Abstract
Rationale
A major issue in the addiction field is the limited number of animal models of the voluntary induction and maintenance of alcohol dependence in outbred rats.
Objectives
To address this issue, we developed a novel apparatus that vaporizes alcohol for 2–10 min after an active nosepoke response.
Methods
Male Wistar rats were allowed to self-administer alcohol vapor for 8 h/day every other day for 24 sessions (escalated) or eight sessions (non-escalated). Escalated and non-escalated rats were then tested for progressive ratio responding. Anxiety-like behavior, somatic signs of withdrawal, and hyperalgesia were assessed during acute withdrawal.
Results
The results showed that rats exhibited excellent discrimination between the active and inactive operanda (>85%), and the escalated rats quickly increased their blood alcohol levels from ~50 to >200 mg% in ~6 weeks. Compared with non-escalated rats, escalated rats exhibited severe addiction-like behavior, including somatic signs of withdrawal, anxiety-like behavior, hyperalgesia, and higher responding on a progressive ratio schedule of reinforcement.
Conclusions
These results demonstrate that outbred rats will voluntarily self-administer alcohol vapor to the point of dependence without the use of forced alcohol administration, sweeteners, food/water restriction, operant pretraining, or behavioral/genetic selection. This novel animal model may be particularly useful for medication development to help unveil the neuronal circuitry that underlies the voluntary induction of alcohol addiction and identify novel molecular targets that are specifically recruited after the voluntary induction and maintenance of alcohol dependence.
Keywords: Addiction, Alcohol, Dependence, Vapor, Withdrawal, Anxiety
Introduction
A major issue in the alcohol field is the limited number of animal models of the voluntary induction and maintenance of alcohol dependence in outbred rats or mice. These animals will readily self-administer alcohol, but its aversive taste and pharmacological properties when ingested considerably limit the amount of alcohol that laboratory animals consume. The highest alcohol self-administration levels are obtained when animals are given intermittent access to alcohol using a two-bottle choice procedure. Even with this model, however, blood alcohol levels (BALs) are generally limited to 10–100 mg% for less than 1–3 h (Nielsen et al. 2008; Rhodes et al. 2005, 2007; Simms et al. 2008; Steensland et al. 2010; Wise 1973), and abstinence from alcohol using this model does not produce the typical somatic and affective signs of withdrawal that are observed in alcohol-dependent humans (Carnicella et al. 2014; George et al. 2012). These animal models are relevant to recreational alcohol use and binge-like drinking because they produce BALs that are comparable to humans (80 mg%; (NIAAA 2004), but they have limited relevance to human alcoholism, which is characterized by BALs in the 100–300 mg% range for most hours of the day, compulsive-like alcohol drinking, and often intense somatic and/or affective withdrawal symptoms. Although no animal model of addiction fully emulates the human condition, the existing models permit investigations of specific elements of the addiction process (Rodd et al. 2004). Multiple methods have been developed to produce BALs in the 100–300 mg% range for ≥12 h per day, associated with somatic and affective withdrawal symptoms, but they use a forced alcohol liquid diet (Gilpin et al. 2009; Lieber and DeCarli 1982; Overstreet et al. 2004), intragastric alcohol intubation (Aujla et al. 2013; Braconi et al. 2010; de Guglielmo et al. 2015; Sidhpura et al. 2010), passive exposure to alcohol vapor (Becker and Lopez 2004; de Guglielmo et al. 2016; Gilpin et al. 2008; Goldstein and Pal 1971; Kallupi et al. 2014; Lopez and Becker 2014; O’Dell et al. 2004; Rimondini et al. 2002; Roberts et al. 1996; Schulteis et al. 1995; Vendruscolo and Roberts 2014), or selected inbred lines of alcohol-preferring rats or mice (Bell et al. 2012; Ciccocioppo et al. 2006; Colombo et al. 2006; Crabbe et al. 2014; Matson and Grahame 2013; McBride and Li 1998; Quintanilla et al. 2006; Sommer et al. 2006). Forced and passive administration models have limited face validity and make it difficult to unveil the neuronal networks that mediate the voluntary induction and maintenance of alcohol dependence. Moreover, inbred lines in genetic studies cannot model the genetic heterogeneity that is found in outbred lines and humans, thus limiting their predictive validity. It is critical to develop a novel animal model of alcohol dependence in outbred rats to unveil the neuronal networks that underlie the voluntary induction and maintenance of dependence and provide novel targets and improved preclinical testing for the development of new pharmacological treatments of alcoholism. We developed a novel apparatus based on a home cage that was equipped with nosepoke holes and connected to an alcohol vaporizer. This apparatus allows the rats to self-administer alcohol vapor. Based on recent studies that demonstrated that intermittent access to alcohol accelerates the pace at which excessive levels of alcohol consumption can be established (Carnicella et al. 2009; Hopf et al. 2010; Hwa et al. 2011; Loi et al. 2010; Rosenwasser et al. 2013; Simms et al. 2008), we validated a new paradigm using intermittent access to alcohol vapor self-administration. With this model, rats self-administer alcohol vapor for 8 h every other day to the point of producing BALs in the 100–300 mg% range. This work describes the characterization and validation of this model by measuring BALs, somatic and affective signs of withdrawal (elevated plus maze (EPM), mechanical hyperalgesia), and compulsive-like responding (progressive ratio (PR)) in Wistar rats.
