Abstract
Sexual intercourse (vaginal and anal) is the predominant mode of HIV (human immune deficiency virus) transmission. Topical microbicides used in an on-demand format (i.e. immediately before or after sex), can be part of an effective tool kit utilized to prevent sexual transmission of HIV. The effectiveness of prevention products is positively correlated with adherence, which is likely to depend on user acceptability of the product. The development of an efficacious and acceptable product is therefore paramount for the success of an on-demand product. Acceptability of on-demand products (e.g. gels, films, and tablets) and their attributes is influenced by a multitude of user-specific factors that span behavioral, life-style, socio-economic, and cultural aspects. In addition, physicochemical properties of the drug, anatomical and physiological aspects of anorectal and vaginal compartments, issues relating to large-scale production and cost can impact product development. These factors together with user preferences determine the design space of an effective, acceptable, and feasible on-demand product. In this review, we summarize the interacting factors that together determine product choice and its target product profile.
Keywords: On-demand products, vaginal microbicides, rectal microbicides, anogenital, HIV, acceptability
1. Background
Human immunodeficiency virus (HIV) infects approximately 36.7 million people worldwide [1]. Globally, more than half of the people living with HIV are women. Sexual transmission is the predominant route of HIV infection. Women are approximately 2–4 times more prone to HIV acquisition than men due to the anatomical and physiological vulnerabilities as well as socio-economic and cultural dynamics [2]. Although therapy with antiretroviral (ARV) drugs has significantly reduced mortality and morbidity rates, new HIV acquisitions are still on the rise in some areas. In the U.S., 39, 513 new HIV cases were documented in 2015 [3]. In addition to the vaginal route, the rectal route of HIV transmission is also a likely contributor to the overall HIV transmission rates in women. Although anal sex is prevalent in men who have sex with men (MSM), recent surveys suggests that women are engaging in receptive anal intercourse (RAI) [4]. Anal sex has about 20 times increased risk of HIV transmission in both MSM and heterosexual partners compared to vaginal sex [5].
One way to curb new HIV acquisition in women is by preventing sexual HIV transmission, which can be achieved with prophylactic use of ARV drugs in high-risk populations. This approach is referred to as pre-exposure prohylaxis (PrEP). In 2012, an oral tablet, Truvada® (emtricitabine and tenofovir disoproxil fumarate), was approved for this indication [6]. Compared to oral administration, higher concentration of ARV drugs can be achieved in the anogenital compartments by local application [7, 8]. These topically applied products are collectively referred to as microbicides. The presence of an ARV agent can inactivate the virus by directly interfering with the virus or the various stages of host cell infection and dissemination before active infection is established, which can lead to HIV prevention. CAPRISA-004 was the first clinical trial utilizing 1% tenofovir (TFV) vaginal gel that showed 39% efficacy in the prevention of HIV acquisition [9]. However, subsequent trials did not show effectiveness, which was largely attributed to the lack of user adherence to product use [10]. Recently, the MTN-020 (ASPIRE) and IPM 027 (The Ring Study) studies have shown a positive correlation between adherence and efficacy [11, 12]. These correlations are consistently observed in several clinical trials emphasizing that adherence is paramount for effective HIV prevention using a microbicide product.
Vaginal microbicide products can be classified into two broad categories, coitally-dependent and coitally-independent, based on their time of intended usage. Coitally-dependent products are also referred to as on-demand products, which are used immediately before and/or after sex. Coitally-independent products are not coupled with coitus and are intended to be used on a regular basis, which can range from once daily to less-frequently with long-acting products such as a monthly intravaginal ring. A number of microbicides developed thus far have been for on-demand use. On-demand products provide greater user control and depending on the product, can be used discretely, thereby empowering women to protect themselves in a cultural setting where condom use with men is non-negotiable. In addition, women may adhere better to coitally-dependent products as compared to coitally-independent products requiring daily dosing regimens. Long-acting microbicide products such as intravaginal rings are promising, however, the MTN-020 (ASPIRE) and IPM 027 (The Ring Study) studies have shown that user adherence is still an issue with long-acting products [11, 12]. Of note, long-acting rectal microbicides have not been fully developed thus far, although some early research is being reported. On-demand products are also advantageous since the long-term effects of excipients and ARV drugs utilized in long-acting products is not well understood. The likelihood of using on-demand or long-acting products will depend on perceived and real advantages. It is imperative that women are presented with a portfolio of safe and effective microbicide products that suit their diverse needs. Women’s needs are dictated by a combination of factors relating to personal preferences, prior use, perceived advantages, interference with sexual activity, and socio-economic and cultural norms.
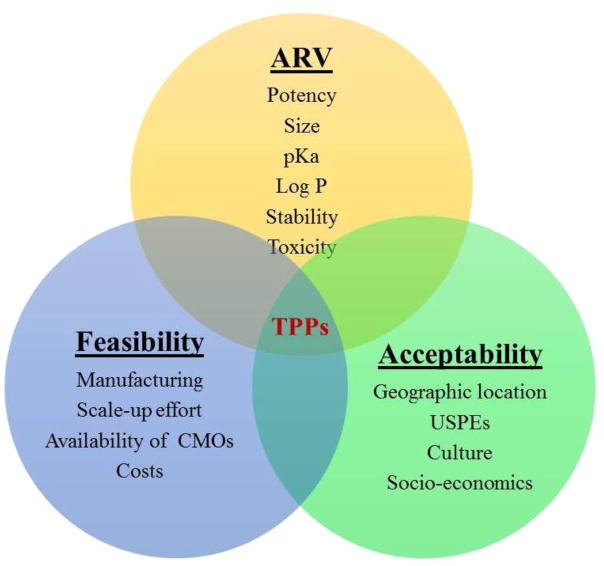
Several microbicide product formats such as gels, films, ovules, tablets, suppositories, enemas, nanofibers, and novel nanoparticle delivery systems are currently under investigation for on-demand use. On-going and recently completed clinical trials using on-demand products are summarized in Tables 1 and 2. The specific design criteria for on-demand products including shape, size, color, odor, feel, ease of administration, spreadability within the vaginal canal, retention, portability, need for an applicator, and stealth characteristics depend on several factors. In this review, these factors, namely anatomical and physiological aspects, user preferences, drug physicochemical and biological characteristics, scale-up and economic considerations are discussed for both vaginal and rectal on-demand microbicides. Importantly, the relationship of these factors to product attributes and ultimately their contribution to future product design is elaborated. Figure 1 depicts the interacting nature of these factors that ultimately define the target product profile (TPP).
Table 1.
Clinical studies currently being pursued using different on-demand products
| Product | Active | Route | Status | Purpose | Reference* |
|---|---|---|---|---|---|
| Enema | TFV | Rectal | Phase 1 (recruiting) | Safety, pharmacokinetics (PK), pharmacodynamics (PD), and acceptability of 3 formulations of a TFV enema |
NCT02750540 DREAM-01 |
| Gel | DPV | Rectal | Phase 1 (planned-Mid 2017) | Safety, PK and acceptability | MTN-026/IPM 038 |
| DPV | Rectal | Phase 1 (planned-Mid 2017) | PK of DPV rectal gel (0.05%) administered via HTI vaginal applicator and a coital simulation device to healthy, HIV-1 uninfected men and transgender women | MTN-033/IPM 044 | |
| Maraviroc | Dual | Phase 1 (completed and awaiting results) | Compare safety, acceptability and distribution of maraviroc gel against oral maraviroc |
NCT02346084 CHARM-03 |
|
| GRFT | Vaginal | Phase 1 (not open) | Safety of GRFT in a carrageenan gel in healthy women | NCT02875119 | |
| Film | TFV | Vaginal | Phase 0 (awaiting results) | Comparative PK of TFV gel and films in healthy women |
NCT02280109 FAME-05 |
| MB66 | Vaginal | Phase1 | Safety study of monoclonal antibodies to reduce vaginal transmission of HSV and HIV |
NCT02579083 VAST |
|
| Placebo | Vaginal | Pre-Phase 1 | Comparison of acceptability and development of administration guidelines between films of different sizes and tactile properties (Hydroxyethyl cellulose and polyvinyl alcohol films) |
NCT02908503 FLAG |
|
| Hydroxyethyl cellulose Placebo |
Vaginal | Pre-Phase 1 | Study acceptability of placebo films using objective adherence markers. Note: Other dosage forms, vaginal inserts, gels, and rings are being explored within this study |
NCT02602366 The Quatro Study |
|
| Tablet | DS003 | Vaginal | Phase 1 | Evaluate safety and assess local and systemic PK | NCT02877979 |
| Insert | Placebo | Vaginal | Phase 1 (results awaiting) | Assess disintegration, safety, and acceptability and development of adherence biomarkers (also included are gels, films, and rings) | NCT02569697 |
| Placebo | Vaginal | Phase 1 (results awaiting) | Study acceptability of placebo inserts (also films, rings, and gels) using objective adherence markers |
NCT02602366 The Quatro Study |
|
| Suppository | Placebo | Dual | Observational (recruiting) | Develop USPE measures for rectal suppositories |
NCT02744261 DRUM-S |
More information can be obtained at https://clinicaltrials.gov/ using the clinical trial identifier number (NCT…)
Table 2.
Major findings from recently completed microbicide clinical studies utilizing on-demand products
| On-demand product | Active | Route | Purpose | Major findings | Reference |
|---|---|---|---|---|---|
| Gel | TFV | Rectal | Extended safety and acceptability study of TFV reduced-glycerin 1% gel and comparison with oral regimen emtricitabine/TFV disoproxil fumarate | Product was safe in MSM and transgender women. Daily gel use was not preferred. Intermittent gel use and oral regimen acceptability was comparable |
NCT01687218 MTN-017 (Cranston et al. 2016)[15] |
| MIV-150/PC-1005 | Vaginal | Safety, PK and acceptability of MV-150 and zinc acetate in a carrageenan (PC-1005) gel | Product was well tolerated (14 days use).11/17 participants liked the gel and 7 recommended reducing the volume. CVLs showed activity against HIV and HPV | NCT02033109 (Friedland et al. 2016)[16] | |
| Film | DPV | Vaginal | Safety, acceptability, PKPD and comparison with a 0.05% DPV gel for one week daily administration | Films were safe and more acceptable than gels. Comparable release between gel and film was seen. Films and gels delivered DPV to tissues in quantities sufficient for ex vivo activity. |
NCT01548560 FAME-02 (Bunge et al. 2016)[17] |
| DPV | Vaginal | PKPD of DPV film and gel in healthy women | No severe adverse effects were observed. DPV film showed comparable PK and ex vivo activity as gel |
NCT01924091 FAME-02B (Robinson et al. 2016)[18] |
Fig. 1.
Drug, product, and user-related factors influencing the target product profile of on-demand microbicide dosage forms. CMOs – Contract Manufacturing Organizations, USPEs – Users’ Sensory Perceptions and Experiences
2. Design considerations for on-demand products
Achieving efficient drug delivery to target tissues and cells is quintessential in order to prevent HIV transmission. Preclinical, clinical, and socio-behavioral studies conducted over the last decade have provided us with a wealth of information. These studies have been instrumental in identifying the factors that significantly affect product use and efficacy. This information is summarized and design criteria are proposed for the development of on-demand microbicide products. When designing on-demand products, having a holistic understanding of these factors could lead to increased product uptake, regulatory approval, and ultimately efficacy.
2.1. Anatomical and physiological considerations
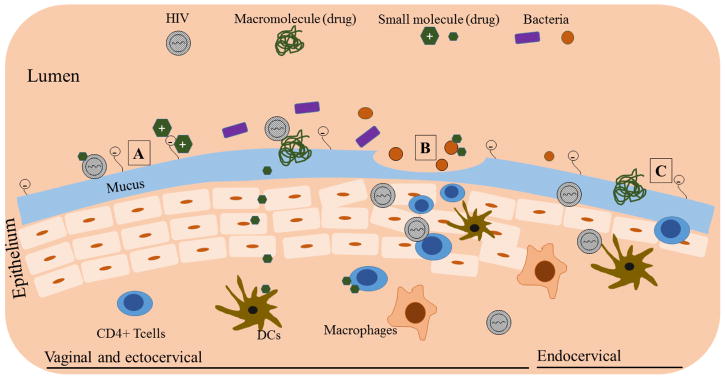
Sexual transmission of HIV through the vagina or rectum occurs in several steps. In vaginal transmission, following HIV exposure to the mucosal surface, cell-free and cell-associated virions traverse the epithelium and infect target cells such as CD4+ T cells [13]. A further recruitment of these target cells occurs due to the activation of the epithelium by virus, which produces chemokines that attract dendritic cells (DCs). These DCs in turn recruit CD4+ T cells. After expansion within the target cells, HIV disseminates to lymph nodes and further to the systemic circulation. HIV transmission via the rectal route is not well understood, however, it likely follows a similar pattern as the vaginal route. Upon viral deposition through semen in the rectal cavity, cell free or cell-associated virus can be involved in transmission [14]. HIV virions can access immune cells by migrating across the epithelial membrane through intercellular junctions and other pathways. Once the seeding population is established, these virions can expand locally and access lymphatic and systemic circulation. Virus can also gain access through microabrasions caused by physical trauma during intercourse (Figure 2). Several factors can influence the susceptibility of the person to HIV infection after viral exposure such as general anogential health, existence of other sexually-transmitted infections (STIs), inflammation, and local pH [13].
