Abstract
IL-34 and colony stimulating factor 1 (CSF1) are two alternative ligands for the CSF1 receptor that play non-redundant roles in the development, survival and function of tissue macrophages and Langerhans cells (LCs). In this study, we investigated the spatio-temporal production of IL-34 and its impact on skin LCs in the developing embryo and adult mice in the steady state and during inflammation using Il34LacZ reporter mice and newly generated inducible Il34-knockout mice. We found that IL-34 is produced in the developing skin epidermis of the embryo, where it promotes the final differentiation of LC precursors. In adult life, LCs required IL-34 to continually self-renew in the steady-state. However, during UV-induced skin damage, LCs regeneration depended on neutrophils infiltrating the skin, which produced large amounts of CSF1. We conclude that LCs require IL-34 when residing in fully differentiated and anatomically intact skin epidermis, but rely on neutrophil-derived CSF1 during inflammation. Our demonstration that neutrophils are an important source of CSF1 during skin inflammation may exemplify the mechanism through which neutrophils promote their subsequent replacement with mononuclear phagocytes.
Keywords: Langerhans cells, cell development, cytokines, IL-34, CSF1, skin inflammation
Introduction
Langerhans cells (LCs) are a unique subset of dendritic cells (DCs) that reside in the epidermis of the skin [1-3]. Because of their location, LCs are considered to be critical inducers of skin immunity, although their precise role in the initiation and control of cutaneous immune responses remains a matter of active debate [4, 5]. Additionally, LCs have been implicated in the pathogenesis of skin tumors independent of immune mechanisms, because of their capacity to transform polyaromatic hydrocarbons into potent carcinogens [6]. The development of LCs encompasses several steps [7-11]. Initially LC progenitors develop in the yolk sac and fetal liver and migrate into the primitive skin that lacks a mature epidermal layer. Towards the end of the embryonic life, the skin develops an epidermal layer and acquires barrier function conferred by the stratus corneum. LC precursors move into the epidermis and differentiate into mature LCs shortly after birth. During the first week of life, LCs undergo a burst of proliferation, after which LCs self-renew by slowly dividing throughout life.
Colony-stimulating factor-1 receptor (CSF1R) is crucial for both development and maintenance of LCs, as demonstrated by the severe defect in LCs in Csf1r−/− mice [12, 13] and wild-type (WT) mice treated with CSF1R-blocking antibodies. CSF1R has two distinct ligands: colony-stimulating factor-1 (CSF1), which is produced by various cell types [14, 15]; and interleukin-34 (IL-34), which is produced by keratinocytes and neurons [15-18]. CSF1 and IL-34 play distinct roles in LCs development. LCs numbers are considerably reduced in newborn but are normal in adult CSF1-deficient mice (Csf1op/op) [12, 13, 19], suggesting that CSF1 plays an early role in LCs development but is not required for LCs maintenance. IL-34-deficient mice (Il34LacZ/LacZ) have a marked defect in LCs at birth that persists in adult mice [17, 18], suggesting that IL-34 promotes LCs development and possibly LCs persistence throughout life.
Following exposure to ultraviolet (UV) light irradiation or other inflammatory stimuli, LCs migrate into skin-draining lymph nodes or die, depleting the LCs niche [20-22]. Regeneration of skin LCs relies on bone marrow-derived monocytes that migrate into the skin and differentiate into LCs [13, 23-25]. While genetic ablation of Csf1r in LCs impairs LCs regeneration following inflammation [18], IL-34 and CSF1 play non-redundant roles. In Il34LacZ/LacZ mice, initial LCs regeneration is intact but the persistence of newly generated LCs is impaired. In contrast, LCs repopulation is delayed in Csf1op/op mice, indicating that CSF1 is essential for the early phase of LCs regeneration [13], although the source of CSF1 during this process is unknown.