Materials and methods
Subjects
Male Wistar rats (Charles River, Wilmington, MA, USA; n = 32) were housed two per cage on a reverse 12 h/12 h light/dark cycle (lights off at 8:00 AM) in a temperature (20–22 °C) and humidity (45–55%) controlled vivarium with ad libitum access to tap water and food pellets (PJ Noyes Company, Lancaster, NH, USA). All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. At the time of testing, the rats’ body weight ranged from 350 to 400 g.
Apparatus
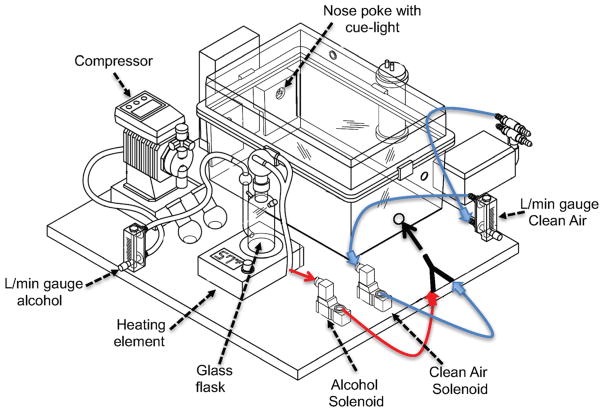
The alcohol inhalation systems that are currently available are designed to expose a large number of animals non-contingently. Alcohol is usually vaporized with a heating element and mixed with air at a concentration in the 20–30 mg/L range to produce BALs that are similar to alcohol-dependent humans (in the 100–300 mg% range). However, classic alcohol vapor exposure apparatuses are unable to vaporize alcohol upon operant fixed-ratio responding. Therefore, we developed a new apparatus that consists of a standard rat home cage that is equipped with a nosepoke hole and a cue light on each side of the chamber for active and inactive responses (Fig. 1). The apparatus was connected to a custom-made alcohol vaporization system, consisting of a heating element, a glass flask, two solenoids (one “normally opened,” connected to clean air; one “normally closed,” connected to the alcohol flask), a gas washing bottle, and a compressor. The apparatus was controlled by a Med Associates smartcard, and each response at the active nosepoke hole triggered the activation of the two solenoids. The one that was “normally open” closed, and the one that was “normally closed” opened, so alcohol vapor could be released into the chamber. After 2, 5, or 10 min (depending on the parameters used), the solenoids turned off, and clean air was pushed into the chamber. All active and inactive responses were recorded on a computer. The home cage was always ventilated with a minimum of 15 L/min of clean air during the experiment.
Fig. 1.
Schematic of the apparatus
Intravenous catheterization
Catheterization was necessary for BAL determination in the first experiment. The animals were anesthetized by inhalation of a mixture of isoflurane, and intravenous catheters were aseptically inserted in the right jugular vein using a modified version of a previously described procedure (Caine and Koob 1993). The vein was punctured with a 22-gauge needle, and the tubing was inserted and secured inside the vein by tying the vein with suture thread. The catheter assembly consisted of an 18-cm length of Micro-Renathane tubing (0.023 in. inner diameter, 0.037 in. outer diameter; Braintree Scientific, Braintree, MA, USA) that was attached to a guide cannula (Plastics One, Roanoke, VA, USA) that was bent at a near right angle, embedded in dental acrylic, and anchored with 2 cm2 mesh. The catheter exited through a small incision on the back, and the base was sealed with a small plastic cap and metal cover cap. This design helped to keep the catheter base sterile and protected. Catheters were flushed daily with heparinized saline (10 units/ml of heparin sodium (American Pharmaceutical Partners, Schaumburg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA)) that contained antibiotic (20 mg/0.2 ml Timetin, GlaxoSmithKline, Philadelphia, PA, USA) once daily for 1 week, then once every 7–14 days, followed by heparinized saline.
Characterization of the kinetics of blood alcohol levels after exposure to alcohol vapor
The concentration of alcohol (obtained from the Department of Environmental Health and Safety, The Scripps Research Institute, La Jolla, CA, USA) that was delivered into the alcohol vapor self-administration chamber was determined by collecting a 5-s sample from the reward port into an airtight 50 ml syringe. Samples were then pushed into a breathalyzer (Intoxicometers, St. Louis, MO, USA) to determine air alcohol levels. To evaluate the effect of exposure to alcohol vapor on BALs, 12 rats were prepared with intravenous catheters. One week later, they were placed in the new apparatus. The animals were passively exposed to alcohol vapor (15 L/min) for 64 min (32 vapor pulses, 1 pulse = 2 min), and blood samples (0.1 ml) were collected from the catheter after 1, 2, 4, 8, 16, 32, and 64 min. After the vapor was turned off, blood samples were collected every 30 min for 3 h until the BALs returned to zero.