Fig. 2.
Interaction of drug molecules with vaginal physiological milieu. A. Vaginal and ectocervical region showing multilayer epithelium. Interaction of positively charged molecules with mucin and physical entrapment of large macromolecules, and permeation of neutral molecules is shown. B. Disrupted epithelium due to processes such as physical injury and inflammation showing viral entry and increased immune cell accumulation. Also shown is the interaction of drug molecules with bacteria (e.g. Gardnerella vaginalis). C. Ectocervical region showing single layer epithelium (similar to rectum), which increases vulnerability of tissue to viral entry. Note: Structures are not to scale
The female anogenital tract presents several barriers to as well as opportunities for the delivery of ARV drugs. Previous reviews have described in detail the anatomical and physiological aspects of the rectum and vagina that influence drug delivery [19, 14]. The attributes of a microbicide product such as physical state, geometry, application volume, formulation composition (excipients), dilution, spreadability, and mucoadhesion must be tailored toward the anatomical and physiological context of use for greater acceptability and efficacy. Herein, we briefly describe these aspects and their effect on the design of vaginally and rectally applied ARV products.
2.1.1. Vaginal anatomy and physiology
The anatomy of the vagina has been discussed in previous reports [20, 21] and is briefly presented in table 3. The walls of the cervicovaginal compartment are made of several layers of stratified squamous epithelium, excluding the endocervix, which presents as a single layer. Immune cells present within the cervicovaginal compartment are targets for HIV infection. The cervicovaginal mucosa and its mucus coating act as a protective barrier for HIV transmission [13]. In addition, the cervicovaginal environment produces several antimicrobial factors such as enzymes, proteins, defensins, cytokines and chemokines. Components of the vaginal microflora produce lactic acid, which maintains an acidic virus-inactivating pH [22]. These vaginal innate protective mechanisms contribute to the low frequency of HIV-1 transmission through vaginal sex (1-in-1250 exposures) [23].
Table 3.
Anatomical and physiological comparison of vagina and anorectum
| Vagina | Anorectum | |
|---|---|---|
| Length | 6–8 cm | Anal canal – 2.5–4 cm Rectum – 10–15 cm |
| Surface Area | 65.73 to 107.07 cm2 | Rectum – 200–400 cm2 |
| Fluid volume | 0.5–0.75 mL | 1–3 mL |
| Epithelial Tissue Type | Vagina and ectocervix – Multilayer squamous epithelium Endocervix – Simple columnar epithelium (single layer) |
Anus – Squamous epithelium Rectum and upper zone in anus – Simple columnar epithelium (single layer) |
| Pressure | 0 mm of Hg | Resting: 40–70mm of Hg During squeeze: 100–200 mm of Hg |
| pH | 4–4.5 | 7–8 |
| Buffer capacity | High | Low |
| Relative HIV target cells(e.g. CD4+ T cells) | Low | High |
The presence of STIs and the use of vaginal products can affect vaginal health and can increase the risk of HIV acquisition. Bacterial vaginosis (BV), characterized by a change in vaginal microbiota from acid-producing lactobacilli to predominantly anaerobic bacteria such as Gardnerella vaginalis and Prevotella species, increases pH and inflammation [24]. The risk of HIV acquisition increases significantly in the presence of BV. It has been recently reported that women participating in CAPRISA-004 trial, which utilized 1% TFV intravaginal gel, showed a positive correlation between lactobacilli species and efficacy [25]. Only 18% women were protected whose vaginal microbiota contained less than 50% lactobacilli compared to 61% protection in women with greater than 50% lactobacilli. Additionally, it has been reported that higher pH and bacteria such as Gardnerella vaginalis can reduce TFV uptake by T cells, thus affecting the efficacy [26]. Also, women with more than 1% of Prevotella bivia in their vaginal microbiota, showed increased inflammation and likelihood of infection. Similarly, the presence of co-infections such as herpes-simplex virus-2 (HSV-2) and human papillomavirus (HPV) were identified as significant co-factors contributing to HIV infection [27, 28]. Vaginal microbicide development should take into account the anatomical and physiological aspects, specifically the effect on innate protective mechanisms, and impact on/of microbiota, in the context of product design.
2.1.2. Anorectal anatomy and physiology
The increased risk for HIV transmission with anal intercourse can be attributed, for the most part, to the anatomical and physiological differences between the rectum and the vagina (Table 3). The anatomical aspects of rectum relevant to microbicide delivery has been recently reviewed [14]. The rectum is physiologically much different from the vagina. The rectum, which is the distal part of the gastrointestinal tract, has a single columnar epithelial lining, thus posing increased vulnerability to HIV infection. Trauma produced during intercourse could easily compromise its epithelial integrity. The pH of the anorectal compartment is slightly basic (7–8) compared to the vagina (4–4.5). It also lacks buffering capacity. Thus, the rectal environment is easily disrupted when products with extreme pH are introduced. One significant difference between the anorectal and vaginal compartments is that the target immune cells are present in much higher quantities in the rectum as compared to in the vagina [14]. Moreover, the T cells localized in the rectum are activated and express increased CCR5, a coreceptor used by HIV-1 for cell attachment. A recent study showed that MSM who engage in condomless RAI had a change in the activation status of immune cells [29]. Additionally, a marked change in the anorectal microbiota to a species (Prevotella) that could increase HIV transmission by increasing inflammation was also observed. Any of these individual factors, or a combination of these factors, could be responsible for the marked increase in the HIV infection risk associated with anal intercourse.
In addition to these risk factors, the anorectal compartment also presents with several innate factors that could act as a natural defense mechanism against HIV infection. Although the effect of rectal flora on HIV transmission is not very well understood, studies are emerging in this area. The microbiota of the anorectum mostly consists of anaerobic gram-positive and gram-negative bacteria that could play an important role in defense against pathogens. Although the rectum is devoid of innate antimicrobial factors that are present in the small intestine, some of the rectal factors such as lysozymes, could aid in defending against pathogens.
The anatomical and physiological features described above contribute to the complexity of developing safe and effective topical microbicide products. Even more challenging is the fact that these factors can display great variability among women depending on their ethnicity, geography, age, menstrual cycle, presence of other STIs, and behavioral and social conditions, thus making the topical microbicide development a daunting task. It is therefore important to not only account for general anatomical and physiological aspects during product design, but also variability among women and utilize this information to design an effective microbicide.
2.1.3. Design Considerations
Anatomical and physiological aspects of a specific route of delivery can affect the choice of dosage form and its design attributes such as shape, physical form, volume, composition, viscosity, and tactile properties. The first attribute that needs to be considered for a topical microbicide product is the physical state of the dosage form. On-demand microbicides have been formulated into liquids, semisolids, and solids with each form possessing advantages and limitations. The product retention in the vagina and rectum will depend on the physical state. For liquid and semisolid preparations, product viscosity and the administered volume are important attributes that dictate leakage from and spreading within the vaginal and rectal compartments [30, 31]. For solid dosage forms such as vaginal tablets, inserts, suppositories, and films, disintegration will impact drug delivered to the mucosal site. Due to the self-cleansing action of vagina [21], the vaginal fluid is replaced regularly, which has implications for product retention and efficiency of drug delivery. Due to the small amount of vaginal and rectal fluid, extent and rate of disintegration for solid products are important factors affecting efficacy because efficient and timely coating of the anogenital tract with the microbicide product is important to reduce the likelihood of exposed vulnerable sites for HIV infection. Therefore, clinically investigated solid on-demand microbicide products have been designed to disintegrate quickly, typically less than 30 minutes. Although the extent of spreading was not studied for all the products, a recent study comparing dapivirine (DPV), a non-nucleoside reverse transcriptase inhibitor, polymeric vaginal film and vaginal gel showed comparable drug release and distribution within the female genital tract [18, 17].
The design of a safe and effective product should take into account their effect on the epithelium. A compromised epithelium can provide viral access to immune cells [32]. The integrity of cervicovaginal mucosa is dependent on a number of elements including age, use of hormonal drugs, and composition of microbiota. The impact of hormonal contraceptives on epithelial thinning or loss of integrity is not conclusive, but animal studies show increased risk of viral transmission with hormonal use [33]. In fact, Depo-Provera is often used in non-human primate HIV challenge models to thin the vaginal epithelium and increase HIV susceptibility [34]. Recent studies have shown the effect of several commonly used excipients in vaginal products (citric acid, glycerin, propylene glycol, and parabens) on epithelial integrity [35, 36]. Semisolid products such as gels can provide lubrication and reduce the friction during intercourse, which can reduce abrasions to the epithelium.
Several other physiological attributes can influence product performance. Vaginal and rectal fluids can dilute the product leading to leakage, reduction of drug concentration in the lumen, and reduction in drug product/drug substance retention in the vagina. On the other hand, these fluids can help with disintegration, dissolution (drug release), and product spreading within the target anatomical compartment for effective protection. There are several enzymes such as aminopeptidases present within vaginal fluid and tissues, which can destabilize labile drugs such as proteins. Newer studies report the presence of efflux and uptake transporters in the female genital tract, which could impact drug disposition [37, 38]. This will ultimately affect drug pharmacokinetics (PK) and the amount of drug delivered in the vaginal tract. In addition, the innate microflora in the vagina contributes to its lower pH through lactic acid production. This low pH environment exhibits protection against several STIs including HIV [22]. The ARV product could alter the vaginal pH or bacterial viability and other innate antimicrobial factors depending on the volume applied and the formulation components. Therefore, pharmaceutical product design should take into account the effect of formulation components on innate microflora, pH, epithelial integrity, transporters, and antimicrobial factors. In addition, recent studies have shown that certain components of microbicide gels and films can affect glycosylation in the vagina [39]. The long-term effect of these changes is currently not understood. A film acceptability and placement study (FLAG) is currently investigating the effects of formulation excipients and volume applied on the vaginal glycome and microbiome (Table 1). There is also a need to understand the pharmacokinetics and pharmacodynamics (PKPD) of ARVs in the context of other vaginal product use. Of significance, drug-drug interactions could impact the effective drug levels in the cervicovaginal tissues and systemic circulation due to their effect on drug metabolizing enzymes, transporters and protein binding. For example, concomitant use of a miconazole vaginal capsule and a DPV ring was shown to increase the systemic exposure of DPV, whereas the levels in the cervicovaginal fluid were shown to be reduced [40]. Although the reduced levels are not biologically significant in this case, this aspect needs to be considered during product design.
Product attributes relating to physical design and physicochemical properties vary between vaginal and rectal applications due to anatomical and physiological differences such as surface area, shape, fluid volume, pH, microbiota, immune cell content, mucosal integrity, and innate biological factors. Although initial rectal microbicide delivery studies utilized products developed and/or employed in vaginal applications, it was quickly realized that direct translation from vaginal products is not optimal. Unsatisfactory results from these studies unequivocally showed that rectal microbicide delivery needs products with varied attributes suitable to the anatomical and physiological environment of the rectal compartment. Previous reviews have summarized preclinical and clinical rectal microbicide studies utilizing vaginal products [41, 14]. Most notably, RMP-02/MTN-006 [42] conducted a rectal safety study using a previously developed vaginal TFV gel. This gel, although safe and acceptable for vaginal administration, showed several gastrointestinal adverse effects such as bloating, diarrhea, flatulence, and nausea. Also observed was damage to the mucosal integrity. These effects were due to the high osmolality of the gel preparation (3111 mOsm/kg). A subsequent study (MTN-007) utilizing a TFV gel with reduced osmolality (836 mOsm/kg) was shown to be safe and acceptable [43]. These results clearly indicate that the product osmolality needs to be adjusted specific to the anorectal environment.