In this study, we investigated the spatio-temporal production of IL-34 and its impact on skin LCs in the developing embryo and adult mice in the steady state and during inflammation. We found that IL-34 promotes LCs differentiation and survival in synchrony with maturation of the skin epidermis. Moreover, we generated a novel inducible Il34-knockout mouse line and demonstrated that IL-34 is required for the maintenance of LCs in the intact epidermis of adult mice. Remarkably, after UV-mediated skin damage, LCs repopulation in the skin depended on infiltrating neutrophils that produced CSF1, whereas IL-34 was dispensable. Collectively, our results demonstrate distinct spatio-temporal roles of IL-34 and CSF1 in LCs development. Renewal of LCs residing in a fully differentiated and anatomically intact skin epidermis requires IL-34, whereas renewal of LCs in the inflamed skin requires neutrophil-derived CSF1.
Results
IL-34 controls LCs differentiation and survival in synchrony with maturation of the skin epidermis
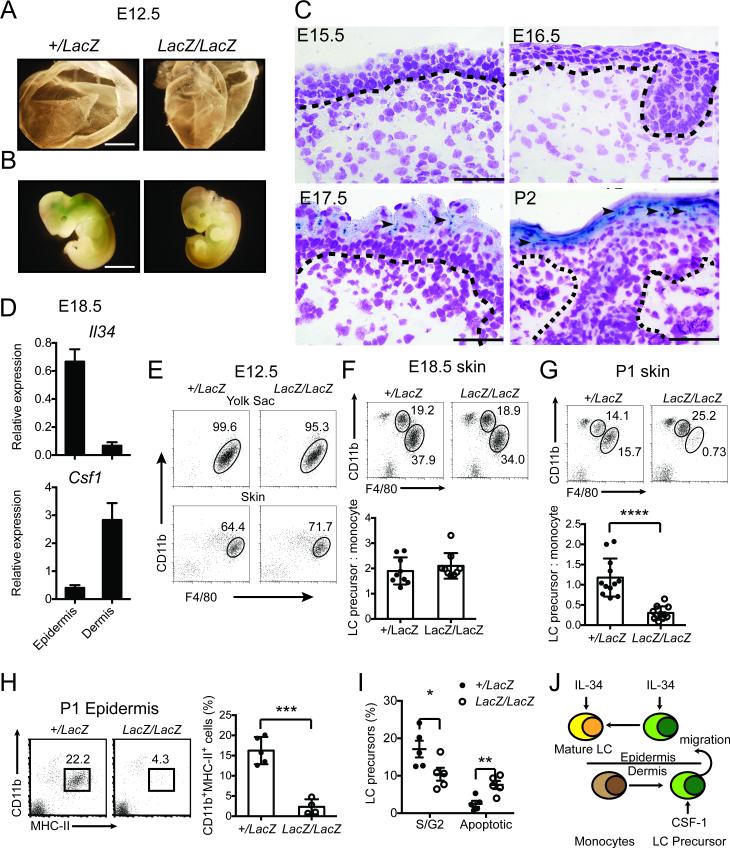
To determine the spatial-temporal expression of IL-34 at successive developmental stages of the skin, we stained Il34+/LacZ embryos with Xgal, which detects expression of the β-galactosidase reporter driven by the IL-34 promoter. Early on (embryonic day 12.5, E12.5), β-galactosidase was expressed neither in the yolk sac nor in the limb buds that comprise the primitive skin (Figure 1A, B). β-galactosidase expression became detectable in the skin at E17.5, concomitant with the appearance of mature epidermis and was sustained after birth (Figure 1C). Real time PCR confirmed that Il34 mRNA is highly expressed in the epidermis of E18.5 embryos, whereas Csf1 mRNA is predominantly expressed in the dermis (Figure 1D). Thus, IL-34 production substantially overlaps with maturation of the skin epidermis.
Figure 1. IL-34 promotes survival and differentiation of LC precursors in synchrony with maturation of the skin epidermis in the embryo.