Chronic intermittent alcohol vapor self-administration model
The animals were trained to self-administer alcohol vapor in 8-h sessions every other day from 10 AM to 6 PM for a total of 24 sessions. Rats did not have access to food or water during the test sessions. For the first 8 sessions, a response in the active nosepoke hole resulted in the activation of two solenoids (one for alcohol vapor opened and one for clean air closed) to expose the animals to alcohol vapor (15 L/min) for 2 consecutive minutes. The illumination of a cue light for 20 s followed a nosepoke response. Any nosepoke in the active hole while the cue light was on did not result in any delivery of alcohol vapor (timeout (TO)). After the 20-s TO, responses in the active nosepoke hole released alcohol vapor for another 2 min, and the cue light was illuminated again for another 20 s. Responses in the opposite hole were recorded but had no consequences. In the next eight sessions, the animals were subjected to the same parameters. The only difference was that the time of vapor exposure after each nosepoke increased to 5 min. In the last eight sessions, the time of vapor exposure after each nosepoke increased to 10 min. In the last 16 sessions (5 and 10 min of vapor per response), the animals progressively escalated their alcohol self-administration. “Escalated” animals were defined as those that were subjected to 24 sessions of alcohol vapor self-administration (with 2, 5, and 10 min of alcohol vapor per response). “Non-escalated” animals were defined as those that only self-administered alcohol vapor for 3 weeks in the 2-min paradigm (2 min of vapor exposure after each active nosepoke).
Progressive ratio responding for alcohol vapor self-administration
Two groups of rats, escalated and non-escalated, were exposed to a PR schedule for three consecutive sessions, during which the number of nosepokes that were necessary to obtain the next reinforcement progressively increased according to the following progression: 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 7, 7, 9, 9, 11, 11, 13, 13, etc. (Leao et al. 2015; Vendruscolo et al. 2012). Each reinforcement consisted of 10 min of alcohol vapor. The PR session stopped after 8 h or when 60 min elapsed after the last reinforcement.
Determination of blood alcohol levels
Blood was sampled for the determination of BALs during vapor exposure and also upon removal from the chambers in sessions 8, 16, and 24 at the end of each phase of the self-administration paradigm. The rats were gently restrained under the technician’s arm, and the tip of the tail (2 mm) was cut with a clean razor blade or drawn from an intravenous catheter.
Blood samples (0.1 ml) were collected in Eppendorf tubes (catalog no. 05-407-13C, Fisher Scientific, Waltham, MA) that contained evaporated heparin and kept on ice. The samples were centrifuged, and serum was decanted into fresh Eppendorf tubes. The serum was then injected into an oxygen-rate alcohol analyzer (Analox Instruments, Stourbridge, UK) for BAL determination.
Withdrawal score
Behavioral signs of withdrawal were measured 8 h after the end of session 24 using a rating scale that was adapted from Macey et al. (1996). Withdrawal signs included ventromedial limb retraction (VLR), irritability to touch (vocalization (VOC)), tail rigidity (TR), abnormal gait (AG), and body tremors. Each sign was assigned a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, and 2 = severe. The sum of the four observation scores (0–8) was used as a quantitative measure of withdrawal severity.
Elevated plus maze
The elevated plus maze is a pharmacologically validated model of anxiety. The apparatus was constructed from black plastic and consisted of two open arms (50 cm × 10 cm) and two closed arms of the same size but with sidewalls. The apparatus was elevated 50 cm above the floor. All arms of the maze were connected by a central area (10 cm × 10 cm). Testing was performed under dim red light. The number of entries into the open and closed arms and time spent on the open and closed arms were recorded for 5 min. The rats were placed in the central area of the maze facing one of the open arms. An arm entry was defined as all four paws entering an arm. A trained observer who was blind to the treatment conditions scored behavior.
Mechanical nociception (von Frey test)
The hindpaw withdrawal threshold was determined using von Frey filaments, ranging from 3.63 to 125.89 g. A test session began after 10 min of habituation to the testing environment. A series of von Frey filaments was applied from below the wire mesh to the central region of the plantar surface of the left hindpaw in ascending order, beginning with the smallest filament (3.63 g). The filament was applied until buckling of the hair occurred, and it remained in place for approximately 2 s. A sharp withdrawal of the hind paw indicated a positive response. The stimulus was incrementally increased until a positive response was observed and then decreased until a negative response was observed to determine a pattern of responses to apply to the statistical method of Dixon (Dixon 1980). The 50% paw withdrawal threshold was determined by the formula Xf + kδ, where Xf is the last von Frey filament employed, k is the Dixon value that corresponds to the response pattern, and δ is the mean difference between stimuli. Once the threshold was determined for the left hindpaw, the same testing procedure was applied to the right hindpaw after 5 min. Paw withdrawal thresholds were recorded before beginning the alcohol vapor self-administration sessions (i.e., when the animal was still naive to alcohol vapor self-administration) and 8 h after the last self-administration session (8 h into withdrawal).