The product shape, volume applied, and viscosity characteristics will depend on the anatomical features of anorectal cavity (Table 3) and the physiological effects they exhibit. The pressure within the rectal cavity is 40–70 mm of Hg and increases to 100–200 mm of Hg due to squeezing, compared to 0 mm of Hg in the vaginal cavity. Therefore, the effect of this pressure difference on differential rheological response and spreading behavior of the same product in the vaginal or rectal compartments needs to be considered during product development. Since the anorectal cavity extends to other parts of gastrointestinal tract (GIT), the susceptible surface area for viral infection is very large, thus necessitating use of a large volume of product. However, increased volume of gel/enema could stimulate defecation. Product volumes less than 35 mL are shown to be appropriate for rectal administration [44]. In contrast, vaginal applications utilize much lower volumes (~4 mL). Based on published macaque and human studies combined with modeling by Katz and colleagues [45], it is predicted that smaller gel volumes (2 mL) with better coating and less leakage can achieve similar efficacy as the currently utilized vaginal gel volume (4 mL). Interestingly, smaller volumes have also been reported for rectal gels. In a recent study, Hendrix and colleagues [31] found that the colonic distribution of two volumes of iso-osmolar rectal gels, 3.5 and 10 mL, in HIV-negative men was similar. Using simulated intercourse, they also showed that both volumes cover the typical GI distribution of ejaculate. Until rectal microbicide trials show efficacy with lower volumes, it is judicious to utilize larger volumes that can theoretically guarantee coverage of vulnerable portion of GIT, while remaining within the acceptability window.
An important component of semisolid and liquid dosage forms is the applicator [46]. To deliver microbicide gels to the vagina, several applicators have been used. For example, the HTI vaginal applicator has been widely utilized in microbicide trials. Applicators for microbicide delivery can influence performance of the microbicide product in terms of dose delivered, surface area covered, and effect on mucosal surface. An ideal applicator should be safe, easy and comfortable to insert, deliver a precise dose, cost-effective, and aesthetically acceptable. Traditional applicators have a single hole, which can lead to suboptimal distribution of gel in the vaginal and cervical mucosa. Recently, Omar et al. developed a vaginal applicator that contains multiple apical and lateral holes and therefore it can release the gel and coat the vaginal and cervical mucosa uniformly [47]. MRI studies confirmed that this newly developed vaginal applicator is superior in terms of vaginal gel and cream distribution. To date, rectal application of microbicide gels has been by traditional vaginal applicators, although they are not ideal for this route of delivery. Gross et al. investigated acceptability of vaginal applicators delivering a N-9 gel in male couples [48]. Most participants (68%) disliked the applicator due to its tampon-like shape, sharp edges of the exposed tip, and rectal fullness attributed to the air released from the applicator [48]. To address these complaints, Carballo-Dieguez et al. developed a rectal-specific applicator that incorporated the Fleet Comfort Tip™, which is commonly used for enema administration [46]. Unfortunately, in this study comparing this rectal applicator with a vaginal applicator, users (MSM) had no specific preference for either applicator. It was identified that users’ preferred characteristics include portability, discreet use, and aesthetic appeal. A recent study by Bauermeister et al. showed that young MSM’s future use of a gel product was highly dependent on their satisfaction with the applicator even if they are satisfied with the gel itself [49], emphasizing the role applicator could play in product acceptability. These results suggest that it is important to optimize vaginal and rectal applicator design to enhance product acceptability. It should be noted that most of the applicator studies for rectal administration have been conducted in the MSM population. Future studies evaluating the acceptability of applicator use in women in the context of RAI are needed because men and women may have varying preferences.
2.2. User preferences
The success of an effective microbicide product is dependent on its acceptability, which is influenced by user preferences. The preference for specific product attributes could be a function of users’ behavioral and life choices, socio-economic conditions, and cultural proclivities. Since an acceptable product is likely to increase adherence, and ultimately the success of HIV prevention efforts, formulation engineers must account for the effect of these factors during product design [50]. Of note, partner preference and awareness also has a significant impact on product acceptability [51, 52]. Due to several failed clinical studies and constant advocacy from microbicide researchers [50, 53], acceptability assessments are now routinely captured within clinical studies. In this section, we discuss how these factors affect a product’s acceptability and identify specific design attributes that could increase acceptability.
2.2.1. Previous experience with products
Acceptability of any product will depend on the prior use or familiarity with similar products. Microbicide researchers have leveraged the fact that potential product users are familiar with lubricant use during intercourse and hence this prior experience could positively influence the acceptability of gels/semisolid preparations. A survey conducted by Giguere et al. showed that most women are familiar with vaginal gel products utilized to treat STIs or for cleansing purposes [54]. As a result, gel-based microbicides were felt to be acceptable dosage forms [55]. In the case of rectal administration, a study conducted in HIV-uninfected males and females identified that gel was the most preferred product compared to an enema or a suppository for rectal delivery [56]. However, well-known problems such as product leakage, messiness, lack of precision in delivered dose, requirement for an applicator, could interfere with acceptability as newer microbicide dosage forms are introduced and women gain experience and exposure to these products.
Since douching before anal intercourse is a common practice, specifically among MSM, microbicides that are formulated as enemas can be promising due to their acceptability based on prior experience [57]. In a qualitative (focus group and interview-based) study conducted in South American MSM and transgender women, participants thought that an enema could provide hygiene and protection [58]. They also preferred enemas over gels due to perceived greater protection from enemas given their ability to go deeper into the body. However, some participants expressed uncomfortable sex after enema use due to dryness as a potential disadvantage and in this case, a preference for gel or condom was shown. Transportation of a large enema tube and the longer preparation time during a spontaneous sexual encounter were other limitations cited by users. Hence, it is important to provide options that are acceptable and meet the needs of users. In other studies, concerns about potential efficacy and safety, as well as the products’ effect on condoms with rectal douching were cited. These concerns may impact acceptability and product uptake [59]. It is clear from these studies that participants have varied experiences and perceptions about enemas, gels, and suppositories. Of note, MSM are more aware of rectal enemas than women are and hence acceptability needs to be studied in both populations.
2.2.2. Sensory perception
Color, taste, shape, physical form, odor, and tactile properties of a product can influence user perception [60, 61]. Tactile properties, loosely described as “feel”, encompass attributes such as product firmness, texture, stickiness, and slipperiness [60]. Hayes and colleagues measured ex vivo acceptability of oval-shaped products (ovules) or suppositories and a hypothetical applicator by assessing user willingness-to-try, perceived effectiveness, and imagined ease of insertion [61]. In a preceding focus group study, the authors identified that most women preferred a long oval and bullet shape compared to tear drop, tampon, or round oval shapes [62]. By systematically varying the size (1, 3, and, 5 g) and firmness as measured by storage modulus, nine different products were prepared. Women in the no applicator group preferred a larger and firmer product, which is easier to hold and insert, compared to the applicator group. Women in this study thought that softer products, as opposed to firmer, are not easy to insert. As noted by the authors, the results may not be directly transferrable to a vaginal administration scenario because participants in this study felt the products in hand (in mano). Nevertheless, these results surely indicate that physical attributes of microbicides can influence acceptability.
For semisolid preparations, rheological properties, volume administered, and measures of retention and spreading are important criteria that can affect user perception such as feel (before, after and during coitus), leakage, interference with sex, and pleasure [63]. Mahan et al. attempted to establish a relationship between sensory perceptions obtained in mano such as thickness, slipperiness, graininess, and rubberiness with rheology using six commercial lubricants [60]. They identified that the storage/loss modulus and consistency index of lubricants correlated well with sensory attributes (stickiness, rubberiness, peaking, and uniform thickness). The study findings are important since the “in hand feel” of the product can influence actual vaginal use. As noted by the authors, these findings need to be evaluated with actual use. It has been shown that increased volume leads to greater leakage [44], which can negatively impact acceptability. Studies have shown product leakage and distribution are related to viscosity and flow of the gel under stress [64, 30, 65]. It has been shown that users feel differently when products with different properties are administered and it is likely that a qualitative relationship exists between the former and the latter. Morrow and colleagues pioneered the work on developing the relationship between users’ sensory perceptions and experiences (USPEs) and biophysical properties of gels. This work has clearly shown how different properties of gels namely rheological properties and volume, can be related to USPEs [50]. A first application of this approach, which was recently published, showed that there were no significant differences in user perception between 3.5 mL and 10 mL gel volumes, which also correlated well with GI distribution of gels in the presence of simulated intercourse [31]. Given that the framework for such relationships between USPEs and biophysical properties has been developed, microbicide researchers can implement this information into newly developed and holistic TPPs.
Solid dosage forms such as inserts, films and nanofibers are being studied to understand the effect of specific product attributes on user preferences (Table 1). In order to understand the effect of film tactile properties on acceptability, a study involving films with two different polymers and sizes (Table 1) is currently ongoing. Another large study being conducted in Sub-Saharan Africa (The Quatro Study) is investigating the acceptability of vaginal dosage forms such as gels, films, inserts, and rings (Table 1). A recent focus group study collected iterative user feedback to identify and optimize drug-eluting nanofibers [66]. Specifically, geometry, texture, dissolution, dosing regimen, and applicator were considered. Women preferred the capped tube geometry with digital insertion and the quick-dissolving circle with an applicator. The authors noted that this information has led to progress in formulation and product design.
It may not be possible to prepare products with a wide spectrum of tactile properties that elicit varied sensory perceptions. However, products that employ excipients with a broad range of properties (e.g. viscosity) could provide products amenable to a variety of tactile properties. For example, carrageenan-based soft gels (or ovules) are amenable to changes in properties (firmness) [61]. Similarly, tactile properties of solid dosage forms can be modified, theoretically, such as films and nanofibers. These modifications can be done by employing varied excipients and/or physical modifications such as thickness, patterns that increase friction to aid in insertion, and size. Several other perceptions mentioned by women such as the feeling of fullness, real and perceived leakiness or messiness, dryness in the rectum and vagina after the use of enema and gels, stickiness, urge to defecate, and interference with sex could affect product acceptability [48] [56] [51, 50].
2.2.3. Other Considerations
Co-existing conditions, behavioral patterns, and cultural and religious aspects can also impact acceptability. A study conducted by Cook et al. [67] in young minority women in the United States diagnosed with BV, showed that women preferred microbicide products that also provide personal hygiene benefit. In some cases, the product’s personal hygiene impact was rated as more important than its pharmacological effect. Some cultures practice dry sex as it is more pleasurable to men due to increased friction. In this population, products that lubricate the vaginal vault such as gels and pessaries may be less acceptable, whereas, dosage forms such as films can be viable alternatives. Cultural factors, lack of education and awareness deter some women from accessing internal parts of vagina and rectum. In a Brazilian study, it was noted that women evaluated within this particular cultural setting were not willing to use product that required them to touch their vagina or insert devices into the vagina [68]. In summary, information obtained from several studies indicate that a strong preference for specific product attributes exists, which is based on perceived and actual factors. This qualitative and quantitative data can guide product design in congruence with other factors discussed in the upcoming sections.
2.3. ARV drug properties
Based on acceptability research, recommendations for design attributes from each product category (dosage form) can be established. However, it is important to realize that incorporating these criteria within the product development framework could be a daunting and sometimes impossible task due to the unfavorable drug properties and interference with efficacy and safety. Several API related factors affect the choice of dosage form, dose volume, and flexibility with manipulation of tactile properties. Importantly, potency, ionization, hydrophobicity, stability, tissue and cell permeability, PK, and toxicity of the API are some of the properties that strongly influence product attributes. In this section, we discuss some of these physicochemical and biological properties and their relationship to microbicide product design. The goal of a drug delivery vehicle is to obtain high drug concentrations in target cells and tissues. The properties of drugs and the formulation can affect drug release and dissolution, entry into tissues and cells, and retention time within the anogenital compartments, target cells and tissues. Figure 2 captures some of the interactions of ARV drugs with components of the vaginal milieu that could interfere with the drug product activity.
Drug solubility is an important property that could influence product preparation, stability, and in vivo performance. Although hydrophobic and hydrophilic drugs can be incorporated into hydrogels, the preparation of hydrophobic dispersed gels can be more complicated. For example, in order to formulate hydrophobic DPV and UC781 in gel formulations [69–72, 18], micronization of API could be required to obtain a uniform drug dispersion in the gel product. A significant difference between water soluble and insoluble drugs is their physical form in the dosage form. Since hydrophobic drugs are dispersed as particles in an aqueous formulation, their dissolution in biological fluids is required before drug uptake by target cells and tissues. This may necessitate the use of excipients such as solubilizers that increase drug solubility, but they can also affect product attributes such as viscosity. These solubilizers can be irritating to the mucosa and exhibit toxic effects on the epithelium. pH-dependent solubility is also another aspect to consider. A drug molecule, once released from the delivery vehicle, should remain dissolved in order to access the target tissues and/or cells. The formulation can be buffered to provide necessary pH for drug solubilization, however, the impact of such a modification on innate antimicrobial potential should be considered. The pKa of a drug impacts its ionization state in the vagina and rectum. Ionized drugs are more soluble, but their tissue and cellular uptake reduces because they are charged (Figure 2). In such cases, pH of the preparation can be altered. Physical modifications such as amorphization can also be used to increase drug solubility. However, amorphous drugs can convert to a more stable crystalline form during storage and use, posing stability issues. It should be noted that both water soluble and hydrophobic drugs have been delivered in the vaginal environment effectively using gels, but the latter may necessitate the use of additional processing steps and/or formulation additives. The delivery of more than one active molecule may further increase the complexity of the product development, especially those drugs that display different physicochemical properties. Previously, the combination of water-soluble TFV and hydrophobic UC 781 has been reported as a gel formulation [70]. Similarly, TFV and DPV were incorporated efficiently in a film formulation [73].