(A-J) Il34+/LacZ and Il34LacZ/LacZ embryos (n=3-12) were examined at different stages of embryogenesis. LacZ expression and frequencies of tissue monocytes, macrophages, LC precursors and mature LCs were examined. (A, B) X-gal staining of E12.5 yolk sac (A) and embryos (B). Original magnification 4X. Scale bar=2mm. (C) X-gal staining of the skin of E15.5, E16.5, E17.5 embryos and P2 neonates. Original magnification 400X. Scale bar= 50μm. (A-C) Images shown are representative of 2 independent experiments with 3-4 embryos per group. (D) Il34 and Csf1 mRNA expression in E18.5 epidermis and dermis. (E) FACS analysis of frequencies of primitive macrophages among CD45+ cells in the yolk sac and skin of E12.5 embryos. (F, G) FACS analysis of frequencies of CD11bhiF4/80lo fetal liver-derived monocytes and CD11bloF4/80hi LC precursors among CD45+ cells in the skin of E18.5 embryos (F) and P1 neonates (G). (H) FACS analysis of LC precursors in the epidermis of P1 neonates. (I) Cell cycle analysis of LC precursors in the skin of P1 neonates. (D-I) Data are shown as mean ±SD (n= 3-5) and are representative of two independent experiments (D, E) or pooled from three independent experiments (F-I). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, Student's t-test. (J) Proposed model for the role of IL-34 and CSF1 in prenatal and perinatal development of LCs..
We next analyzed the impact of IL-34 deficiency on LC precursors and mature LCs before and after development of the epidermis. At E12.5, we examined primitive macrophages (defined as CD11b+F4/80+ cells) and found that they were equally represented in the yolk sac and in the total skin of Il34LacZ/LacZ and Il34+/LacZ embryos (Figure 1E). At E18.5, we examined fetal liver-derived monocytes (CD11bHIF4/80LO cells) and LC precursors (CD11bLOF4/80HI cells) as defined by Hoeffel et al. [7] (Supporting information Fig. 1). Equivalent numbers of both populations were present in the skin of Il34LacZ/LacZ and Il34+/LacZ embryos (Figure 1F). Thus, IL-34 deficiency has no detectable effect on LC precursors before the complete maturation of the epidermis. In contrast, LC precursors (CD11bLOF4/80HI cells) and mature LCs (CD11b+MHCII+ cells) were markedly reduced in the skin of Il34LacZ/LacZ neonates in comparison to Il34+/LacZ neonates (Figure 1G, H). Histological analysis confirmed a selective reduction of CD11b+ LCs in the epidermis of Il34LacZ/LacZ 2-day-old (P2) neonates and E18.5 embryos compared to Il34+/LacZ controls (Table 1, Supporting information Fig. 2A, B), suggesting that LCs require IL-34 once they migrate into the epidermis. Consistent with these results, cell cycle analysis revealed less proliferation and more apoptosis among LC precursors in the skin of Il34LacZ/LacZ neonates than in the skin of Il34+/LacZ neonates (Figure 1I). We conclude that IL-34 is produced as soon as the skin epidermis matures and that it becomes essential for LCs when they move into the epidermis to take up their final residence. In contrast, IL-34 deficiency has no detectable impact on LC precursors before maturation of the epidermis, most likely because LC precursors are confined to the prospective dermis of the immature skin, where CSF1 can support their survival (Figure 1J).
Table 1.
Impact of IL-34 deficiency on CD11b+ cells in the epidermis of E18.5 embryos and 2-day-old (P2) neonates
| E18.5 |
P2 |
||||
|---|---|---|---|---|---|
| +/LacZ | LacZ/LacZ | P value | +/LacZ | LacZ/LacZ | P value |
| 2.93±1.79 | 0.75±1.29 | 0.01 | 4.08±1.87 | 0.67±1.30 | 0.04 |
IL-34 maintains LCs homeostasis
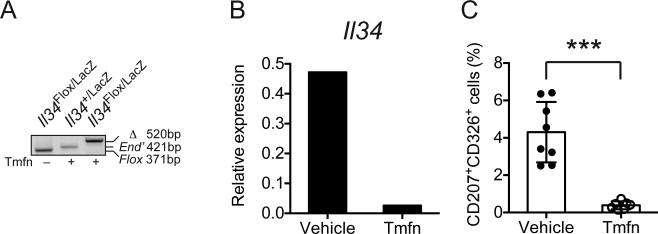
Although Il34LacZ/LacZ mice lack LCs at birth and throughout life [17, 18], it is unclear whether IL-34 is essential only for LCs development or also for LCs maintenance in the epidermis. To answer this question, we generated an inducible Il34-knockout mouse that harbors one Il34LacZ allele and one Il34 floxed allele (Il34Flox) as well as a transgene encoding Cre recombinase fused to a triple mutant form of the human estrogen receptor under control of the human ubiquitin C (UBC) promoter (UBC-Cre/ERT2). Administration of Tamoxifen to these mice effectively deleted Il34 in the skin, resulting in marked reduction of Il34 mRNA (Figure 2A) as well as an almost complete depletion of LCs within 10 days (Figure 2B). We conclude that IL-34 is essential for maintenance of LCs in steady state and that induction of IL-34 deficiency in the skin leads to rapid depletion of LCs.