Statistical analysis
Self-administration and elevated plus maze data were analyzed by appropriate analyses of variance (ANOVAs), followed by the Newman-Keuls post hoc test. Progressive ratio and mechanical nociception data were analyzed by Student’s t test. Data from mechanical nociception testing during withdrawal were analyzed by Student’s t test by comparing the withdrawal results with the baselines during the naive state. Withdrawal signs were analyzed by the nonparametric Mann-Whitney U statistic, followed by Dunn’s multiple-comparison test.
Results
Characterization of the kinetics of blood alcohol levels after exposure to alcohol vapor in the new apparatus
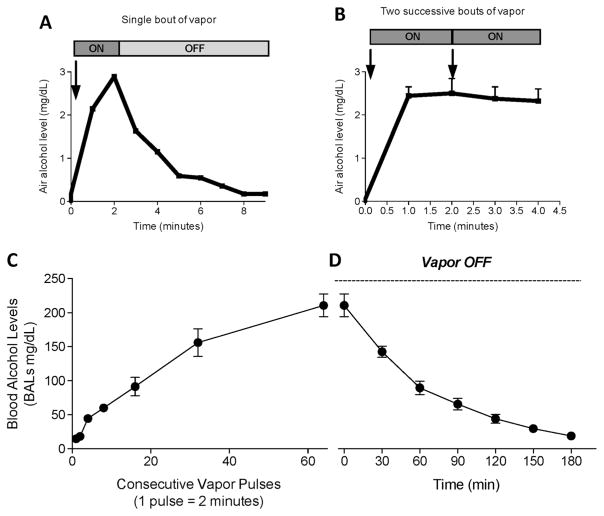
After a single nosepoke, air alcohol levels reached 2–3 mg/dl within 2 min of alcohol exposure and significantly decreased when the alcohol vapor was turned off, producing a return of alcohol vapor levels to undetectable levels after ~8 min (Fig. 2a). We then measured the stability of air alcohol levels after multiple nosepokes. If another response occurred in the active nosepoke hole before the end of the first 2 min, then air alcohol levels remained constant at ~2.5 mg/dl (Fig. 2b). For technical reasons, alcohol vapor in this test was stopped at the 3-min time-point, but it was normally maintained for 2 min after the second nosepoke.
Fig. 2.
a Air alcohol levels after one nosepoke (2 min of vapor). b Air alcohol levels after two consecutive nosepokes (2 + 2 min of vapor). Blood alcohol levels (c) during 1 h of consecutive vapor pulses (1 pulse = 2 min) and d) in the successive 3 h after discontinuing alcohol vapor. The data are expressed as mean ± SEM
The next experiment characterized the kinetics of BALs after exposure to various pulses of alcohol. Each vapor pulse (2 min) corresponded to one nosepoke (under a fixed-ratio 1 schedule). After the equivalent of ~20 nosepokes, the rats had BALs that were relevant to heavy drinking (> 80 mg%), reaching nearly 200 mg% after ~60 consecutive nosepokes (every 2 min; Fig. 2c). At this point, we evaluated the clearance of alcohol and determined that ~3 h was necessary to return BALs to near zero (Fig. 2d).