The labile nature and large size of macromolecules such as probiotics, proteins, and monoclonocal antibodies (mAbs) introduce additional hurdles to their formulation. Despite these challenges, candidate topical microbicide products containing macromolecules have been developed. Examples of such products include RC101 films, RANTES nanoparticles, griffithsin (GRFT) nanofibers, mAb gels, and lactobacillus jensenii (releases modified cyanovirin-N) containing vaginal disintegration tablets [74–77]. Creative strategies are required to successfully develop formulations for these problematic drug candidates. In some cases, they may need to be altered in order to increase their stability either in the formulation or in the dynamic rectal or vaginal environment. Lactobacilli have been formulated into several dosage forms that utilized stabilization through specialized lyophilization processes and other processes such as preservation by vaporization [78, 76]. Alternatively, labile macromolecular drugs can be packaged into nanoparticles for targeting and intracellular uptake [75]. Macromolecules have low diffusion rates and hence may require longer times to penetrate the epithelium and reach the target immune cells. This may necessitate the use of excipients in the product that increase retention and aid in cellular uptake, such as mucoadhesive polymers.
The chemical and structural makeup of ARV drugs could make them susceptible to degradation/metabolism and potential inactivation. Factors such as metabolizing enzymes (e.g. aminopeptidases and cytochrome P450), transporters, and pH which can affect drug effectiveness should be considered. Recent findings regarding TFV uptake by vaginal microbiota [26] indicate that products with increased dose may need to be prepared in order to maintain the protective concentrations in the biological milieu.
2.4. Manufacturing, scale up, and economic aspects
The manufacturability and scale-up of on-demand products is a crucial factor that needs to be thoroughly investigated during early product development stage. Different dosage forms present different sets of manufacturing challenges. Table 4 shows the unit operations involved in the manufacture of different on-demand microbicide products and their scale-up considerations. The lack of equipment at cGMP-compliant production scale or increased production costs to employ specialized equipment can negatively affect the product cost. For example, products that involve simple and well-understood mixing procedures are easier to scale up compared to those that utilize heat and size reduction methods such as drying and sonication.
Table 4.
Scale-up considerations of different on-demand microbicide products
| Product | Unit Operations | Scale up considerations | Potential problems |
|---|---|---|---|
| Solutions | Mixing |
|
Content uniformity |
| Ointments | Size reduction and Mixing |
|
Content uniformity |
| Gels | Mixing |
|
Content uniformity and viscosity due to inefficient polymer wetting under mixing |
| Films | Solvent cast: Mixing, Coating, and Drying Hot melt extrusion: Size reduction, Dry powder mixing, Extrusion, and Shaping |
|
Uniform drying, precipitation of polymers, solid state changes, and content uniformity |
| Tablets/Inserts | Traditional: Mixing, Granulation, Milling, and Compaction Freeze dried: Lyophilization |
|
Undesirable solid state changes, content uniformity, friability, and hardness |
| Suppositories | Heating/Melting, Mixing, and Moulding |
|
Content uniformity and mechanical properties |
Scalability of a manufacturing process requires a thorough understanding of the process and product variable interactions at different scales. For example, in a mixing unit operation, variables such as geometry of mixing vessel, paddle, and homogenizer, mixing speed, temperature, and order of addition can impact drug content uniformity [79]. These effects are scale-dependent and direct linear translation from laboratory scale to production scale is challenging. Importantly, dispersion of hydrophobic drugs in an aqueous environment can be quite challenging. Laboratory techniques can utilize particle size reduction methods such as sonication, to disperse the hydrophobic drugs, however, these methods may not be viable in a large-scale setting.
Large-scale production of nanofibers can be challenging due to the need to optimize a large number of process variables and significant differences in laboratory and production scale equipment. The low throughput of fiber production, generally in grams per hour, is also another limitation. However, different formats of electrospinning such as multi-nozzle, free surface and centrifugation based formats are expected to increase the throughput to kilograms per hour [80]. Krogstad and Woodrow [80] have shown a feasibility scale-up study of TFV quick dissolving fibers from a laboratory needle instrument (mg/h) to a production scale (g/h) wire instrument. The critical quality attributes (CQAs) such as fiber morphology, drug loading and release were comparable between both scales, showing scale up feasibility.
One of the critical processing operations important for scale up of polymeric thin films manufactured using solvent casting technique is drying. Film preparation at the laboratory scale generally utilizes drying in a vacuum oven or heating from the bottom of the surface on which polymer is coated. However, production-scale coating machines are equipped with heated air for drying, which is applied from the top. This difference in heating can significantly affect the drying process leading to differences in residual solvent and possibly tactile properties. Due to the differences in drying, abnormalities such as blushing, which is formation of white specks on the film, can be observed at the production scale but not in the laboratory. These challenges may preclude use of certain polymers or give rise to challenges in obtaining tactile properties similar to those achieved at the laboratory scale.
Differences in material attributes such as particle size, residual solvents, and polymer viscosity due to changes in lots and manufacturers can cause significant challenges during scale up and can potentially affect batch-to-batch consistency. Currently, there is a paradigm shift in pharmaceutical manufacturing from batch to continuous format. The salient features of continuous manufacturing such as reduced number of steps, in-process quality control, and real time release are expected to reduce product cost. Microbicide dosage forms such as tablets, gels, and films are amenable for continuous manufacturing.
An important component of a microbicide product are the excipients. Excipients in microbicide products have functional roles that impact rheology, bioadhesion, pH, visual appeal, and tactile properties. Moreover, since excipients make up the bulk of the product, they should not negatively impact product efficacy. At the minimum, a well characterized excipient from the FDA’s GRAS (Generally Regarded as Safe) list should be used. Of note, this does not guarantee safety because the long-term effect of these excipients might not have been studied in the vaginal and rectal environment. Novel drug delivery systems such as nanoparticles that utilize specialized polymers may not be amenable for scale up, primarily, because the polymer(s) cannot be acquired from a commercial source. Unavailability of toxicological characterization of newer excipients is another factor that will impede utilization of these excipients and advancement for human use.
Successful uptake of a microbicide product depends on its cost. Especially, in resource-poor countries, cost is a major determining factor. Some of the major costs associated with microbicide products include manufacturing costs of the product and applicator (if needed), packaging, shipping, and distribution. In order to reduce shipping costs, it is ideal to manufacture the product closer to the site of consumption. A previous cost-analysis study for on-demand TFV gels identified that manufacturing costs were the major contributor to the total price of the microbicide product [81]. In the clinical trial scenario, 51% of the overall cost was attributed to the product cost when the unit price of TFV gel was assumed to be $0.25. For a production scale, a $0.13 per dose projected cost was estimated when a multi-use tube and a reusable applicator was considered. However, the price increased to $0.20 and $0.66 depending on whether single use paper or plastic applicators were included in the analysis, indicating the impact of an applicator on the product price. Of note, although paper applicators and multi-use tubes are cost effective, their use may be prohibited by user acceptability. This projected cost may be further increased for dual compartment products that utilize a separate applicator for vaginal and rectal administration. Development of dual-use applicators is therefore a cost-effective alternative. Production volume also dictates the product costs. The cost of the 1% TFV gel used in FACTS 001 clinical trials was $1.67. However, the cost can be significantly reduced to $0.17 when large production volumes (millions of units) is considered [81]. Polymeric films may be a cost-effective alternative to dosage forms that require applicators such as gels and enemas. Although a systematic cost-analysis is not reported for microbicide films, several factors that can contribute to lower costs of films include smaller foot print, multi-dose packaging, and lack of applicator. The cost of specialized dosage forms such as nanofibers can be high. Blakney et al. estimated that a unit dose of nanofibers weighing 300 mg would cost $0.5–$3.00 at production scale [82]. The cost of a product also depends on excipients and API. Products with widely used, easily available, and low cost GRAS category excipients can have reduced cost. Products with macromolecules such as proteins, mAbs, and nanoparticles may not be cost effective due to high costs involved in synthesis and purification, compared to small molecule drugs. However, efforts are underway to develop cost-effective synthesis (e.g. plant produced), purification, and extraction procedures for biologics and other macromolecules [83].
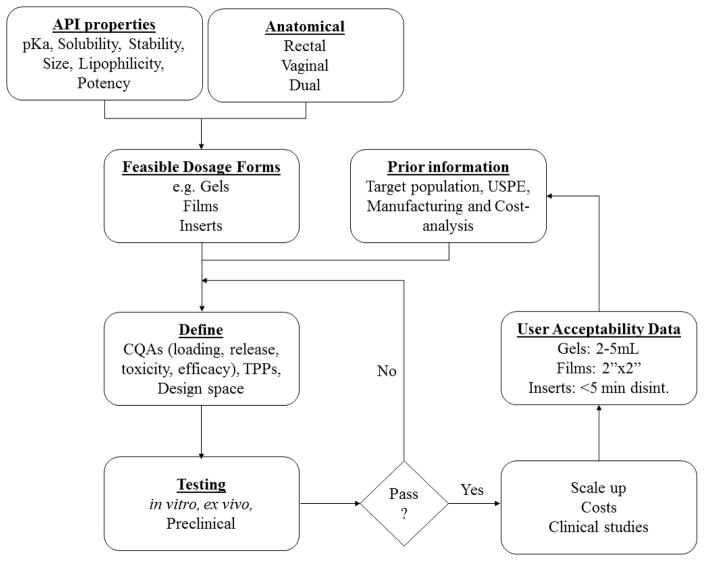
In summary, unlike several other pharmaceutical applications, development of microbicide products is increasingly complicated due to the factors discussed in the above sections. Nevertheless, it is imperative that these considerations for product development efforts be rationalized through a solid scientific framework and practical feasibility assessment. Before developing products, it is prudent to conduct a theoretical exercise to arrive at feasible dosage forms, which takes into account all influencing aspects. Figure 3 shows a putative theoretical framework for microbicide product development.
Fig. 3.
A putative theoretical flow diagram showing the influence of various factors on microbicide product development and optimization
3. On-demand vaginal, rectal, and dual compartment dosage forms
Initial topical PrEP products utilized surfactants, buffering gels, and polyanion-based products, which failed to show efficacy [84]. The next generation products employed potent anti-HIV compounds with specific mechanisms of action. In this review, we focus on several on-demand vaginal dosage forms utilizing a variety of ARV drug classes such as entry inhibitors, fusion inhibitors, protease inhibitors, integrase inhibitors, and reverse transcriptase inhibitors.
Before discussing the details of each dosage form, here we describe important criteria to be considered for a safe and effective on-demand product. Given that on-demand products need to deliver drugs in sufficient quantities to inactivate the virus within a short span, drug release from the product and its dissolution becomes highly crucial. An immediate release dosage form can be ideal for this purpose. However, in order for the product to be effective, drug levels in the mucosal tissues, cells, and fluid may need to be sustained for longer periods of time after sex. This requirement could be a characteristic of the active moiety itself, which will depend on its physicochemical properties and susceptibility to vaginal milieu. Alternatively, it can be controlled by utilizing appropriate design of the dosage form to achieve continuous drug release and longer product retention for sustained protection after the sexual act. Additionally, it is important for the product to distribute throughout the vaginal and rectal mucosal surface, so no vulnerable sites are exposed. The product needs to have minimum impact on the innate factors such as pH, innate protective factors, and microflora. The product should not exhibit irritation or any deleterious effect on the epithelium, which could increase the susceptibility to infection. In addition, the product should be easy to administer (not messy or leaky), discreet, low cost, and not negatively interfere with pleasure. Several dosage forms can achieve these characteristics to different extent. Therefore, the choice of the dosage form significantly depends on satisfying the criteria that affects its efficacy and safety, at the same time maintaining user acceptability. The following sections will focus on the products developed thus far, highlighting their advantages and limitations with respect to on-demand use.