Figure 2. LCs survival in the adult requires constant IL-34 signaling.
(A-C) UBC-Cre/ERT2.Il34Flox/LacZ mice (n=4) were treated with Tamoxifen (Tmfn) i.p. for 7 days. Ear skin was analyzed 9 days after the last treatment. UBC-Cre/ERT2.Il34+/LacZ mice were used as a negative control. (A) Efficacy of Cre-mediated recombination after Tamoxifen treatment was assessed by a PCR assay that detected endogenous wild-type Il34 allele (End), floxed Il34 allele (Flox) and Cre excised allele (Δ). Blot is representative of three independent experiments. (B) Ablation of Il34 mRNA after Tamoxifen treatment was confirmed by real time PCR. (C) Frequencies of CD207+ (Langerin+) CD326+ (Epcam+) LCs after Tmfn treatment were measured by flow cytometry. Data are shown as mean±SD (n=6-8 mice/group) and are pooled from two independent experiments. ***p<0.001, Student's t-test.
LCs regeneration after UV-induced skin injury requires neutrophil-derived CSF1
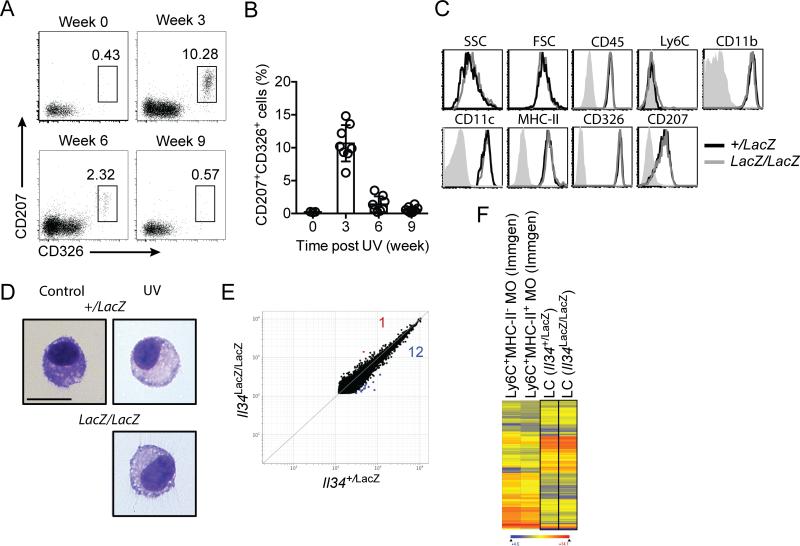
It has been shown that regeneration of LCs from circulating monocytes after UV-induced damage is IL-34 independent, whereas maintenance of newly generated LCs requires IL-34 [17, 18]. Accordingly, LCs repopulated the epidermis of Il34LacZ/LacZ mice 3 weeks after UV-induced injury of ear skin but disappeared after 9 weeks (Figure 3A, B). A transient increase in Csf1 mRNA in UV-damaged skin has been reported [18], which suggests that CSF1 may be sufficient for the generation of monocyte-derived LCs in the absence of IL-34. However, it is possible that LCs generated in the presence or absence of IL-34 are functionally distinct. To address this question, we compared various features of LCs repopulating WT and Il34LacZ/LacZ mice 21 days after UV treatment and found them virtually indistinguishable in terms of phenotypic markers (Fig. 3C), morphology (Fig. 3D) and gene expression profiles (Fig. 3E, F). Thus, these data indicate that IL-34 does not play a relevant role in the regeneration of LCs after UV-induced skin injury.