Alcohol vapor self-administration paradigm
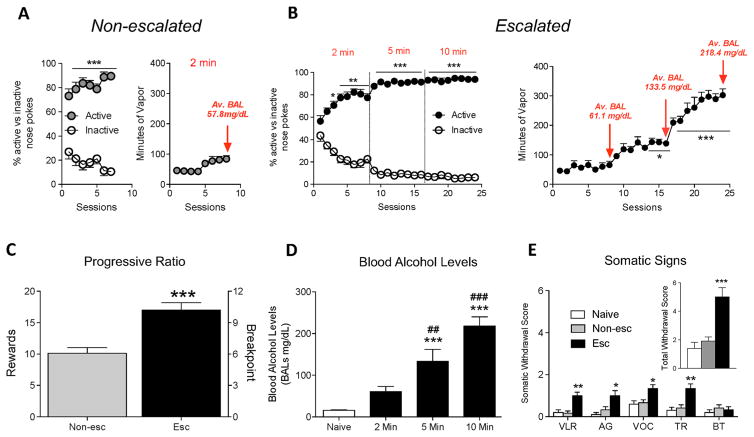
The rats discriminated very well between the active and inactive nosepoke operanda (Fig. 3a, b, left panels). In the non-escalated group, the mixed factorial ANOVA, with lever as the between-subjects factor and session as the within-subjects factor, revealed a significant lever × session interaction (F7,98 = 5.722, p < 0.001). In the first session, the animals emitted 66.8% (73.8 ± 3.9) active nosepokes vs. 33.2% (35.6 ± 4.3) inactive nosepokes. The percentage of active vs. inactive nosepokes increased as the number of sessions increased. In the last four sessions, the animals exhibited strong preference for the active nosepoke hole (90%, 55.5 ± 3.3 vs. 5.9 ± 2.6). In the escalated group, the mixed factorial ANOVA, with lever as the between-subjects factor and session as the within-subjects factor, revealed a significant lever × session interaction (F23,506 = 31.39, p < 0.001). In the first session, the animals emitted 55.3% (83.9 ± 6.1) active nosepokes vs. 43.7% (35.8 ± 3.8) inactive nosepokes. The percentage of active vs. inactive nosepokes increased as the number of sessions increased and as the time of vapor exposure increased after each nosepoke. The animals emitted 77.3% active nosepokes (49.7 ± 4.7 vs. 14 ± 5.1) at the end of the 2-min phase (p < 0.01, vs. inactive nosepokes), 91.3% (54.1 ± 10.9 vs. 5.6 ± 2.5) at the end of the 5-min phase (p < 0.001, vs. inactive nosepokes), and 93.8% (52.4 ± 6.9 vs. 3.1 ± 2.8) at the end of the 10-min phase (p < 0.001, vs. inactive nosepokes).
Fig. 3.
a Left discrimination between active and inactive nosepokes throughout the self-administration paradigm in non-escalated rats. Right mean ± SEM minutes of vapor during the self-administration paradigm in non-escalated rats. b Left discrimination between active and inactive nosepokes throughout the self-administration paradigm in escalated rats. Right mean ± SEM minutes of vapor during the self-administration paradigm in escalated rats. The animals presented escalation of intake starting from session 13 (*p < 0.05, vs. last day of 2 min) and continued increasing their intake (***p < 0.001, vs. last day of 2 min) until stabilization from sessions 21 to 24. c Progressive ratio responding for alcohol vapor self-administration. ***p < 0.001, vs. non-escalated group. The data are expressed the mean ± SEM of rewards (left y axis) or breakpoint (right y axis). d Increase in BALs in the different phases of the self-administration paradigm. ***p < 0.001, vs. naive; ##p < 0.01, ###p < 0.001, vs. 2 min phase. e Somatic withdrawal signs measured 8 h after the last session. Inset overall withdrawal severity (sum of somatic withdrawal scores across the five behavioral signs of alcohol withdrawal). *p < 0.05, **p < 0.01, ***p < 0.001, vs. naive. VLR ventromedial limb retraction, AG abnormal gait, VOC vocalization, TR tail rigidity, BT body tremors
The animals presented escalation of alcohol vapor self-administration compared with the last day of the 2-min phase (F11,23 = 35.91, p < 0.001, one-way ANOVA). The Newman-Keuls post hoc test revealed that animals escalated their exposure to alcohol vapor from session 13 to session 16 (p < 0.05, 5 min phase) and from session 17 to session 24 (p < 0.001, 10 min phase; Fig. 3b, right panel).
Progressive ratio responding for alcohol vapor self-administration
Student’s t test indicated that escalated animals had a significantly higher breakpoint for alcohol vapor self-administration compared with non-escalated animals (t14 = 4.604, p < 0.01; Fig. 3c). This experiment demonstrated that animals that escalated their exposure to alcohol vapor following chronic intermittent alcohol vapor self-administration exhibited higher motivation for alcohol compared with non-escalated rats.
Blood alcohol levels
Blood samples for BAL determination were collected at the end of each phase. The ANOVA revealed a significant increase in BALs over time (F3,11 = 27.04, p < 0.001). The Newman-Keuls post hoc test showed that at the end of the 5- and 10-min phases, the animals presented an increase in BALs compared with their naive state (both p < 0.001) and their non-escalated state (2-min phase; p < 0.01 and p < 0.001, respectively; Fig. 3d).
Withdrawal score
Eight hours after the last session, escalated and non-escalated rats were subjected to a withdrawal score test. The Mann-Whitney test indicated significant differences in withdrawal signs between groups. Withdrawal from alcohol vapor affected VLR (Mann-Whitney U = 13.99, p < 0.001), AG (U = 8.96, p < 0.05), VOC (U = 8.74, p < 0.5), and TR (U = 11.51, p < 0.01), with no significant differences in body tremors (U = 0.34, p > 0.05). Dunn’s multiple-comparison test indicated that escalated rats exhibited increases in VLR (p < 0.01), AG (p < 0.05), VOC (p < 0.05), and TR (p < 0.01) compared with non-escalated and naive rats (Fig. 3e). The sum of the five rating scores revealed a significant difference between groups in overall withdrawal severity (U = 15.63, p < 0.001). Dunn’s multiple-comparison test indicated that escalated rats exhibited an increase in withdrawal severity (p < 0.001; Fig. 3e, inset), whereas non-escalated rats did not present any somatic signs of withdrawal.