3.1. Solutions
Pharmaceutical solutions are products in which ingredients (solutes) are homogeneously dissolved (molecularly dispersed) in a solvent, generally an aqueous phase. Microbicide solution formulations include enemas or douches. A typical formulation of an enema includes the drug, solubilizers, thicknening agents, tonicity-adjusting agents such as sodium chloride, and preservatives. The product attributes such as dose, pH, and osmolality can affect the safety, efficacy, and acceptability of this product. Hendrix and colleagues compared colorectal distribution of radiolabeled isoosmolar (294 mOsm/kg), hyperosmolar (2100 mOsm/kg), and hypoosmolar (0 mOsm/kg) TFV enemas [57]. Although all products showed similar acceptability, the isoosmolar enema showed better colonic distribution. Moreover, the hyperosmolar enema induced epithelial sloughing, and the hypoosmolar enema resulted in increased permeability and systemic levels of TFV. TFV and its prodrugs have been formulated into enemas [85]. Villinger et al. [86] compared isoosmolar and hypoosmolar enema formulations of tenofovir disoproxil fumarate (TDF) at 1.76 and 5.28 mg/ml in monkeys after single dose rectal administration. The tissue drug concentration was higher with the hypoosmolar enema compared to iso-osmolar formulation at the initial time points evaluated. Importantly, TFV-diphosphate level in CD4+ cells was significantly higher with the hypoosmolar enema. The hypoosmolar high dose enema was also effective in preventing HIV infection by SHIV/SIV. Although no toxic effects were observed in this study, clinical applicability of this formulation will depend on a multi-dose safety assessment. A clinical study comparing the safety, acceptability, and PK of enemas with varied osmolality is planned (Table 1).
3.2. Ointments and Creams
Ointments are oleaginous preparations where the drug is either dispersed or dissolved in the base. Ointments can provide greater retention and less leakiness compared to solutions or hydrogels. However, some ointment bases can be sticky, retained for longer times, and not easily removed with washing. These attributes may potentially result in decreased product acceptability. Terameprocol vaginal ointment has been tested for safety and PK in clinical studies [87]. Although encouraging results were obtained, this product was not further investigated because patients preferred a clear hydrogel compared to a white ointment.
Creams are emulsion-based preparations containing oil and water phases. Vaginal creams have been used to deliver antimicrobial agents, antifungal agents, and hormones. One of the challenges of cream-based formulations is drug dispersion and long-term stability. Since creams are a dispersion of immiscible phases, they tend to destabilize by phase separation. Similar to hydrogels, creams can be leaky, cause messiness, and require an applicator for administration. Although microbicide creams have not been reported in the literature, they offer a viable platform for many ARV drugs.
3.3. Gels
To date, gel products represent the most widely studied topical microbicide dosage form for on-demand use. Antimisiaris and Moutas have previously summarized the various gel preparations evaluated in clinical safety and efficacy studies [88]. Gels, also referred to as hydrogels, are semisolid preparations defined as a hydrophilic network of water absorbent polymers dispersed in water. There are several advantages associated with the use of gels for vaginal or rectal drug delivery. They are relatively easy to prepare and scale up. A wide variety of ARV drugs can be incorporated into gel platforms including hydrophilic, hydrophobic, and large biomolecules [72, 18, 42, 7, 9, 43, 10, 74]. Due to their lubricating property, on-demand application of a gel product prior to intercourse may be preferred in those individuals currently using sexual lubricants. This lubricating property could also help reduce abrasions caused during intercourse. Gels with suitable attributes can be applied for both rectal and vaginal use [89].
Typically, gel preparations are composed of gelling agents such as carbomers (carbopol) and hydroxy ethyl cellulose (HEC), pH modifying agents, glycerin, and preservatives. Key product attributes including viscosity, osmolality, pH, and administration volume can impact the acceptability, safety, and efficacy of gel products. As discussed previously, the osmolality of a gel preparation can affect epithelial integrity, extent of distribution, and cellular uptake. An important aspect of microbicide gels that has been widely investigated through experimental and computational modeling is their rheology. The flow of gel product has a significant impact on its efficacy because vaginal spreading, retention, and drug release is directly dependent on this property [64, 30, 90, 65]. It is therefore essential to incorporate rheology as part of the gel product design. Importantly, a gel product’s rheology needs to be evaluated during different stages of product use i.e. release from the applicator, spreading within vagina and rectum, leakage and spreading under squeezing forces and in the presence of semen and varying amount of vaginal fluid. Katz and group have shown through computational modeling that the product volume applied as well as its rheological properties greatly affect vaginal product distribution, mucosal coverage, and leakage [64, 30, 65]. The discussion in section 2 has focused on acceptability parameters and their relation with gel rheological properties. A combination of these aspects should drive gel development efforts.
There are several gel products currently under investigation (Table 1). Of these, gels incorporating macromolecules such as proteins, multipurpose technology (MPT) products that combine HIV and other STI prevention, and dual compartment products, represent some of the most recent activities within this class. A combination microbicide gel containing MIV-150 and zinc acetate formulated in a carrageenan gel showed efficacy against HIV-1, HSV-2, and HPV in macaques after vaginal and rectal challenges [91]. A phase-1 study showed that this product is safe, and the cervicovaginal lavages (CVLs) show in vitro activity against HIV-1 and HPV [16]. In order to protect women who engage in vaginal sex and RAI in the same sexual encounter, Ham et al. developed dual compartment gels (DuoGel™) [90]. A series of IQP-0528 dual compartment gels were prepared and an optimum formulation satisfying a defined TPP was selected. The attributes of DuoGel™s such as osmolality (256–271 mOsm/kg) and pH (6.0) were favorable for rectal and vaginal compartments. The selection of candidate DuoGel™ was based on the drug permeability and spreading behavior that was theoretically computed as a combination of better distribution in the rectum and less vaginal leakage. In a subsequent study [92], the candidate DuoGel™ (IQB-3002) was evaluated in rhesus macaques after vaginal administration and shown to deliver effective drug concentrations required for HIV-1 inhibition even with a reduced macaque dose of 1.5 mL. Dual chamber gels are promising, but the clinical acceptability, safety, and efficacy remains to be tested.
Because gels are generally designed to be retained for a short time in the vagina, most of the gel products developed thus far have been for on-demand use. However, thermogelling and bioadhesive gels have been investigated, which could provide sustained retention and long-term protection [93]. Despite the issues concerning leakiness and messiness, hydrogels are being investigated for topical delivery of ARV drugs either by the vaginal or rectal route.
3.4. Inserts (Suppositories, Tablets, and Ovules)
Suppositories are solid dosage forms and they are prepared using bases that either melt at body temperature (lipid-based; e.g., Whitepsol) or dissolve in the vagina or rectum (hydrophilic; e.g., polyethylene glycol). Suppositories are easy to manufacture and have a high drug loading capacity. Fat and water-soluble bases can be utilized to incorporate hydrophilic and hydrophobic drugs, including macromolecules such as proteins. The major disadvantage of suppositories is their stability especially during storage and shipping in tropical environments where temperatures can reach as high as 50 °C. In such cases, bases that dissolve rather than melt can be utilized. In addition, in an acceptability study, women showed low preference for suppositories due to perceived messiness and interference with sex. However, suppositories have a long-standing history of use for rectal administration of drugs. The efficiency and extent of rectal distribution achieved with ARV suppositories remains to be tested. Suppositories for rectal administration incorporating either TFV or the integrase inhibitor MK-2048 have been recently reported [94, 95]. The TFV suppository was shown to have favorable release and high TFV-DP concentration was found in the rectal fluid and tissues of nonhuman primates post administration, demonstrating the clinical potential of this delivery system. Vaginal suppositories have been previously used to deliver probiotics for general vaginal health. Microbicides formulated into vaginal suppositories have also been reported [96]. Zaveri et al. has shown incorporation of TFV into semisoft vaginal suppositories prepared using a carrageenan base, which offered evidence of anti-HIV activity [96]. Similar to gels, suppositories can be prepared as dual compartment products. Recently, a dual compartment IQP-0528 suppository with a bullet shape and fast melting time (2 minutes) was reported [97]. These suppositories showed faster disintegration (two times quicker) in vaginal fluid simulant compared to rectal fluid simulant. However, the osmolality was higher post disintegration (3104 mOsm/kg) and warrants further investigation to assess any effects on the epithelial integrity. The product attributes such as shape, size, firmness, melting, disintegration, and dissolution can affect the acceptability of suppositories.
Tablets are one of the oldest and most widely used solid dosage form for oral application. Typical components of tablets include bulking agents (e.g. lactose), disintegrating agents, and manufacturing aids (e.g. magnesium stearate). They have been vaginally used for applications involving both local and systemic action. Since oral delivery of anti-HIV drugs is primarily through the use of tablets, their translation to the vaginal route brings with it a sense of familiarity. The advantages of tablets include well-understood scale up and manufacturing processes, wide availability in resource-poor settings, and cost effectiveness. However, tablets have not been considered highly acceptable dosage forms vaginal applications due to the tendency to leave behind granules that can leak, be uncomfortable, and interfere with sex. Rapidly disintegrating vaginal tablets as microbicides have been reported [98–100]. Clark et al. and Periera et al. reported rapidly disintegrating vaginal tablets combining TFV and emtricitabine, which achieved similar drug levels as the 1% TFV gel after vaginal administration [98, 101]. There were no toxic effects on vaginal microflora. These tablets were designed to disintegrate in vivo in less than 30 minutes, and demonstrated an in vitro disintegration time of < 2 min. Tablets have also been applied as MPT products. McConville et al. developed multilayer vaginal tablets incorporating DPV, acyclovir, and levenorgestrel for combined contraception, HIV and STI prevention. This study showed the feasibility of in vitro release modification of each drug.
CONRAD (Contraceptive Research and Development) is developing fast disintegrating vaginal inserts. Results presented at HIV R4P 2016 described an improved fast disintegrating insert, which was optimized with respect to disintegration time based on user feedback. The first sign of in vivo disintegration was observed at 7 minutes versus 37 minutes for previous inserts. However, time to complete disintegration was still undesirable, (around 90 minutes). Fourteen of 48 participants showed leakage and some women mentioned that leakage could affect their future use. Due to the improved disintegration time, 75% of women liked the product as opposed to 46% in the previous study. In another study presented by CONRAD, freeze-dried (FD) vaginal inserts were developed using an oral FD (Zydis®) technology. These inserts were found to disintegrate completely in less than 30 minutes in vivo. TFV containing FD inserts were non-toxic, showed similar drug permeability as 1% TFV gel in an ex vivo model, and they were amenable to co-formulation of TDF and elvitegravir. In an acceptability study, all participants (n=12) had a favorable opinion of the product. Using a similar approach, researchers at PATH and Population Council are investigating FD GRFT inserts that readily disintegrate (< 90 secs) in VFS and form an in situ gel with comparable in vitro anti-HIV activity to reference standard. Fast disintegrating vaginal inserts represent a viable alternative to vaginal tablets and suppositories due to their portability and their favorable disintegration profile. However, due to the technology involved in manufacturing these inserts, their production and cost need further evaluation.
3.5. Films
Polymeric thin films or strips are a solid dosage form that combine the properties of semisolid and solid products. Films are a network of polymer(s) entanglements with the drug either dispersed or dissolved in the matrix. There are several advantages of vaginal films such as portability, low preparation cost, discrete use, minimal interference with sex, and low product volume [102]. Vaginal films can be manufactured using solvent cast or hot-melt extrusion methods [103, 104]. The major ingredients of film dosage forms include film forming polymer(s), release modifying polymer(s), disintegrating agent(s), pH modifiers, humectants, and plasticizers. Several studies comparing films and other dosage forms such as gels, soft gel capsules, suppositories, and tablets have shown preference for films by most women [105, 17, 106]. Size, texture, color, dryness/stickiness, dissolved versus dispersed drug, disintegration, and drug loading can affect acceptability and efficacy of film products.
Studies have been conducted to assess safety, acceptability, PK and ex vivo efficacy of vaginal films in women. In these studies, DPV vaginal film and gel showed a comparable drug release and genital tract distribution. However, the film affected the innate vaginal components [107] to a lesser extent than the gel [17, 18]. Films, in general, are proven to provide similar protection as gels in ex vivo challenge models [18]. Although films generally have high drug loading capacity (~50%), given the small volume for film dosage forms the total amount of drug that can be incorporated may be limited. Dosing amount and restrictions on dosage size could be a limitation for film dosage forms. A wide array of physicochemically diverse ARV drugs have been incorporated into films namely TFV [73, 70], DPV [17, 73, 108], EfdA [109], efavirenz [110], RC-101 [77], VRC01 anti-HIV mAb [111], and si-RNA and nanoparticles [112].