Figure 3. IL-34 is essential for long-term maintenance of LCs after UV injury.
(A, B) Frequencies of LCs in the epidermis of Il34LacZ/LacZ mice at various time points post UV treatment. Data are shown as mean±SD (n=8-10) and are pooled from two independent experiments. (C, D) Comparison of phenotypic markers by flow cytometry (C) and cellular morphology by cytospin (D) of LCs in Il34+/LacZ and Il34LacZ/LacZ mice 21 days post UV treatment. Data shown are representative of two independent experiments. Original magnification 400X. Scale bar= 10μm. (E, F) Gene expression analysis of FACS-sorted LCs from Il34LacZ/LacZ or Il34+/LacZ mice 21 days post UV treatment. (E) Scatter plot shows direct comparison of genes with expression value greater than 120 between LCs from Il34LacZ/LacZ and Il34+/LacZ mice. (F) Heat map shows very similar expression of a set of genes in LCs purified from Il34LacZ/LacZ and Il34+/LacZ mice after UV irradiation, as compared to Ly6C+ MHC class II− monocytes and Ly6C+ MHC class II+ monocytes, which were used as reference. This set of genes includes genes that are at least 4-fold enriched in either LCs (GSE) or Ly6C+ blood monocytes (www.immgen.org, GSE15907). Gene expression data shown are representative of the average of two independent experiments.
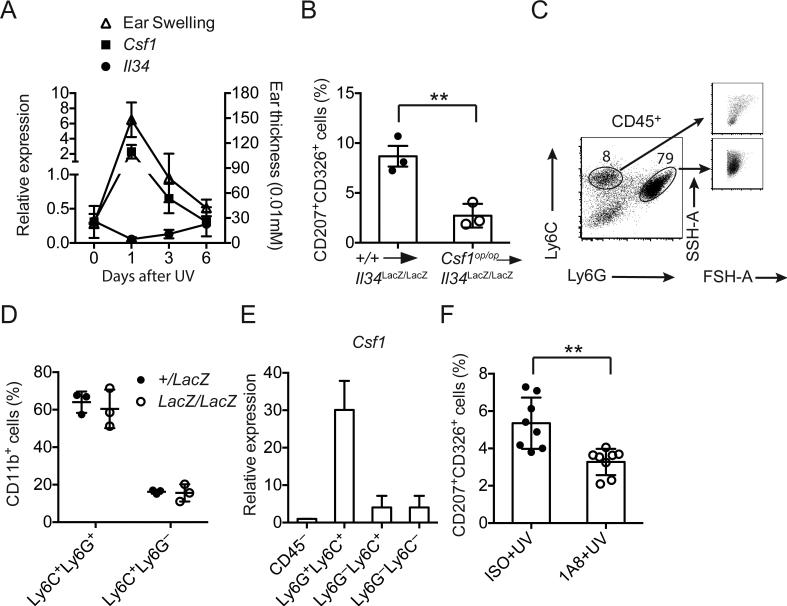
We next asked: what is the cellular source of CSF1 for LCs regeneration? We found that CSF1 expression peaked one week after skin injury, coincident with the appearance of inflammation and swelling, whereas IL-34 expression decreased, likely due to keratinocytes injury (Fig. 4A). To determine the origin of CSF1 during LCs repopulation, we reconstituted Il34LacZ/LacZ mice with bone marrow from WT or CSF1-deficient (Csf1op/op) mice after lethal irradiation. We then exposed mice to UV irradiation and determined the frequencies of LCs in the epidermis after 21 days. Repopulation of LCs in Il34LacZ/LacZ mice reconstituted with Csf1op/op bone marrow was significantly attenuated (Figure 4B), indicating that the CSF1 required for inducing the generation of LCs after inflammation derives at least in part from cells of hematopoietic origin. Major CD45+ leukocyte populations present in the skin after UV radiation included Ly6G+Ly6C+ neutrophils and Ly6C+ monocytes, both of which were recruited in an IL-34-independent fashion (Fig. 4C, D). We noticed that neutrophils represented the major population of CD45+ leukocytes in the skin and expressed more Csf1 mRNA than all other cell types in the skin (Fig. 4E), suggesting that neutrophils may be the major source of CSF1 for LCs regeneration. Indeed, systemic depletion of neutrophils in Il34LacZ/LacZ mice during UV-mediated skin injury using an anti-Ly6G antibody significantly attenuated generation of LCs (Fig. 4F), even though the efficiency of depletion in the skin was approximately 50% (data not shown). We conclude that that the generation of monocyte-derived LCs after UV-induced damage is dependent on neutrophil-derived CSF1.