Alcohol withdrawal-induced anxiety-like behavior and mechanical hypersensitivity
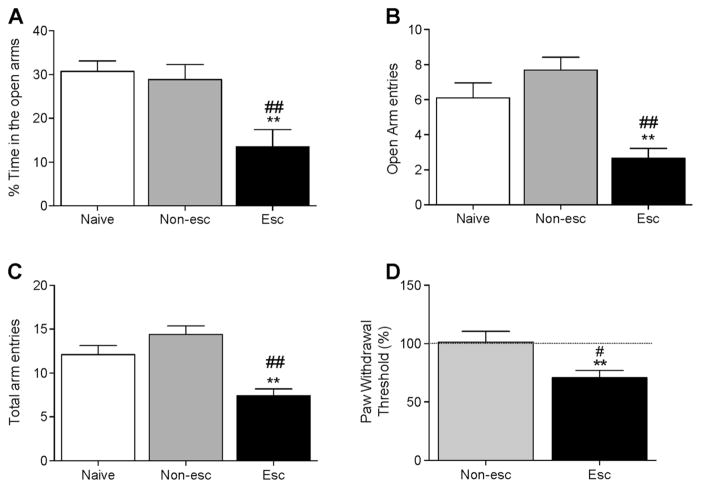
During acute withdrawal (8 h after the self-administration session), the escalated rats exhibited more anxiety-like behaviors compared with non-escalated and naive rats. The one-way ANOVA revealed significant differences between groups in the percentage of time spent on the open arms (F2,29 = 8.170, p < 0.01), entries into the open arms (F2,29 = 13.71, p < 0.001), and total arm entries (F2,29 = 15.69, p < 0.001) in the elevated plus maze. No differences in entries into the closed arms were observed. The Newman-Keuls post hoc test indicated that escalated animals spent less time on the open arms during acute withdrawal (p < 0.01; Fig. 4a), made fewer entries into the open arms (p < 0.01; Fig. 4b), and made fewer arm entries in general (p < 0.01; Fig. 4c) compared with non-escalated and naive rats. Non-escalated rats did not exhibit any signs of anxiety-like behavior during withdrawal.
Fig. 4.
Elevated plus maze test during withdrawal in alcohol vapor-escalated vs. non-escalated and naive rats. a Percent time spent on the open arms. b Number of open arms entries. c Number of total arm entries. The data indicate higher anxiety-like behavior in rats that escalated alcohol vapor self-administration. d Development of mechanical hypersensitivity after 24 sessions of alcohol vapor self-administration. The data are expressed as mean ± SEM. **p < 0.01, vs. naive rats; #p < 0.05, ##p < 0.01, vs. non-escalated rats
A significant increase in mechanical hypersensitivity was observed in escalated rats, with a significant decrease in pain thresholds during acute withdrawal compared with non-escalated (t14 = 2.879, p < 0.05; Fig. 4d) and their naive state (t7 = 4.729, p < 0.01; Fig. 4d). Alcohol withdrawal did not affect pain thresholds in non-escalated rats (Fig. 4d).
Discussion
The present study characterized a novel animal model for the induction and maintenance of alcohol dependence in outbred rats using chronic intermittent alcohol vapor self-administration. To our knowledge, the only other report of alcohol vapor self-administration in rodents was a study by Kantak and Luzzo (2007) in mice. They showed that mice self-administered alcohol vapor, but the BALs that were achieved (~13 ± 3 mg%) were too low to be relevant to binge drinking (80 mg%) or alcohol use disorder (100–200 mg%) in humans. Using a novel apparatus, we found that rats self-administered alcohol vapor to the point of reaching BALs in the 150–250 mg% range. We first demonstrated that our apparatus produced air alcohol levels in the 2–3 mg/dl range, similar to the level that is known to produce alcohol dependence after passive exposure (Gilpin et al. 2008, 2009); Fig. 2a, b). Our findings also demonstrate the reliability and feasibility of alcohol vapor self-administration in the home cage. When the rats were placed in the new apparatus and were passively exposed to alcohol vapor, after the equivalent of ~20 nosepokes (40 min of exposure), they had BALs that were relevant to heavy drinking (>80 mg%), reaching nearly 200 mg% after ~60 nosepokes (120 min of exposure; Fig. 2c). At this point, we evaluated the clearance of alcohol vapor and determined that ~3 h is necessary to reach BALs that are close to zero. These results demonstrate that the novel chronic intermittent alcohol vapor self-administration system leads to BALs that are relevant to alcohol dependence.