To date, microbicide films were formulated as a coitally-dependent product due to their rapid disintegration time. Currently they are being explored for extended release of an integrase inhibitor for weekly administration (FAME, AI120249). The versatility of the film platform allows for modification of drug release profile and disintegration. Further a range of drug candidates can be incorporated into films including both small molecule hydrophilic and hydrophobic drugs, protein and peptide drugs, and probiotics. Films have not been investigated for rectal delivery.
3.6. Nanofibers
Nanofibers are nanometer sized polymer fibers that can be produced in three dimensional structures such as meshes, sheets, and tubes [82]. Microbicide nanofibers have been manufactured using electrospinning methods, where electrostatic forces draw the charged polymer fluid into thin fibers. Nanofibers exhibit high surface area, tunable porosity, and functional properties. Because nanofibers can be formatted into various three dimensional structures, they can be theoretically used for rectal and dual compartment administration.
The utility of nanofibers in the microbicide field is relatively new and several preclinical studies have been reported. A typical nanofiber formulation contains drug, polymers (e.g. PVP and PEO) and a suitable solvent (e.g. ethanol and water). Specific excipients to manipulate the charge, release, and tactile properties can be included in the formulation mixture. Process and material variables including solution flow rate, voltage, viscosity and composition of the polymer mixture can be varied to produce products with a multitude of properties [113–115]. For example, the release-modifying polymer, poly-L-lactic acid, has been incorporated in the formulation mixture to tune drug release [116, 114]. Nanofibers can be prepared in a core-shell format with the release-modifying polymer in the shell, similar to enteric-coated tablets. Recently, Ball et al. reported coaxial electrospun core-shell nanofibers with tunable drug release. Nanofibers can be loaded with high drug amounts, up to 60% w/w [114]. Availability of a wide variety of materials that can be used in electrospinning-based nanofiber production renders this technique useful for microbicide applications.
Several nanofiber formulations incorporating ARV drugs such as maraviroc, AZT, acyclovir, cyclodextrins, etravirine, TFV, and GRFT have been reported [116–118]. Nanofibers have also been applied as a combined barrier and chemical method for HIV prevention and contraception [116]. Additionally nanofibers have been engineered to release ARVs under stimuli such as pH. Huang et al. reported cellulose acetate phthalate nanofibers that dissolve at basic pH in the presence of semen. Bi-botti and colleagues recently reported electrospun nanofibers using a thiolated hyaluronic acid polymer for stimuli-sensitive dug release [119]. This novel platform released more than 80% of its payload within 1 h in the presence of seminal hyaluronidase. Grooms et al. loaded GRFT on the surface of adhesive fibers to impede HIV-1 entry into the epithelium via physical and chemical barriers [120]. This study showed the utility of fibers for biomolecule incorporation and the ability to introduce physicochemical modifications. Although studies demonstrated the feasibility of incorporating ARVsin nanofibers, clinical realization is still limited due to the lack of cGMP facilities for large-scale production. Although there is limited acceptability data available for nanofibers, since nanofibers can be delivered in a film format, in addition to an acceptable tube-like format, the data from film acceptability studies can be extrapolated and utilized. Specific product attributes that are preferred by users based on perception scores could lead to the development of nanofibers with optimized TPPs.
3.7. Nanomedicine
Nanoparticle-based novel dosage forms are being introduced into the microbicide field, especially for vaginal delivery. In this section, the application of nanomedicine pertaining to on-demand dosage forms is discussed. Interested readers can access reviews published in this area [121, 122]. Nanomedicine or nanoparticles are structures with size less than 1000 nm in each direction. Nanomedicine formulations have been used in the biomedical field for drug delivery and diagnostic imaging [123]. Nanomedicine is a broad term encompassing different categories including liposomes, nanoemulsions, micelles, solid lipid nanoparticles, polymeric and inorganic nanoparticles, and nanoshells. Some nanoparticles may contain intrinsic anti-HIV activity such as dendrimers [124].
In topical anti-HIV products, nano-based approaches have been applied for several reasons which include increased drug solubility, increased cellular uptake, protection from biological factors (e.g., metabolizing enzymes), increased stability, drug targeting, mucus and tissue penetration, and alteration of mucoadhesion and drug release [121]. Nanoparticle technology can address barriers to successful protection from on-demand products. These include protection of labile drugs, retention in the vagina, prolonged activity, intracellular concentration, and tissue permeability. For example, mucus-penetrating nanoparticles have been developed to increase vaginal retention and distribution. When compared with drug delivered through a traditional gel, these nanoparticles showed better epithelial coverage [125]. A significant advantage of nanoparticles will be to increase target tissue and cell concentrations as well as targeting to specific cells. This aspect was shown in vitro using poly(lactic-co-glycolic acid) nanoparticles encapsulating a microbicide candidate, PSC-RANTES [75]. Compared to unformulated drug, a 5-fold increase in tissue uptake was observed with nanoparticles. Although not investigated in this study, encapsulation in the nanoparticles could potentially protect the drug in the vaginal environment. Recently, nanolipogels containing maraviroc and TDF were fabricated [126]. These nanolipogels contain nanoparticles with a hydrogel-core and lipid-shell. Additionally, nanoparticles have been combined with traditional dosage forms such as gels [93] and films [110]. One of these platforms, VivaGel™, which is a dendrimer-based gel, has shown acceptability and high activity against HIV-1 and HSV-2 in human studies [124].
Although the drug delivery benefits of nanoparticles are evident, safety must be investigated thoroughly. For example, the high surface activity of nanoparticles may give rise to cell lysing properties, so their effect on the vaginal epithelium and protective lactobacilli must be evaluated. Nanoparticles should also be assessed for their immunomodulatory effects, which can significantly impact toxicity and efficacy. In addition to using well-characterized excipients, simple and scalable processing methods can help realize scale-up of vaginally-applied nanoparticles.
4. Future perspectives
In line with the developed relationship between microbicide gel acceptability (USPE) and biophysical properties, there is a need to develop similar associations for other on-demand products. These studies have been initiated for vaginal films, inserts, and rectal suppositories (Table 1). These relationships, in conjunction with the factors relating to safety, efficacy, cost, manufacturing and scale up feasibility, should dictate product design efforts. It is also necessary to introduce marketing and educational programs for products, such as instructional videos, in order to increase awareness and acceptability. These programs are important to increase the uptake of a specific product that is biologically safe and effective, especially in the setting where acceptability may be less favorable due to perceived limitations.
The impact of microbicides on the microbiota, glycome and other innate factors must be explored. There is a need to determine if a relationship between these innate factors exists and, if so, the implications for HIV transmission and vaginal or rectal health. In order to delineate adherence and innate physiological differences contributing to the observed efficacy, there is a need to develop novel objective adherence markers. Efforts have begun in this area. Drug-free adherence markers are being explored for gels, films, rings, and inserts. Towards this end, CONRAD and Northwestern University researchers developed HEC and glycerin specific spectroscopic assays to detect polymers in vaginal swabs from participants using gels and films [127]. The sensitivity of the assay showed 70–100% detection up to 12 h post-administration. Efforts are also underway to develop markers for long-acting rings. Approaches include changes in the ring spectral profile and composition that are dependent on the duration of use. These encouraging studies suggest that adherence can be measured using non-invasive and potentially cost-effective assays. This quantitative data combined with user reported adherence can shed light on measuring true adherence and efficacy relationships. Going forward, there is a need to develop more sensitive and specific assays that can detect small levels of the analyte, which gives flexibility to measure levels long after product administration. There is also a need to develop analytical methods to assess properties associated with ease of administration and product use (e.g. tactile properties) because clinical or focus group based acceptability studies are not cost-effective in the early stages of product development. Research on alternate manufacturing methods to aid in easy scale-up and lower production costs should be investigated. Product development efforts should be in-line with current FDA guidances and vision. The use of quality-by-design (QbD) approaches to understand the manufacturing process and obtain quantitative relationships between product attributes and process and formulation variables should be intensified. Further, the incorporation of continuous manufacturing aspects can reduce production costs.
To increase the future market use of microbicide products, potential users must be presented with a range of products which potentially have combination indications. Therefore, research and development efforts to fabricate novel dosage forms is required. Additionally, MPTs combining HIV prevention with contraception or prevention of other STIs such as HSV-2 and HPV must be investigated. Combination products that may indirectly affect anti-HIV efficacy such as those combining ARV drugs and probiotics to reduce BV should also be explored. Since inflammation is linked to an increased risk of HIV acquisition, products combining anti-inflammatory drugs and ARV drugs may be beneficial. Since vaginal and anal sex can occur within a single sexual act, dual compartment products can provide women with increased opportunity for protection and warrant further research efforts.
The development of microbicides is a complex endeavor impacted by a multitude of qualitative and quantitative interacting variables. Identification and association of these variables with product attributes is essential to develop useful products. Specifically, the impact of user perceptions, physicochemical properties, cost, and scalability must be realized and incorporated into product development plans. It is clear that no single product caters to the needs of all women. This demands provision of a broad portfolio of microbicide products in order to increase user acceptability and compliance which directly correlates with product efficacy, and ultimately contributes to the end goal of eradicating new HIV infections.
Acknowledgments
The authors are very thankful to Dr. Lindsay Ferguson Kramzer for critical reading and useful suggestions to improve the quality of this review.
Footnotes
Conflicts of Interest
Lisa C. Rohan declares that she has no conflicts of interest. Sravan Kumar Patel declares that he has no conflicts of interest.