Figure 4. LCs regeneration following UV-induced skin damage requires neutrophil-derived CSF1.
(A) Induction of inflammation (as measured by skin thickness) and the expression of Csf1 and Il34 mRNA were determined in the ears WT mice after UV treatment. (B) Il34LacZ/LacZ mice were reconstituted with bone marrow cells from WT or Csf1op/op mice after lethal irradiation. LCs regeneration was determined 21 days after UV treatment by flow cytometry. (C, D) Ly6G+Ly6C+ neutrophils and Ly6C+ monocytes represent ~90% of the CD45+ cells that are recruited to the UV damaged skin. (C) Right panels show differences in size (FSH-A) and granularity (SSH-A) between Ly6G+Ly6C+ neutrophils and Ly6C+ monocytes. (D) IL-34 deficiency does not affect the percentages of infiltrating Ly6G+Ly6C+ neutrophils and Ly6C+ monocytes after UV irradiation. (E) qPCR analysis of Csf1 mRNA expression in several population of cells in UV damaged skin. Infiltrating neutrophils express highest level of Csf1. (F) Anti-Ly6G 1A8 mAb injection attenuates the regeneration of LCs in Il34LacZ/LacZ mice in response to UV. Il34LacZ/LacZ mice were treated with 200 μg 1A8 mAb i.p. on day 5 post UV treatment and every other day for 15 days. Frequencies of LCs were determined 21 days post UV. (A-F) Data are shown as mean±SD (n=3-8) and are representative of two independent experiments. **p<0.01, Student's t-test.
Discussion
In this study, we defined the spatio-temporal expression of IL-34 and its impact on the generation of LCs in the embryo and adult in the steady-state and during inflammation. It was previously shown that IL-34 deficiency affects the skin content of LC precursors [18]; however, the specific stage of LCs differentiation at which IL-34 is required was not delineated. We showed that IL-34 is produced in the developing epidermis in the embryo and is selectively required during the final stage of LCs development, when LC precursors migrate into the epidermis and differentiate into mature LCs. Using a newly generated inducible Il34-knockout mouse, we demonstrated that LCs are immediately affected by the induced depletion of IL-34. Thus, in adult life, LCs continually require IL-34 for self-renewal in the steady-state.
After UV-induced skin damage, however, LCs regeneration was independent of IL-34 and relied on CSF1. Moreover, we noted that the morphological, phenotypic and transcriptional profiles of monocyte-derived LCs repopulating the skin in the presence or absence of IL-34 were almost superimposable. We postulate that once LC progenitors have differentiated in the epidermis, their features are largely driven by the tissue microenvironment, which dictates the final functional profile regardless of whether their initial generation was IL-34- or CSF1-dependent. Importantly, we found that neutrophils infiltrating the skin were a major source of CSF1 required for LCs reconstitution from circulating monocytes. Our study demonstrates for the first time that neutrophils are substantial producers of CSF1 during skin inflammation. Since Csf1op/op mice have been shown to have reduced cutaneous expression of Il34 mRNA [26], it is possible that neutrophil-derived CSF1 also contributes to subsequent production of IL-34 during the repair phase. The capacity of neutrophils to produce large amounts of CSF1 is consistent with the neutrophil RNA profile reported by the Immgen consortium (http://www.immgen.org). Moreover, neutrophil production of CSF1 has been noted in another context, specifically during activation of neutrophils by sodium caseinate, which is used in a mouse experimental model of peritoneal inflammation [27, 28]. The demonstration that neutrophils are an important source of CSF1 illustrates a mechanism through which neutrophils promote their subsequent replacement with mononuclear phagocytes during skin inflammation. In conclusion, our study shows that LCs depend on IL-34 when residing in a fully differentiated and anatomically intact skin epidermis, but rely on neutrophil-derived CSF1 in inflamed skin.
