cerutti A. Role of group 3 innate lymphoid cells in antibody production. Curr Opin Immunol. 2015;33:36–42. doi: 10.1016/j.coi.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HY, Lee HJ, Chang YJ, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–6. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klose CS, Kiss EA, Schwierzeck V, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–5. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 70.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med. 2001;194:393–406. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldburger JM, Rossi S, Hollander GA, Rodewald HR, Reith W, Acha-Orbea H. Promoter IV of the class II transactivator gene is essential for positive selection of CD4+ T cells. Blood. 2003;101:3550–9. doi: 10.1182/blood-2002-06-1855. [DOI] [PubMed] [Google Scholar]
- 73.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 74.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–25. doi: 10.1016/j.jaci.2013.07.020. quiz 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietra G, Vitale C, Pende D, et al. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother. 2015 doi: 10.1007/s00262-015-1744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]