References
- 1.HIV/AIDS Data and Statistics. World Health Organization; 2017. [Accessed January 12 2017]. http://www.who.int/hiv/data/en/ [Google Scholar]
- 2.Higgins JA, Hoffman S, Dworkin SL. Rethinking Gender, Heterosexual Men, and Women’s Vulnerability to HIV/AIDS. Am J Public Health. 2010;100(3):435–45. doi: 10.2105/Ajph.2009.159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HIV AIDS Statistics Overview. Centers for Disease Control and Prevention; [Accessed January 8 2017]. http://www.cdc.gov/hiv/statistics/overview/ [Google Scholar]
- 4.O’Leary A, DiNenno E, Honeycutt A, Allaire B, Neuwahl S, Hicks K, et al. Contribution of Anal Sex to HIV Prevalence Among Heterosexuals: A Modeling Analysis. AIDS and Behavior. 2017:1–9. doi: 10.1007/s10461-016-1635-z. [DOI] [PubMed] [Google Scholar]
- 5.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcolm RK, Lowry D, Boyd P, Geer L, Veazey RS, Goldman L, et al. Pharmacokinetics of a CCR5 inhibitor in rhesus macaques following vaginal, rectal and oral application. J Antimicrob Chemother. 2014;69(5):1325–9. doi: 10.1093/jac/dkt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees HD-MS, Baron D, Lombard C, Gray G, Myer L, Panchia R, Schwartz J, Doncel G. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women [abstract 26LB]. Program and abstracts of the 2015 Conference on Retroviruses and Opportunistic Infections (CROI); Seattle: CROI2015. [Google Scholar]
- 11.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375(22):2121–32. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med. 2016;375(22):2133–43. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 13.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8(6):447–57. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes R, Sarmento B, das Neves J. Formulation and delivery of anti-HIV rectal microbicides: advances and challenges. J Control Release. 2014;194:278–94. doi: 10.1016/j.jconrel.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Cranston RD, Lama JR, Richardson BA, Carballo-Dieguez A, Kunjara Na Ayudhya RP, Liu K, et al. MTN-017: A Rectal Phase 2 Extended Safety and Acceptability Study of Tenofovir Reduced-Glycerin 1% Gel. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedland BA, Hoesley CJ, Plagianos M, Hoskin E, Zhang S, Teleshova N, et al. First-in-Human Trial of MIV-150 and Zinc Acetate Coformulated in a Carrageenan Gel: Safety, Pharmacokinetics, Acceptability, Adherence, and Pharmacodynamics. Jaids-J Acq Imm Def. 2016;73(5):489–96. doi: 10.1097/QAI.0000000000001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunge KE, Dezzutti CS, Rohan LC, Hendrix CW, Marzinke MA, Richardson-Harman N, et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. Jaids-J Acq Imm Def. 2016;71(5):498–505. doi: 10.1097/Qai.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson JA, Marzinke MA, Bakshi RP, Fuchs EJ, Radebaugh CL, Aung W, et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B) AIDS Res Hum Retroviruses. 2016 doi: 10.1089/AID.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson LM, Rohan LC. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Therapeutic delivery. 2011;2(12):1535–50. doi: 10.4155/tde.11.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 22.Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68(9):2015–25. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. Aids. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. INFECTIOUS DISEASE Vaginal microbiome affects HIV risk. Science. 2016;353(6297):331. doi: 10.1126/science.353.6297.331. [DOI] [PubMed] [Google Scholar]
- 26.Taneva ECS, Cheshenko N, Srinivasan S, Fredricks DN, Herold BC, editors. HIV R4P. Chicago, IL: 2016. Modulation of Tenofovir (TFV) Pharmacokinetics (PK) and Antiviral Activity by Vaginal Microbiota: Implications for Topical Preexposure Prophylaxis. [Google Scholar]
- 27.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. The Journal of infectious diseases. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 28.Villegas G, Calenda G, Zhang S, Mizenina O, Kleinbeck K, Cooney ML, et al. In Vitro Exposure to PC-1005 and Cervicovaginal Lavage Fluid from Women Vaginally Administered PC-1005 Inhibits HIV-1 and HSV-2 Infection in Human Cervical Mucosa. Antimicrobial agents and chemotherapy. 2016;60(9):5459–66. doi: 10.1128/AAC.00392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley C, Kraft C, de Man TJ, Duphare C, Lee H, Yang J, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal immunology. 2016 doi: 10.1038/mi.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Yuan A, Chuchuen O, Ham A, Yang KH, Katz DF. Vaginal deployment and tenofovir delivery by microbicide gels. Drug Deliv Transl Re. 2015;5(3):279–94. doi: 10.1007/s13346-015-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weld ED, Hiruy H, Guthrie KM, Fava JL, Vargas SE, Buckheit K, et al. A Comparative Pre-Phase I Study of the Impact of Gel Vehicle Volume on Distal Colon Distribution, User Experience, and Acceptability. AIDS Res Hum Retroviruses. 2016 doi: 10.1089/AID.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbai NS, Wand H, Ramjee G. Biological factors that place women at risk for HIV: evidence from a large-scale clinical trial in Durban. Bmc Womens Health. 2016:16. doi: 10.1186/s12905-016-0295-5. ARTN 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler K, Ritter JM, Ellis S, Morris MR, Hanson DL, McNicholl JM, et al. A Depot Medroxyprogesterone Acetate Dose That Models Human Use and Its Effect on Vaginal SHIV Acquisition Risk. Jaids-J Acq Imm Def. 2016;72(4):363–71. doi: 10.1097/Qai.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal models for microbicide studies. Curr HIV Res. 2012;10(1):79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu M, Zhou T, Dezzutti CS, Rohan LC. The Effect of Commonly Used Excipients on the Epithelial Integrity of Human Cervicovaginal Tissue. AIDS Res Hum Retroviruses. 2016;32(10–11):992–1004. doi: 10.1089/AID.2016.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gali Y, Delezay O, Brouwers J, Addad N, Augustijns P, Bourlet T, et al. In Vitro Evaluation of Viability, Integrity, and Inflammation in Genital Epithelia upon Exposure to Pharmaceutical Excipients and Candidate Microbicides. Antimicrobial agents and chemotherapy. 2010;54(12):5105–14. doi: 10.1128/Aac.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M, Patel SK, Zhou T, Rohan LC. Drug transporters in tissues and cells relevant to sexual transmission of HIV: Implications for drug delivery. J Control Release. 2015;219:681–96. doi: 10.1016/j.jconrel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grammen C, Baes M, Haenen S, Verguts J, Augustyns K, Zydowsky T, et al. Vaginal Expression of Efflux Transporters and the Potential Impact on the Disposition of Microbicides in Vitro and in Rabbits. Mol Pharmaceut. 2014;11(12):4405–14. doi: 10.1021/mp5005004. [DOI] [PubMed] [Google Scholar]
- 39.Koppolu S, Wang L, Dezzutti CS, Isaacs CE, Moncla B, Hillier SL, et al. Impact of Microbicide Formulation on Glycosylation and Innate Immunity in Cervicovaginal Fluid. The FASEB Journal. 2016;30(1 Supplement):lb120-lb. [Google Scholar]
- 40.Nel A, Haazen W, Russell M, Nuttall J, Van Niekerk N, Treijtel N. Drug-drug Interactions between the Dapivirine Vaginal Ring (Ring-004) and Miconazole Nitrate Vaginal Capsule (Gyno-Daktarin (R)) Aids Res Hum Retrov. 2014;30:A144-A. [Google Scholar]
- 41.Rohan LC, Yang H, Wang L. Rectal pre-exposure prophylaxis (PrEP) Antiviral Res. 2013;100(Suppl):S17–24. doi: 10.1016/j.antiviral.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28(11):1412–21. doi: 10.1089/AID.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007) PLoS One. 2013;8(4):e60147. doi: 10.1371/journal.pone.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carballo-Dieguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability. Results of a volume escalation trial. Sex Transm Dis. 2007;34(4):224–9. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- 45.Katz D, Ham A, Smith J, Guthrie K, Simons M, Gao Y, et al. HIV Research for Prevention (R4P) Chicago, IL: 2016. Do Microbicide Gel Volume and Properties Matter? Effects on Deployment, PK and User Sensory Perceptions and Experiences. [Google Scholar]
- 46.Carballo-Dieguez A, Giguere R, Dolezal C, Bauermeister J, Leu CS, Valladares J, et al. Rectal-Specific Microbicide Applicator: Evaluation and Comparison with a Vaginal Applicator Used Rectally. Aids and Behavior. 2014;18(9):1734–45. doi: 10.1007/s10461-014-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omar RF, Trottier S, Brousseau G, Ouellet C, Danylo A, Ong T, et al. Universal vaginal applicator for the uniform distribution of vaginal gel and cream formulations: a magnetic resonance imaging study. J Obstet Gynaecol Can. 2014;36(1):42–50. doi: 10.1016/S1701-2163(15)30682-4. [DOI] [PubMed] [Google Scholar]
- 48.Gross M, Celum CL, Tabet SR, Kelly CW, Colleti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999;26(10):572–8. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Bauermeister J, Giguere R, Dolezal C, Leu CS, Febo I, Cranston RD, et al. To Use a Rectal Microbicide, First Insert the Applicator: Gel and Applicator Satisfaction among Young Men Who Have Sex with Men. Aids Educ Prev. 2016;28(1):1–10. doi: 10.1521/aeap.2016.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–83. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Primrose RJ, Zaveri T, Bakke AJ, Ziegler GR, Moskowitz HR, Hayes JE. Drivers of Vaginal Drug Delivery System Acceptability from Internet-Based Conjoint Analysis. Plos One. 2016;11(3) doi: 10.1371/journal.pone.0150896. ARTN e0150896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanham M, Wilcher R, Montgomery ET, Pool R, Schuler S, Lenzi R, et al. Engaging Male Partners in Women’s Microbicide Use: Evidence from Clinical Trials and Implications for Future Research and Microbicide Introduction. Aids Res Hum Retrov. 2014;30:A9-A. doi: 10.7448/IAS.17.3.19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE, Hoffman S, Gai F, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008;17(3):383–92. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 54.Giguere R, Carballo-Dieguez A, Ventuneac A, Mabragana M, Dolezal C, Chen BA, et al. Variations in microbicide gel acceptability among young women in the USA and Puerto Rico. Cult Health Sex. 2012;14(2):151–66. doi: 10.1080/13691058.2011.630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008;84(6):483–7. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pines HA, Gorbach PM, Weiss RE, Hess K, Murphy R, Saunders T, et al. Acceptability of Potential Rectal Microbicide Delivery Systems for HIV Prevention: A Randomized Crossover Trial. Aids and Behavior. 2013;17(3):1002–15. doi: 10.1007/s10461-012-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leyva FJ, Bakshi RP, Fuchs EJ, Li LY, Caffo BS, Goldsmith AJ, et al. Isoosmolar Enemas Demonstrate Preferential Gastrointestinal Distribution, Safety, and Acceptability Compared with Hyperosmolar and Hypoosmolar Enemas as a Potential Delivery Vehicle for Rectal Microbicides. Aids Res Hum Retrov. 2013;29(11):1487–95. doi: 10.1089/aid.2013.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galea JT, Kinsler JJ, Imrie J, Nurena CR, Sanchez J, Cunningham WE. Rectal douching and implications for rectal microbicides among populations vulnerable to HIV in South America: a qualitative study. Sex Transm Infect. 2014;90(1):33–5. doi: 10.1136/sextrans-2013-051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Javanbakht M, Murphy R, Gorbach P, LeBlanc MA, Pickett J. Preference and practices relating to lubricant use during anal intercourse: implications for rectal microbicides. Sex Health. 2010;7(2):193–8. doi: 10.1071/Sh09062. [DOI] [PubMed] [Google Scholar]
- 60.Mahan ED, Morrow KM, Hayes JE. Quantitative perceptual differences among over-the-counter vaginal products using a standardized methodology: implications for microbicide development. Contraception. 2011;84(2):184–93. doi: 10.1016/j.contraception.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li BD, Zaveri T, Ziegler GR, Hayes JE. User Preferences in a Carrageenan-Based Vaginal Drug Delivery System. Plos One. 2013;8(1) doi: 10.1371/journal.pone.0054975. ARTN e54975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li BD, Zaveri T, Ziegler GR, Hayes JE. Shape of vaginal suppositories affects willingness-to-try and preference. Antivir Res. 2013;97(3):280–4. doi: 10.1016/j.antiviral.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrow KM, Underhill K, van den Berg JJ, Vargas S, Rosen RK, Katz DF. User-identified gel characteristics: a qualitative exploration of perceived product efficacy of topical vaginal microbicides. Arch Sex Behav. 2014;43(7):1459–67. doi: 10.1007/s10508-013-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Funke C, MacMillan K, Ham A, Szeri AJ, Katz DF. Coupled gel spreading and diffusive transport models describing microbicidal drug delivery. Chem Eng Sci. 2016;152:12–20. doi: 10.1016/j.ces.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katz DF, Yuan A, Gao Y. Vaginal drug distribution modeling. Adv Drug Deliv Rev. 2015;92:2–13. doi: 10.1016/j.addr.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guthrie KM, Vargas SE, Guillen M, Rosen RK, Steger A, Getz ML, et al. HIV Research for Prevention (R4P) Chicago, IL: 2016. Incorporation of Early End-user Feedback into the Iterative Design of Fiber-based Microbicides: Considering Adherence from Inception. [Google Scholar]
- 67.Cook RL, Downs JS, Marrazzo J, Switzer GE, Tanriover O, Wiesenfeld H, et al. Preferred Characteristics of Vaginal Microbicides in Women with Bacterial Vaginosis. J Womens Health. 2009;18(8):1163–7. doi: 10.1089/jwh.2008.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy E, de Padua KS, Hebling EM, Osis MJD, Zaneveld LJD. Women’s preferences for vaginal antimicrobial contraceptives. V: Attitudes of Brazilian women to the insertion of vaginal products. Contraception. 2003;67(5):391–5. doi: 10.1016/S0010-7824(03)00026-X. [DOI] [PubMed] [Google Scholar]