Materials and Methods
Mice and Treatment
Il34LacZ/LacZ and Il34Flox/LacZ mice were generated as previously described [17]. UBC-Cre/ERT2 mice were purchased from the Jackson Laboratory. C57BL/6 WT control mice were bred in house. All animal studies were approved by the Washington University Animal Studies Committee. Tamoxifen working solution was prepared by first dissolving 100mg of Tamoxifen citrate (Sigma) in 1mL 100% ethanol before diluting into sunflower oil to a final concentration of 10mg/mL. Mice were injected with 200uL of Tamoxifen working solution daily i.p. for 7 days. To induce skin inflammation, mice were treated with UVB for 30min as previously described [13].
Bone marrow chimeras
Bone marrow cells were isolated from WT and Csf1op/op mice and retro-orbitally injected into lethally irradiated Il34LacZ/LacZ mice. Chimeric mice were housed for 6 weeks before UV treatment.
Immunohistochemistry
Sections for immunohistochemistry were obtained from formalin-fixed paraffin-embedded or frozen tissues. For morphology, H&E stained slides were evaluated. Five micron cryostat sections were air dried overnight, fixed in acetone for 10 min and stained by using the β-Galactosidase Reporter gene staining kit (Sigma) as described previously. Nuclei were counterstained with fast red or giemsa. A similar method was also used to detect β-Galactosidase reporter activity in embryos. Four-micron sections from fixed tissues were stained using anti-CD11b mAb (M1/70) and reactivity revealed using Rat-on-mouse HRP-Polimer (Biocare Medical) followed by diaminobenzidine (DAB). Nuclei were counterstained with haematoxylin. To examine LCs morphology, sorted LCs were centrifuged onto glass slides, air dried overnight and counterstained with Hema3 Stat Pack (Fisher Diagnostics). All images were acquired using an Olympus DP70 camera mounted on an Olympus Bx60 microscope.
Cell isolation
Yolk sacs and limb buds were digested with collagenase D (100ug/mL, Sigma) for 30 min and vortexed to achieve single cell suspension. Epidermis was isolated as previously described [17]. To examine skin infiltrating monocytes and neutrophils, ear skin was cut into fine pieces and digested with collagenase VIII (4830U/mL, Sigma) and DNase I (0.1mg/mL, Sigma) for 60min.
Flow cytometry analysis
LCs and other cell types in single cell suspension were incubated with HB-197 supernatant (ATCC) for Fc receptor blockade and stained for CD11b, CD11c, CD326 (eBioscience), CD45, F4/80, Ly6C, MHC-II (BD Pharmingen), Ly6G (BioLegend). For intracellular staining with CD207 (L31, eBioscience), cells after surface staining were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Bioscience). Cells were processed on a FACSCalibur or FACSCanto II (BD Bioscience) and analyzed with FlowJo (Treestar).