We then evaluated whether chronic alcohol exposure in this model is associated with the development of addiction-like behaviors. We developed a new paradigm based on intermittent access to alcohol vapor self-administration, with 8-h sessions every other day. The length of each session was slightly different from what is normally employed with passive vapor exposure (14 h on/10 h off for rats and 16 h on/8 h off for mice). The every-other-day schedule was chosen because intermittent access to two-bottle choice has been shown to lead to higher levels of responding than every-day access (George et al. 2012; Simms et al. 2008). The 8-h time point was based on preliminary data that demonstrated that a shorter duration of access may not be sufficient to reliably produce BALs in the 200 mg/dl range, which was previously shown to induce physical withdrawal signs in rats (Macey et al. 1996). Significant somatic signs of withdrawal were detected in escalated animals 8 h after the end of the last self-administration session (Fig. 3d). The reason we used 8 h instead of 14 h (chronic intermittent exposure model) was to allow future studies to test two cohorts per day to increase throughout using reversed light cycles (in the present study, we used only one cohort per day). Our results indicate that the length of the vapor bout was a major factor that drove the escalation of alcohol vapor self-administration. We observed a significant increase in BALs and minutes of vapor exposure when the length of the vapor pulses was increased from 2 to 5 min and when it was increased from 5 to 10 min.
In our protocol, the two groups of rats were subjected to different periods of alcohol vapor self-administration (8 sessions for non-escalated and 24 sessions for escalated rats). This was employed to simulate the recreational use of alcohol in non-dependent individuals and avoid the escalation of alcohol vapor self-administration in our non-escalated rats. We cannot exclude the possibility that alcohol vapor self-administration escalated after 24 sessions at 2 min, but we did not detect BALs >60 mg% or any signs of escalation after 3 weeks in any of the animals that were tested with these parameters.
After 24 sessions in our new paradigm, escalated rats also presented the emergence of a negative emotional state during withdrawal, with an increase in anxiety-like behavior 8 h after the end of the daily session, similar to passive alcohol vapor exposure (Valdez et al. 2002). In the elevated plus maze, the significant reduction of the total number of entries in escalated rats might indicate a general reduction of locomotor activity. However, the analysis of closed arm entries did not indicate any differences between escalated, non-escalated, and naive rats. Additionally, evidence of a general reduction of motor function during alcohol withdrawal has been reported in rats (Kumar et al. 2013; Williams et al. 2012). The negative emotional state during acute withdrawal was also reflected by the development of mechanical hyperalgesia. These data are consistent with previous studies that evaluated passive alcohol exposure (Edwards et al. 2012) and chronic intermittent voluntary alcohol drinking (Fu et al. 2015). At the end of the chronic intermittent alcohol vapor self-administration paradigm, escalated rats were subjected to 3 sessions under a PR schedule of reinforcement and were compared with non-escalated animals to measure differences in the motivation to self-administer alcohol vapor, reflected by an exponential increase in the effort required to obtain a reward. Escalated rats exhibited an increase in motivation for alcohol, indicated by a higher breakpoint compared with non-escalated rats.
A major goal in the alcohol field has been to develop animal models that employ the voluntary induction of compulsive-like responding to the point of alcohol dependence and addiction in rats. This has been achieved with oral administration in Indiana alcohol-preferring P rats and Wistar rats that are given 8 months of access (Bell et al. 2006; Spanagel and Holter 1999). Previous work from our laboratory showed that vapor inhalation using passive chronic intermittent exposure to alcohol vapor is a very efficient way to produce alcohol dependence (George and Koob 2010; Gilpin 2012; Gilpin et al. 2008, 2009). The chronic intermittent exposure model has often been criticized for its use of passive exposure and the possibility that high levels of alcohol vapor may be aversive and cause stress in the animals. However, the present data indicate that alcohol vapor at the same air level as in the CIE model is not aversive and instead is reinforcing in adult Wistar rats. Rats will voluntarily self-administer alcohol vapor to the point of dependence using the alcohol vapor self-administration paradigm. Using this new model, animals can reach BALs that are similar to BALs that are achieved in the chronic intermittent exposure model. We suggest that this new method of dependence induction may substitute for the passive exposure that is commonly used in the chronic intermittent exposure model. Future experiments will investigate this possibility and evaluate alcohol drinking after dependence that is induced by alcohol vapor self-administration.
The alcohol vapor self-administration model also has direct face validity to the human condition because of reports of alcohol vapor self-administration in humans. Alcohol vapor inhalation apparatuses, such as AWOL (www.awolspirit.com) and Vaportini (www.vaportini.com), have been marketed for several years and are increasingly popular in young individuals. Interestingly, a walk-in cloud bar, where guests can inhale spirits at a concentration of 1:3, has recently been closed in London (pending a new location; http://bompasandparr.com/projects/view/alcoholic-architecture1/), and a bar in Austin, Texas offers “Vapshots” (http://www.minibaraustin.com/vaporshot).