- 69.Anton PA, Saunders T, Elliott J, Khanukhova E, Dennis R, Adler A, et al. First Phase 1 Double-Blind, Placebo-Controlled, Randomized Rectal Microbicide Trial Using UC781 Gel with a Novel Index of Ex Vivo Efficacy. Plos One. 2011;6(9) doi: 10.1371/journal.pone.0023243. ARTN e23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cost M, Dezzutti CS, Clark MR, Friend DR, Akil A, Rohan LC. Characterization of UC781-Tenofovir Combination Gel Products for HIV-1 Infection Prevention in an Ex Vivo Ectocervical Model. Antimicrobial agents and chemotherapy. 2012;56(6):3058–66. doi: 10.1128/Aac.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ventuneac A, Carballo-Dieguez A, McGowan I, Dennis R, Adler A, Khanukhova E, et al. Acceptability of UC781 Gel as a Rectal Microbicide Among HIV-Uninfected Women and Men. Aids and Behavior. 2010;14(3):618–28. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW. Pharmacokinetics of 2 Dapivirine Vaginal Microbicide Gels and Their Safety Vs. Hydroxyethyl Cellulose-Based Universal Placebo Gel. Jaids-J Acq Imm Def. 2010;55(2):161–9. doi: 10.1097/QAI.0b013e3181e3293a. [DOI] [PubMed] [Google Scholar]
- 73.Akil A, Devlin B, Cost M, Rohan LC. Increased Dapivirine Tissue Accumulation through Vaginal Film Codelivery of Dapivirine and Tenofovir. Mol Pharmaceut. 2014;11(5):1533–41. doi: 10.1021/mp4007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris GC, Wiggins RC, Woodhall SC, Bland JM, Taylor CR, Jespers V, et al. MABGEL 1: First Phase 1 Trial of the Anti-HIV-1 Monoclonal Antibodies 2F5, 4E10 and 2G12 as a Vaginal Microbicide. Plos One. 2014;9(12) doi: 10.1371/journal.pone.0116153. ARTN e116153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted Delivery of PSC-RANTES for HIV-1 Prevention using Biodegradable Nanoparticles. Pharm Res-Dordr. 2009;26(3):502–11. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lagenaur LA, Swedek I, Lee PP, Parks TP. Robust Vaginal Colonization of Macaques with a Novel Vaginally Disintegrating Tablet Containing a Live Biotherapeutic Product to Prevent HIV Infection in Women. Plos One. 2015;10(4) doi: 10.1371/journal.pone.0122730. ARTN e0122730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, et al. Formulation Development of Retrocyclin 1 Analog RC-101 as an Anti-HIV Vaginal Microbicide Product. Antimicrobial agents and chemotherapy. 2011;55(5):2282–9. doi: 10.1128/Aac.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bronshtein V. Google Patents. 2016. Preservation by vaporization. [Google Scholar]
- 79.Yu LX. Pharmaceutical quality by design: Product and process development, understanding, and control (vol 25, pg 10, 2008) Pharm Res-Dordr. 2008;25(10):2463. doi: 10.1007/s11095-008-9667-3. [DOI] [PubMed] [Google Scholar]
- 80.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int J Pharmaceut. 2014;475(1–2):282–91. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terris-Prestholt F, Foss AM, Cox AP, Heise L, Meyer-Rath G, Delany-Moretlwe S, et al. Cost-effectiveness of tenofovir gel in urban South Africa: model projections of HIV impact and threshold product prices. Bmc Infect Dis. 2014:14. doi: 10.1186/1471-2334-14-14. Artn 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antivir Res. 2013;100:S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Cerini F, Gaertner H, Madden K, Tolstorukov I, Brown S, Laukens B, et al. A scalable low-cost cGMP process for clinical grade production of the HIV inhibitor 5P12-RANTES in Pichia pastoris. Protein Expres Purif. 2016;119:1–10. doi: 10.1016/j.pep.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexandre KB, Mufhandu HT, London GM, Chakauya E, Khati M. Progress and Perspectives on HIV-1 microbicide development. Virology. 2016;497:69–80. doi: 10.1016/j.virol.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Lyu Y. Development of Tenofovir Prodrugs as Rectal Microbicides for HIV Prevention. University of Pittsburgh; 2016. [Google Scholar]
- 86.Villinger F. HIV Research for Prevention (HIVR4P) Chicago: 2016. Hypo-osmolar Formulation of TFV Enemas Promotes Uptake and Transformation of TFV to TFV-DP in Tissues and Prevents SHIV/SIV Infection. [Google Scholar]
- 87.Khanna N, Dalby R, Connor A, Church A, Stern J, Frazer N. Phase I clinical trial of repeat dose terameprocol vaginal ointment in healthy female volunteers. Sex Transm Dis. 2008;35(6):577–82. doi: 10.1097/OLQ.0b013e31816766af. [DOI] [PubMed] [Google Scholar]
- 88.Antimisiaris SG, Mourtas S. Recent advances on anti-HIV vaginal delivery systems development. Adv Drug Deliv Rev. 2015;92:123–45. doi: 10.1016/j.addr.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemoth. 2012;67(9):2139–42. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ham AS, Nugent ST, Peters JJ, Katz DF, Shelter CM, Dezzutti CS, et al. The rational design and development of a dual chamber vaginal/rectal microbicide gel formulation for HIV prevention. Antiviral Res. 2015;120:153–64. doi: 10.1016/j.antiviral.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnable P, Calenda G, Bonnaire T, Menon R, Levendosky K, Gettie A, et al. MIV-150/zinc acetate gel inhibits cell-associated simian-human immunodeficiency virus reverse transcriptase infection in a macaque vaginal explant model. Antimicrobial agents and chemotherapy. 2015;59(7):3829–37. doi: 10.1128/AAC.00073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pereira LE, Mesquita PM, Ham A, Singletary T, Deyounks F, Martin A, et al. Pharmacokinetic and Pharmacodynamic Evaluation following Vaginal Application of IQB3002, a Dual-Chamber Microbicide Gel Containing the Nonnucleoside Reverse Transcriptase Inhibitor IQP-0528 in Rhesus Macaques. Antimicrobial agents and chemotherapy. 2015;60(3):1393–400. doi: 10.1128/AAC.02201-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Date AA, Shibata A, McMullen E, La Bruzzo K, Bruck P, Belshan M, et al. Thermosensitive Gel Containing Cellulose Acetate Phthalate-Efavirenz Combination Nanoparticles for Prevention of HIV-1 Infection. J Biomed Nanotechnol. 2015;11(3):416–27. doi: 10.1166/jbn.2015.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dobard C, Sharma S, Jhunjhunwala K, CD, Martin A, Holder A, et al. HIV Research for Prevention (R4P) Chicago, IL: 2016. Pharmacokinetic Evaluation of Rectal Tenofovir Suppositories in Macaques. [Google Scholar]
- 95.Jhunjhunwala K, Sant V, Wang L, Graebing P, Marshall L, Cummins J, Jr, et al. HIV Research for Prevention (R4P) Chicago, IL: 2016. Development of a Suppository Dosage Form for the Integrase Inhibitor, MK-2048. [Google Scholar]
- 96.Zaveri T, Hayes JE, Ziegler GR. Release of tenofovir from carrageenan-based vaginal suppositories. Pharmaceutics. 2014;6(3):366–77. doi: 10.3390/pharmaceutics6030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ham A, Katz DF, Patel K, Beveridge E, Hawse A, Peters J, et al. HIV Research for Prevention (R4P) Chicago, IL: 2016. Designing Microbicide Suppositories for Rectal or Vaginal Administration. [Google Scholar]
- 98.Clark MR, Peet MM, Davis S, Doncel GF, Friend DR. Evaluation of rapidly disintegrating vaginal tablets of tenofovir, emtricitabine and their combination for HIV-1 prevention. Pharmaceutics. 2014;6(4):616–31. doi: 10.3390/pharmaceutics6040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McConville C, Major I, Devlin B, Brimer A. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Eur J Pharm Biopharm. 2016;104:171–9. doi: 10.1016/j.ejpb.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Joshi SN, Dutta S, Kumar BK, Katti U, Kulkarni S, Risbud A, et al. Expanded safety study of Praneem polyherbal vaginal tablet among HIV-uninfected women in Pune, India: a phase II clinical trial report. Sex Transm Infect. 2008;84(5):343–7. doi: 10.1136/sti.2007.029207. [DOI] [PubMed] [Google Scholar]
- 101.Pereira LE, Clark MR, Friend DR, Garber DA, McNicholl JM, Hendry RM, et al. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrobial agents and chemotherapy. 2014;58(5):2665–74. doi: 10.1128/AAC.02336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan MD, Kramzer LF, Hillier SL, Chang JC, Meyn LA, Rohan LC. Preferred Physical Characteristics of Vaginal Film Microbicides for HIV Prevention in Pittsburgh Women. Arch Sex Behav. 2016 doi: 10.1007/s10508-016-0816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garg S, Goldman D, Krumme M, Rohan LC, Smoot S, Friend DR. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antivir Res. 2010;88:S19–S29. doi: 10.1016/j.antiviral.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 104.Repka MA, Repka SL, McGinity JW. Google Patents. 2002. Bioadhesive hot-melt extruded film for topical and mucosal adhesion applications and drug delivery and process for preparation thereof. [Google Scholar]
- 105.Nel AM, Mitchnick LB, Risha P, Muungo LTM, Norick PM. Acceptability of Vaginal Film, Soft-Gel Capsule, and Tablet as Potential Microbicide Delivery Methods Among African Women. J Womens Health. 2011;20(8):1207–14. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- 106.Coggins C, Elias CJ, Atisook R, Bassett MT, Ettiegne-Traore V, Ghys PD, et al. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. Aids. 1998;12(11):1389–91. doi: 10.1097/00002030-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 107.Moncla B, Bunger K, Meyn LA, Rohan L, Hillier S. HIV Research for Prevention (R4P) Chicago, IL: 2016. The Differential Effects of Polyvinyl Alcohol Based (PVA) Films and Gels on Vaginal Glycomic Signatures. [Google Scholar]
- 108.Akil A, Parniak MA, Dezzutti CS, Moncla BJ, Cost MR, Li MG, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Re. 2011;1(3):209–22. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang W, Parniak MA, Sarafianos SG, Cost MR, Rohan LC. Development of a vaginal delivery film containing EFdA, a novel anti-HIV nucleoside reverse transcriptase inhibitor. Int J Pharmaceut. 2014;461(1):203–13. doi: 10.1016/j.ijpharm.2013.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cunha-Reis C, Machado A, Barreiros L, Araujo F, Nunes R, Seabra V, et al. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J Control Release. 2016;243:43–53. doi: 10.1016/j.jconrel.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 111.Costanzo CJ. Immune defense of the female lower reproductive tract and the use of monoclonal antibody-based topical microbicide films to protect against HIV infection: SCHOOL OF MEDICINE Thesis IMMUNE DEFENSE OF THE FEMALE LOWER REPRODUCTIVE TRACT AND THE USE OF MONOCLONAL ANTIBODY-BASED TOPICAL MICROBICIDE FILMS TO PROTECT AGAINST HIV INFECTION by COREY J. COSTANZO BS. Boston University; 2015. [Google Scholar]
- 112.Gu J, Yang S, Ho EA. Biodegradable Film for the Targeted Delivery of siRNA-Loaded Nanoparticles to Vaginal Immune Cells. Mol Pharm. 2015;12(8):2889–903. doi: 10.1021/acs.molpharmaceut.5b00073. [DOI] [PubMed] [Google Scholar]
- 113.Teo WE, Inai R, Ramakrishna S. Technological advances in electrospinning of nanofibers. Sci Technol Adv Mat. 2011;12(1) doi: 10.1088/1468-6996/12/1/013002. Artn 013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. Journal of Controlled Release. 2015;220:584–91. doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carson D, Jiang YH, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm Res-Dordr. 2016;33(1):125–36. doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. Plos One. 2012;7(11) doi: 10.1371/journal.pone.0049792. ARTN e49792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ball C, Chou SF, Jiang Y, Woodrow KA. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Mat Sci Eng C-Mater. 2016;63:117–24. doi: 10.1016/j.msec.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang CB, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33(3):962–9. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Agrahari V, Meng J, Ezoulin MJ, Youm I, Dim DC, Molteni A, et al. Stimuli-sensitive thiolated hyaluronic acid based nanofibers: synthesis, preclinical safety and in vitro anti-HIV activity. Nanomedicine (Lond) 2016;11(22):2935–58. doi: 10.2217/nnm-2016-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP, et al. Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold To Prevent HIV Infection. Antimicrobial agents and chemotherapy. 2016;60(11):6518–31. doi: 10.1128/Aac.00956-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.das Neves J, Nunes R, Rodrigues F, Sarmento B. Nanomedicine in the development of anti-HIV microbicides. Adv Drug Deliv Rev. 2016;103:57–75. doi: 10.1016/j.addr.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez-Rodriguez J, Vacas-Cordoba E, Gomez R, De La Mata FJ, Munoz-Fernandez MA. Nanotech-derived topical microbicides for HIV prevention: the road to clinical development. Antiviral Res. 2015;113:33–48. doi: 10.1016/j.antiviral.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 123.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3(2):133–49. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, et al. SPL7013 Gel (VivaGel (R)) Retains Potent HIV-1 and HSV-2 Inhibitory Activity following Vaginal Administration in Humans. Plos One. 2011;6(9) doi: 10.1371/journal.pone.0024095. ARTN e24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, et al. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect Against Herpes Simplex Virus. Sci Transl Med. 2012;4(138) doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramanathan R, Jiang Y, Read B, Golan-Paz S, Woodrow KA. Biophysical characterization of small molecule antiviral-loaded nanolipogels for HIV-1 chemoprophylaxis and topical mucosal application. Acta Biomater. 2016;36:122–31. doi: 10.1016/j.actbio.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adedipe O, Jacot T, Thurman A, Doncel GF, Clark M. Can Infrared Spectroscopy (IR) Coupled with Chemometrics Be Used for Objective Measure of Adherence to Microbicide Products? HIV Research for Prevention (R4P) 2016 [Google Scholar]