Quantitative PCR and microarray analysis
RNA was extracted from skin using the RNeasy Fibrous Tissue Mini kit (QIAGEN) as recommended by the manufacturer. cDNA was synthesized from RNA with Superscript III first-strand synthesis system for RT-PCR (Invitrogen), and relative levels of RNA expression were determined by qPCR. SYBR Green PCR master mix (Bio-Rad Laboratories) and an ABI7000 machine (Applied Biosystems) were used according to the manufacturer's instructions. Follo wing oligos were used: Il34 Forward: 5’-ACTCAGAGTGGCCAACATCACAAG-3’, Il34 reverse: 5’-ATTGAGACTCACCAAGACCCACAG-3’, Csf1 forward: 5’-TACAAGTGGAAGTGGAGGAGCCAT-3’, Csf1 reverse: 5’-AGTCCTGTGTGCCCAGCATAGAAT-3’, Hprt Forward: 5’-GCAGTACAGCCCCAAAAT-3’, Hprt Reverse: 5’-AACAAAGTCTGGCCTGTATCCAA-3’. For Mouse Gene 1.0 ST Arrays, RNA was extracted from sorted LCs using the Arturus PicoPure RNA Isolation Kit (Applied BioSystems), amplified with a WT Expression kit (Ambion) and labeled, fragmented and hybridized with a WT Terminal Labeling and Hybridization kit (Affymetrix). Data were processed by robust multiarray average (RMA) normalization, and expression values were modeled with ArrayStar software (DNASTAR).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad software).
Supplementary Material
Acknowledgements
We would like to thank I. Kang and K. Choi for the assistance in embryo isolation. Y.W. is supported by Lilly Innovation Fellowship Award (Eli Lilly and Company). M.B. is supported by Fondazione Beretta (Brescia, Italy). T.K.U. is supported by T32 training grant from NCI (5T32CA009547-30). M.C. is supported by NIH R21 AI105728 and the Alvin J. Siteman Cancer Research Fund (#09-FY14-01).
Footnotes
Authorship Contributions
Y.W. designed, performed, analyzed experiments and wrote the manuscript; M.B. and W.V. carried out immunohistochemical analysis; T.K.U. performed bone marrow transplant. S.G. generated the UBC-Cre/ERT2.Il34Flox/LacZ mice and setup timed-mating experiments; M.Co. supervised research and wrote the manuscript.
Conflict of Interest
Yaming Wang is an employee of Lilly & Company.
References
- 1.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, Malissen B. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 2010;88:366–375. doi: 10.1038/icb.2010.34. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity? Immunol Cell Biol. 2010;88:351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 6.Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, Kwong BY, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, et al. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 15.Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur J Immunol. 2014;44:1575–1581. doi: 10.1002/eji.201344365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 20.Bergstresser PR, Toews GB, Streilein JW. Natural and perturbed distributions of Langerhans cells: responses to ultraviolet light, heterotopic skin grafting, and dinitrofluorobenzene sensitization. J Invest Dermatol. 1980;75:73–77. doi: 10.1111/1523-1747.ep12521261. [DOI] [PubMed] [Google Scholar]
- 21.Aberer W, Schuler G, Stingl G, Honigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J Invest Dermatol. 1981;76:202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 22.Kolgen W, Both H, van Weelden H, Guikers KL, Bruijnzeel-Koomen CA, Knol EF, van Vloten WA, et al. Epidermal langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. J Invest Dermatol. 2002;118:812–817. doi: 10.1046/j.1523-1747.2002.01742.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sere K, Baek JH, Ober-Blobaum J, Muller-Newen G, Tacke F, Yokota Y, Zenke M, et al. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity. 2012;37:905–916. doi: 10.1016/j.immuni.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Chopin M, Seillet C, Chevrier S, Wu L, Wang H, Morse HC, 3rd, Belz GT, et al. Langerhans cells are generated by two distinct PU.1-dependent transcriptional networks. J Exp Med. 2013;210:2967–2980. doi: 10.1084/jem.20130930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago E, Mora L, Bautista M, Montesinos JJ, Martinez I, Ramos G, Zambrano IR, et al. Granulocyte colony-stimulating factor induces neutrophils to secrete macrophage colony-stimulating factor. Cytokine. 2001;15:299–304. doi: 10.1006/cyto.2001.0937. [DOI] [PubMed] [Google Scholar]
- 28.Santiago-Osorio E, Mora L, Bautista M, Montesinos JJ, Martinez I, Ramos-Mandujano G, Zambrano R, et al. Sodium caseinate induces secretion of macrophage colony-stimulating factor from neutrophils. Immunobiology. 2010;215:332–339. doi: 10.1016/j.imbio.2009.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.