A known limitation of the present alcohol vapor self-administration model is whole-body exposure in rats compared with nose-only exposure in humans. Indeed, one possibility is that a fraction of the alcohol vapor may be absorbed through the skin, leading to a longer pharmacokinetic effect of alcohol. However, although we cannot exclude this possibility entirely, the dense fur of the animal likely limits this absorption process. Evidence suggests that most of the alcohol is absorbed through inhalation. Inhalation is a very potent route of drug administration, characterized by fast absorption from the nasal mucosa and extensive lung capillaries (Meng et al. 1997). Toxicological studies have shown that skin absorption represents less than 10% of the total dose received from combined skin and inhalation exposure (Hayes and Bakand 2010). The alcohol vapor self-administration model may thus provide a unique opportunity to investigate the biological and psychological consequences of acute and chronic exposure to alcohol vapor and unveil the neuronal networks that mediate the voluntary induction and maintenance of alcohol dependence.
In summary, the present results demonstrate that chronic intermittent alcohol vapor self-administration produces alcohol dependence in outbred rats without the use of forced alcohol administration, natural or artificial sweeteners, food/water restriction, operant pretraining, or behavioral/genetic selection. The results also suggest that chronic exposure to alcohol vaporizers in humans may be associated with a higher risk of developing alcohol abuse and addiction. Finally, this novel animal model may be particularly useful for medication development and may further unveil the neuronal circuitry that underlies the voluntary induction of alcohol addiction and identify novel molecular targets that are specifically recruited after the voluntary induction and maintenance of alcohol dependence. Finally, this novel animal model of the voluntary induction and maintenance of alcohol dependence in rats may be valuable for studying alcohol use disorder and developing better animal models of cannabis, cocaine, nicotine, methamphetamine, and toluene abuse and dependence.
Acknowledgments
The authors thank Michael Arends for proofreading the manuscript.
Funding This study was supported by the National Institutes of Health grants AA006420, AA020608, and AA022977 (OG). MDC is the owner of La Jolla Alcohol Research inc. (LJARi). OG is a consultant for the National Institute of Health, LJARi, and Simply-Lab LLC. OG, MDC, and GdG have co-invented and co-developed the apparatus described in this manuscript.
Footnotes
Author’s contributions GdG and OG were responsible for the study concept and design. GdG, MC, and OG developed the new apparatus. GdG, MK, and MC contributed to the acquisition of animal data. GdG and OG drafted the manuscript. All authors critically reviewed the content and approved the final version for publication.
Compliance with ethical standards All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Disclosure The other authors declare no conflict of interest.
References
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcohol Clin Exp Res. 2010;34:538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Belknap JK, Spence SE, Cameron AJ, Schlumbohm JP, Huang LC, Barkley-Levenson AM, Ford MM, Phillips TJ. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav. 2014;13:236–246. doi: 10.1111/gbb.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol. 2015;7:136–144. [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW. Neuropeptide Y (NPY) in the extended amygdala is recruited during the transition to alcohol dependence. Neuropeptides. 2012;46:253–259. doi: 10.1016/j.npep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;Chapter 9(Unit 9):29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2015;20:643–651. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O. Recruitment of a neuronal ensemble in the central nucleus of the amygdala is required for alcohol dependence. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:9446–9453. doi: 10.1523/JNEUROSCI.1395-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A, Bakand S. Inhalation toxicology. EXS. 2010;100:461–488. doi: 10.1007/978-3-7643-8338-1_13. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Vendruscolo LF, Carmichael CY, George O, Koob GF, Gilpin NW. Neuropeptide YY(2)R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict Biol. 2014;19:755–757. doi: 10.1111/adb.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Luzzo C. Ethanol vapor self-administration in adult C57BL/6J male mice. Drug Alcohol Depend. 2007;86:123–131. doi: 10.1016/j.drugalcdep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Kumar J, Hapidin H, Bee YT, Ismail Z. Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav Brain Funct. 2013;9:43. doi: 10.1186/1744-9081-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, Hope BT, Koob GF, George O. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. J Neurosci. 2015;35:6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Operant ethanol self-administration in ethanol dependent mice. Alcohol. 2014;48:295–299. doi: 10.1016/j.alcohol.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol. 2013;18:921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Meng Y, Lichtman AH, Bridgen DT, Martin BR. Inhalation studies with drugs of abuse. NIDA Res Monogr. 1997;173:201–224. [PubMed] [Google Scholar]
- NIAAA. NIAAA Newsletter No. 3. 2004. NIAAA Council approves definition of binge drinking. [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol. 2006;11:310–323. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol. 2013;18:496–507. doi: 10.1111/j.1369-1600.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, Kloss ND, Bilimoria JL, Smith CE, Walker BM. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology. 2012;223:75–88. doi: 10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]